Abstract
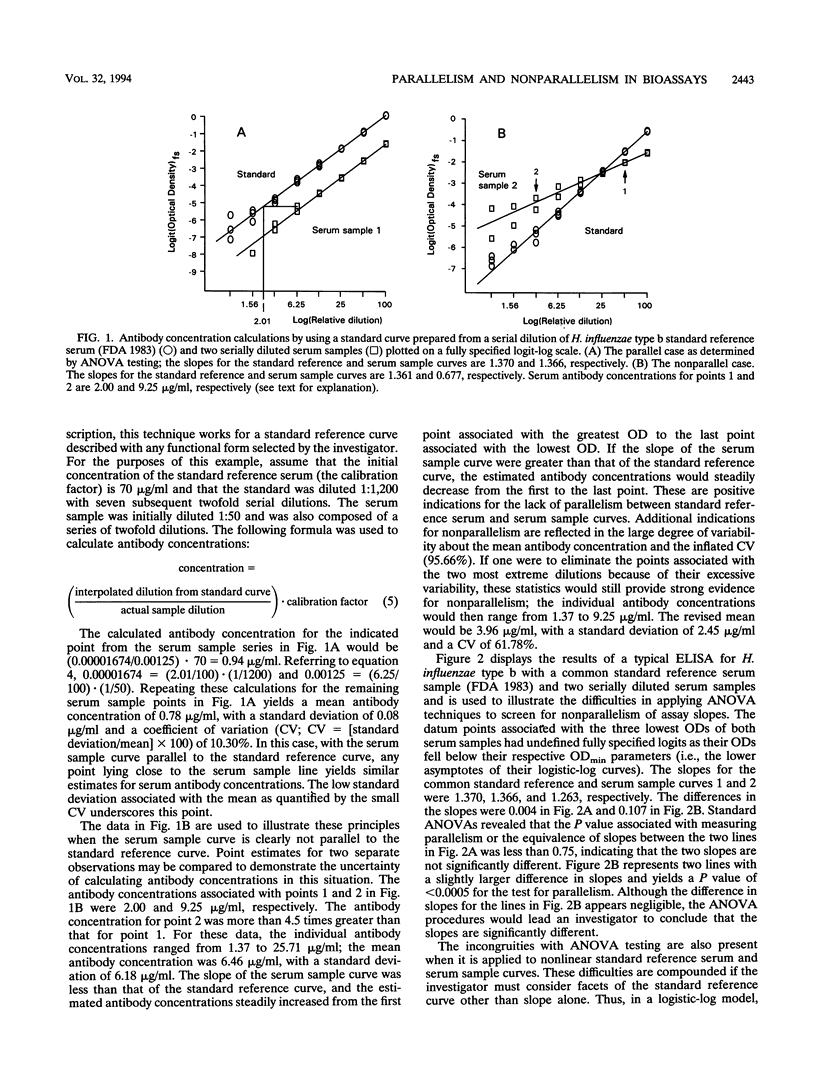
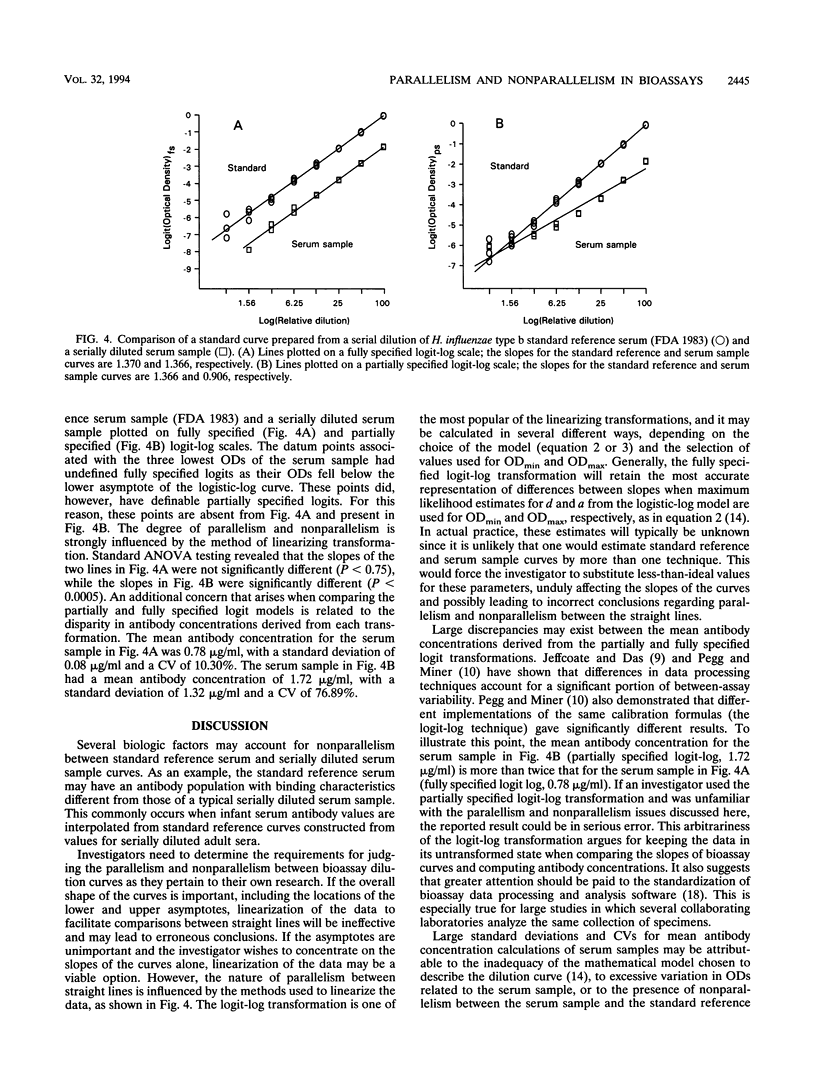
There is a lack of consensus among investigators who use a variety of immunoassay techniques (e.g., enzyme-linked immunosorbent assay [ELISA] and radioimmunoassay) regarding the protocols for describing and forming standard reference or calibration curves and interpolating serum antibody concentrations. This confounds the issue of detecting the presence or absence of parallelism between standard reference serum and serially diluted serum sample curves. These curves must be parallel to support the assumption that the antibody-binding characteristics are similar enough to allow the determination of antibody levels in the diluted serum sample. There is no universal and widely adopted strategy for assessing parallelism in bioassays, and without an assurance of parallelism, investigators are not able to calculate reliable estimates for antibody concentrations in serum samples. Furthermore, single-point (dilution) serum assays do not provide information related to parallelism and nonparallelism, and this, too, may lead to considerable error when calculating antibody concentrations. When assay methodology, technique, and precision improve to the extent that standard reference serum and serially diluted serum sample curves are fit with little error, standard analysis of variance techniques are overly sensitive to negligible departures from parallelism. We present a series of guidelines that compose a protocol for assessing parallelism between bioassay dilution curves that are applicable to data derived from ELISAs. These criteria should be applicable, with minor modifications, to most immunoassay experimental situations and, most importantly, are not dependent on the mathematical model used to form the standard reference curve. These guidelines have evolved in our laboratories over the past 4 years during the performance of thousands of ELISAs for antibodies to the capsular polysaccharides of Neisseria meningitidis groups A and C and Haemophilus influenzae type b.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakere G., Frasch C. E. Specificity of antibodies to O-acetyl-positive and O-acetyl-negative group C meningococcal polysaccharides in sera from vaccinees and carriers. Infect Immun. 1991 Dec;59(12):4349–4356. doi: 10.1128/iai.59.12.4349-4356.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlone G. M., Frasch C. E., Siber G. R., Quataert S., Gheesling L. L., Turner S. H., Plikaytis B. D., Helsel L. O., DeWitt W. E., Bibb W. F. Multicenter comparison of levels of antibody to the Neisseria meningitidis group A capsular polysaccharide measured by using an enzyme-linked immunosorbent assay. J Clin Microbiol. 1992 Jan;30(1):154–159. doi: 10.1128/jcm.30.1.154-159.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney D. J. Radioligand Assay. Biometrics. 1976 Dec;32(4):721–740. [PubMed] [Google Scholar]
