Abstract
Objective
This retrospective study of 215 patients with 383 symptomatic osteoporotic vertebral compression fractures (VCFs) treated by percutaneous vertebroplasty (PVP), was performed to evaluate the clinical outcomes, and to analyze the various clinical factors affecting these results.
Methods
The authors assessed the clinical outcome under the criteria such as the pain improvement, activity, requirement of analgesics, and the patient's satisfaction, and determined the relation to various peri- and intra-operative factors, and postoperative imaging findings.
Results
The outcome was determined as 84.2% in relief of pain, 72.0% in change in activity, 65.7% in analgesics use, and 84.7% of satisfaction rate. More severe focal back pain, high uptake bone scan, and the lower mean T-score were related to the better pain relief following PVP. The longer the duration between fracture and PVP, the less severe focal back pain, low uptake bone scan, and leakage of PMMA into the paravertebral space were related to the less improvement in activity. Female and low uptake bone scan showed a correlation with more analgesic use. The longer the duration between fracture and PVP, low uptake bone scan, and the higher the mean T-score were correlated with the less the patients satisfaction.
Conclusion
Our study suggests that PVP may be more effective in the acute phase of VCFs, more severe focal pain, and far advanced osteoporosis on BMD. Leakage of PMMA into the paravertebral spcae also could be affecting the surgical results.
Keywords: Osteoporosis, Vertebral compression fracture, Vertebroplasty
INTRODUCTION
Osteoporotic vertebral compression fractures (VCFs) cause not only severe pains but also functional and social problems such as sleep disorder, restriction on social activities and mental diseases20,22,23). Therefore, it needs to treat actively, however, the traditional management has been almost exclusively conservative because of concern about the risk related to extensive stabilization surgery.
Percutaneous vertebroplasty (PVP), pioneered by Dermond et al.7) in late 1980, consists of fluoroscopy guided procedures to inject polymethylmetacrylate (PMMA) into the osteoporotic or neoplastic fractured vertebral body. This procedure was expected to eliminate or reduce the pain postoperatively by the effectual stabilization of fractured body, and then it has been widely accepted as a fascinating minimally invasive surgery for osteoporotic VCFs.
Since then, many published previous results have demonstrated significant pain relief after PVP. However, most of reports have just focused on the change of pain pre- and postoperatively, which tend to depend on only subjective assessments. Therefore, it is necessary to make a detailed investigation in various clinical viewpoints for defining the exact therapeutic effect of PVP in osteoporotic VCFs. Moreover, although it is supposed that the factors which influenced on the surgical outcome of PVP would be the age of fracture, the degree of osteoporosis, and the surgical complication such as extavasations of PMMA, there are few clinical studies to assess the statistical relation to the clinical outcome4,6,12,15,19,21).
This retrospective study of 215 patients, with back pain secondary to osteoporotic VCFs and treated by PVP, was designed to evaluate the clinical outcomes in terms of the pain improvement, mobility, requirement of analgesics, and the patient' satisfaction. The authors also analyzed the various clinical factors affecting these results using Spearman correlation analysis and multivariate polytomous logistic regressions test.
MATERIALS AND METHODS
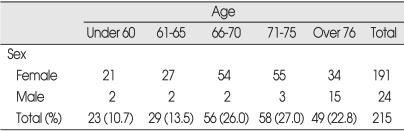
A total 215 patients with 383 symptomatic osteoporotic VCFs were consecutively treated with PVP by one physician (C.K.P.), who could be followed-up for more than 6 months without interruption after PVP, were included in this study. The mean age of the patients was 70.37 years (range 47-92 years) at the time of the operation (Table 1). The follow-up period ranged from 6 months to 22 months (average 15 months).
Table 1.
