Abstract
Acetylcholine released from parasympathetic excitatory nerves activates contraction in detrusor smooth muscle. Immunohistochemical labeling of guinea pig detrusor with anti-c-Kit and anti-VAChT demonstrated a close structural relationship between interstitial cells of Cajal (ICC) and cholinergic nerves. The ability of guinea pig bladder detrusor ICC to respond to the acetylcholine analog, carbachol, was investigated in enzymatically dissociated cells, loaded with the Ca2+ indicator fluo 4AM. ICC fired Ca2+ transients in response to stimulation by carbachol (1/10 μM). Their pharmacology was consistent with carbachol-induced contractions in strips of detrusor which were inhibited by 4-DAMP (1 μM), an M3 receptor antagonist, but not by the M2 receptor antagonist methoctramine (1 μM). The source of Ca2+ underlying the carbachol transients in isolated ICC was investigated using agents to interfere with influx or release from intracellular stores. Nifedipine (1 μM) or Ni2+ (30–100 μM) to block Ca2+ channels or the removal of external Ca2+ reduced the amplitude of the carbachol transients. Application of ryanodine (30 μM) or tetracaine (100 μM) abolished the transients. The phospholipase C inhibitor, U-73122 (2.5 μM), significantly reduced the responses. 2-Aminoethoxydiethylborate (30 μM) caused a significant reduction and Xestospongin C (1 μM) was more effective, almost abolishing the responses. Intact in situ preparations of guinea pig bladder loaded with a Ca2+ indicator showed distinctively different patterns of spontaneous Ca2+ events in smooth muscle cells and ICC. Both cell types responded to carbachol by an increase in frequency of these events. In conclusion, guinea pig bladder detrusor ICC, both as isolated cells and within whole tissue preparations, respond to cholinergic stimulation by firing Ca2+ transients.
Keywords: confocal microscopy, c-Kit
there is an increasing body of evidence that the urinary bladder contains, in addition to bulk smooth muscle, a population of cells with many characteristics of the interstitial cells of Cajal (ICC) which are responsible both for the generation of peristaltic activity and the transmission of signals from enteric nerves to smooth muscle cells in the gastrointestinal tract (10, 20). Studies of ICC-like cells in tissues of the urinary tract are at a comparatively early stage; however, there is substantial evidence that they are present in the urethra, ureters, and the urinary bladder (for a review, see Ref. 3). These new cells have been described as interstitial cells (IC), ICC, cells resembling interstitial cells of Cajal (ICC-like cells), or myofibroblasts; however, while urinary tract ICC share many morphological and physiological properties of gut ICC, specific functions analogous to those of gut ICC have not been attributed to the similar cells in the urinary tract. The Fifth International Symposium on Interstitial Cells of Cajal (Ireland, 2007) recommended that all such cells in the urinary tract be classified as ICC and we adopted this nomenclature in the present paper (we previously described these cells as IC or ICC-like cells).
Morphological studies on guinea pig and human bladder have identified ICC in the suburothelial/lamina propria region [lamina propria ICC (ICC-LP)], on the boundary of smooth muscle bundles in the detrusor [intramuscular ICC (ICC-IM)], and in the spaces between the muscle bundles [interbundle interstitial cells (ICC-IB)]. These ICC have been identified in the urinary bladder using antibodies to the Kit receptor, an established ICC marker, both in fixed tissue preparations and when enzymatically dispersed (16). Vimentin antibodies have also been used to successfully label ICC in bladder and have identified ICC-LP in enzymatic cell dispersals (24). While the precise physiological roles of bladder ICC have yet to be established, information on their spontaneous activity, the complement of ion channels they possess, and their ability to fire Ca2+ signals in response to stimulation by agonists is accumulating (17, 18, 24, 25, 28). ICC-LP, also described as myofibroblasts, have been shown to exhibit spontaneous changes in membrane potential and respond to application of exogenous ATP by firing inward currents and Ca2+ transients (25, 28). These cells form close connections with each other in the lamina propria region and may be involved in sensory transmission. Detrusor ICC comprising both ICC-IM and ICC-IB also exhibit spontaneous Ca2+ signals and show increases in intracellular Ca2+ concentration in response to agonist stimulation. They also possess inward L-type and non-L-type Ca2+ channels and K+ channels including large-conductance Ca2+-activated K+ and voltage-dependent delayed rectifier.
