Abstract
In this study, the authors used the Facial Action Coding System (FACS; P. Ekman & W. V. Friesen, 1978) to examine the immediate facial responses of abstinent smokers exposed to smoking cues. The aim was to investigate whether facial expressions thought to be linked to ambivalence would relate to more traditional measures of ambivalence about smoking. The authors adapted N. A. Heather's (1998) definition of ambivalence about smoking, which emphasizes difficulty in refraining from smoking despite intentions to do so. Ambivalence expressed during smoking cue exposure was operationalized as the simultaneous occurrence of positive and negative affect-related facial expressions. Thirty-four nicotine-deprived dependent smokers were presented with in vivo smoking cues, and their facial expressions were coded using FACS. Participants also completed self-report measures related to ambivalence about smoking. Smokers who displayed ambivalent facial expressions during smoking cue exposure reported significantly higher scores on measures of smoking ambivalence than did those who did not display ambivalent facial expressions.
Keywords: ambivalence, smoking cue reactivity, facial expression, FACS
An extensive literature reveals that smokers show increased reactivity to laboratory smoking cues (see Carter & Tiffany, 1999). These responses interest researchers in part because it is assumed that reactions to cues in the laboratory relate to naturally occurring addictive processes. Indeed, several studies have reported associations between various measures of cue-elicited reactivity and clinical outcome (Abrams, Monti, Carey, Pinto, & Jacobus, 1988; Niaura, Abrams, Demuth, Pinto, & Monti, 1989; Waters et al., 2003).
Ambivalence and conflict have been recognized as central features of drug addiction (American Psychiatric Association, 1994; Cox & Klinger, 1988; Heather, 1998; Miller & Rollnick, 1991; Shaffer, 1992). An individual experiencing both approach and avoidance inclinations about drug use is thought to be ambivalent (Breiner, Stritzke, & Lang, 1999). Behaviorally, ambivalence has been defined as repeated failures to refrain from substance use despite intentions to do so (Heather, 1998). It is important to note that one's level of ambivalence is related to readiness to change a health-related behavior (Lipkus et al., 2005; Stritzke, Breiner, Curtin, & Lang, 2004), with the most ambivalence experienced during the contemplative stage of behavior change (see Armitage, Povey, & Arden, 2003; Prochaska, DiClemente, & Norcross, 1997). Therefore, inducing ambivalence may motivate behavior change (e.g., motivational interviewing; Miller & Rollnick, 1991), but ultimately it is the resolution of this ambivalence that may prevent relapse (Armitage et al., 2003).
Despite the emphasis on ambivalence in models of drug addiction, most cue-reactivity research has considered cue-elicited craving to reflect only a desire to acquire or use a drug (see Sayette et al., 2000). Recently, though, there has been a move toward an ambivalence framework of cue-elicited drug craving, which allows for competing inclinations to approach and avoid drug consumption (e.g., Breiner et al., 1999). Although approach and avoidance reactivity have related to difficulty quitting smoking and the desire to do so (Stritzke et al., 2004), investigators have tended to analyze these components separately rather than focusing on the joint increase in both approach and avoidance inclinations (i.e., ambivalence). In addition, most studies have relied solely on self-reports of these conflicting inclinations (e.g., Avants, Margolin, Kosten, & Cooney, 1995).
Many theorists agree that there is a need to investigate the concurrent nonverbal responses associated with these cue-elicited responses (Abrams, 2000; Anton, 1999; McEvoy, Stritzke, French, Lang, & Ketterman, 2004). This is especially true for the emotional experience of ambivalence, as it is posited to be short-lived and unstable (J. T. Larsen, McGraw, & Cacioppo, 2001). Indeed, people are likely limited in their ability to consciously acknowledge and report on their own ambivalence (Bassili, 1996; Cacioppo, Gardner, & Berntson, 1999). For this reason, systematic coding of facial expressions may be a particularly useful approach to examining the ambivalence experienced during cue exposure (referred to herein as cue-induced ambivalence, or AMB), as expressive-behavioral assessments arguably offer a more basic and direct measure of emotion than do self-report formats (Barlow, 2002). The most comprehensive of these approaches is the Facial Action Coding System (FACS; Ekman & Friesen, 1978), which is an anatomically based system that allows all possible facial displays, referred to as action units (AUs), to be coded (Ekman, Friesen, & Hager, 2002). Although this technique is labor intensive, it provides an objective, reliable, and fairly unobtrusive method of measuring facial behavior over extremely rapid time frames (Sayette, Cohn, Wertz, Perrott, & Parrott, 2001). FACS can detect barely visible signs of emotion (Ekman & Friesen, 1975), and its use during cue exposure can provide information that might not be available via self-report.
