Abstract
Background & Aims
Raf-1 kinase is a key regulator of a number of cellular processes, which promote the maintenance of a healthy colon epithelium. This study addresses the role of Raf in epithelial cell survival in response to dextran sulfate sodium (DSS)-induced injury and inflammation.
Methods
Inducible intestinal epithelium-specific Raf knockout mice were generated and subjected to acute colitis followed by a short recovery period. Colon sections were analyzed by in situ oligo ligation or immunostaining for Ki67, phospho-ERK and NF-κB p65. Western blot analysis and TUNEL assays were performed on Raf siRNA-transfected YAMC cells following DSS treatment.
Results
We report that Raf protects against epithelial injury and inflammation and promotes recovery from acute dextran sulfate sodium (DSS)-induced colitis by both MEK-dependent and –independent pathways. Furthermore, we demonstrate that Raf induces novel cell survival responses through activating NF-κB in a MEK-independent manner.
Conclusions
These novel findings indicate a protective role for Raf in colon epithelium following ulcerative damage through inhibiting cell apoptosis and promoting proliferation with important implications for responses such as inflammation-associated carcinogenesis.
Introduction
The intestinal epithelium is a single cell layer that forms the only barrier between the body and the luminal gastrointestinal contents. Maintenance of this cell layer, which turns over every 3–5 days, is dependent upon tight regulation of proliferation and apoptosis1. Coordinated activation of signaling molecules involved in the regulation of these processes is essential to maintain intestinal homeostasis.
In the current study, we test the hypothesis that Raf-1 protects the colon epithelium during injury and inflammation through activation of an NF-κB-dependent survival mechanism. The most widely studied family member of the Raf family of serine/threonine kinases, Raf-1, is involved in key cellular processes including proliferation, differentiation, and survival. Its function is best understood in the context of the highly conserved mitogen-activated protein kinase (MAPK) cascade, in which Raf phosphorylates its substrate MEK, which in turn activates ERK to promote proliferation and differentiation. In addition, Raf regulates MAPK-independent responses to promote cell survival through various cell context-dependent mechanisms. Raf appears essential for mammalian development as Raf-1 knockout mice die between days E9.5–10.5 due to placental defects and Fas-mediated apoptosis in the fetal liver2, 3. In contrast to the Fas-dependent mechanism shown in the liver, Raf-null macrophages are more sensitive to S. typhimurium-induced apoptosis through caspase-1 activation4. Raf functions as an anti-apoptotic regulator in cardiomyocytes through inhibition of the pro-apoptotic kinase, ASK-15.
Using the IL-10−/− mouse model, which develops spontaneous colitis associated with unchecked TNF production6, we have shown that Raf threonine phosphorylation is increased during active disease7. In the current study, we utilize a well-characterized model of chemically induced colitis8–10 to better understand the role of Raf in regulating response to epithelial injury and mucosal inflammation. Short-term administration of dextran sulfate sodium (DSS) induces acute colitis characterized by epithelial damage, disruption of crypt architecture, and severe inflammatory infiltrate9, 11. While the exact mechanisms by which DSS induces colitis are not well characterized, the physiological response involves increased local production of cytokines11, 12 resulting from increased apoptosis due to loss of barrier function13 or failure to recognize bacterial-derived signals14, 15. Recovery from the acute colitis involves a process during which the damaged epithelium undergoes hyperproliferation to re-establish the epithelial barrier15.
Our data show that intestinal epithelium-specific Raf knockout (Raf KOIE) mice develop worse colitis than their wildtype counterparts. Raf KOIE mice exhibit significantly higher levels of colon epithelial apoptosis and decreased enterocyte proliferation following DSS treatment compared to wildtype. Furthermore, Raf expression is required for ERK and NF-κB activation in DSS-treated mouse colon epithelial cells. While MEK activation is insufficient to promote colon epithelial cell survival, constitutive NF-κB activation rescues DSS-induced apoptosis in the absence of Raf expression. NF-κB activation is attenuated in colon epithelial cells following DSS-induced injury in Raf KOIE mice compared to wildtype. These results demonstrate that Raf regulates NF-κB activation and cell survival in a MEK-independent manner to protect against intestinal epithelial injury and inflammation.
Materials and Methods
Mice and DSS colitis
Mice harboring a floxed Raf allele were crossed with villin-Cre ERT2 (inducible) mice. All mice were maintained on a C57BL/6 background. Polymerase chain reaction was performed to confirm the genotype of homozygous Rafflx; vilCre ERT2 mice (as previously described2, 16). Cre recombination in Rafflx; vilCre ERT2 mice was induced at 6 weeks of age by intraperitoneal injection of 1 mg tamoxifen (Sigma) daily for 5 days. Sunflower oil (Sigma (St. Louis, MO)) vehicle was used as the negative control. Experiments began ten days after the last tamoxifen injection to allow effective Cre recombination of the floxed allele. A 3% aqueous solution of dextran sulfate sodium (DSS) (36–50 kD, MP Biomedicals (Solon, OH)) was filtered and administered ad libitum to mice for four or eight days. A subset of these mice was sacrificed at four days and others were allowed to recover on regular water for three or seven days as indicated. As a control, mice were given regular drinking water throughout the treatment period. Mice were weighed and stool samples tested (Hemoccult, Beckman Coulter (Fullerton, CA)) for fecal blood daily. All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee at Vanderbilt University.
Mucosal isolation
Colon or small intestine was scraped with a glass slide as previously described17 and lysates were extracted in 10 volumes of RIPA buffer. Epithelium and stroma were isolated with Cell Recovery Solution (BD Biosciences (San Jose, CA)) for 16 h at 4°C followed by manual shaking and lysis in RIPA buffer18.
Cell culture, lysis and Western blot analysis
Conditionally-immortalized young adult mouse colon cells (YAMC)19 were cultured as previously described7 and plated at a density of 2×105 (6-well plate) or 5×104 (4-well chamber slide, Nunc (Rochester, NY)) and transfected with 50 nmol NF-κB p65, Raf-1 and/or IκBα siRNA SMARTpool or non-targeting siRNA duplexes (Dharmacon (Layafette, CO)) with Lipofectamine RNAi/MAX (Invitrogen (Carlsbad, CA)) according to manufacturer’s directions. Cells were treated with murine TNF 100 ng/ml (Peprotech (Rocky Hill, NJ)) for 15 min or with 5% DSS for 30 min to 7 h. MEK inhibitors, PD98059 (20μM) and U0126 (10μM) were obtained from Calbiochem (San Diego, CA). Cell lysates were prepared by scraping in RIPA buffer and passed through a 27g needle to shear DNA. Western blot analysis was performed as described in Supplementary Methods.