Immunohistochemical labeling and confocal microscopy showed that the ICC-IM and ICC-IB in the detrusor lie in close proximity to intramural nerves (16). Similar cells in the gastrointestinal tract are involved in both inhibitory and excitatory neurotransmission, acting as intermediaries in the relay of signals from nerves to smooth muscle (for a review, see Refs. 10, 20). It is unclear whether a similar situation is present in the bladder; however, it has been reported that enzymatically dispersed detrusor ICC respond to cholinergic stimulation by firing Ca2+ transients (16). The major excitatory innervation to the bladder is parasympathetic and activation of cholinergic nerves evokes contraction of bladder smooth muscle in the micturition response. Given the structural relationship between nerves and ICC in the bladder wall, it was therefore of interest to investigate the Ca2+ responses of detrusor ICC to cholinergic stimulation both in isolated cells and within tissue preparations.
Preliminary findings have been communicated to The Physiological Society.
METHODS
Immunohistochemistry.
Guinea pigs (250–500 g) of either sex were killed by cervical dislocation in accordance with United Kingdom Animal Scientific Procedures Act (1986) and were approved by local University animal welfare and ethics committee. Bladders were removed, opened longitudinally, and the mucosa was removed by sharp dissection. Detrusor preparations were fixed in acetone (4°C), washed in PBS, blocked in 1% BSA, and incubated with primary antibodies, anti-c-Kit (rabbit), and anti-vesicular acetylcholine transferase (goat) (both from Santa Cruz Biotechnology, 1:200) for 24 h. After being washed to remove excess antibody, tissues were incubated in secondary fluorescent antibodies (Alexa 488 and Alexa 594, 1:200, Molecular Probes) for 1 h, washed in PBS, and mounted on glass slides for examination with epi-fluorescent microscopy. Slides were imaged with a Bio-Rad 1024 confocal/multiphoton imaging system mounted on a Nikon upright microscope using a 60× Plan Apo 1.2 numerical aperture water immersion objective. Fluorophores were excited with the 488- or 568-nm line of a krypton-argon laser, a Ti-sapphire multiphoton laser tuned to 750 nm (for DAPI imaging), and the resulting emission fluorescence was collected through appropriate filters to photomultiplier tubes. Sequential imaging of fluorophores was carried out to minimize any bleed-through.
Control experiments were carried out as follows: 1) omission of the primary antibody to control for the specificity of the secondary antibody, 2) omission of all antibodies to control for autofluorescence of the tissue, 3) anti-c-Kit with the secondary antibody for vAChT, i.e., anti-goat (irrelevant secondary control), and 4) anti-vAChT with secondary antibody for anti-c-Kit, i.e., anti-rabbit. Significant fluorescence was not observed in any of the control samples.
Organ bath tension recordings.
After removal of the mucosa, strips of detrusor (10 × 2 × 2 mm) were mounted in organ baths, to an initial tension of 1 g. Tissues were perfused with Krebs solution at 35°C and allowed to equilibrate for 1 h. The strips typically relaxed as spontaneous contractile activity developed and this activity was maintained for several hours. Drugs were applied via the perfusion system (1–2 ml/min). Data were acquired using Intracept Chart Software and analyzed using Microsoft Excel and Prism (Graphpad) software.
Isolated cell fluorescent microscopy.
Cells were enzymatically isolated from the detrusor region as previously described (16), loaded with 1–2 μM fluo 4AM (Molecular Probes) at room temperature, plated to the bottom of a recording chamber, and washed for at least 30 min with PSS solution before being studied with confocal microscopy. A mixed population of cells was obtained, including typical spindle-shaped smooth muscle cells and branched cells that have previously been identified as ICC using c-Kit antibodies. In addition to the bath perfusion, the cell of interest was superfused via a drug delivery system placed 200 μm away enabling the solution to be replaced with one containing a drug in less than 10 s. All experiments were carried out at room temperature. Cells were imaged with a confocal microscope (Nikon C1) mounted on an upright (e90i) microscope using a water-dipping objective lens (×60W Fluor numerical aperture 1.0). The fluorophore was excited at 488 nm with an argon-ion laser and the resulting emission was collected via a 515/30-nm filter to a photomultiplier tube. Data were collected as time series with acquisition rates between 1 and 10 frames/s, typically in a 512 pixel by 64 line box using Nikon EZ-C1 software. Images were analyzed with WinFluor software (Dr. J. Dempster, University of Strathclyde), Microsoft Excel and Prism (Graphpad). Statistical comparisons were made using the Student's t-test, taking the P < 0.05 level as significant. n Refers to the number of cells and summary data are expressed as means ± SE. Data are expressed as F/F0; where the fluorescence F of an event is expressed as a ratio of background F0.
Whole tissue confocal microscopy.
