Abstract
Objective
Direct surgical clipping of paraclinoid aneurysms poses technical challenges to even very experienced neurosurgeons, making endovascular treatment an alternative treatment modality in many centers. We have therefore retrospectively evaluated the safety and efficacy of endovascular detachable coil embolization of paraclinoid aneurysms.
Methods
From June 1997 to June 2007, 65 patients underwent endovascular detachable coiling for 67 paraclinoid aneurysms (of which 9 were ruptured and 58 were unruptured) in our institute. Their medical records, radiological images and readings, and operation records were reviewed retrospectively.
Results
After the initial embolization procedure, complete occlusion was achieved in 29 (43.3%) of the aneurysms treated by endovascular detachable coiling. Six aneurysms required retreatment, with two each requiring one, two, or three additional endovascular procedures. Fifty-five (82.1%) aneurysms were measured by three-dimensional time of flight (TOF) magnetic resonance images (MRI) or transfemoral cerebral angiography (TFCA) at a mean follow-up of 29.7 months (range from 4 to 94 months), with 39 aneurysms (70.9%) showing complete occlusion. Thromboembolic events (3.8%) were the most frequent complication. Rupture did not occur during or after any of the procedures. According to the Glasgow Outcome Scale (GOS), 98.4% of the patients treated by coil embolization had a score of 4 or 5.
Conclusion
Our results indicate that endovascular detachable coiling is a safe and effective treatment modality in paraclinoid aneurysms.
Keywords: Paraclinoid, Aneurysms, Endovascular
INTRODUCTION
Paraclinoid aneurysms can be defined as intracranial aneurysms that arise from the internal carotid artery distal to the roof of the cavernous sinus but proximal to the posterior communicating artery1,5,27). Direct surgical clipping of paraclinoid aneurysms, however, poses technical challenges to even very experienced neurosurgeons. Microsurgical treatment has been associated with high morbidity and mortality rates owing to the difficulty of proximal control and of safe intracranial exposure.15,24,25,31,35,47) During the past several decades, however, the development of microsurgical techniques and further advancement of anatomical knowledge have facilitated surgical approaches and greatly improved microsurgical outcomes in patients with paraclinoid aneurysms13,17,20,25,27,34,50,53,54) although successful treatment of paraclinoid aneurysms continuously presents neurosurgical challenges. Endovascular treatment, an alternative treatment modality for paraclinoid aneurysms, has been performed in many centers. We have therefore retrospectively evaluated the safety and efficacy of endovascular detachable coil embolization in paraclinoid aneurysms at our center.
MATERIALS AND METHODS
This study included all saccular aneurysms arising from the internal cerebral artery (ICA) between the roof of the cavernous sinus and the origin of the posterior communicating artery that were treated by detachable coil embolization between June 1997 and June 2007 at our institution. A total of 159 consecutive patients with paraclinoid aneurysms were treated by either endovascular or microsurgical treatment. Selection of the treatment modality for each patient was discussed by neurosurgeons (B.K., J.A.) and neurointerventionists (C.C., D.K.). Of the 71 consecutive patients with paraclinoid aneurysms treated by endovascular procedures, 6 were excluded as they had been treated by parent artery trapping with balloon or coil. The remaining 65 patients underwent endovascular procedures with detachable coils for 67 paraclinoid aneurysms (of which 9 were ruptured and 58 were unruptured). Indications for endovascular detachable coiling for paraclinoid aneurysms included : 1) small aneurysms with narrow neck (n=26); 2) aneurysms reasonable to embolization (1 < dome to neck ratio < 2)(n=28); 3) poor clinical status unprofitable for surgery due to brain swelling (n=1); 5) trial for endovascular detachable coiling (dome to neck ratio < 1) (n=12). Paraclinoid aneurysms were defined as lesions arising from the clinoid (C5) and ophthalmic (C6) segments of the ICA7). Clinoid segment aneurysm variants were differentiated into anterolateral and medial variants according to their site of origin and direction of projection11). Ophthalmic segment subtypes were divided into ophthalmic artery, superior hypophyseal artery and dorsal wall aneurysms11,13,14).