The potential value of FACS to reveal unique information about clinical outcomes has been demonstrated in several areas of psychopathology. For instance, facial expressions during an intake interview outperformed clinical ratings by expert clinicians in predicting improvement for patients with mood disorders (Ekman, Matsumoto, & Friesen, 2005). In addition, FACS has revealed important links between particular facial expressions and schizophrenia, depression, adolescent psychopathology, and cardiovascular disease (see Ekman & Rosenberg, 2005). These studies suggest that facial expressions may provide unique and meaningful information related to clinical outcomes beyond what is available via self-report. Past research also has identified patterns of positive and negative affect associated with cigarette cravings (e.g., Sayette & Hufford, 1995), but to date there has been no examination of AMB using FACS.
The use of FACS to examine AMB also may help to evaluate theories of basic emotion (J. T. Larsen, Norris, & Cacioppo, 2003). There is an ongoing debate about the link between positive and negative emotions and, in particular, about whether these two classes of emotions can be experienced simultaneously (Diener, 1999). The idea that smokers exposed to smoking cues may experience positive and negative affect simultaneously is compatible with the evaluative space model of the affect system (Cacioppo & Berntson, 1994; Cacioppo et al., 1999). In contrast, the more traditional circumplex model cannot accommodate the cooccurrence of particular positive and negative emotions (e.g., happy and sad) at any one time (Russell & Carroll, 1999). Although consensus has yet to be reached, this debate has implications for addiction theories. Smoking researchers typically use scales that are based on the circumplex model of affect (e.g., Shiffman, Waters, & Hickox, 2004), and this makes it impossible to find evidence for the simultaneous experience of positive and negative affect (see Sayette et al., 2000). To our knowledge, research on the co-occurrence of positive and negative emotions has yet to use FACS (J. T. Larsen et al., 2003).
In summary, we sought to use FACS to code smokers' facial reactivity during in vivo cue exposure to examine a particular response that involved the simultaneous activation of expressions linked to both positive and negative affect. We hypothesized that smokers evincing AMB in the laboratory would report significantly higher scores on more traditional real-world measures associated with ambivalence about smoking than would those who did not display AMB. Specifically, we predicted that smokers displaying AMB would report significantly more difficulty in refraining from smoking, coupled with significantly greater interest in quitting smoking, than would smokers who did not show AMB.
Method
Participants
Thirty-four smokers (19 men and 15 women) ages 21-35 years participated in the study. These participants made up the entire nicotine-deprived heavy smoker group described previously in Sayette et al., 2003. Their ethnic background was as follows: 82% Caucasian, 9% African American, and 9% other. Exclusion criteria included medical conditions that contraindicated nicotine ethically and illiteracy. Participants had to report smoking an average of 21 or more cigarettes/day for at least 24 continuous months (Shiffman, Paty, Kassel, Gnys, & Zettler-Segal, 1994). They had to have carbon monoxide levels that did not exceed 16 parts per million (M = 9.38, SD = 3.58) to ensure that they abstained from smoking for 7 hr prior to the experiment. Participants' mean age was 25.21 years (SD = 4.42). They averaged 14.35 years of formal education (SD = 1.98), 9.49 years of smoking (SD = 4.97), 24.41 cigarettes per day (SD = 5.33), and 6.53 prior quit attempts (SD = 3.14).
Procedure
Telephone screening and instructions
Participants who responded to advertisements recruiting smokers for a research study underwent a phone interview to exclude those not meeting selection criteria. Eligible smokers were asked to attend a 2-hr lab session. Participants were instructed to refrain from smoking for at least 7 hr and were told that breath samples would test whether they had abstained. They were told to bring a pack of their preferred brand of cigarettes with them.
Laboratory setup
Participants underwent the cue exposure manipulation while seated in a comfortable chair behind a desk. Facing the desk was a mounted video camera. Participants were told that the camera and intercom facilitated communication and helped the investigator determine whether instructions were understood throughout the study.