Small preparations of guinea pig bladder containing only a few smooth muscle bundles (∼3 × 3 mm) were obtained by fine dissection and pinned to the Sylgard base of a recording chamber. Tissues were placed in an incubator for 30 min at 35°C before being loaded with Oregon Green BAPTA-AM (Molecular Probes; 5 μM) and pluronic (0.06%) at room temperature for 1 h (loading at 35°C often resulted in the tissue becoming overloaded with indicator). After being washed in HEPES-buffered Krebs solution for at least 30 min, tissues were imaged with a water-dipping objective lens (×60 W Fluor numerical aperture 1.0) either with a confocal microscope or an EMCCD camera (Nikon) running at 25 frames/s. The camera-based imaging system was operated with WinFluor software. Movement artifacts due to spontaneous contraction of the tissue were minimized by extensive pinning of the preparation. Analysis of images was performed using WinFluor, Microsoft Excel and Prism software. Experiments were carried out at room temperature.
Solutions.
The solutions (in mM) were as follows: 1) Hanks solution (PSS): 130 Na+, 5.8 K+, 135 Cl−, 0.44 H2PO4−, 0.34 HPO42−, 4.16 HCO3−, 0.4 SO42−, 1.8 Ca2+, 0.9 Mg2+, 10 HEPES, 10 glucose, 2.9 sucrose, pH buffered to 7.40 with NaOH; 2) Krebs solution: 146.2 Na+, 5.9 K+, 133.3 Cl−, 1.2 H2PO4−, 25 HCO3−, 2.5 Ca2+, 1.2 Mg2+, 11 glucose, pH was buffered to 7.4 by gassing with 95% O2-5% CO2; and 3) HEPES-buffered Krebs solution: 125.3 Na+, 5.8 K+, 133.3 Cl−, 0.77 H2PO4−, 1.8 Ca2+, 1.0 Mg2+, 11 glucose, 10 HEPES.
Drugs.
Nifedipine, atropine, methoctramine, 4-DAMP, 2-aminoethoxydiethylborate (2-APB), ryanodine, xestospongin C, and tetracaine were all purchased from Sigma. U73122 was obtained from Tocris. Nifedipine was dissolved in ethanol; Xestospongin and U73122 were dissolved in DMSO. Dilutions were made from stock solutions such that the percentage of vehicle in the final concentration did not exceed 0.1%.
RESULTS
Immunohistochemistry.
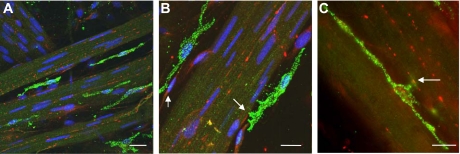
Whole mount preparations of guinea pig bladder were labeled with anti-vesicular acetylcholine transferase (vAChT) to label cholinergic nerve fibers (red), and anti-c-Kit to label ICC (green). Nuclei were counterstained with DAPI (blue). Figure 1 shows the location of ICC on the boundary of smooth muscle bundles in close proximity to cholinergic nerve fibers with points of contact highlighted by arrows. These images are typical of similar experiments in bladder tissue from at least five animals.
Fig. 1.
Immunohistochemical micrographs showing relationship between cholinergic nerves labeled with anti-vesicular acetylcholine transferase (red) and anti-c-Kit (green). Nuclei in A and B were counterstained with DAPI. Arrows show points of close contact between cholinergic nerve fibers and interstitial cells (B, C). Scale bars represent 10 μm in each case.
Muscarinic receptor-mediated contractions.
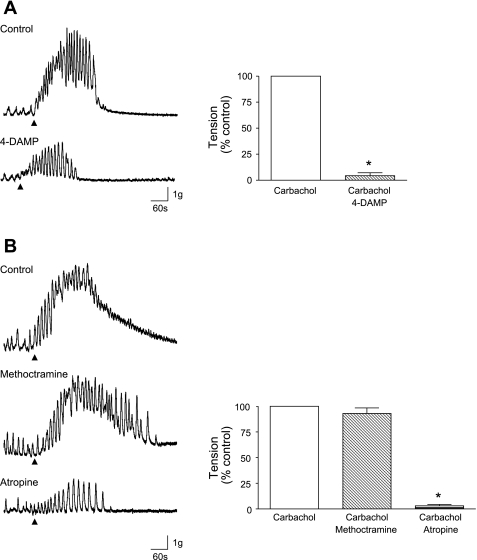
Application of 1 μM carbachol caused a large contraction of the tissue and spontaneous contractions were often superimposed on the response. The effects of carbachol were repeatable after the preparations were washed for 15 min. The M3 muscarinic receptor antagonist 4-DAMP (1 μM) reduced the carbachol response (control 1 ± 0 reduced to 0.04 ± 0.03 by the drug, n = 16 strips from 6 animals, P = 0.0001; Fig. 2A). The M2 antagonist methoctramine (1 μM) had little effect (control 1 ± 0 reduced to 0.93 ± 0.05, P = 0.1979, n = 18 strips from 6 animals; Fig. 2B). The general muscarinic receptor antagonist atropine (1 μM) abolished the responses to carbachol (control 1 ± 0 reduced to 0.03 ± 0.01, n = 18 strips from 6 animals, P = 0.0001).