- Gheesling L. L., Carlone G. M., Pais L. B., Holder P. F., Maslanka S. E., Plikaytis B. D., Achtman M., Densen P., Frasch C. E., Käyhty H. Multicenter comparison of Neisseria meningitidis serogroup C anti-capsular polysaccharide antibody levels measured by a standardized enzyme-linked immunosorbent assay. J Clin Microbiol. 1994 Jun;32(6):1475–1482. doi: 10.1128/jcm.32.6.1475-1482.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardabasso V., Munson P. J., Rodbard D. A versatile method for simultaneous analysis of families of curves. FASEB J. 1988 Mar 1;2(3):209–215. doi: 10.1096/fasebj.2.3.3350235. [DOI] [PubMed] [Google Scholar]
- Guardabasso V., Rodbard D., Munson P. J. A model-free approach to estimation of relative potency in dose-response curve analysis. Am J Physiol. 1987 Mar;252(3 Pt 1):E357–E364. doi: 10.1152/ajpendo.1987.252.3.E357. [DOI] [PubMed] [Google Scholar]
- Jeffcoate S. L., Das R. E. Interlaboratory comparison of radioimmunoassay results. Variation produced by different methods of calculation. Ann Clin Biochem. 1977 Sep;14(5):258–260. doi: 10.1177/000456327701400170. [DOI] [PubMed] [Google Scholar]
- Pegg P. J., Miner E. M. The effect of data reduction technic on ligand assay proficiency survey results. Am J Clin Pathol. 1982 Mar;77(3):334–337. doi: 10.1093/ajcp/77.3.334. [DOI] [PubMed] [Google Scholar]
- Phipps D. C., West J., Eby R., Koster M., Madore D. V., Quataert S. A. An ELISA employing a Haemophilus influenzae type b oligosaccharide-human serum albumin conjugate correlates with the radioantigen binding assay. J Immunol Methods. 1990 Dec 31;135(1-2):121–128. doi: 10.1016/0022-1759(90)90264-v. [DOI] [PubMed] [Google Scholar]
- Plikaytis B. D., Carlone G. M., Edmonds P., Mayer L. W. Robust estimation of standard curves for protein molecular weight and linear-duplex DNA base-pair number after gel electrophoresis. Anal Biochem. 1986 Feb 1;152(2):346–364. doi: 10.1016/0003-2697(86)90420-3. [DOI] [PubMed] [Google Scholar]
- Plikaytis B. D., Turner S. H., Gheesling L. L., Carlone G. M. Comparisons of standard curve-fitting methods to quantitate Neisseria meningitidis group A polysaccharide antibody levels by enzyme-linked immunosorbent assay. J Clin Microbiol. 1991 Jul;29(7):1439–1446. doi: 10.1128/jcm.29.7.1439-1446.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbard D. Statistical quality control and routine data processing for radioimmunoassays and immunoradiometric assays. Clin Chem. 1974 Oct;20(10):1255–1270. [PubMed] [Google Scholar]
- Story M. J., Winson J. A., Beyer P. L., Boehm G. A new parallelism acceptance criterion for validating large plate bioassay results. J Biol Stand. 1986 Jul;14(3):249–254. doi: 10.1016/0092-1157(86)90010-7. [DOI] [PubMed] [Google Scholar]