The medical records, radiological images and readings, and procedural reports of all patients were retrospectively reviewed. Initial patient clinical status was assessed using the Hunt and Hess scale, and clinical outcomes were assessed using the Glasgow Outcome Scale (GOS). Aneurysm fundus and neck sizes were taken at the point of maximum width or length. A fundus was classified into tiny if <5 mm in size, small if 5 to 10 mm, and large if ≥10 mm. Neck size was divided to narrow if <4 mm and broad if ≥4 mm arbitrarily. Outcomes in each aneurysm were assessed as : class 1, complete obliteration; class 2, residual neck; or class 3, residual aneurysm43,45,46). Routine follow-up evaluation by three dimensional time of flight (TOF) magnetic resonance image (MRI) was performed after 6 months if the aneurysms were completely obliterated, but after 3 months if the aneurysms were incompletely occluded, and every year thereafter. Conventional digital subtraction angiography (DSA) was also performed to confirm coil compaction or recurrence of the aneurysm.
RESULTS
The 65 patients evaluated were 13 (20.0%) men and 52 (80.0%) women, of mean age 52.3 years (range, 32 to 73 years). Their clinical presentations are shown in Table 1. Nine patients (13.8%) presented with subarachnoid hemorrhage from a ruptured paraclinoid aneurysm; of these, five were classified as Hunt and Hess Grade II, three as Grade III, and one as Grade IV. Twenty-three patients (35.4%) presented with non-specific headache, and 33 (40.8%) with incidental paraclinoid aneurysms; of the latter, 5 suffered from prior subarachnoid hemorrhage (SAH) caused by other ruptured aneurysm and 6 presented with unrelated transient ischemic attack (TIA) or ischemic stroke.
Table 1.
Clinical presentation of 65 patients with paraclinoid aneurysms
SAH : subarachnoid hemorrhage, TIA : transient ischemic attack
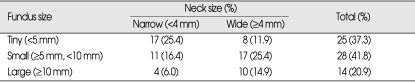
The 67 aneurysms were classified into five anatomic subgroups (Table 2). Fifteen (22.4%) were clinoid medial or anterolateral aneurysms, 10 (14.9%) were ophthalmic aneurysms, 27 (40.3%) were superior hypophyseal aneurysms, and 8 (11.9%) were dorsal wall aneurysms. The fundus and neck sizes of these paraclinoid aneurysms are shown in Table 3. There were 17 tiny aneurysms with narrow necks, 8 tiny aneurysms with wide necks, 11 small aneurysms with narrow necks, 17 small aneurysms with wide necks, 4 large aneurysms with narrow necks, and 10 large aneurysms with wide necks.
Table 2.
Subtypes of the 67 paraclinoid aneurysms
Table 3.
Fundus and neck sizes of the 67 paraclinoid aneurysms
Immediate post-procedural angiograms showed complete occlusion in 29 aneurysms (43.3%), residual neck in 26 aneurysms (38.8%), and residual sac in 12 aneurysms (18.9%). Six aneurysms (9.0%) required retreatment, with two each requiring one, two, or three additional endovascular procedures.
Radiological follow-up was performed for 55 aneurysms, with mean follow-up of 29.7 months; 3D-TOF MRI was performed for 44 aneurysms; 3D-TOF MRI with conventional angiography was performed for 9 aneurysms; conventional DSA was performed for 1 aneurysm as a mean of radiological follow-up. Routine follow-up evaluation by 3D TOF MRI or conventional DSA was generally performed after 6 months if the aneurysms were completely obliterated, but after 3 months if the aneurysms were incompletely occluded, and every year thereafter. Conventional digital subtraction angiography (DSA) was also performed to confirm coil compaction or recurrence of the aneurysm.