Fig. 2.
A: tension recordings from guinea pig detrusor strips showing contractile response to carbachol (1 μM). Arrow heads indicate application of carbachol. This was significantly reduced by the M3 receptor antagonist 4-DAMP (1 μM). Summary data are shown for n = 6 tissues. B: carbachol-induced contraction was not blocked by the M2 antagonist methoctramine (1 μM) but was abolished by atropine (1 μM). Summary data for methoctramine (n = 7) and atropine (n = 6) are shown in the graph. *Statistical significance.
Muscarinic receptor-mediated Ca2+ transients in isolated ICC.
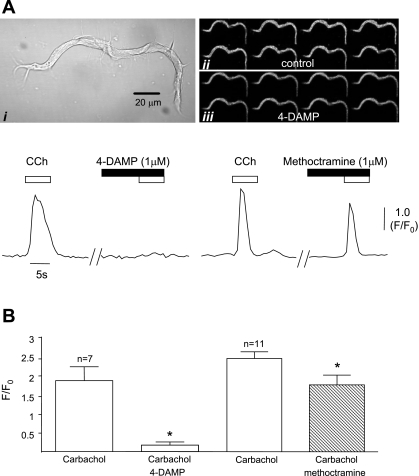
Cells with the typical branched morphology of ICC were selected for the following series of experiments. These were previously identified as ICC by labeling with c-Kit antibodies (16). Application of 0.1, 1, or 10 μM carbachol to isolated ICC induced an increase in intracellular [Ca]i2+, although lower concentrations sometimes induced oscillatory activity rather than eliciting individual Ca2+ transients. One micromolar carbachol was selected for the following experiments as this was used in the mechanical experiments described above and in our previous published work on isolated ICC. These responses were repeatable and comprised large transients (F/F0 2.36 ± 0.02) lasting 2.5 ± 0.19 s (n = 27). Previous work showed that the carbachol responses were sensitive to the muscarinic blocker atropine; this finding was confirmed in the present investigation with atropine (1 μM) blocking carbachol responses in eight cells; control amplitude F/F0 was reduced from 2.5 ± 0.72 to 0.03 ± 0.007 (P = 0.011). The identity of the muscarinic receptors mediating the carbachol-induced Ca2+ transients was further examined using the pharmacological antagonists of M2 and M3 receptors used in the contractile experiments above. Exposure of cells to 4-DAMP (1 μM) inhibited the carbachol responses (Fig. 3A) by 90% (n = 7, P = 0.004), with complete inhibition seen in five cells (Fig. 3B). Methoctramine (1 μM; Fig. 3B) reduced the carbachol responses (n = 11); however, this effect was somewhat variable with little change seen in five cells, whereas reductions were seen in six cells. Overall, the mean control amplitude was reduced from 2.4 ± 0.17 to 1.73 ± 0.25 (P = 0.005; Fig. 3B).
Fig. 3.
Ai: typical morphology of bladder interstitial cell. Aii–iii: time series fluorescence micrograph showing Ca2+ response to 1 μM carbachol which was blocked by the M3 antagonist 4-DAMP (1 μM). Traces show intensity time series of responses to carbachol and the effects of 4-DAMP and the M2 antagonist methoctramine (1 μM). Fluorescence (F) of an event is expressed as a ratio of background (F0). CCh, carbachol. B: graph summarizing the effect of 4-DAMP and methoctramine on 7 and 11 cells, respectively. *Statistical significance.
Role of Ca2+ influx.