Distribution of patients (M=24, F=191)
All patients demonstrated acute agonizing or chronic severe focal back pain, which did not respond to bed rest and analgesics. Osteoporotic VCFs were confirmed by plain radiography, bone mineral densitometry (BMD), CT and bone scan imaging. The degree of focal back pain was assessed using a visual analogue scale (VAS : 0=no pain, 10 : worst pain possible). Limited CT scanning at the operated vertebrae was performed in all patients to determine leakage of the PMMA cement within 24 hrs. The degree of vertebral compression was ranged from 15% to 75% of the expected normal height of the affected vertebral body on lateral radiographs (average 45.5%). 77.3% (296 vertebrae) of VCFs was observed at the level between D9 and L3, 15.7% (60 vertebrae) was at the lower lumbar region (L4 and L5), and the remaining 7.0% (27 vertebrae) was above D9. Bone scan imaging showed high uptake (greater or equal to the sacroiliac joint) in 352 vertebrae (92%) and low uptake (lesser than the sacroiliac joint but greater than normal adjacent vertebrae) in 31 (8%). The average T-score of the spine in BMD was -3.324 (range, -1.6 to -6.2). The precise chronology of pain and the age of VCFs were well defined in some patients but uncertain in others. However, even in the uncertain cases, the patients presented with a history of back pain for specified duration. The duration of pain before PVP was used as a surrogate measure for the age of VCF. The duration ranged from 6 days to 6 months : within 2 weeks in 7 cases, 3.3% of the all cases; 2 to 4 weeks in 41, 19.1%; 4 to 8 weeks in 68, 31.6%; 2 to 4 months in 62, 28.8%; 4 to 6 months in 37, 17.2%. Postopeartive CT scan revealed PMMA leakage into the epidural space of the spinal canal in 157 patients (29.5%), the paravertebral space in 18 (3.4%), and the adjacent intradiscal space in 12 (2.3%). However, no patients presented neurological deficit immediately or late, or needed surgical intervention. No other serious complications such as osteomyelitis or pulmonary embolism occurred in any of our patients.
The authors used pre-established protocols to analyze the possible clinical predictors affecting the treatment results of PVP. The variables employed in the protocols were classified into three groups : 'preoperatve factors', 'intraoperative factors', and 'postoperative image finding'.
Preoperative Factors
First, the age of fracture, the duration from fracture to PVP was divided into five groups ranging from less than two weeks to over 4 months. Second, severity of pain, assessed with VAS score at admission. Third, the number of fractured vertebrae, treated with. Fourth, the levels of fractured vertebrae were divided into four groups as 'upper thoracic vertebrae' (above 8th thoracic vertebra), 'lower thoracic vertebrae' (between 9th and 12th thoracic vertebrae), 'upper lumbar vertebrae' (between 1st and 3rd lumbar vertebrae), and 'lower lumbar vertebrae' (4th and 5th lumbar vertebrae). Fifth, the degree of compression was divided into three groups based on plain radiography as 'less than 30% of the expected normal height of the affected vertebrae', 'from 31% to 70%', and 'more than 70%'. Sixth, the patients were divided into two groups based on results of bone scan imaging as high uptake at fractured vertebrae, and low uptake. Seventh, the mean value of T-score in the lumbar region obtained by BMD was correlated to treatment results.
Intraoperative factors
Depending on the total amount of PMMA injected into the affected vertebrae, patients were divided into 4 groups as 'less than 2 cc', '2-3.9 cc', '4-5.9 cc', and 'more than 6 cc'. Comparative analysis was also made between two groups, that is, one undergoing PVP by bipedicular and the other by unipedicular approach.
Postoperative imaging findings
The patterns of the injected PMMA observed on the axial images of the postoperative CT were analyzed in four ways to determine correlation with the treatment results. First, the cases were divided into two groups depending on the extent of PMMA filling up the vertebral body as one group filling with more than 50% of the vertebrae, and the other with less than 50%. Second, according to the types of epidural leakage of PMMA, we divided the patients into three groups as, 'into the central spinal canal', 'into the neural foramen', and 'no leakage'. In cases with epidural leakage, the extent of leakage was expressed with percentages by dividing the area of the leaked PMMA to the total area of the spinal canal or by dividing the width of neural foramen. Cross sectional areas of the vertebral body and the injected PMMA, and of the spinal canal and leaked PMMA were measured by determining the pixel quantity in the region of interest through the distribution of histogram on the digital image obtained by the scanner, using graphic software (Adobe Photoshop version 7.0), and then each quantity was expressed with percentage. Third, the cases with the leakage of PMMA into the paravertebral muscles were divided into two groups, one group with leakage and the other without. Lastly, the cases of the leakage into the adjacent intervertebral disc space were also divided into two groups in the same manner.