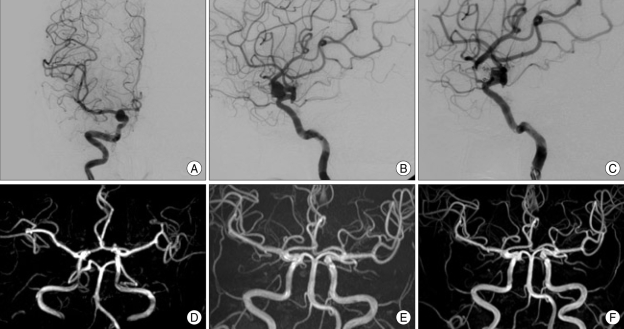
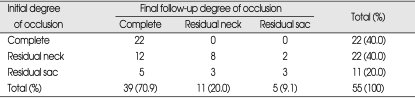
The follow-up evaluation showed that 12 aneurysms initially demonstrating residual neck (class II) and 5 demonstrating residual sac (class III) showed complete occlusion (Fig. 1). Of these, two aneurysms with incomplete occlusion after coil embolization were treated for coil compaction or recurrence of aneurysms. The other 15 aneurysms with incomplete occlusion after initial treatment occluded spontaneously. At the last necessary radiological follow-up, of the 55 evaluable aneurysms, 39 (70.9%) showed complete occlusion (class I), 11 (20%) showed residual neck (class II), and 5 (9.1%) showed residual sac (class III) (Table 4).
Fig. 1.
Preoperative anteroposterior (A) and lateral (B) right carotid artery (CA) angiogram, revealing a dorsal wall aneurysm arising from the distal portion of the ophthalmic artery. Final lateral angiogram (C) after detachable coil embolization confirmed a small residual neck of the aneurysm. About 6 months later, follow-up 3D time of flight (TOF) magnetic resonance images (MRI) (D) shows that the small residual neck of the dorsal wall aneurysm is suspicious. Post-embolization 3D TOF MRI (E) taken one-and-half year later shows no definite residual sac. Post-embolization magnetic resonance angiography (F) performed at two-and-half year later confirms no definite residual sac or neck.
Table 4.
Radiological outcomes of initial embolization and final radiological follow-up in 55 paraclinoid aneurysms
There were 6 procedural related complications (7.6%) among the 79 procedures (Table 5). The patient with middle cerebral artery territory infarction after embolization suffered from dense left upper extremity weakness (motor grade I/V) but recovered with slight motor deficit (motor grade IV+/V). Other complications except this did not affect morbidity directly. There was no permanent neurological morbidity. Rupture of aneurysms did not occur during any of the procedures.
Table 5.
Procedure-related complications in the embolization of paraclinoid aneurysms
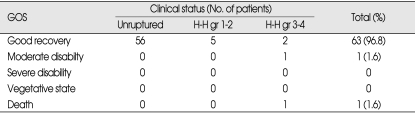
According to the Glasgow Outcome Scale (GOS), 64 patients (98.4%) treated by coil embolization had good recovery or moderate disability (Table 6). One patient died from diffuse brain swelling originating from dense SAH.
Table 6.
Clinical outcomes of paraclinoid aneurysms with respect to clinical status at presentation
GOS : Glasgow Outcome Scale, H-H : Hunt&Hess, gr : grade
DISCUSSION
Paraclinoid aneurysms, which account for approximately 1.5-8% of all intracranial aneurysms15,25,35,37), occur more frequently in women than in men5,18,27,28). Patients with paraclinoid aneurysms are likely to have multiple lesions, particularly at sites such as the posterior communicating artery, the contralateral paraclinoid artery, and the cavernous ICA segment3,13,15,25,52,54). A significant number of paraclinoid aneurysms are found incidentally13,27,51), and they frequently present with large size4,27,32). Similar to previous reports, we found that 52 out of our 65 patients (80%) were women, and paraclinoid aneurysms were found incidentally in 35 (53.8%) patients.
There have been many definitions and classifications of aneurysms arising in proximity to the anterior clinoid process, with some classifications according to their anatomic relationship to the carotid artery, optic nerves, and chiasm2,3,5,13,20,27,33,42,52,54). We included all aneurysms arising from the ICA between the roof of the cavernous sinus and the origin of the posterior communicating artery. According to the recent anatomical segmentation of the ICA7), this portion of the ICA corresponds to the clinoid (C5) and ophthalmic (C6) segments. Aneurysms arising on the distal cavernous ICA were excluded because no definite methods could differentiate these aneurysms intradurally or extradurally.