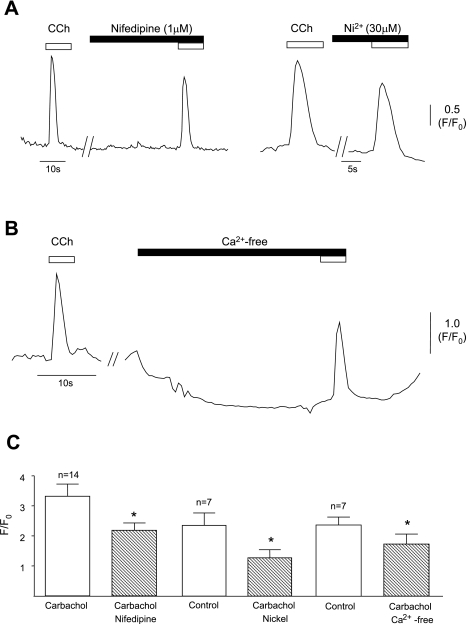
The source of Ca2+ underlying the carbachol-induced transients was first investigated using agents to interfere with Ca2+ influx. Influx via voltage-dependent L-type Ca2+ channels was investigated using the L-type Ca2+ channel blocker nifedipine (Fig. 4A). The amplitude of carbachol-induced transients was reduced by 1 μM nifedipine from 3.32 ± 0.41 to 2.18 ± 0.25, P = 0.001, n = 14. There was no effect on basal Ca2+. Ni2+ (30 μM), a blocker of other Ca2+ channels, including T-type (Fig. 4A), reduced transient amplitude from 2.32 ± 0.4 to 1.26 ± 0.26, P = 0.017, n = 7 and a higher concentration (100 μM) had a greater effect (1.75 ± 0.30 to 0.2 ± 0.1, P = 0.028, n = 4). A reduction in basal Ca2+ was seen with 100 μM Ni2+. Removal of external Ca2+ (Ca2+ substituted by Mg2+) lowered the baseline (1.02 ± 0.02 to 0.62 ± 0.07) and reduced the transient amplitude from 2.29 ± 0.26 to 1.68 ± 0.32 (P = 0.04, n = 7; Fig. 4B).
Fig. 4.
A: typical examples of the effect of nifedipine (1 μM) and nickel (30 μM) on the carbachol-induced Ca2+ transients. B: initial response to carbachol is shown in normal Ca2+ after which the cell was exposed to Ca2+-free external solution. Note the fall in basal fluorescence in the absence of external Ca2+; however, the cell was still able to respond to carbachol by firing a Ca2+ transient. C: summary data for the effect of nifedipine (n = 14), nickel (n = 7), and Ca2+-free (n = 7). *Statistical significance.
Intracellular Ca2+ stores.
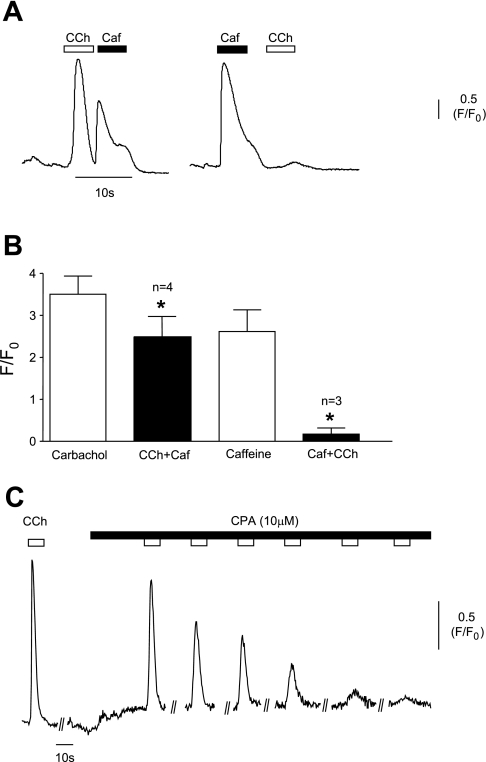
The possibility that carbachol was releasing Ca2+ from internal stores was initially addressed using experiments involving caffeine and carbachol. Initial application of carbachol caused a large Ca2+ transient and subsequent application of 10 mM caffeine also elicited an increase in [Ca2+]. Exposure to carbachol after an initial application of caffeine, on the other hand, failed to elicit a further Ca2+ transient (Fig. 5A). Summary data for both experiments are shown in Fig. 5B. These experiments suggested that carbachol was releasing Ca2+ from internal stores and that either the release was from ryanodine-sensitive stores or that carbachol was acting on a common IP3/ryanodine store. Application of cyclopiazonic acid (CPA; 10 μM) caused a gradual reduction in the amplitude of the carbachol transients until they were abolished (Fig. 5C), indicating that release of Ca2+ from internal stores was a major source. Mean amplitude in five cells was reduced from 3.12 ± 0.32 to 0.006 ± 0.004, P = 0.001. Basal Ca2+ was generally elevated in the presence of CPA, although this was not statistically significant.
Fig. 5.
A: experiment showing that carbachol evoked a large Ca2+ transient and that caffeine (Caf; 10 mM) could evoke a further response when applied immediately after. Pretreatment with caffeine, however, prevented carbachol from evoking a Ca2+ transient. B: summary data for these experiments are shown graphically. C: typical record of the cellular response to carbachol in the continued presence of cyclopiazonic acid (CPA; 10 μM) showing gradual reduction of the response. *Statistical significance.