Apoptosis assays
Apoptotic cells in tissue sections were detected using in situ oligo ligation (ISOL) and active caspase-3 staining as previously described7. Details are provided in Supplementary Methods.
Immunohistochemistry
Slides were stained as described in Supplementary Methods. Phospho-ERK staining was quanitified by determining the average number of positive cells per crypt in 50 midcolonic crypts. The percentage of Ki-67 positive cells was determined with the Ariol SL-50 automated image analysis system (Applied Imaging, San Jose, CA).
Statistical analyses
For each experiment, the analysis of variance model was used and the overall effect was tested at P=0.05 to control the type I error rate. All data analyses were performed using R 2.6.020.
Results
Raf protects against DSS-induced crypt injury and inflammation
Raf is a key regulator of cell proliferation and differentiation, and appears to be an important mediator of cell survival21. As Raf-1 deletion is embryonic lethal2, we tested the role of Raf in colitis using intestinal epithelium-specific Raf knockout (Raf KOIE) mice generated by crossing Rafflx/flx mice (wildtype)4 with mice expressing a tamoxifen-inducible Cre recombinase under the control of a villin promoter16. Recombination of the Raf allele in the inducible villin-Cre ERT2 system was detected by Western blot analysis of isolated epithelial and stromal fractions. Raf expression was effectively knocked out in the intestinal epithelium, but not the stroma, of both the colon and small intestine in Raf KOIE mice (Figure 1A). In contrast, B-Raf expression in the intestinal epithelium was not affected.
Figure 1. Raf KOIE mice exhibit increased crypt injury and inflammation resulting from DSS colitis.
Epithelial and stromal fractions were isolated from the (A) colon and small intestine of oil- or tamoxifen-injected Rafflx/flx; villin-Cre mice. Western blot analysis was performed with antibodies against Raf-1, B-Raf, E-cadherin, and actin. (B) H&E staining of colon sections from wildtype and Raf KOIE mice treated with water, 3% DSS for 4 days, or 4 days plus a 3 day recovery period. Horizontal lines represent the mean. Scale bar = 500 or 125μm (C) Combined scores grading severity of inflammation and extent of crypt damage in wildtype or Raf KOIE mice following DSS treatment. (D) Crypt height in wildtype and Raf KOIE mice following DSS treatment. Horizontal lines represent the median value.
While other conditional Raf knockout mice have striking phenotypic changes5, Raf KOIE mice show normal architecture of the intestinal and colonic epithelium (Figure 1B) and no change in body weight compared to wildtype (data not shown). Unchallenged Raf KOIE mice also display no basal defects in cell proliferation or migration up the crypt-villus axis (data not shown). B-Raf expression is detectable in the colon epithelium, both in cultured cells and in vivo, indicating that regulation of Raf signaling may be functionally redundant in the context of cell maintenance.
Injury models have been used to delineate the requirement for specific gene products and/or signaling pathways in response to tissue damage and disease. To assess the role of Raf in intestinal injury and inflammation, 3% DSS was administered via drinking water to 8 week old wildtype and Raf KOIE mice. Mice received either water alone (control) or 3% DSS for four days to induce colitis, while some DSS-treated mice were allowed to recover drinking water without DSS for a three-day period. In response to DSS, both wildtype and Raf KOIE colonic epithelium suffered loss of crypt architecture and mucosal inflammation primarily in the midcolon (Figure 1B). The proximal and terminal colon showed little crypt damage. However, injury and inflammation scores were significantly higher in Raf KOIE mice (Figure 1C). Raf expression contributed to attenuation of DSS-induced inflammatory response as Raf KOIE mice had increased inflammatory infiltrate, greater depth of inflammation, and higher percent colon involved in inflammation following acute injury. Representative images of specific injury scores are provided in Supplementary Figure 1. We observed that Raf KOIE mice were 2.5 (95% CI (1.2,5.4)) times more likely to have blood present in the stool compared to wildtype during days 2–4 of DSS treatment (data not shown). Wildtype mice showed increased crypt height compared to Raf KOIE during recovery, whereas both genotypes had shortened crypts following injury (Figure 1D). These data indicate that Raf expression in the intestinal epithelium protects against epithelial damage and the associated inflammatory response after acute DSS treatment.
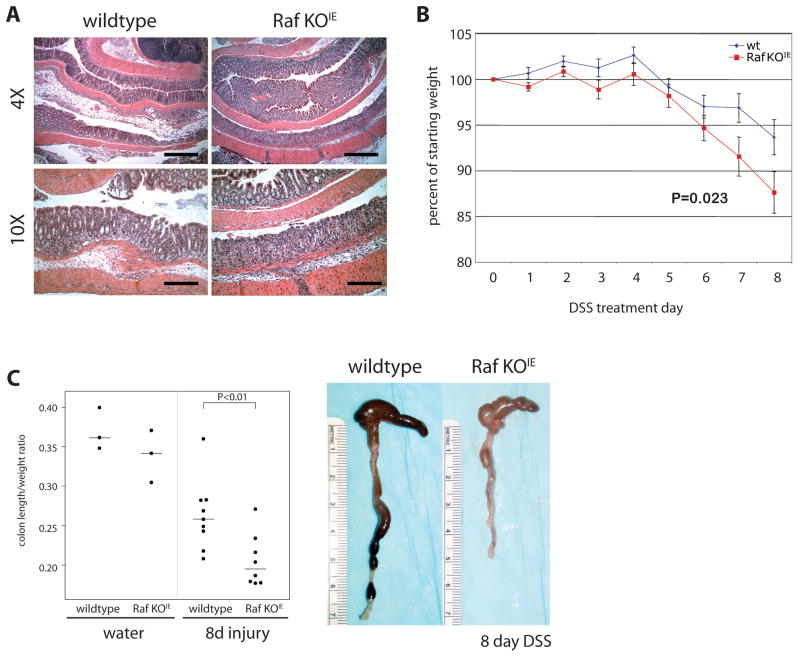
No clinical signs of colitis were apparent in wildtype or Raf KOIE mice after 4 days of DSS treatment. To assess if loss of Raf resulted in clinical differences in response to a more severe injury, the length of DSS adminstration was extended to 8 days. In response to prolonged DSS exposure, both wildtype and Raf KOIE mice demonstrated extensive crypt injury and inflammation (Figure 2A). Mice began losing weight at treatment day 6, and by day 8, Raf KOIE mice had lost significantly more weight than wildtype (Figure 2B). Furthermore, Raf KOIE mice had decreased colon length compared to wildtype, another indication of colonic inflammation (Figure 2C). These data indicate that Raf expression in the intestinal epithelium protects against epithelial damage and the associated inflammatory response after acute DSS treatment.