Paraclinoid aneurysms were specifically classified as described previously11,13,14), with the two clinoid segment variants differentiated according to their site of origin and direction of fundus. The anterolateral variant arises from the anterolateral surface of the clinoid segment as it obliquely ascends toward the dural ring, whereas the medial variant extends from the medial surface of the clinoid segment and enlarges toward the sphenoid sinus and sella. Three aneurysm subtypes originate from the ophthalmic segment. Those showing a clear association with the ophthalmic artery were defined as ophthalmic artery aneurysms. Those arising from the inferior or inferomedial surface of the ICA in close association with origin of the superior hypophyseal artery were defined as superior hypophyseal artery aneurysm. Those arising along the dorsal surface of the ICA distinctly distal to the ophthalmic artery origin were defined as dorsal wall aneurysms. According to another specific subclassification of paraclinoid aneurysms1), those arising distal to the origin of the ophthalmic artery and proximal to the posterior communicating artery were defined as superior hypophyseal aneurysms projecting superiorly or ventral paraclinoid aneurysms projecting posteroinferiorly; those arising at the junction of the ophthalmic artery and the ICA were defined as true ophthalmic aneurysms. Medial infraophthalmic and supracavernous aneurysms are designated carotid cave aneurysms if they arise from the ICA in the clinoid space (carotid cave)33). Superior hypophyseal aneurysms13,14) therefore include superior hypophyseal and ventral paraclinoid aneurysms1), and clinoid aneurysms13,14) include carotid cave and transitional aneurysms1). Subclassification according to location, however, is not important for the endovascular treatment of paraclinoid aneurysms. Rather, it is important to preoperatively localize the lesion in relation to the distal dural ring (DDR), which will discriminate it intradurally or extradurally. Thus, for endovascular procedures, a new classification of paraclinoid aneurysms according to their relationship to the DDR and distinguishing intradural and extradural aneurysms, may be required for adequate treatment. High-resolution MRI or CT analysis may discriminate intradural from extradural paraclinoid aneurysms.
In general, direct surgical clipping of the aneurysm at the neck and preserving the circulation through the parent vessel have been the procedure of choice for the treatment of paraclinoid aneurysms. However, mortality rates range from 20% to 60%, with good outcomes reported from 40% to 71%15,25,31,35,47). This led to the development of indirect techniques, including common carotid artery ligation, ICA occlusion with a balloon and with or without extracranial-intracranial bypass (EIAB), or aneurysm trapping with or without EIAB19,27,31,37,50). These indirect techniques, however, are associated with increased risks of ischemic complications and do not always provide favorable outcomes21,27,29,41,49).
Advances in microcatheter technology, endovascular techniques, and embolization materials have increased the popularity of neurointerventional therapy for paraclinoid aneurysms8). The major advantage of endovascular occlusion of cerebral aneurysms is that it is less invasive and more economical compared with clipping. Additionally, a prospective, randomized, controlled trial, found that patients who underwent endovascular coiling of ruptured intracranial aneurysms had a 6.9% absolute reduction in the risk of dependency or death at 1 year, compared with those who underwent surgical clipping39). Nevertheless, direct surgical clipping is used for some specific paraclinoid aneurysms inappropriate for endovascular detachable coil embolization. In addition, the durability of direct surgical treatment may favor direct clipping for young patients with paraclinoid aneurysms.
The follow-up of the paraclinoid aneurysms that showed incomplete occlusion after detachable endovascular coiling revealed that some showed spontaneous complete occlusion, some showed no change from previous images, and some showed coil compaction or aneurysm recurrence. We found that the mean diameters of the fundus (5.89±2.738 mm vs 10.50±6.143 mm, p=0.076) and aneurysm neck (3.90±1.014 mm vs. 4.89±1.389 mm, p=0.064) were smaller in paraclinoid aneurysms with spontaneous complete occlusion than in those with coil compaction or aneurysm recurrence. However, there was no statistical significance. We found that age, sex, primary aneurysm origin, and initial occlusion status were not associated with spontaneous complete occlusion. Three-dimensional morphology of the residual portion (e.g., eccentric or concentric residual filling of contrast agent) and coil compaction density, which were not included as independent variables for spontaneous complete occlusion, may be associated with spontaneous complete occlusion of aneurysms treated incompletely by coiling.