Ca2+ release from IP3-sensitive stores.
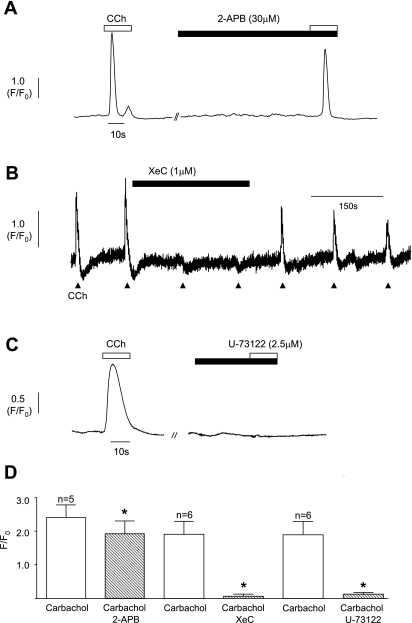
The contribution of Ca2+ release from IP3 intracellular stores was initially investigated using 2-APB, a drug often used to block release from IP3-sensitive stores. Thirty micromolar 2-APB (Fig. 6A) reduced the carbachol-induced transients from 2.41 ± 0.37 to 1.92 ± 0.38, n = 6, P = 0.02. Increasing the concentration of 2-APB to 100 μM was more effective, reducing mean transient amplitude from 2.34 ± 0.31 to 1.53 ± 0.22, P = 0.001, n = 6. However, in some of the cells tested, application of 100 μM 2-APB caused a marked increase in basal Ca2+ making it impossible to assess its effect on the carbachol responses. Another agent widely used to block IP3 receptors, xestospongin-C (1 μM), consistently blocked the carbachol responses (Fig. 6B) from 1.89 ± 0.39 to 0.06 ± 0.06 (n = 6, P = 0.006) with little effect on basal Ca2+. The effect of inhibition of phospholipase C was tested using U-73122 (2.5 μM). This agent blocked the carbachol transients (Fig. 6C) with no effect on basal Ca2+ in six cells (1.91 ± 0.39 to 0.11 ± 0.07, P = 0.005).
Fig. 6.
A: typical example of the effect of 2-aminoethoxydiethylborate (2-APB; 30 μM) on carbachol-evoked Ca2+ transients. B: Xestospongin C (XeC; 1 μM) blocked the carbachol responses; the effect was reversible on washout. C: example of the effect of the phospholipase C inhibitor U-73122 (2.5 μM). D: summary graph of the effect of 2-APB (5 cells), xestospongin C (6 cells), and U-73122 (6 cells). *Statistical significance.
Contribution of Ca2+ release from ryanodine-sensitive stores.
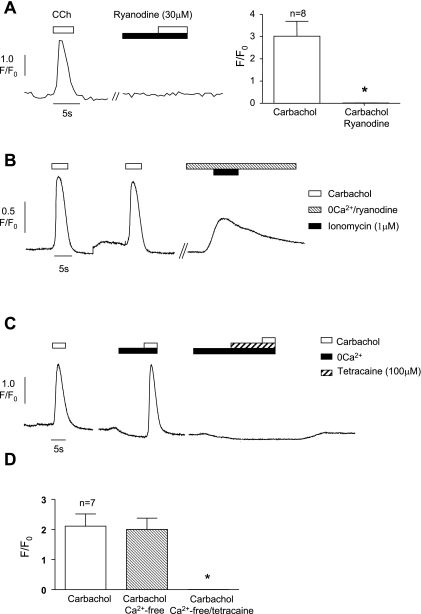
Ryanodine and tetracaine were used to assess the effect of blocking ryanodine receptors on the carbachol-induced transients. Ryanodine (30 μM) abolished the carbachol responses in eight cells (3.02 ± 0.67 to 0.01 ± 0.004, P = 0.003; Fig. 7A). The effects of ryanodine were irreversible. The increase in basal Ca2+ seen in the presence of ryanodine (0.93 ± 0.03 to 1.22 ± 0.12, P = 0.06) may indicate gradual depletion of the Ca2+ store content and therefore may indirectly block the carbachol-induced transients. This possibility was addressed by treating the cells with ryanodine for 60 s (as for the experiments described above) and then applying ionomycin (1 μM) in the absence of external Ca2+ to empty the stores. Ionomycin, in the continued presence of ryanodine, caused a substantial release of Ca2+ indicating that ryanodine treatment had not emptied the stores (Fig. 7B). Similarly, exposure of cells to tetracaine (100 μM) abolished the carbachol responses (2.03 ± 0.57 to 0.01 ± 0.01, n = 8, P = 0.009) without changing basal Ca2+. As others showed that tetracaine may block L-type Ca2+ channels (23), the experiments were repeated in external Ca2+-free conditions (in this set of experiments, the overall reduction of the carbachol response by Ca2+-free was not statistically significant, perhaps indicating that there was variability in the contribution of Ca2+ influx in some cells). The example shown in Fig. 7C shows that tetracaine still abolished the carbachol responses ruling out an effect via Ca2+ channels and indicating that it was working by blocking ryanodine receptors.