Baseline assessment
Experimental sessions began between 3:00 p.m. and 5:00 p.m. On participants' arrival, written informed consent was obtained. To confirm abstinence, participants reported the last time they smoked, and a carbon monoxide reading was recorded. Participants presented their pack of cigarettes and lighter to the experimenter, then completed a baseline assessment.
Cue exposure
Prior to cue exposure, participants were instructed on how to perform a simple response-time task, which involved clicking a mouse button whenever a tone sounded (Sayette et al., 2003). Next, a tray holding an inverted plastic bowl was placed on the desk. Participants then lifted the bowl, which revealed a role of tape. They were asked to hold the tape and look at it. After 34 s, participants rated their urge to smoke on a scale ranging from 0 (labeled absolutely no urge to smoke at all)to 100 (labeled strongest urge to smoke I've ever experienced). Two min later, the experimenter replaced the tray and bowl with a second tray and bowl. Participants then lifted the bowl, which revealed their pack of cigarettes, an ashtray, and a lighter. They were told to remove one cigarette from the pack and light it without putting it in their mouth. They then held the lit cigarette and looked at it. After 31 s, they again rated their urge to smoke. They then extinguished the cigarette and completed several additional measures reported elsewhere (for further details, see Sayette et al., 2003). Finally, participants completed a form about the study's purpose, were debriefed, and were paid $45.
Baseline Measures
Demographic information and smoking history and patterns were assessed with standard forms (see Sayette et al., 2003) prior to starting the experiment. Several measures putatively associated with smoking ambivalence were included in the baseline battery. Heather (1998) defined ambivalence as repeated failures to refrain from substance use despite intentions to do so. Therefore, questions related to (a) difficulty experienced when attempting to refrain from smoking and (b) interest in quitting smoking were examined. (Participants did not directly report their ambivalence about smoking; rather, consistent with Heather's approach, their responses to the aforementioned questions allowed us to draw inferences about their ambivalence.)
Difficulty refraining from smoking
Difficulty refraining from smoking was assessed using two variables, past severity of withdrawal and difficulty abstaining. Severity of past withdrawal symptoms experienced when attempting to refrain from smoking was assessed by asking participants to recall their experience when they had quit smoking, cut down on smoking, or gone without smoking for a while (see Shiffman et al., 2004). As noted by Shiffman et al. (2004), this wording was chosen so that withdrawal history could be obtained from those who have not previously succeeded in quitting smoking. Withdrawal symptoms were assessed on scales ranging from 1 to 5 applied to six individual symptoms (craving, irritability, nervousness, difficulty concentrating, physical symptoms, and sleep disturbance), which were averaged to form a reliable composite (α = .77). Participants who endorsed at least one previous quit attempt (n = 25) also rated the following question on a 4-point scale (1 = easy, 2 = slightly difficult, 3 = difficult, 4 = very difficult): “How hard was it for you to quit smoking on your most recent attempt?”
Interest in quitting smoking
Participants were asked to rate their current interest in quitting on a 10-point scale (1 = not at all interested and 10 = extremely interested).
Cue Exposure Measure: Facial Coding
Facial expressions were coded by a FACS-certified coder during three time periods of cigarette cue exposure and two time periods of control cue exposure. One hundred fifty consecutive frames (5s) were coded when participants initially saw the cigarette and when each participant initially touched the cigarette. Three hundred consecutive frames were also coded when each participant initially held the cigarette. During control cue exposure, 150 consecutive frames were coded when participants initially saw the tape and when each participant initially touched the tape. Specific AUs and AU combinations were classified as positive affect-related AUs (positive AUs) or negative affect-related AUs (negative AUs) on the basis of a review of FACS literature. The following AUs and AU configurations were coded as positive: 12 and 6 + 12 (smile with cheek raise), both of which could be accompanied by 1 + 2 (inner and outer brow raise), 25 (lips part), or 26 (jaw drop; Ekman, Friesen, & Ancoli, 1980; Sayette & Parrott, 1999). For expressions to be considered positive, AU 12 (the contraction of zygomatic major, in which the corners of the lips are raised) had to receive a minimum intensity rating of b using Friesen and Ekman's (1992) a to e intensity scale. Negative AUs were defined by the presence of at least one of the following AUs: 9 (nose wrinkle), 10 (upper lip raise), unilateral 14 (dimpler), 15 (lip corner depress), 20 (lip stretch), and 1 + 4 (pulling the medial portion of the eyebrows upward and together). These AUs are thought to appear during the expression of negative emotion (Ekman & Friesen, 1982, 1986; Ekman et al., 1980; Sayette & Parrott, 1999). For negative AUs, a minimum intensity rating of b was required to meet criteria (Friesen & Ekman, 1992).