Since the introduction of the Guglielmi Detachable Coil (GDC), endovascular treatment of intracranial aneurysms has been shown to be feasible for both ruptured and unruptured lesions, with complication rates of less than 10% and delayed re-bleeding rates of 1-2%6,9,10,12,16,22,23,30,36,38,40,44,48). The morbidity and mortality rates following coil embolization were found to be equal to or better than those of published surgical series of similar aneurysms, indicating that an endovascular approach should be considered in the treatment of paraclinoid aneurysms26,46,51). In our series, the complication rate was less than 9%, which is somewhat lower than that of surgery15,25,31,35,47). Although embolization has been reported to be a safe and efficient alternative modality for paraclinoid aneurysms, aneurysm recurrence and repeated procedures are great disadvantages. Long-term analysis of detachable coil embolization is therefore necessary. One of the limitations of our study was the lack of a surgical control group that may preclude direct comparison of endovascular coiling with surgical clipping. However, when compared with historical controls, detachable coil embolization was a safer and more efficient treatment modality than direct clipping if indications of embolization was applied appropriately to paraclinoid aneurysms15,25,31,35,47).
CONCLUSION
Our experience with coil embolization for the treatment of intracranial aneurysms suggests that procedural risks are fairly low. As our study demonstrates, detachable coil embolization is an efficient and safe treatment modality for paraclinoid aneurysms. However, to prove the efficacy of endovascular detachable coiling compared with microsurgery in paraclinoid aneurysms, radominzed multicenter case-control study is necessary.
References
- 1.al-Rodhan NR, Piepgras DG, Sundt TM., Jr Transitional cavernous aneurysms of the internal carotid artery. Neurosurgery. 1993;33:993–996. doi: 10.1227/00006123-199312000-00006. discussion 997-998. [DOI] [PubMed] [Google Scholar]
- 2.Aldrich F. Anterior (dorsal) paraclinoid aneurysm: case report. Surg Neurol. 1991;35:374–376. doi: 10.1016/0090-3019(91)90048-e. [DOI] [PubMed] [Google Scholar]
- 3.Almeida GM, Shibata MK, Bianco E. Carotid-ophthalmic aneurysms. Surg Neurol. 1976;5:41–45. [PubMed] [Google Scholar]
- 4.Arnautovic KI, Al-Mefty O, Angtuaco E. A combined microsurgical skull-base and endovascular approach to giant and large paraclinoid aneurysms. Surg Neurol. 1998;50:504–518. doi: 10.1016/s0090-3019(97)80415-6. discussion 518-520. [DOI] [PubMed] [Google Scholar]
- 5.Batjer HH, Kopitnik TA, Giller CA, Samson DS. Surgery for paraclinoidal carotid artery aneurysms. J Neurosurg. 1994;80:650–658. doi: 10.3171/jns.1994.80.4.0650. [DOI] [PubMed] [Google Scholar]
- 6.Bavinzski G, Killer M, Gruber A, Reinprecht A, Gross CE, Richling B. Treatment of basilar artery bifurcation aneurysms by using Guglielmi detachable coils: a 6-year experience. J Neurosurg. 1999;90:843–852. doi: 10.3171/jns.1999.90.5.0843. [DOI] [PubMed] [Google Scholar]
- 7.Bouthillier A, van Loveren HR, Keller JT. Segments of the internal carotid artery: a new classification. Neurosurgery. 1996;38:425–432. doi: 10.1097/00006123-199603000-00001. discussion 432-433. [DOI] [PubMed] [Google Scholar]
- 8.Brilstra EH, Rinkel GJ, van der Graaf Y, van Rooij WJ, Algra A. Treatment of intracranial aneurysms by embolization with coils: a systematic review. Stroke. 1999;30:470–476. doi: 10.1161/01.str.30.2.470. [DOI] [PubMed] [Google Scholar]