Fig. 7.
A: typical experiment showing the lack of effect of carbachol in the presence of ryanodine (30 μM). Summary of the effect of ryanodine on 8 cells. B: 2 repeatable responses to carbachol before treatment with ryanodine in the absence of external Ca2+. Subsequent application of ionomycin was able to cause an increase in intracellular Ca2+. C: carbachol-induced Ca2+ transient in normal solution and then repeated in external Ca2+-free solution. Application of tetracaine (100 μM) abolished the carbachol response. D: summary of the effect of tetracaine in 7 cells. *Statistical significance.
Cholinergic stimulation of ICC in whole tissue preparations.
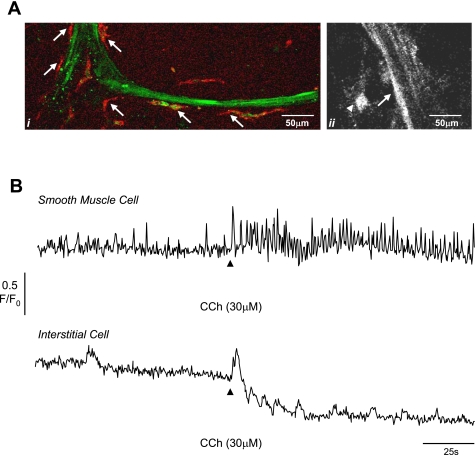
Whole tissue preparations of guinea pig bladder containing a few smooth muscle bundles were loaded with a Ca2+ indicator and imaged using a confocal microscope. ICC were readily identified on the boundary of the bundles or in the interstitial spaces between bundles, both by their location and their branched morphology, consistent with our previous work (16, 17). The loading of cells with fluorescent indicator enabled their morphology to be examined and cells of interest identified. To confirm that we were correctly identifying ICC, we labeled several of the loaded preparations with c-Kit antibody and Alexa 594 secondary antibody. Figure 8Ai shows that the cells on the edge of the bundle which we identified as ICC were labeled with c-Kit, whereas the smooth muscle cells were not labeled. We did not label ICC with antibodies in the following Ca2+-imaging experiments to avoid any interference with the cellular physiology.
Fig. 8.
Ai: preparation showing smooth muscle bundle loaded with fluo 4AM (green) and labeled with anti-c-Kit (red) showing the interstitial cells (arrows) on the edge of the bundle. Aii: smooth muscle bundle and interstitial cell to left border of bundle. B: intensity time series from smooth muscle cell (arrow in Aii) and interstitial cell (arrow head in Aii) showing the effect of 30 μM carbachol. Carbachol increased the frequency of spontaneous transients in the smooth muscle cell and caused an additional increase in intracellular Ca2+ in the interstitial cell followed by several oscillations. The reduction in baseline in the interstitial cell record is due to movement of the tissue. This record is typical of 5 experiments described in the text.
After the preparations were loaded, they were washed with Krebs solution, to remove excess indicator and areas of the tissue containing ICC and smooth muscle cells were imaged at room temperature (Fig. 8Aii). Spontaneous Ca2+ activity was typically present in both ICC and smooth muscle cells; however, the signaling patterns were distinctively different, consistent with the findings of Hashitani et al. (7). The smooth muscle cells fired regular Ca2+ events of 2.3 ± 0.2-s duration, amplitude 0.21 ± 0.03 dF/F0 at a frequency of 9.2 ± 1.7/min (n = 8), whereas the ICC fired transients of 12.2 ± 2.4-s duration, amplitude 0.49 ± 1.2 dF/F0 less frequently at 1.3 ± 0.3/min. In addition, integrated, coordinated flashes in the whole smooth muscle bundle were encountered (amplitude F/F0 0.61 ± 0.07, duration 3.5 ± 0.5 s, frequency 3.8 ± 0.9/min, n = 7). Application of carbachol initially caused contraction of the tissue so that the tissue moved outside of the field of focus. However, extensive pinning of the tissue meant that we were able to record the effects of cholinergic stimulation without movement artefacts. Figure 8B shows the results of one such experiment. Carbachol (30 μM) increased the frequency of events in smooth muscle cells from 11 ± 1/min to 21 ± 2.9/min (n = 5) and caused large Ca2+ transients (0.34 ± 0.02 amplitude) in ICC followed by several oscillations.