Figure 2. Loss of Raf expression results in worsened clinical signs of colitis.
(A) H&E staining of wildtype and Raf KOIE mice following 8 day DSS treatment. Scale bars = 500 or 250μm (B) Graphical representation of the average percent of weight loss for wildtype and Raf KOIE mice compared to the weight at the start of DSS treatment. (C) Colon length is represented as a ratio (cm: g) compared to the starting weight of mice prior to DSS administration. Horizontal bars represent the median value.
Raf expression confers intestinal epithelial cell survival during DSS colitis
Maintenance of the intestinal epithelial barrier is dependent upon the tight regulation of proliferation and apoptosis. To assess whether the increased severity of crypt damage in Raf KOIE mice was due to decreased intestinal epithelial survival, we measured DSS-induced apoptosis in intact crypts surrounding regions of ulceration in wildtype and Raf KOIE mice by ISOL (Figure 3). Acute DSS exposure induced colon epithelial cell death in both wildtype and Raf KOIE mice; however, apoptosis was significantly higher in the absence of Raf expression following 1 and 4 day DSS treatment. These data were confirmed by active-caspase-3 staining (P=0.002, P=0.008)(data not shown). Taken together, the results indicate that Raf plays an anti-apoptotic role during DSS-induced injury and inflammation.
Figure 3. Raf protects from apoptosis in response to damage in the intestinal epithelium.
(A) Apoptotic cells were detected by in situ oligo ligation (ISOL) in colon sections of DSS-treated wildtype and Raf KOIE mice following 1 or 4 days injury or 3 days recovery. Scale bar = 50μm (B) The average number of ISOL-positive cells were represented graphically. Horizontal lines represent the median.
Raf promotes hyperproliferation during epithelial regeneration
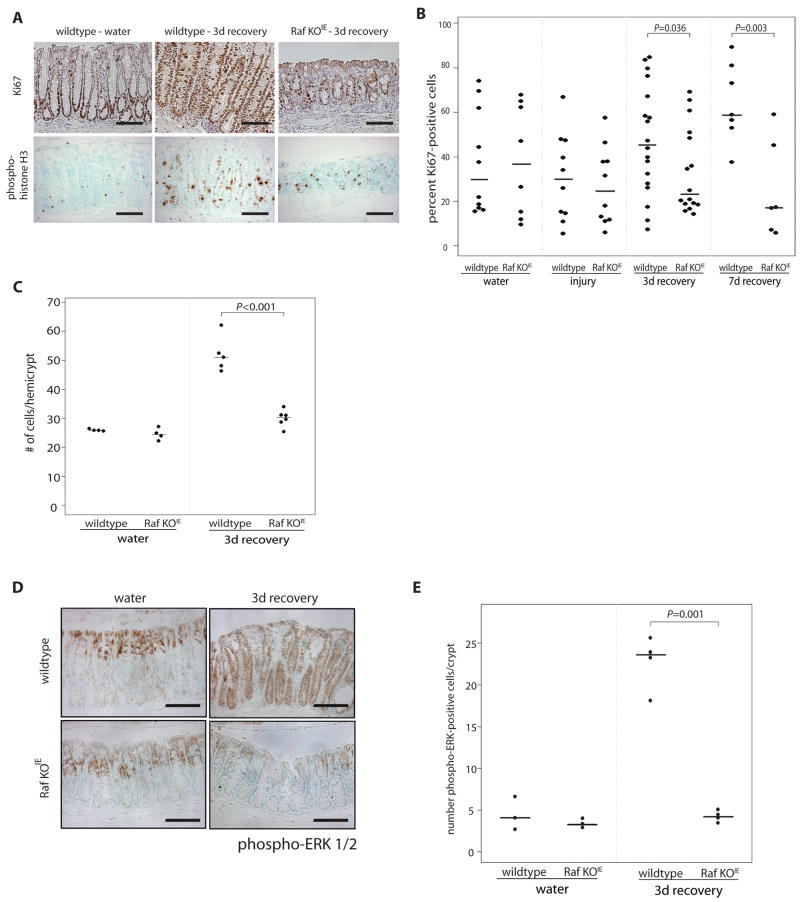
Previous studies have demonstrated that hyperproliferation of enterocytes occurs to regenerate damaged epithelium during recovery from acute colitis15. To determine the role of Raf in this phenomenon, we examined colonic epithelial cell proliferation during the recovery phase of DSS colitis in wildtype and Raf KOIE mice by immunohistochemistry for Ki-67 and phospho-histone H3. During recovery, wildtype mice have long, highly proliferative colonic crypts compared to Raf KOIE, which display fewer proliferative cells in shorter, poorly organized crypts (Figure 4A). Quantification of Ki-67 positive cells demonstrated hyperproliferation in the midcolon of wildtype mice in response to initial injury; in contrast, the number of proliferating cells in Raf KOIE mice during recovery is similar to that of mice receiving water alone (Figure 4B). Cell proliferation was decreased during DSS-induced injury, however this decrease was not Raf-dependent (Supplementary Figure 2). The number of cells per hemicrypt was also increased in wildtype mice compared to Raf KOIE mice after 4 days of DSS (Figure 4C). In situ hybridization for Lgr5 demonstrated that Raf expression does not drastically effect the stem cell population in unchallenged mice or during epithelial regeneration (Supplementary Figure 3).
Figure 4. Raf is required for the epithelial hyperproliferative response following DSS-induced injury.
(A) Proliferating cells were detected by Ki67 and phospho-histone H3 staining in colon sections of wildtype and Raf KOIE mice during 3 day recovery from DSS-induced colitis. (B) The percentage of Ki67 positive cells in the midcolon are represented graphically. (C) The number of cells per hemicrypt are represented graphically. (D) Phospho-ERK staining of colon sections from control and Raf KOIE mice during 3 day recovery. Scale bars = 125μm. (E) The average number of phospho-ERK-positive cells per crypt are represented graphically. Horizontal lines represent the median.
To determine the role of canonical Raf signaling in cell proliferation, we assessed the localization of active ERK in wildtype and Raf KOIE mice during recovery. In both wildtype and Raf KOIE untreated mice, phosphorylated ERK is localized to the nucleus of differentiated cells lining the surface epithelium (Figure 4D). In the hyperproliferating crypts of recovering wildtype, but not Raf KOIE mice, active ERK is visible as nuclear and diffuse cytoplasmic staining throughout the crypt axis (Figure 4E). Western blot analysis confirmed the absence of Raf expression in the epithelium following injury, eliminating the possibility that Raf expression and signaling are restored during recovery (data not shown). These data indicate that Raf is required for both an anti-apoptotic role and for rapid epithelial regeneration following DSS colitis.