AMB was defined as the simultaneous occurrence of both a positive AU and a negative AU (as described above). These expressions had to remain on the face for at least 10 frames to ensure reliable coding of each AU (Sayette et al., 2001). We did not require that the onset of positive and negative AUs had to occur within the same frame, as long as they both remained visible simultaneously. Reliability was tested using comparison coding by a second FACS-certified coder of a random sample of 15% of the total coding periods. Kappa coefficients showed that POS AUs (.90) and NEG AUs (.69) were coded reliably.
Results
Facial data are presented for 33 smokers (1 smoker was not recorded because of experimenter error). Twenty-four percent displayed AMB during smoking cue exposure (see Figure 1 for an example of a prototypical AMB expression). The mean duration of AMB was 55.88 frames (SD = 44.54). Because the duration of AMB expressions was not normally distributed (skew = 5.74), AMB was coded categorically. In most (75%) of the AMB expressions, the onset of positive and negative AUs occurred in the exact same frame (i.e., 1/30 s). For the remaining 25%, a positive AU was evinced first, which remained on the face while a negative AU was also displayed. It is important to note that AMB reactions were specific to the cigarette cue, as none of the participants displayed this facial configuration during control cue exposure. All but one of the AMB expressions evinced during cigarette cue exposure occurred during the first two coding intervals (i.e., the identical coding periods of control cue exposure where zero AMB expressions were observed).
Figure 1.
Example of a prototypical cue-induced ambivalence expression (action units = 6 + 10 + 12 + 15).
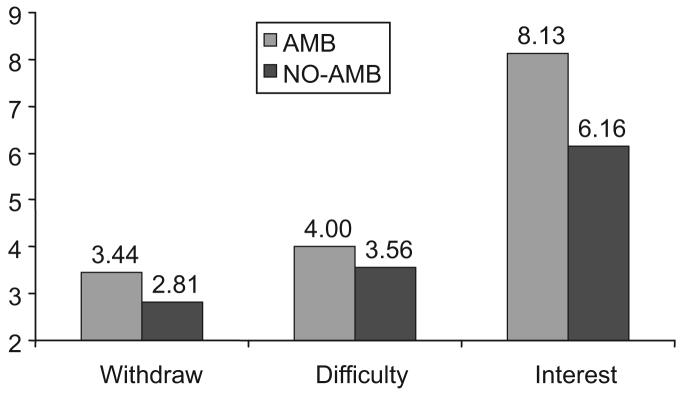
We conducted t tests to contrast smokers who did (AMB smokers) and those who did not (NO-AMB smokers) display AMB on increased desire to quit smoking and increased difficulty doing so. As expected (Heather, 1998), these two components of smoking ambivalence were independent (for desire to quit and withdrawal symptoms, r = .03, p > .85; for desire to quit and difficulty abstaining, r = .15, p > .47), and they were analyzed separately. Levene's test for the equality of variances indicated that the sample variances were unequal for two of the three dependent variables (for difficulty abstaining, F = 14.95, p = .001; for interest in quitting, F = 11.16, p = .002). To address this homoscedasticity, we used the Welch-Aspen t test for these two variables (Glass & Hopkins, 1995). Figure 2 presents smoking ambivalence scores for AMB and NO-AMB smokers. AMB smokers indicated that they had significantly more severe withdrawal symptoms when refraining from smoking than did NO-AMB smokers, t(31) = 2.18, p < .04, and AMB smokers who endorsed one previous quit attempt (n = 5) reported significantly more difficulty abstaining than did NO-AMB smokers who endorsed one previous quit attempt (n = 20), Welch-Aspen t(19) = 2.93, p < .01. AMB smokers also reported significantly greater interest in quitting than did NO-AMB smokers, Welch-Aspen t(28) = 2.44, p < .02.