Treatment results
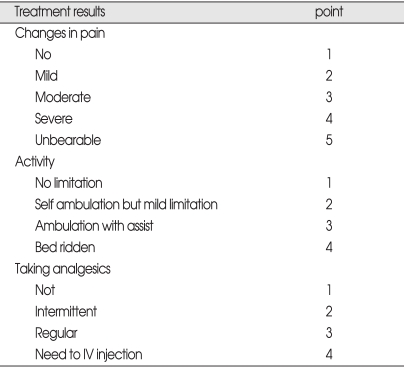
Three specific outcome variables including 'change in pain', 'degree of activity', and 'analgesics use' were investigated with questionnaires of the pre-established protocol at admission and more than 6 months after the PVP procedure by personal interviews or telephone calls from a third party, who was not involved in the treatment (Table 2). Pre- and post-treatment questionnaires were compared. Treatment results were intentionally categorized into four grades; 'good' for a case showing decrease of 1 point, 'no change' showing no change, and 'worse' showing increase of the point. As for patient's satisfaction, the other outcome variable, the respondents were divided into 4 groups as 'very satisfied', 'somewhat satisfied', 'somewhat dissatisfied' and 'very dissatisfied'.
Table 2.
Original points used for three outcome variables
Statistical analysis
Statistical association of each outcome variable with the predictor variables was determined using Spearman correlation analysis. Multivariate polytomous logistic regressions were applied to determine the independent effect of the predictor variables after adjusting confounding effects of the variables. Logistic regression models were selected by choosing those variables showing statistical significance in a univariate analysis.
All statistical analyses were performed using SAS for Windows version 6.2, and the case, whose p-value was 0.05 or less, was regarded to be statistically significant.
RESULTS
Factors affecting relief of pain
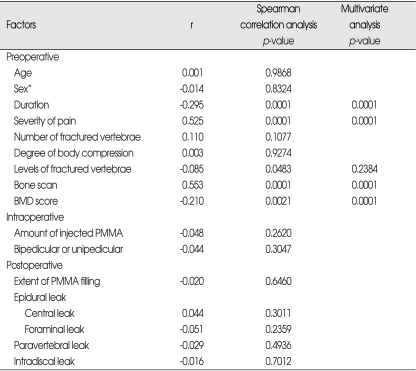
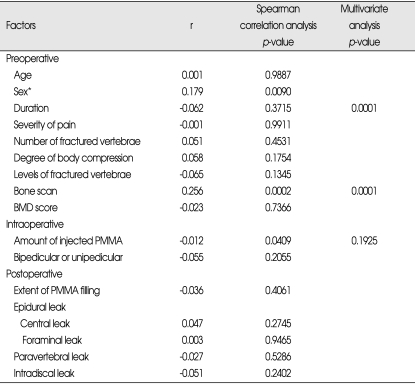
With regards to long-term effects of PVP on focal back pain related to VCFs, there were 'good' in 95 cases (44.2%), 'fair' in 86 cases (40.0%), 'no change' in 28 cases (13%), and 'worse' in 6 cases (2.7%). Spearman correlation analysis revealed that only the preoperative factors had a relationship with changes in pain, but neither intraoperative factors nor postoperative image findings showed significant relationship, The sooner the patient received PVP after fracture (p=0.0001), the more severe focal back pain (p=0.0001), the higher the level of the fractured vertebrae (p=0.0483), high uptake bone scan (p=0.0001), and the lower mean T-score on the BMD (p=0.0021), the better pain relief following PVP was observed at follow-up.