- 9.Byrne JV, Sohn MJ, Molyneux AJ, Chir B. Five-year experience in using coil embolization for ruptured intracranial aneurysms: outcomes and incidence of late rebleeding. J Neurosurg. 1999;90:656–663. doi: 10.3171/jns.1999.90.4.0656. [DOI] [PubMed] [Google Scholar]
- 10.Casasco AE, Aymard A, Gobin YP, Houdart E, Rogopoulos A, George B, et al. Selective endovascular treatment of 71 intracranial aneurysms with platinum coils. J Neurosurg. 1993;79:3–10. doi: 10.3171/jns.1993.79.1.0003. [DOI] [PubMed] [Google Scholar]
- 11.Cawley CM, Zipfel GJ, Day AL. Surgical treatment of paraclinoid and ophthalmic aneurysms. Neurosurg Clin N Am. 1998;9:765–783. [PubMed] [Google Scholar]
- 12.Cognard C, Weill A, Spelle L, Piotin M, Castaings L, Rey A, et al. Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology. 1999;212:348–356. doi: 10.1148/radiology.212.2.r99jl47348. [DOI] [PubMed] [Google Scholar]
- 13.Day AL. Aneurysms of the ophthalmic segment. A clinical and anatomical analysis. J Neurosurg. 1990;72:677–691. doi: 10.3171/jns.1990.72.5.0677. [DOI] [PubMed] [Google Scholar]
- 14.Day AL. Clinicoanatomic features of supraclinoid aneurysms. Clin Neurosurg. 1990;36:256–274. [PubMed] [Google Scholar]
- 15.Drake CG, Vanderlinden RG, Amacher AL. Carotid-ophthalmic aneurysms. J Neurosurg. 1968;29:24–31. doi: 10.3171/jns.1968.29.1.0024. [DOI] [PubMed] [Google Scholar]
- 16.Eskridge JM, Song JK. Endovascular embolization of 150 basilar tip aneurysms with Guglielmi detachable coils: results of the Food and Drug Administration multicenter clinical trial. J Neurosurg. 1998;89:81–86. doi: 10.3171/jns.1998.89.1.0081. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson GG, Drake CG. Carotid-ophthalmic aneurysms: the surgical management of those cases presenting with compression of the optic nerves and chiasm alone. Clin Neurosurg. 1980;27:263–307. doi: 10.1093/neurosurgery/27.cn_suppl_1.263. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez Zubillaga A, Guglielmi G, Vinuela F, Duckwiler GR. Endovascular occlusion of intracranial aneurysms with electrically detachable coils: correlation of aneurysm neck size and treatment results. AJNR Am J Neuroradiol. 1994;15:815–820. [PMC free article] [PubMed] [Google Scholar]
- 19.Fox AJ, Vinuela F, Pelz DM, Peerless SJ, Ferguson GG, Drake CG, et al. Use of detachable balloons for proximal artery occlusion in the treatment of unclippable cerebral aneurysms. J Neurosurg. 1987;66:40–46. doi: 10.3171/jns.1987.66.1.0040. [DOI] [PubMed] [Google Scholar]
- 20.Fox JL. Microsurgical treatment of ventral (paraclinoid) internal carotid artery aneurysms. Neurosurgery. 1988;22:32–39. doi: 10.1227/00006123-198801010-00005. [DOI] [PubMed] [Google Scholar]
- 21.Gelber BR, Sundt TM., Jr Treatment of intracavernous and giant carotid aneurysms by combined internal carotid ligation and extra- to intracranial bypass. J Neurosurg. 1980;52:1–10. doi: 10.3171/jns.1980.52.1.0001. [DOI] [PubMed] [Google Scholar]
- 22.Guglielmi G, Vinuela F, Dion J, Duckwiler G. Electrothrombosis of saccular aneurysms via endovascular approach. Part 2: Preliminary clinical experience. J Neurosurg. 1991;75:8–14. doi: 10.3171/jns.1991.75.1.0008. [DOI] [PubMed] [Google Scholar]
- 23.Guglielmi G, Vinuela F, Duckwiler G, Dion J, Lylyk P, Berenstein A, et al. Endovascular treatment of posterior circulation aneurysms by electrothrombosis using electrically detachable coils. J Neurosurg. 1992;77:515–524. doi: 10.3171/jns.1992.77.4.0515. [DOI] [PubMed] [Google Scholar]