Figure 2.
Mean smoking ambivalence scores for smokers who did (AMB) and those who did not (NO-AMB) display cue-induced ambivalence. Scores for Withdraw (representing withdrawal symptoms) ranged from 1 to 5, scores for Difficulty (representing difficulty abstaining from smoking) ranged from 1 to 4, and scores for Interest (representing interest in quitting smoking) ranged from 1 to 10.
We also examined the association between reporting high levels on the self-report measures (i.e., scoring high on both withdrawal and desire to quit) and AMB. (Note that we used withdrawal rather than difficulty abstaining in the following analysis because we had responses for all participants only for the former variable.) We categorized (using a median split) participants as being either high on both withdrawal and desire to quit (n = 10) or low on at least one of the components (n = 23). There was a significant point-biserial correlation between this categorization of the self-report measures and AMB (r = .40, p = .02). This reinforces the view that AMB was related to smoking ambivalence.
To determine whether AMB might just have been a proxy for nicotine dependence, we conducted t tests to contrast AMB and NO-AMB smokers on several widely used measures of nicotine dependence. AMB smokers were not different from NO-AMB smokers on variables related to dependence—that is, the Fagerström Test for Nicotine Dependence (AMB smoker M = 3.5, SD = 1.4, NO-AMB smoker M = 4.28, SD = 1.7), t(31) = 1.19, p = .24; latency to first cigarette of the day (AMB smoker M = 13.8 min, SD = 8.6, NO-AMB smoker M = 22.2 min, SD = 20.1), t(31) = 1.14, p = .26; and number of cigarettes smoked per day (AMB smoker M = 23 cigarettes, SD = 5.1, NO-AMB smoker M = 24.4 cigarettes, SD = 5.1), t(31) = 0.69, p = .49—suggesting that AMB was not simply capturing increased nicotine dependence.
Discussion
Smokers who reacted to in vivo cigarette cues with concurrent positive and negative affect-related facial expressions reported significantly higher scores on our measures of smoking ambivalence than did smokers who did not display this facial configuration. Specifically, those smokers displaying this AMB response reported increased severity of withdrawal symptoms when abstaining from smoking and more difficulty quitting smoking in their most recent quit attempt while also reporting a higher current interest in quitting than did those who did not express AMB. These findings were strengthened by the observation of a significant point-biserial correlation between AMB and the self-report measures. Finally, AMB was related to smoking ambivalence specifically and was not merely a proxy for nicotine dependence.
Current theoretical models note the importance of ambivalence in addiction (e.g., Heather, 1998), and accumulating data suggest the appropriateness of assessing conflicting (i.e., approach and avoidance) reactions during craving episodes (e.g., Breiner et al., 1999; Stritzke et al., 2004). Past studies on ambivalence about smoking are limited, however, because they have relied on self-report instruments. Because people are likely limited in their ability to consciously acknowledge and report on their own ambivalence (Bassili, 1996; Cacioppo et al., 1999), the use of FACS may provide an especially sensitive and reliable index of smoking ambivalence. Facial affect has long been recognized as providing important information about emotional experiences that may not be captured by traditional self-report assessments (e.g., Ekman et al., 2005). Although FACS is demanding, the coding system has predicted important clinical outcomes in several other areas of psychopathology (Ekman & Rosenberg, 2005). The present findings highlight the potential utility of FACS to detect information related to ambivalence about smoking, which potentially could be used to predict smoking outcomes.
In addition to advancing understanding of ambivalence in drug addiction, the current findings also relate to a basic question regarding emotion research. Specifically, this study suggests that it is possible for displays of positive and negative affect-related facial expressions to appear simultaneously. The onset of positive and negative AUs occurred in the exact same frame for most of the AMB expressions, suggesting that a rapid sequencing of expressions did not reflect AMB (Ekman, 1993). To the extent that facial expressions indicate underlying feeling states (Ekman et al., 1980; Ekman & Rosenberg, 2005), these findings converge with those of prior work (e.g., Cacioppo et al., 1999) to challenge emotion models that exclusively emphasize the bipolarity of negative and positive emotions (e.g., Russell & Carroll, 1999). Emotion researchers have noted the importance of cross-validating self-report data with nonverbal measures of emotions (Cacioppo et al., 1999; R. J. Larsen & Diener, 1992), and our use of FACS provided a microanalytic technique sensitive enough to assess these complex responses in the laboratory as they unfold over time (Rosenberg & Ekman, 1994).