Multivariate polytomous logistic regression analysis indicated that the level of fractured vertebrae lost statistical significance (p=0.2384) (Table 3).
Table 3.
Factors affecting relief of pain
Spearman correlation coefficients / Prob > |R| under Ho : Rho=0 / N=215. *Chi-square test for trend
Factors affecting activity
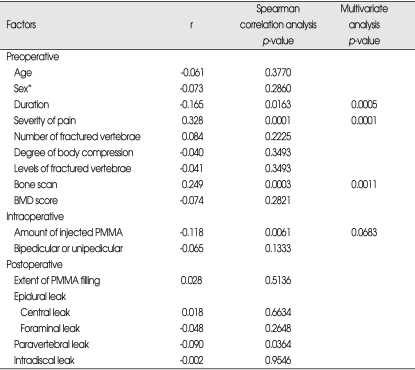
With regard to long-term effects of PVP on activities following VCFs, 76 cases (35.3%) were observed as 'good', 79 cases (36.7%) as fair, 53 cases (24.7%) as 'no change', and 7 (3.3%) as 'worse'. Spearman correlation analysis showed that the longer the duration between fracture and PVP (p=0.0163), the less severe focal back pain was (p=0.0061), low uptake bone scan (p=0.0003), the smaller amount of injected PMMA (p=0.061), and leakage of PMMA into the paravertebral space (p=0.0364), the less improvement or the worse in activity following PVP was observed at follow-up.
However, multivariate polytomous logistic regression analysis revealed that the age of fracture (p=0.0005), the severity of pain (p=0.0001) and the low uptake bone scan (p=0.0011) among the preoperative factors, and the leakage into the paravertebral space (p=0.0483) among the postoperative image findings remained significant, but amount of injected PMMA, one of the intraoperative factors, lost statistical significance (p=0.0683) (Table 4).
Table 4.
Factors affecting activity
Spearman correlation coefficients / Prob > |R| under Ho : Rho=0 / N=215. *Chi-square test for trend
Factors affecting analgesics use
With regard to long-term effects of PVP on taking analgesics following VCFs, 68 cases (31.7%) were classified as 'good', 73 (34.0%) as 'fair', 60 (27.9%) as 'no change', and 14 (6.4%) as 'worse'. Spearman correlation analysis showed that the fair sex (p=0.0090), low uptake bone scan (p=0.0002) and the small amount of injected PMMA (p=0.0409) had statistical significance. However, multivariate polytomous logistic regression analysis indicated that the amount of injected PMMA lost its statistical significance (p=0.1925) (Table 5).
Table 5.
Factors affecting analgesic use
Spearman correlation coefficients / Prob > |R| under Ho: Rho=0 / N=215. *Chi-square test for trend
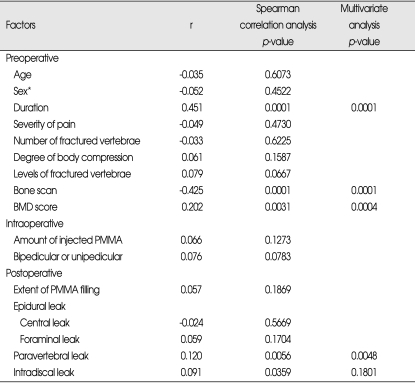
Patient's satisfaction
Seventy-six cases (34.9%) were disclosed as 'very satisfied', 106 (49.3%) as 'somewhat satisfied', 21 (9.8%) as 'somewhat dissatisfied', and 13 (6.0%) as 'very dissatisfied' based on the survey determining the degree of satisfaction at the operation result. Spearman correlation analysis showed that among the preoperative factors, the longer the duration between fracture and PVP (p=0.0001), low uptake bone scan (p=0.0001), and the higher the mean T-score (p=0.0031), the less the patients were satisfied with the operation result. Among the postoperative image findings, the cases with leakage of PMMA into the paravertebral space (p=0.0056) and into the adjacent intervertebral disc space (p=0.0359) showed also statistical significance. However, multivariate polytomous logistic regression analysis revealed that the leakage of PMMA into the adjacent intervertebral disc space lost statistical significance (p=0.1801) (Table 6).