- 24.Guidetti B, La Torre E. Carotid-ophthalmic aneurysms. A series of 16 cases treated by direct approach. Acta Neurochir (Wien) 1970;22:289–304. doi: 10.1007/BF01402996. [DOI] [PubMed] [Google Scholar]
- 25.Guidetti B, La Torre E. Management of carotid-ophthalmic aneurysms. J Neurosurg. 1975;42:438–442. doi: 10.3171/jns.1975.42.4.0438. [DOI] [PubMed] [Google Scholar]
- 26.Gurian JH, Vinuela F, Guglielmi G, Gobin YP, Duckwiler GR. Endovascular embolization of superior hypophyseal artery aneurysms. Neurosurgery. 1996;39:1150–1154. doi: 10.1097/00006123-199612000-00016. discussion 1154-1156. [DOI] [PubMed] [Google Scholar]
- 27.Heros RC, Nelson PB, Ojemann RG, Crowell RM, DeBrun G. Large and giant paraclinoid aneurysms: surgical techniques, complications, and results. Neurosurgery. 1983;12:153–163. doi: 10.1227/00006123-198302000-00004. [DOI] [PubMed] [Google Scholar]
- 28.Hoh BL, Carter BS, Budzik RF, Putman CM, Ogilvy CS. Results after surgical and endovascular treatment of paraclinoid aneurysms by a combined neurovascular team. Neurosurgery. 2001;48:78–89. doi: 10.1097/00006123-200101000-00014. discussion 89-90. [DOI] [PubMed] [Google Scholar]
- 29.Hopkins LN, Grand W. Extracranial-intracranial arterial bypass in the treatment of aneurysms of the carotid and middle cerebral arteries. Neurosurgery. 1979;5:21–31. doi: 10.1227/00006123-197907010-00004. [DOI] [PubMed] [Google Scholar]
- 30.Horowitz MB, Levy E, Kassam A, Purdy PD. Endovascular therapy for intracranial aneurysms: a historical and present status review. Surg Neurol. 2002;57:147–158. doi: 10.1016/s0090-3019(01)00701-7. discussion 159. [DOI] [PubMed] [Google Scholar]
- 31.Hosobuchi Y. Direct surgical treatment of giant intracranial aneurysms. J Neurosurg. 1979;51:743–756. doi: 10.3171/jns.1979.51.6.0743. [DOI] [PubMed] [Google Scholar]
- 32.Kattner KA, Bailes J, Fukushima T. Direct surgical management of large bulbous and giant aneurysms involving the paraclinoid segment of the internal carotid artery: report of 29 cases. Surg Neurol. 1998;49:471–480. doi: 10.1016/s0090-3019(97)00374-1. [DOI] [PubMed] [Google Scholar]
- 33.Kobayashi S, Kyoshima K, Gibo H, Hegde SA, Takemae T, Sugita K. Carotid cave aneurysms of the internal carotid artery. J Neurosurg. 1989;70:216–221. doi: 10.3171/jns.1989.70.2.0216. [DOI] [PubMed] [Google Scholar]
- 34.Korosue K, Heros RC. "Subclinoid" carotid aneurysm with erosion of the anterior clinoid process and fatal intraoperative rupture. Neurosurgery. 1992;31:356–359. doi: 10.1227/00006123-199208000-00024. discussion 359-360. [DOI] [PubMed] [Google Scholar]
- 35.Kothandaram P, Dawson BH, Kruyt RC. Carotid-ophthalmic aneurysms. A study of 19 patients. J Neurosurg. 1971;34:544–548. doi: 10.3171/jns.1971.34.4.0544. [DOI] [PubMed] [Google Scholar]
- 36.Kuether TA, Nesbit GM, Barnwell SL. Clinical and angiographic outcomes, with treatment data, for patients with cerebral aneurysms treated with Guglielmi detachable coils: a single-center experience. Neurosurgery. 1998;43:1016–1025. doi: 10.1097/00006123-199811000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Locksley HB. Natural history of subarachnoid hemorrhage, intracranial aneurysms and arteriovenous malformations. Based on 6368 cases in the cooperative study. J Neurosurg. 1966;25:219–239. doi: 10.3171/jns.1966.25.2.0219. [DOI] [PubMed] [Google Scholar]