Limitations and Future Directions
The current study was subject to several limitations. First, the sample size was small. Given the time demands associated with FACS coding and the preliminary nature of the present study, we chose to initially investigate this measure of AMB in a sample that was especially likely to experience ambivalence (i.e., nicotine-deprived heavy smokers who were exposed to a potent smoking cue). In future studies, researchers might attempt to replicate these findings with a larger sample size and to assess facial reactivity in nonabstinent states. Larger studies also could be used to examine the impact of potential covariates, such as nicotine dependence. In addition, larger samples presumably would permit analyses of potential differences between AMB expressions that do versus those that do not start at the same frame.
Second, participants were relatively young and were not actively trying to quit smoking at the time of recruitment. Nevertheless, participants did report a wide range of interest in quitting, something not typically observed in participants presenting for treatment because of ceiling effects (e.g., Gwaltney, Shiffman, & Sayette, 2005). It would be important, though, to replicate these findings in a treatment-seeking sample and in other groups of smokers (e.g., nonsmokers, former smokers, or tobacco chippers. If one is studying treatment seekers, the chief variable of interest would likely be restricted to difficulty quitting).
Third, this study used a retrospective design to examine prior difficulties refraining from smoking. Despite this methodological shortcoming, relations were observed between AMB and our measures indexing difficulty refraining from smoking. We believe that this is the first study to link a facial expression evinced during a laboratory-based cue exposure to an index of real-world difficulty controlling smoking. In future studies, researchers could use a prospective design to examine the link between AMB in the laboratory and subsequent smoking relapse. Such research would add to an emerging body of literature that has found other nonverbal measures of cue reactivity to predict smoking relapse (e.g., Waters et al., 2003).
Fourth, the mechanisms underlying why some participants displayed AMB in response to the cigarette cue are not well understood. As noted by Breiner et al. (1999), pathways that influence the desire to approach or avoid substance use include historical factors (e.g., past reinforcement), current factors (e.g., access to alternative valued reinforcers), and expectancies about smoking. Perhaps an ambivalent expression during cue exposure reveals that the smoker has both a negative response linked to the threat of smoking despite knowing it is harmful and a positive reaction associated with a lingering attraction to smoking. Alternatively, the positive and negative reactions evinced by these smokers may not relate equally to the smoking cue. For instance, negative reactions may have captured irritation with not being able to smoke the cigarette on lighting it rather than a negative response to the smoking cue itself. Although our data cannot rule out this alternative explanation, participants expressing this particular facial response reported significantly higher scores on measures of interest in quitting smoking and difficulty quitting smoking than did participants not displaying this mixed expression. Nevertheless, future studies that attempt to elucidate these mechanisms are indicated.
Acknowledgments
This research was supported by Grant R01 DA10605 from the National Institute on Drug Abuse. We thank Eric Donny and Jeff Cohn for their helpful comments and the staff of the Alcohol and Smoking Research Laboratory for their assistance.