Table 6.
Factors affecting patient's satisfaction
Spearman correlation coefficients / Prob > |R| under Ho: Rho=0 / N=215. *Chi-square test for trend
Age, degree of vertebral body compression, number of fractured vertebrae, the level of VCFs, the amount of the injected PMMA, injection by bipedicular or unipedicular approach, the extent of PMMA filling up the vertebral body, or the leakage of PMMA into the posterior epidural space or adjacent intradiscal space had no statistical correlation with any outcome variables.
DISCUSSION
This study demonstrates that the age of the fracture, bone scan imaging findings, the degree of pain as well as the severity of osteoporosis all influence the treatment outcome. There was strong correlation between the severity of pre-procedural pain score and the post-procedural clinical response; the more severe the pain, the more significant was the improvement following PVP.
In patients with VCFs, which resulted from severe osteoporosis, diagnosed by T-score of BMD, better long-term treatment results could be obtained compared to patients with no or mild osteoporosis. Heini and his colleagues12) demonstrated in their study of biomechanical study with fresh human cadaver spines that the lower the initial BMD, the more pronounced was the augmentation effect of PMMA, which reliably and significantly raised the stiffness and maximal tolerable force until failure in osteoporotic vertebral bodies12). In contrast, no significant increase was achieved in non-porotic specimens. To our knowledge, the effect of severity of osteoporosis on long-term outcome of PVP treatment has not been specifically measured to date.
Kaufmann et al.15) reported that the effect of PVP on the pain relief after OVCFs was attenuated in patients with older fractures. Maynard et al.18) reported that increased activity on OVCFs revealed by bone scan imaging had high predictive value of positive clinical response to PVP. But, they concerned about a limitation of work-up bias in the results caused by exclusion of patients with negative bone scan from their series. Moreover, these results have been achieved in a small series and short-term follow-up. These were confirmed by our larger and longer-term follow-up series, including patients with low uptake bone scan.
Other techniques, including CT and MRI, might be useful in selecting patients. In particular, MRI offers the advantage over bone scan imaging such as accurate assessment of bony deformity and the presence or absence of edema. In our series, MRI was optional, but CT and bone scan imaging were exclusively included : CT for assessing disruption of bony continuity in the posterior wall of vertebral body in order to avoid leakage of PMMA through the bony disruption into the spinal canal, and bone scan for distinguishing painful fractures from healed fractures.
It appeared that the fair sex and low uptake on bone scan imaging had negative effects on the treatment results with regard to analgesics use in this study. The mechanism of the relationship of the fair sex and low uptake bone scan with unfavorable treatment outcome regarding 'analgesics use' has not been defined yet. The fair sex and the patients with low uptake bone scan (older fractures) might have become more habituated to or dependent on a certain level of analgesia before the procedures than male sex and the patients with high uptake bone scan (younger fractures), and thus responded less to PVP with regard to analgesic use. Expected improvement in patient activity after PVP might be also rather limited in such patients with lower pre-procedural activity levels as with chronic back pain resulting from older age of fractures.
Other preoperative clinical factors assessed at study entry such as patient's age, the number of fractured vertebrae treated, or level of fractured vertebrae did not predict the response to PVP. Cortet et al.4) have demonstrated in small series of 16 cases that treatment efficacy of PVP in patients with osteoporosis VCFs was not influenced by sex, number of vertebrae treated, or BMD at baseline in 6 months follow-up study. O'Brien et al.19) have insisted that reduced vertebral body height might not be an exclusion criterion for PVP in osteoporotic VCFs or a factor inducing unfavorable surgical results. However, in our study, the degree of reduced vertebral body height was not related to all parameters of the clinical outcome.