Raf protects from DSS-induced apoptosis through NF-κB activation
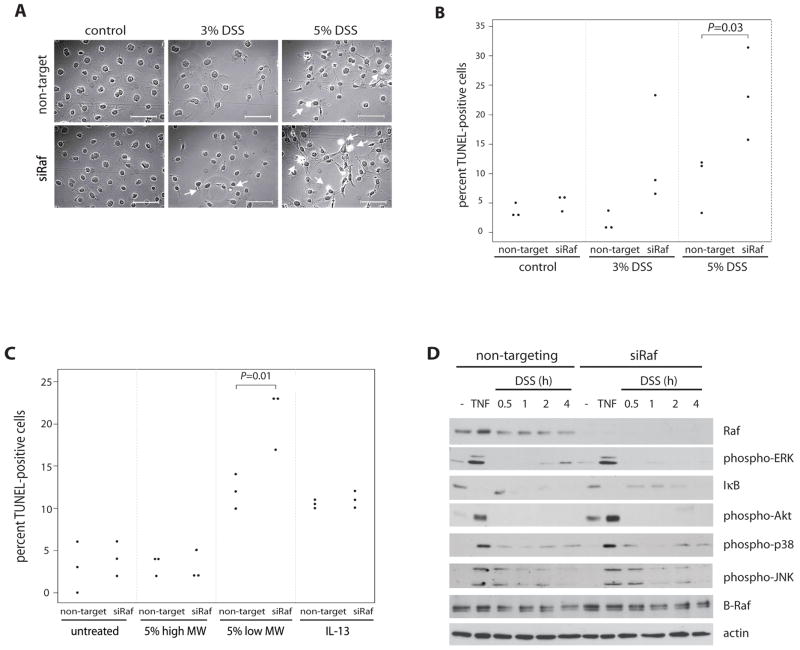
To assess the role of Raf on the activation of pro- and anti-apoptotic signaling pathways promoting colon epithelial cell survival during injury and inflammation, we utilized RNA interference in cell culture. YAMC cells were transfected with non-targeting or Raf-1 siRNA and then treated with 3 or 5% DSS. A time course of DSS exposure demonstrated increased apoptosis at 7 h (data not shown). TUNEL staining showed that apoptosis was significantly increased in response to 5% DSS in YAMC cells transfected with Raf siRNA compared to non-targeting siRNA (Figure 5A&B). To confirm that these apoptotic effects were specific to our DSS treatment, siRNA-transfected YAMC cells were treated with 5% high molecular weight DSS (500kD), which did not induce colitis or epithelial apoptosis in wildtype or Raf KOIE mice (data not shown). Similar to our in vivo findings, high molecular weight DSS failed to induce apoptosis in cell culture compared to treatment with 5% low molecular weight DSS (Figure 5C). IL-13 was used as a negative control to demonstrate Raf-independent apoptosis22.
Figure 5. Raf is required for intestinal epithelial cell survival in response to DSS.
YAMC cells were transfected with non-targeting or Raf siRNA and treated with 3 or 5% DSS for 7 h. (A) Apoptosis was determined by TUNEL assay (Scale = 100μm) and (B) the percent of TUNEL-positive cells was assessed. (C) Transfected cells were treated with IL-13 (10 ng/ml), 5% high (500kD) or low (36–50kD) molecular weight DSS for 7 h and the number of apoptotic cells were quantified by TUNEL assay. (D) Non-targeting or Raf siRNA-transfected YAMC cells were treated with 5% DSS for the times indicated. Western blots with whole cell lysates from DSS- or TNF- (100 ng/ml, 15 min) treated siRNA-transfected cells were probed for Raf, B-Raf, phospho-ERK, IκB, phospho-Akt, phospho-p38, phospho-JNK, and actin antibodies.
To address the mechanisms involved in this effect, lysates from cells treated at various time points prior to cell death were subjected to Western blot analysis for pro- and anti-apoptotic signaling pathways. TNF, which promotes both cell survival and death in these cells23, served as a positive control. Raf siRNA is specific to Raf-1 and does not block expression of B-Raf isoform (Figure 5D). 5% DSS stimulated the pro-apoptotic pathways p38 and JNK regardless of Raf expression. In contrast, DSS-induced ERK phosphorylation and IκB degradation required Raf (Figure 5D). Interestingly, DSS did not induce Akt phosphorylation, suggesting that in this model Raf is only required for ERK and NF-κB activation.
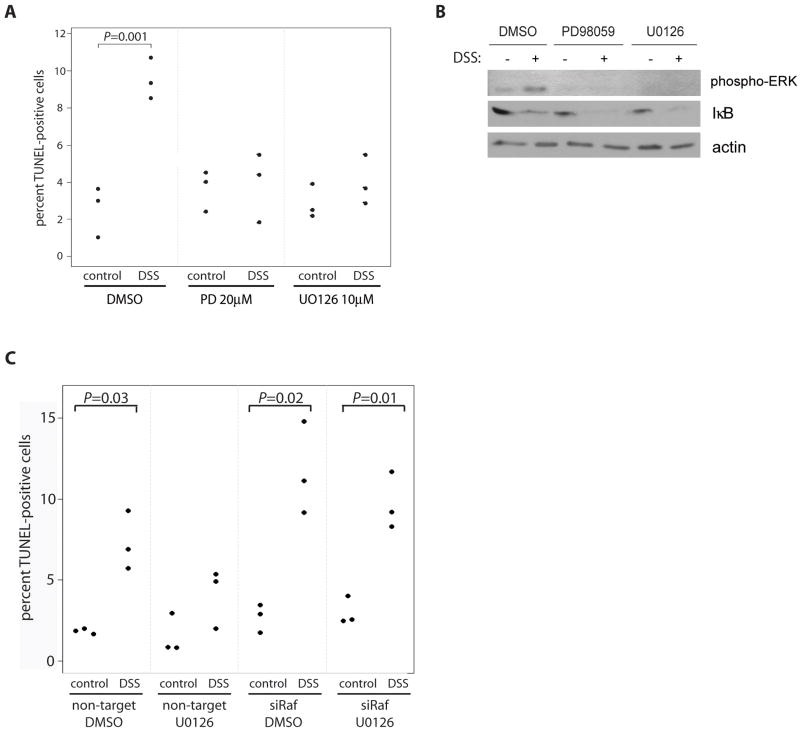
To determine whether Raf activation of MEK/ERK signaling or NF-κB mediates cell survival in response to DSS, TUNEL was performed on YAMC cells either co-treated with a MEK inhibitor, PD98059 (20μM) or U0126 (10μM), and 5% DSS for 7 h or transfected with siRNA directed against IκBα to induce activation of NF-κB. While both MEK inhibitors blocked ERK activation in response to DSS (Figure 6B), neither increased susceptibility to DSS-induced apoptosis (Figure 6A), indicating that MEK inhibitors protect cells from apoptosis following DSS exposure. Inhibition of MEK using U0126 did not block DSS-induced apoptosis in the absence of Raf expression (Figure 6C) further confirming that Raf-mediated cell survival occurs independent of MEK activation.