References
- Abrams DB. Transdisciplinary concepts and measure of craving: Commentary and future directions. Addiction. 2000;95:237–246. doi: 10.1080/09652140050111807. [DOI] [PubMed] [Google Scholar]
- Abrams DB, Monti PM, Carey KB, Pinto RP, Jacobus SI. Reactivity to smoking cues and relapse: Two studies of discriminant validity. Behavioral Research and Therapy. 1988;26:225–233. doi: 10.1016/0005-7967(88)90003-4. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed. Author; Washington, DC: 1994. [Google Scholar]
- Anton RF. What is craving? Models and implications for treatment. Alcohol Research & Health. 1999;23(3):165–173. [PMC free article] [PubMed] [Google Scholar]
- Armitage CJ, Povey R, Arden MA. Evidence for discontinuity patterns across the stages of a change: A role for attitudinal ambivalence. Psychology and Health. 2003;18:373–386. [Google Scholar]
- Avants KS, Margolin A, Kosten TR, Cooney NL. Differences between responders and nonresponders to cocaine cues in the laboratory. Addictive Behaviors. 1995;20:215–224. doi: 10.1016/0306-4603(94)00066-2. [DOI] [PubMed] [Google Scholar]
- Barlow DH. Anxiety and its disorders. 2nd ed. Guilford Press; New York: 2002. [Google Scholar]
- Bassili JN. Meta-judgmental versus operative indexes of psychological attributes: The case measures of attitude strength. Journal of Personality and Social Psychology. 1996;71:637–653. [Google Scholar]
- Breiner MJ, Stritzke WGK, Lang AR. Approaching avoidance: A step essential to the understanding of craving. Alcohol Research and Health. 1999;23:197–206. [PMC free article] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG. Relationship between attitudes and evaluative space: A critical review, with emphasis on the separability of positive and negative substrates. Psychological Bulletin. 1994;115:401–423. [Google Scholar]
- Cacioppo JT, Gardner WL, Berntson GG. The affect system has parallel and integrative processing components: Form follows function. Journal of Personality and Social Psychology. 1999;76:839–855. [Google Scholar]
- Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- Cox WM, Klinger EA. A motivational model of alcohol use. Journal of Abnormal Psychology. 1988;97:168–180. doi: 10.1037//0021-843x.97.2.168. [DOI] [PubMed] [Google Scholar]
- Diener E. Introduction to the special section on the structure of emotion. Journal of Personality and Social Psychology. 1999;76:803–804. doi: 10.1037//0022-3514.76.5.803. [DOI] [PubMed] [Google Scholar]
- Ekman P. Facial expression and emotion. American Psychologist. 1993;48:384–392. doi: 10.1037//0003-066x.48.4.384. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Unmasking the face: A guide to recognizing emotions from facial clues. Prentice Hall; Englewood Cliffs, NJ: 1975. [Google Scholar]
- Ekman P, Friesen WV. Facial action coding system. Consulting Psychologists Press; Palo Alto, CA: 1978. [Google Scholar]
- Ekman P, Friesen WV. Rationale and reliability for EMFACS coders. 1982. Unpublished manuscript. [Google Scholar]
- Ekman P, Friesen WV. A new pan-cultural facial expression of emotion. Motivation and Emotion. 1986;10:159–168. [Google Scholar]
- Ekman P, Friesen WV, Ancoli S. Facial signs of emotional experience. Journal of Personality and Social Psychology. 1980;39:1125–1134. [Google Scholar]
- Ekman P, Friesen WV, Hager JC. The Facial Action Coding System. Consulting Psychologists Press; Palo Alto, CA: 2002. [Google Scholar]
- Ekman P, Matsumoto D, Friesen WV. Facial expression in affective disorders. In: Ekman P, Rosenberg E, editors. What the face reveals: Basic and applied studies of spontaneous expression using the Facial Action Coding System (FACS) 2nd ed. Oxford University Press; New York: 2005. pp. 331–342. [Google Scholar]
- Ekman P, Rosenberg E. What the face reveals: Basic and applied studies of spontaneous expression using the facial action coding system (FACS) Oxford University Press; New York: 2005. [Google Scholar]
- Friesen WV, Ekman P. Changes in FACS scoring. University of California; San Francisco: 1992. Unpublished manuscript. [Google Scholar]
- Glass GV, Hopkins KD. Statistical methods in education and psychology. Allyn & Bacon; Boston: 1995. [Google Scholar]
- Gwaltney CJ, Shiffman S, Sayette MA. Situational correlates of abstinence self-efficacy. Journal of Abnormal Psychology. 2005;114:644–660. doi: 10.1037/0021-843X.114.4.649. [DOI] [PubMed] [Google Scholar]