Among the outcome variables of the intraoperative factors and postoperative image findings, only leakage of PMMA into the paravertebral space had relationship with the long-term treatment results. It has been reported that one of the most serious surgical complications, which had unfavorable influence upon both immediate and late surgical outcome, was leakage of PMMA into the paravertebral muscles. The mechanisms for leakage of PMMA into the paravertebral space can be considered in two ways : leaking through the broken cortex of the vertebral body into the paravertebral tissues during injection and direct injection to the paravertebral muscles caused by injection of PMMA following improper placement of needle, especially in small vertebrae like the mid and upper thoracic ones. We have experienced two cases of leakage into paravertebral muscles. The leaked PMMA spread rapidly along the psoas muscle during injection, and the patients complained of agonizing flank pain a minute later, maybe due to thermal effects of polymerization o PMMA. Postoperatively, they could not ambulate well for several months because of consistent severe flank pain. In contrast, the cases with leakage of PMMA into the adjacent tissues the broken cortex did not present such serious symptoms. Leakage of PMMA into the paravertebtal space exerted unfavorable influence upon the changes in activity and patient's satisfaction postoperatively in this study.
The other intraoperative factors and postoperative imaging such as amount of PMMA injected, bi- or uni-pedicluar approach, the extent of PMMA filling in the vertebral body, or epidural and intradiscal leakage of PMMA did not appear to have relationship with long-term outcome. Gangi et al.10) have reported the clinical experiences of PVP in bone metastasis or multiple myeloma. In their results, there was no correlation between the degree of pain relief and the extent of PMMA filling or between pain relief and amount of PMMA injected per vertebra. Cortet et al.4) have stated that the control of PMMA volume seemed to be the most critical point for avoiding complications, because excessive PMMA volume injection could result in not only PMMA leakages but also the treatment failure including adjacent vertebra fractures.2,3,9,16) These clinical factors have been also investigated in various biomechanical studies with postmortem subjects. Liebschner et al.17) have shown that large fill volumes of PMMA in damaged vertebra might not be the most biomechanical optimal configuration, which could result in substantial increase in stiffness too much beyond the intact level. Dean et al.5) have revealed that the magnitude of strength in the vertebral body did not correlate with volume of PMMA injected. Moreover, Tohmeh et al.24) have demonstrated that there were no significant differences in strength of vertebral bodies injected between by uni- and bi-pedicular approaches.
With regard to correlation between epidural leakage and outcome, we have analyzed therapeutic results with leakage of PMMA into the epidural space in 92 of 347 treated vertebrae in 64 of the 159 patients, and reported that epidural leakage of PMMA might attenuate the immediate therapeutic effects of PVP on pain relief, but it had no significant effects on the late outcome, 3 months postoperatively in previous report21).
Our success rate is 181/215 (84.2%) in relief of pain, 155/215 (72.0%) in change in activity, 141/215 (65.7%) in analgesics use, and 182/215 (84.7%) of satisfaction rate, if the grades, 'good' and 'fair' are considered as success, which is comparable with the results of what has been previously reported as 75-90%1,4,7,8,11,13,14,25).
The cohort of our study represents a challenging series using several variables and relatively large sample size. Moreover, this study is limited by review biases inherent in many retrospective observational studies, even if a pre-established protocol was used. In some cases, it was hard to assess the postoperative status and to determine reliability of their answers. Although we tried to reduce the effects of biases statistically, but it was not possible to know exactly the extent to which factors such as the placebo effect, natural history of the disease, and general condition of the patients may have biased conclusions made from these data. Inclusion and exclusion criteria for PVP merit further study, preferably with prospective randomized trials.
We believe that careful patient selection is essential to the success of PVP. Our study suggests that PVP may be considered early in the acute phase for the treatment of VCFs who present severe focal pain, and far advanced osteoporosis on BMD. The earlier PVP is performed in these patients, the earlier they return to usual activity and improve in analgesics use. Moreover, the risk of various complications, especially leakage of PMMA into the paravertebral and epidural spaces should be avoided to improve surgical results. In experienced hands and with appropriately selected patients, PVP could be a safe and efficacious procedure in terms of not only pain relief and improvement of disability associated with osteoporotic VCFs but also patient satisfaction.