Figure 6. MEK inhibitors protect against DSS-induced apoptosis.
YAMC cells were treated with MEK inhibitors, PD98059 (20μM) or U0126 (10μM), and 5% DSS for 7 h. (A) Apoptosis was detected by TUNEL and (B) whole cell lysates were blotted with anti-phospho-ERK, IκB, and actin. (C) YAMC cells were transfected with non-targeting or Raf siRNA and treated with U0126 (10μM) and 5% DSS for 7h.
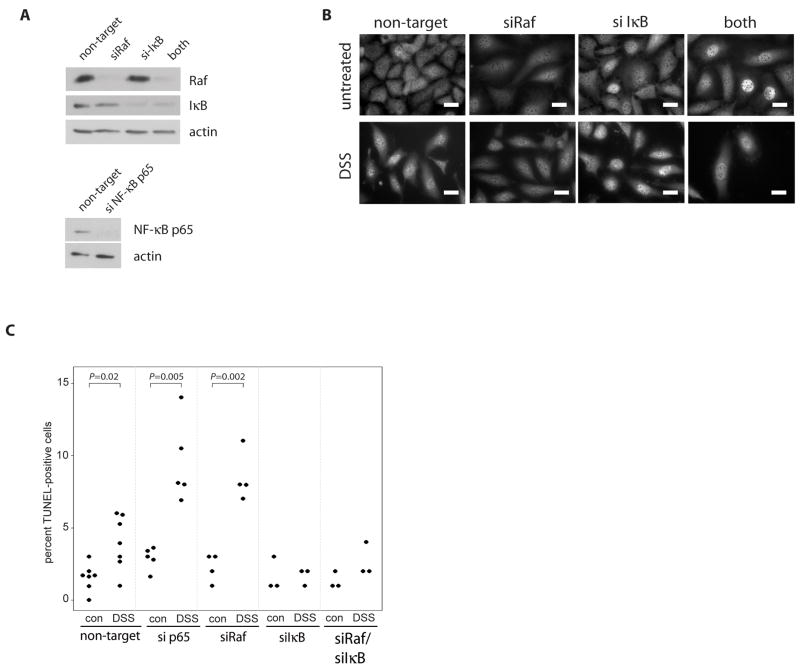
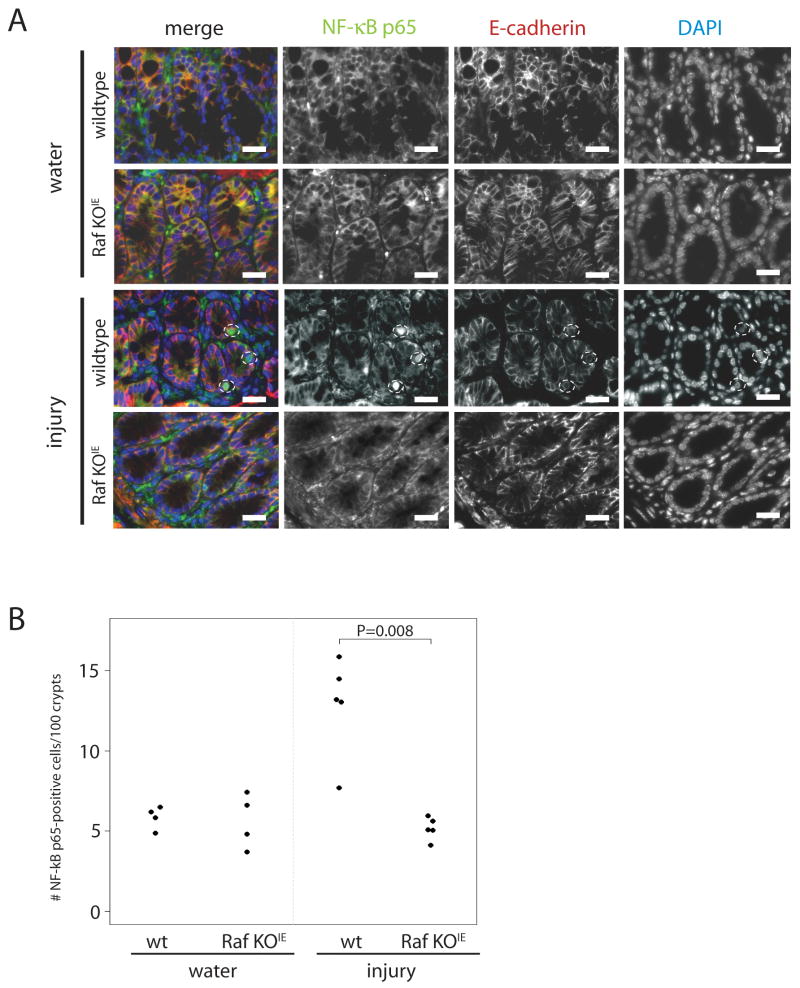
Using siRNA to knock down expression of NF-κB p65, Raf and/or IκBα, we showed that NF-κB signaling is involved in Raf-induced cell survival (Figure 7). Deletion of IκBα promoted constitutive translocation of NF-κB p65 subunit to the nucleus (Figure 7B). Furthermore, DSS-induced NF-κB p65 translocation was partially inhibited in the absence of Raf. Following treatment with 5% DSS for 7 h, TUNEL staining shows that either absence of NF-κB p65 or Raf absence promotes DSS-induced apoptosis to a similar extent. Furthermore, constitutive activation of NF-κB with IκBα siRNA rescues Raf siRNA-transfected cells from DSS-induced apoptosis (Figure 7C). This requirement for Raf in NF-κB activation was confirmed in vivo, as nuclear NF-κB p65 was increased in the colon epithelium following DSS injury in wildtype, but not Raf KOIE mice (Figure 8). Taken together, these data demonstrate that Raf regulates NF-κB activation in a MEK-independent manner to promote colon epithelial cell survival in response to injury.
Figure 7. NF-κB activation rescues DSS-induced apoptosis in the absence of Raf expression.
(A) Western blot analysis was performed on NF-κB p65, Raf or IκBα siRNA-transfected YAMC cells to detect Raf, IκB, NF-κB p65 and actin expression. (B) NF-κB p65 nuclear translocation was detected in untreated or 5% DSS-treated (2 h) non-targeting, IκBα, or Raf siRNA-transfected cells by immunocytochemistry. Scale bar = 20μm (C) Apoptosis in 5% DSS-treated p65, Raf and/or IκBα siRNA-transfected YAMC cells was assessed by TUNEL assay.