- Heather NA. A conceptual framework for explaining drug addiction. Journal of Psychopharmacology. 1998;12:3–7. doi: 10.1177/026988119801200101. [DOI] [PubMed] [Google Scholar]
- Larsen JT, McGraw AP, Cacioppo JT. Can people feel happy and sad at the same time? Journal of Personality and Social Psychology. 2001;81:684–696. [PubMed] [Google Scholar]
- Larsen JT, Norris CJ, Cacioppo JT. Effects of positive and negative affect on electromyographic activity over zygomaticus major and corrugator supercilii. Psychophysiology. 2003;40:776–785. doi: 10.1111/1469-8986.00078. [DOI] [PubMed] [Google Scholar]
- Larsen RJ, Diener E. Promises and problems with the circumplex model of emotion. In: Clark MS, editor. Review of personality and social psychology: Emotion. Vol. 13. Sage; Newbury Park, CA: 1992. pp. 25–29. [Google Scholar]
- Lipkus IM, Pollak KI, McBride CM, Schwartz-Bloom R, Lyna P, Bloom PN. Assessing attitudinal ambivalence towards smoking and its association with desire to quit among teen smokers. Psychology and Health. 2005;20:373–387. [Google Scholar]
- McEvoy PM, Stritzke WGK, French DJ, Lang AR, Ketterman RL. Comparison of three models of alcohol craving in young adults: A cross-validation. Addiction. 2004;99:482–497. doi: 10.1111/j.1360-0443.2004.00714.x. [DOI] [PubMed] [Google Scholar]
- Miller WR, Rollnick S. Motivational interviewing. Guilford Press; New York: 1991. [Google Scholar]
- Niaura RS, Abrams DB, Demuth B, Pinto R, Monti PM. Responses to smoking-related stimuli and early relapses to smoking. Addictive Behaviors. 1989;14:419–428. doi: 10.1016/0306-4603(89)90029-4. [DOI] [PubMed] [Google Scholar]
- Prochaska JO, DiClemente CC, Norcross JC. In search of how people change: Applications to addictive behaviors. In: Marlatt GA, VandenBos GR, editors. Addictive behaviors: Reading on etiology, prevention, and treatment. American Psychological Association; Washington, DC: 1997. pp. 671–696. [Google Scholar]
- Rosenberg E, Ekman P. Coherence between expressive and experiential systems. Cognition and Emotion. 1994;8:201–229. [Google Scholar]
- Russell JA, Carroll JM. On the bipolarity of positive and negative affect. Psychological Bulletin. 1999;125:3–30. doi: 10.1037/0033-2909.125.1.3. [DOI] [PubMed] [Google Scholar]
- Sayette MA, Cohn JF, Wertz JM, Perrott MA, Parrott DJ. A psychometric evaluation of the Facial Action Coding System for assessing spontaneous expression. Journal of Nonverbal Behavior. 2001;25:167–185. [Google Scholar]
- Sayette MA, Hufford MR. Urge and affect: A facial coding analysis of smokers. Experimental and Clinical Psychopharmacology. 1995;3:417–423. [Google Scholar]
- Sayette MA, Parrott DJ. Effects of olfactory stimuli on urge reduction in smokers. Experimental and Clinical Psychopharmacology. 1999;7:151–159. doi: 10.1037//1064-1297.7.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Shiffman S, Tiffany ST, Niaura RS, Martin CS, Shadel WG. The measurement of drug craving. Addiction. 2000;95(8 Suppl 2):S189–S210. doi: 10.1080/09652140050111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayette MA, Wertz JM, Martin CS, Cohn JF, Perrott MA, Hobel J. Effects of smoking opportunity on cue-elicited urge: A facial coding analysis. Experimental and Clinical Psychopharmacology. 2003;11:218–227. doi: 10.1037/1064-1297.11.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer HJ. The psychology of stage change: The transition from addiction to recovery. In: Lowinson JH, Ruiz P, Millman RB, Langrod JG, editors. Substance abuse: A comprehensive textbook. 2nd ed. Williams & Wilkins; Baltimore: 1992. pp. 100–105. [Google Scholar]
- Shiffman S, Paty JA, Kassel JD, Gnys M, Zettler-Segal M. Smoking behavior and smoking history of tobacco chippers. Experimental and Clinical Psychopharmacology. 1994;2:126–142. [Google Scholar]
- Shiffman S, Waters AJ, Hickox M. The Nicotine Dependence Syndrome Scale: A multidimensional measure of nicotine dependence. Nicotine & Tobacco Research. 2004;6:327–348. doi: 10.1080/1462220042000202481. [DOI] [PubMed] [Google Scholar]
- Stritzke WG, Breiner MJ, Curtin JJ, Lang AR. Assessment of substance cue reactivity: Advances in reliability, specificity, and validity. Psychology of Addictive Behaviors. 2004;18:148–159. doi: 10.1037/0893-164X.18.2.148. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Attentional bias predicts outcome in smoking cessation. Health Psychology. 2003;22:378–387. doi: 10.1037/0278-6133.22.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]