CONCLUSION
Our study demonstrated that PVP could be good therapeutic modality for treating osteoporotic VCFs including pain improvement, increased mobility, and less requiring analgesics. Also, PVP may be more effective when it is performed to the patients who has acute phase of osteoporotic VCFs, severe focal back pain, and severe osteoporosis. The leakage of PMMA could reduce therapeutic effect of PVP. To make surgical outcome of PVP better, appropriate patients selection and careful procedure to prevent PMMA leakages should be required.
References
- 1.Amar AP, Larsen DW, Esnaashari N, Albuquerque FC, Lavine SD, Teitelbaum GP. Percutaneous transpedicular polymethylmethacrylate vertebroplasty for the treatment of spinal compression fractures. Neurosurgery. 2001;49:1105–1114. discussion 1114-1105. [PubMed] [Google Scholar]
- 2.Baroud G, Vant C, Wilcox R. Long-term effects of vertebroplasty: adjacent vertebral fractures. J Long Term Eff Med Implants. 2006;16:265–280. doi: 10.1615/jlongtermeffmedimplants.v16.i4.10. [DOI] [PubMed] [Google Scholar]
- 3.Boger A, Heini P, Windolf M, Schneider E. Adjacent vertebral failure after vertebroplasty: a biomechanical study of low-modulus PMMA cement. Eur Spine J. 2007;16:2118–2125. doi: 10.1007/s00586-007-0473-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortet B, Cotten A, Boutry N, Flipo RM, Duquesnoy B, Chastanet P, et al. Percutaneous vertebroplasty in the treatment of osteoporotic vertebral compression fractures : an open prospective study. J Rheumatol. 1999;26:2222–2228. [PubMed] [Google Scholar]
- 5.Dean JR, Ison KT, Gishen P. The strengthening effect of percutaneous vertebroplasty. Clin Radiol. 2000;55:471–476. doi: 10.1053/crad.2000.0478. [DOI] [PubMed] [Google Scholar]
- 6.Deen HG, Aranda-Michel J, Reimer R, Putzke JD. Preliminary results of balloon kyphoplasty for vertebral compression fractures in organ transplant recipients. Neurosurg Focus. 2005;18:e6. [PubMed] [Google Scholar]
- 7.Deramond H, Depriester C, Galibert P, Le Gars D. Percutaneous vertebroplasty with polymethylmethacrylate. Technique, indications, and results. Radiol Clin North Am. 1998;36:533–546. doi: 10.1016/s0033-8389(05)70042-7. [DOI] [PubMed] [Google Scholar]
- 8.Dunnagan SA, Knox MF, Deaton CW. Osteoporotic compression fracture with persistent pain. Treatment with percutaneous vertebroplasty. J Ark Med Soc. 1999;96:258–259. [PubMed] [Google Scholar]
- 9.Frankel BM, Monroe T, Wang C. Percutaneous vertebral augmentation: an elevation in adjacent-level fracture risk in kyphoplasty as compared with vertebroplasty. Spine J. 2007;7:575–582. doi: 10.1016/j.spinee.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 10.Gangi A, Dietemann JL, Schultz A, Mortazavi R, Jeung MY, Roy C. Interventional radiologic procedures with CT guidance in cancer pain management. Radiographics. 1996;16:1289–1304. doi: 10.1148/radiographics.16.6.8946536. discussion 1304-1306. [DOI] [PubMed] [Google Scholar]
- 11.Garfin SR, Yuan HA, Reiley MA. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine. 2001;26:1511–1515. doi: 10.1097/00007632-200107150-00002. [DOI] [PubMed] [Google Scholar]