Figure 8. Raf promotes NF-κB activation in the colon epithelium in response to DSS-induced injury.
(A) Representative images are shown of immunofluorescence for NF-κB p65 (green), E-cadherin (red) and DAPI-stained nuclei (blue) in colon sections of wildtype and Raf KOIE mice following water treatment or DSS-induced injury. Scale bars = 20μM (B) The number of NF-κB p65-positive cells are represented graphically. Horizontal bars represent the median.
Discussion
In this study, we demonstrate a MEK-independent requirement for Raf in anti-apoptotic signaling in response to colonic epithelial injury. DSS-induced cell damage promotes Raf-dependent activation of both MEK/ERK/MAPK and NF-κB signaling in mouse colon epithelial cells both in vivo and in vitro. In the absence of Raf expression, DSS-induced apoptosis is increased in cell culture and in intact colon epithelium. Interestingly, ERK activation promotes apoptosis in response to DSS-induced injury, suggesting that this pathway is not involved in Raf-stimulated cell survival. However, Raf may directly or indirectly promote ERK activation to enhance epithelial cell proliferation during recovery from colitis. In contrast, loss of NF-κB p65 expression induced apoptosis following DSS exposure, and constitutive activation of NF-κB signaling rescued Raf-null colon epithelial cells from apoptosis. In vivo studies indicate that NF-κB nuclear translocation is decreased in Raf KOIE mice following DSS-induced injury compared to wildtype. Thus, MEK-independent Raf anti-apoptotic signaling is mediated through NF-κB to protect against acute colitis.
Raf KOIE mice develop more severe colitis, with more extensive crypt damage and inflammation during acute injury, than wildtype mice. Furthermore, Raf prevents colon epithelial cell apoptosis during DSS colitis. Cell culture models of DSS treatment have been utilized to identify the requirement for specific signaling pathways by both genetic and pharmacological inhibitors. DSS treatment of cultured colon epithelial cells and macrophages identifies intracellular signaling pathways of biological responses relevant to inflammatory diseases. For example, Caco-2 cells exhibit decreased barrier function following DSS treatment24, 25, similar to DSS-treated mice24. Furthermore, IL-1 production is increased by DSS-treated murine peritoneal macrophages through MAPK signaling26. In our studies, knockdown of Raf expression in YAMC cells decreased both ERK and NF-κB activation in response to DSS (Figure 5D). Given previous reports of MEK/ERK anti-apoptotic function7, 27, we hypothesized that Raf activation of this pathway was responsible for promoting cell survival following DSS treatment. However, our findings show that MEK inhibition protects cells from DSS-induced apoptosis (Figure 6). These data are consistent with previous studies showing ERK promotes hydrogen peroxide-induced cell death by activating caspase-3 in renal epithelial cells28, 29. In the same cell type, E. coli toxin promoted ERK-induced apoptosis in a caspase-independent manner30. Furthermore, these results are consistent with in vivo experiments using the MEK inhibitor, U0126, showing decreased inflammation and renal epithelial cell apoptosis during cisplatin-induced acute renal failure31. However, our own work on TNF signaling suggests MEK contributes to anti-apoptotic response to cytokines7. Thus, our findings here expand the known functions of MAPK signaling in the colon epithelium by demonstrating that Raf stimulates colon epithelial cell proliferation in response to injury, and that MEK signaling triggers a pro-apoptotic response to DSS. Thus, Raf promotes colon epithelial cell survival in a MEK-independent manner.
In addition to promoting MEK/ERK signaling, Raf expression is required for IκB degradation and subsequent NF-κB activation following DSS exposure (Figures 5D, 7B and 8). Several studies have targeted NF-κB as a potential therapeutic target for IBD; however, NF-κB promotes both apoptosis and cell survival in a cell context-dependent manner. NF-κB p65 antisense oligonucleotides decreased the severity of colitis in trinitrobenzene sulfonic acid and IL-10−/− colitis models32. In contrast, disruption of NF-κB activation specifically in the intestinal epithelium demonstrates a protective effect against apoptosis and colitis. Intestinal deletion of IκB kinase β (IKKβ), one of three IKK isoforms involved in NF-κB activation, increases susceptibility to DSS colitis and apoptosis, yet targeted deletion of IKKβ in myeloid cells did not affect cell survival33. RelA-intestinal epithelial-deficient mice are more sensitive to DSS colitis and with increased epithelial apoptosis in response to injury34, further supporting a role for NF-κB in cell survival in response to injury and inflammation.
In these studies we show that Raf regulates NF-κB activation in a MEK-independent manner in intestinal epithelial cells (Figures 6–8). Our findings bring in vivo relevance to a previous report that a Raf mutant (Raf-BXB T481A) which cannot bind MEK, and thus blocks ERK activation, still activates a NF-κB reporter construct in fibroblasts35.
Baumann et al. demonstrated that NF-κB is required for Raf-mediated oncogenic transformation of fibroblasts. In T-cells, Raf promoted activation of a NF-κB reporter gene independent of MEK/ERK activation, but dependent upon Raf kinase activity36. In our study, IκB degradation, NF-κB p65 nuclear translocation, and colon epithelial cell survival in the presence of DSS were Raf-dependent. We hypothesize that Raf may function in conjunction with other known upstream regulators of NF-κB signaling such as NIK or IKK, since knockdown of Raf expression did not completely block NF-κB p65 nuclear translocation (Figure 7B). Thus, these data provide the first evidence that Raf mediates NF-κB activation in vivo using a disease model and in vitro, using non-transformed cells.
During recovery from initial injury, we found that wildtype and Raf KOIE mice injury and inflammation scores were similar, with significant increases in epithelial apoptosis seen in both wildtype and Raf KOIE mice (Figure 3B). In fact, increased infiltrating activated macrophages are required to promote regeneration of the damaged epithelium by eliciting colon epithelial progenitor cell responses37. No apparent difference in the number of macrophages or neutrophils was detected by immunohistological staining between wildtype and Raf KOIE mice following injury or recovery (data not shown). Although similar defects in injury repair have been reported in the DSS colitis model for TLR4 and Cox-2 knockout mice14, 15, we found no change in expression of either protein in the colon in the absence of Raf or in response to DSS (data not shown).