- 38.McDougall CG, Halbach VV, Dowd CF, Higashida RT, Larsen DW, Hieshima GB. Endovascular treatment of basilar tip aneurysms using electrolytically detachable coils. J Neurosurg. 1996;84:393–399. doi: 10.3171/jns.1996.84.3.0393. [DOI] [PubMed] [Google Scholar]
- 39.Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267–1274. doi: 10.1016/s0140-6736(02)11314-6. [DOI] [PubMed] [Google Scholar]
- 40.Murayama Y, Vinuela F, Duckwiler GR, Gobin YP, Guglielmi G. Embolization of incidental cerebral aneurysms by using the Guglielmi detachable coil system. J Neurosurg. 1999;90:207–214. doi: 10.3171/jns.1999.90.2.0207. [DOI] [PubMed] [Google Scholar]
- 41.Nishioka H. Report on the cooperative study of intracranial aneurysms and subarachnoid hemorrhage. Section VII. I. Evaluation of the conservative management of ruptured intracranial aneurysms. J Neurosurg. 1966;25:574–592. doi: 10.3171/jns.1966.25.5.0574. [DOI] [PubMed] [Google Scholar]
- 42.Nutik S. Carotid paraclinoid aneurysms with intradural origin and intracavernous location. J Neurosurg. 1978;48:526–533. doi: 10.3171/jns.1978.48.4.0526. [DOI] [PubMed] [Google Scholar]
- 43.Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34:1398–1403. doi: 10.1161/01.STR.0000073841.88563.E9. [DOI] [PubMed] [Google Scholar]
- 44.Raymond J, Roy D. Safety and efficacy of endovascular treatment of acutely ruptured aneurysms. Neurosurgery. 1997;41:1235–1245. doi: 10.1097/00006123-199712000-00002. discussion 1245-1246. [DOI] [PubMed] [Google Scholar]
- 45.Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001;32:1998–2004. doi: 10.1161/hs0901.095600. [DOI] [PubMed] [Google Scholar]
- 46.Roy D, Raymond J, Bouthillier A, Bojanowski MW, Moumdjian R, L'Esperance G. Endovascular treatment of ophthalmic segment aneurysms with Guglielmi detachable coils. AJNR Am J Neuroradiol. 1997;18:1207–1215. [PMC free article] [PubMed] [Google Scholar]
- 47.Sengupta RP, Gryspeerdt GL, Hankinson J. Carotid-ophthalmic aneurysms. J Neurol Neurosurg Psychiatry. 1976;39:837–853. doi: 10.1136/jnnp.39.9.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Solander S, Ulhoa A, Vinuela F, Duckwiler GR, Gobin YP, Martin NA, et al. Endovascular treatment of multiple intracranial aneurysms by using Guglielmi detachable coils. J Neurosurg. 1999;90:857–864. doi: 10.3171/jns.1999.90.5.0857. [DOI] [PubMed] [Google Scholar]
- 49.Spetzler RF, Schuster H, Roski RA. Elective extracranial-intracranial arterial bypass in the treatment of inoperable giant aneurysms of the internal carotid artery. J Neurosurg. 1980;53:22–27. doi: 10.3171/jns.1980.53.1.0022. [DOI] [PubMed] [Google Scholar]
- 50.Sundt TM, Jr, Piepgras DG. Surgical approach to giant intracranial aneurysms. Operative experience with 80 cases. J Neurosurg. 1979;51:731–742. doi: 10.3171/jns.1979.51.6.0731. [DOI] [PubMed] [Google Scholar]
- 51.Thornton J, Aletich VA, Debrun GM, Alazzaz A, Misra M, Charbel F, et al. Endovascular treatment of paraclinoid aneurysms. Surg Neurol. 2000;54:288–299. doi: 10.1016/s0090-3019(00)00313-x. [DOI] [PubMed] [Google Scholar]
- 52.Thurel C, Rey A, Thiebaut JB, Chai N, Houdart R. [Carotid-ophthalmic aneurysms] Neurochirurgie. 1974;20:25–39. [PubMed] [Google Scholar]
- 53.Yasargil MG, Fox JL. The microsurgical approach to intracranial aneurysms. Surg Neurol. 1975;3:7–14. [PubMed] [Google Scholar]
- 54.Yasargil MG, Gasser JC, Hodosh RM, Rankin TV. Carotid-ophthalmic aneurysms: direct microsurgical approach. Surg Neurol. 1977;8:155–165. [PubMed] [Google Scholar]