- 12.Heini PF, Berlemann U, Kaufmann M, Lippuner K, Fankhauser C, van Landuyt P. Augmentation of mechanical properties in osteoporotic vertebral bones: a biomechanical investigation of vertebroplasty efficacy with different bone cements. Eur Spine J. 2001;10:164–171. doi: 10.1007/s005860000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen ME, Dion JE. Percutaneous vertebroplasty in the treatment of osteoporotic compression fractures. Neuroimaging Clin N Am. 2000;10:547–568. [PubMed] [Google Scholar]
- 14.Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159:1215–1220. doi: 10.1001/archinte.159.11.1215. [DOI] [PubMed] [Google Scholar]
- 15.Kaufmann TJ, Jensen ME, Schweickert PA, Marx WF, Kallmes DF. Age of fracture and clinical outcomes of percutaneous vertebroplasty. AJNR Am J Neuroradiol. 2001;22:1860–1863. [PMC free article] [PubMed] [Google Scholar]
- 16.Kim SH, Kang HS, Choi JA, Ahn JM. Risk factors of new compression fractures in adjacent vertebrae after percutaneous vertebroplasty. Acta Radiol. 2004;45:440–445. doi: 10.1080/02841850410005615. [DOI] [PubMed] [Google Scholar]
- 17.Liebschner MA, Rosenberg WS, Keaveny TM. Effects of bone cement volume and distribution on vertebral stiffness after vertebroplasty. Spine. 2001;26:1547–1554. doi: 10.1097/00007632-200107150-00009. [DOI] [PubMed] [Google Scholar]
- 18.Maynard AS, Jensen ME, Schweickert PA, Marx WF, Short JG, Kallmes DF. Value of bone scan imaging in predicting pain relief from percutaneous vertebroplasty in osteoporotic vertebral fractures. AJNR Am J Neuroradiol. 2000;21:1807–1812. [PMC free article] [PubMed] [Google Scholar]
- 19.O'Brien J, Brennan D, Taylor D, O'Byrne J, Eustace S. Percutaneous vertebroplasty--initial clinical experience in osteoporotic and myelomatous compression fractures. Ir J Med Sci. 2006;175:50–53. doi: 10.1007/BF03169001. [DOI] [PubMed] [Google Scholar]
- 20.Ross PD. Clinical consequences of vertebral fractures. Am J Med. 1997;103:30S–42S. doi: 10.1016/s0002-9343(97)90025-5. discussion 42S-43S. [DOI] [PubMed] [Google Scholar]
- 21.Ryu KS, Park CK, Kim MC, Kang JK. Dose-dependent epidural leakage of polymethylmethacrylate after percutaneous vertebroplasty in patients with osteoporotic vertebral compression fractures. J Neurosurg. 2002;96:56–61. doi: 10.3171/spi.2002.96.1.0056. [DOI] [PubMed] [Google Scholar]
- 22.Schlaich C, Minne HW, Bruckner T, Wagner G, Gebest HJ, Grunze M, et al. Reduced pulmonary function in patients with spinal osteoporotic fractures. Osteoporos Int. 1998;8:261–267. doi: 10.1007/s001980050063. [DOI] [PubMed] [Google Scholar]
- 23.Silverman SL. The clinical consequences of vertebral compression fracture. Bone. 1992;13(Suppl 2):S27–S31. doi: 10.1016/8756-3282(92)90193-z. [DOI] [PubMed] [Google Scholar]
- 24.Tohmeh AG, Mathis JM, Fenton DC, Levine AM, Belkoff SM. Biomechanical efficacy of unipedicular versus bipedicular vertebroplasty for the management of osteoporotic compression fractures. Spine. 1999;24:1772–1776. doi: 10.1097/00007632-199909010-00004. [DOI] [PubMed] [Google Scholar]
- 25.Watts NB, Harris ST, Genant HK. Treatment of painful osteoporotic vertebral fractures with percutaneous vertebroplasty or kyphoplasty. Osteoporos Int. 2001;12:429–437. doi: 10.1007/s001980170086. [DOI] [PubMed] [Google Scholar]