The proliferation defect during recovery in Raf KOIE mice is likely due to impaired MEK-dependent pathways in the colonic crypt. Increased levels of phosphorylated ERK are present in the cytoplasm of wildtype cells throughout the crypt axis during recovery, whereas activated ERK is restricted to the surface epithelium in Raf KOIE mice similar to untreated mice (Figures 4C&D). These data are consistent with reports in various cell types in which transient ERK activation in the cytoplasm promotes proliferation, while sustained ERK activation in the nucleus induces growth arrest and differentiation23, 38, 39. Thus, Raf-1 specific activation of MEK may be required for maximal proliferation following DSS-induced injury. Supporting this conclusion, a recent report shows that even in the presence of activated Raf, loss of MEK1/2 expression inhibits hyperproliferation in the mouse epidermis40. Taken together, these findings suggest that Raf directly or indirectly regulates MEK/ERK-mediated colon epithelial proliferation in response to injury.
Overall, these findings establish a novel role for Raf kinase in both the anti-apoptotic and hyperproliferative epithelial responses to injury in the colon. Our model suggests Raf initiates MEK-independent NF-κB activation to reduce the apoptotic response to injury, while potentially increasing ERK activation to enhance proliferation and rapidly restore the colon epithelium during acute colitis. Given the link between these responses and colitis-associated carcinogenesis, our findings suggest future studies are needed to better define the role of Raf-1 in response to chronic injury. In this regard, pharmacological inhibition of Raf signaling is a current target of cancer therapeutic drug development41. These findings have important implications for human disease, as IBD patients are at an increased risk to develop colon cancer with the greatest risk to those with repeated cycles of inflammation42. A key challenge is to understand how Raf activates NF-κB-dependent pathways in intestinal epithelial cells following injury and to determine if there is a pathogenic role in colitis-carcinogenesis transition.
Supplementary Material
Acknowledgments
Grant Support/Assistance: This work was supported by NIH Award DK56008 and DK66176 (DBP), by NIH CBMS (T32GM008554) training grant (KLE) and by the Vanderbilt University DDRC (NIH Grant DK58404 and DK066176). DIC imaging was performed using the VUMC Cell Imaging Shared Resource (supported by NIH Grants CA68485, DK20593, DK58404, HD15052, DK59637 and EY08126). ARIOL image analysis was performed with assistance from Frank Revetta and the Vanderbilt Ingram Cancer Center. We thank Mark Frey for thoughtful discussions and suggestions.
Abbreviations
- ASK-1
apoptosis signal regulating kinase-1
- DAB
diamino benzidine
- DAPI
4′,6-diamidino-2-phenylindole
- DSS
dextran sulfate sodium
- EDTA
ethylenediaminetetraacetic acid
- EGTA
ethylene glycol tetraacetic acid
- ERK
extracellular signal regulated kinase
- HRP
horseradish peroxidase
- IκB
inhibitor of κB
- IKK
inhibitor of κB kinase
- IL
interleukin
- ISOL
in situ oligo ligation
- KOIE
intestinal epithelium-specific knockout
- MAPK
mitogen activated protein kinase
- NF-κB
nuclear factor-κB
- SDS
sodium dodecyl sulfate
- siRNA
small interfering RNA
- TNF
tumor necrosis factor
- TLR
toll-like receptor
- TUNEL
terminal deoxynucleotidyl transferase nick end labeling
- YAMC
young adult mouse colon
Footnotes
The authors have no financial interests to disclose. No conflicts of interest exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hall PA, Coates PJ, Ansari B, Hopwood D. Regulation of cell number in the mammalian gastrointestinal tract: the importance of apoptosis. J Cell Sci. 1994;107(Pt 12):3569–77. doi: 10.1242/jcs.107.12.3569. [DOI] [PubMed] [Google Scholar]
- 2.Mikula M, Schreiber M, Husak Z, Kucerova L, Ruth J, Wieser R, Zatloukal K, Beug H, Wagner EF, Baccarini M. Embryonic lethality and fetal liver apoptosis in mice lacking the c-raf-1 gene. EMBO J. 2001;20:1952–1962. doi: 10.1093/emboj/20.8.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubiolo C, Piazzolla D, Meissl K, Beug H, Huber JC, Kolbus A, Baccarini M. A balance between Raf-1 and Fas expression sets the pace of erythroid differentiation. Blood. 2006;108:152–9. doi: 10.1182/blood-2005-09-3866. [DOI] [PubMed] [Google Scholar]
- 4.Jesenberger V, Procyk KJ, Ruth J, Schreiber M, Theussl HC, Wagner EF, Baccarini M. Protective role of Raf-1 in Salmonella-induced macrophage apoptosis. J Exp Med. 2001;193:353–64. doi: 10.1084/jem.193.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamaguchi O, Watanabe T, Nishida K, Kashiwase K, Higuchi Y, Takeda T, Hikoso S, Hirotani S, Asahi M, Taniike M, Nakai A, Tsujimoto I, Matsumura Y, Miyazaki J, Chien KR, Matsuzawa A, Sadamitsu C, Ichijo H, Baccarini M, Hori M, Otsu K. Cardiac-specific disruption of the c-raf-1 gene induces cardiac dysfunction and apoptosis. J Clin Invest. 2004;114:937–43. doi: 10.1172/JCI20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–20. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan F, John SK, Wilson G, Jones DS, Washington MK, Polk DB. Kinase suppressor of Ras-1 protects intestinal epithelium from cytokine-mediated apoptosis during inflammation. J Clin Invest. 2004;114:1272–80. doi: 10.1172/JCI21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 9.Cooper H, Murthy S, Shah R, Sedergran D. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 10.Kitajima S, Takuma S, Morimoto M. Tissue distribution of dextran sulfate sodium (DSS) in the acute phase of murine DSS-induced colitis. J Vet Med Sci. 1999;61:67–70. doi: 10.1292/jvms.61.67. [DOI] [PubMed] [Google Scholar]
- 11.Dieleman LA, Palmen MJ, Akol H, Bloemena E, Pena AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385–91. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egger B, Bajaj-Elliott M, MacDonald TT, Inglin R, Eysselein VE, Buchler MW. Characterisation of acute murine dextran sodium sulphate colitis: cytokine profile and dose dependency. Digestion. 2000;62:240–8. doi: 10.1159/000007822. [DOI] [PubMed] [Google Scholar]
- 13.Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. 2007;132:1359–74. doi: 10.1053/j.gastro.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 14.Fukata M, Michelsen KS, Eri R, Thomas LS, Hu B, Lukasek K, Nast CC, Lechago J, Xu R, Naiki Y, Soliman A, Arditi M, Abreu MT. Toll-like receptor-4 is required for intestinal response to epithelial injury and limiting bacterial translocation in a murine model of acute colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1055–65. doi: 10.1152/ajpgi.00328.2004. [DOI] [PubMed] [Google Scholar]
- 15.Fukata M, Chen A, Klepper A, Krishnareddy S, Vamadevan AS, Thomas LS, Xu R, Inoue H, Arditi M, Dannenberg AJ, Abreu MT. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131:862–77. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.el Marjou F, Janssen KP, Chang BH, Li M, Hindie V, Chan L, Louvard D, Chambon P, Metzger D, Robine S. Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis. 2004;39:186–93. doi: 10.1002/gene.20042. [DOI] [PubMed] [Google Scholar]
- 17.Polk DB. Shc is a substrate of the rat intestinal epidermal growth factor receptor tyrosine kinase. Gastroenterology. 1995;109:1845–1851. doi: 10.1016/0016-5085(95)90751-3. [DOI] [PubMed] [Google Scholar]
- 18.Polk DB. Epidermal growth factor receptor-stimulated intestinal epithelial cell migration requires phospholipase C activity. Gastroenterology. 1998;117:493–502. doi: 10.1016/s0016-5085(98)70532-3. [DOI] [PubMed] [Google Scholar]
- 19.Whitehead RH, VanEeden PE, Noble MD, Ataliotis P, Jat PS. Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc Natl Acad Sci, USA. 1993;90:587–591. doi: 10.1073/pnas.90.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Team RDC. A language and environment for statistical computing. 2.6.0 ed. Vienna. Austria: R Foundation for Statistical Computing; 2007. [Google Scholar]
- 21.Baccarini M. Second nature: biological functions of the Raf-1 “kinase”. FEBS Lett. 2005;579:3271–7. doi: 10.1016/j.febslet.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 22.Heller F, Florian P, Bojarski C, Richter J, Christ M, Hillenbrand B, Mankertz J, Gitter AH, Burgel N, Fromm M, Zeitz M, Fuss I, Strober W, Schulzke JD. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterology. 2005;129:550–64. doi: 10.1016/j.gastro.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Kaiser GC, Yan F, Polk DB. Conversion of TNFa from antiproliferative to proliferative ligand in mouse intestinal epithelial cells by regulating mitogen-activated protein kinase. Exp Cell Res. 1999;249:349–358. doi: 10.1006/excr.1999.4488. [DOI] [PubMed] [Google Scholar]
- 24.Walsh-Reitz MM, Huang EF, Musch MW, Chang EB, Martin TE, Kartha S, Toback FG. AMP-18 protects barrier function of colonic epithelial cells: role of tight junction proteins. Am J Physiol Gastrointest Liver Physiol. 2005;289:G163–71. doi: 10.1152/ajpgi.00013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294:G208–16. doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- 26.Kwon KH, Ohigashi H, Murakami A. Dextran sulfate sodium enhances interleukin-1 beta release via activation of p38 MAPK and ERK1/2 pathways in murine peritoneal macrophages. Life Sci. 2007;81:362–71. doi: 10.1016/j.lfs.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 27.Ray RM, Bhattacharya S, Johnson LR. Protein phosphatase 2A regulates apoptosis in intestinal epithelial cells. J Biol Chem. 2005;280:31091–100. doi: 10.1074/jbc.M503041200. [DOI] [PubMed] [Google Scholar]
- 28.Zhuang S, Yan Y, Daubert RA, Han J, Schnellmann RG. ERK promotes hydrogen peroxide-induced apoptosis through caspase-3 activation and inhibition of Akt in renal epithelial cells. Am J Physiol Renal Physiol. 2007;292:F440–7. doi: 10.1152/ajprenal.00170.2006. [DOI] [PubMed] [Google Scholar]
- 29.Nowak G. Protein kinase C-alpha and ERK1/2 mediate mitochondrial dysfunction, decreases in active Na+ transport, and cisplatin-induced apoptosis in renal cells. J Biol Chem. 2002;277:43377–88. doi: 10.1074/jbc.M206373200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen M, Jahnukainen T, Bao W, Dare E, Ceccatelli S, Celsi G. Uropathogenic Escherichia coli toxins induce caspase-independent apoptosis in renal proximal tubular cells via ERK signaling. Am J Nephrol. 2003;23:140–51. doi: 10.1159/000069853. [DOI] [PubMed] [Google Scholar]
- 31.Jo SK, Cho WY, Sung SA, Kim HK, Won NH. MEK inhibitor, U0126, attenuates cisplatin-induced renal injury by decreasing inflammation and apoptosis. Kidney Int. 2005;67:458–66. doi: 10.1111/j.1523-1755.2005.67102.x. [DOI] [PubMed] [Google Scholar]
- 32.Neurath MF, Pettersson S, Meyer zum Buschenfelde KH, Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- 33.Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 34.Steinbrecher KA, Harmel-Laws E, Sitcheran R, Baldwin AS. Loss of Epithelial RelA Results in Deregulated Intestinal Proliferative/Apoptotic Homeostasis and Susceptibility to Inflammation. J Immunol. 2008;180:2588–2599. doi: 10.4049/jimmunol.180.4.2588. [DOI] [PubMed] [Google Scholar]
- 35.Pearson G, Bumeister R, Henry DO, Cobb MH, White MA. Uncoupling Raf1 from MEK1/2 impairs only a subset of cellular responses to Raf activation. J Biol Chem. 2000;275:37303–37306. doi: 10.1074/jbc.C000570200. [DOI] [PubMed] [Google Scholar]
- 36.Baumann B, Weber CK, Troppmair J, Whiteside S, Israel A, Rapp UR, Wirth T. Raf induces NF-kappa B by membrane shuttle kinase MEKK1, a signaling pathway critical for transformation. Proc Natl Acad Sci USA. 2000;97:4615–4620. doi: 10.1073/pnas.080583397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pull SL, Doherty JM, Mills JC, Gordon JI, Stappenbeck TS. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci U S A. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marshall CJ. Specificity of receptor tyrosine kinase signaling: Transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 39.Pouyssegur J, Volmat V, Lenormand P. Fidelity and spatio-temporal control in MAP kinase (ERKs) signalling. Biochem Pharmacol. 2002;64:755–63. doi: 10.1016/s0006-2952(02)01135-8. [DOI] [PubMed] [Google Scholar]
- 40.Scholl FA, Dumesic PA, Barragan DI, Harada K, Bissonauth V, Charron J, Khavari PA. Mek1/2 MAPK kinases are essential for Mammalian development, homeostasis, and Raf-induced hyperplasia. Dev Cell. 2007;12:615–29. doi: 10.1016/j.devcel.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 42.Vagefi PA, Longo WE. Colorectal cancer in patients with inflammatory bowel disease. Clin Colorectal Cancer. 2005;4:313–9. doi: 10.3816/ccc.2005.n.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.