DISCUSSION
The findings of this study demonstrate that bladder ICC fire Ca2+ transients in response to cholinergic stimulation and investigated the sources of Ca2+ underlying the generation of these transients. Experiments on ICC in situ demonstrated that they responded to application of carbachol in a manner consistent with freshly isolated ICC. Immunohistochemical experiments demonstrated the close proximity of cholinergic nerve fibers to ICC, providing morphological support for the hypothesis that ICC participate in cholinergic signaling in the bladder. Isolated smooth muscle cells and ICC present in the enzymatic cell suspension responded differently to stimulation with carbachol. While both cell types fired Ca2+ transients, this was accompanied by contraction in smooth muscle cells, whereas the ICC were not observed to contract, despite the large increases in intracellular Ca2+ that were generated. This is consistent with our previous studies on these cells where agonist stimulation or depolarization via a patch electrode did not induce contraction of ICC (16, 17).
Muscarinic receptor subtypes.
M2 and M3 muscarinic receptors are present in the mammalian detrusor, with the numbers of M2 significantly exceeding those of M3 (9, 26, 31). In human and guinea pig bladder, the proportion of M2 and M3 is ∼3:1, although the M3 subtype is dominant in the excitation of smooth muscle contraction via Gq/11 activating hydrolysis of phosphoinositide, IP3 production, and elevation of intracellular Ca2+. Conversely, M2 receptors may indirectly enhance M3-mediated contraction, by opposing the normal effects of β-adrenoceptor G protein-coupled activation of adenylyl cyclase which lead to cAMP production and bladder relaxation (1, 8, 31). In the present investigation, the contractile response of guinea pig detrusor strips to carbachol was antagonized by the M3 receptor blocker 4-DAMP and partially reduced by the M2 antagonist methoctramine. In isolated ICC, carbachol-induced Ca2+ transients were blocked by the general muscarinic antagonist atropine or with 4-DAMP. The M3 subtype was apparently dominant in mediating the response as blockade of M2 receptors with methoctramine caused only a small reduction. The latter experiments, however, provide evidence that M2 receptors are present on bladder ICC. A similar situation has been reported for mouse gastric ICC where 4-DAMP antagonized the carbachol-induced increase in frequency of pacemaker currents but methoctramine was less effective (11).
Ca2+ influx.
The source of Ca2+ underlying the carbachol-induced Ca2+ transients in isolated ICC was investigated using a number of pharmacological agents. Ca2+ entry was clearly an important component, as blockade of membrane Ca2+ channels with nifedipine or Ni2+, or the removal of extracellular Ca2+, caused a significant reduction of the carbachol responses. Ca2+ influx is an important component of the response to cholinergic stimulation in other cells. Isolated human bladder smooth muscle cells were reported to fire Ca2+ transients in response to carbachol, which were reduced up to 40% in external Ca2+-free conditions but unaffected by the L-type Ca2+ channel blocker nicardipine (29). Similar Ca2+ responses to carbachol have been seen in guinea pig detrusor smooth muscle cells (30) where carbachol also produced a membrane hyperpolarization followed by a small depolarization that occurred after the peak of the Ca2+ transient, indicating that depolarization via activation of L-type Ca2+ channels was not responsible for the transient. We do not currently have information on any changes in membrane potential in guinea pig bladder ICC due to carbachol stimulation; however, the reduction of our transients by nifedipine and Ni2+ may suggest that some membrane depolarization could also be occurring.
Application of carbachol after prior exposure to caffeine (at a concentration to deplete the internal store) failed to elicit a Ca2+ transient, whereas pretreatment with carbachol did not prevent further Ca2+ release by caffeine. This contrasts with findings in mouse bladder smooth muscle cells where pretreatment with carbachol caused a reduction in the caffeine-induced transient and pretreatment with caffeine similarly reduced the amplitude of the carbachol-induced transient (22). In guinea pig bladder ICC, it would seem that carbachol causes release from intracellular stores which are also sensitive to caffeine. Further support for the internal stores being a major source of Ca2+ for the agonist-induced signals came from experiments with CPA (SR Ca2+-ATPase blocker) which caused elevation of [Ca2+]i, and additionally blocked the carbachol responses. Release of Ca2+ from intracellular stores could potentially occur by at least two pathways; classical activation of muscarinic receptors coupled to G proteins activating phospholipase C leading to production of diacylglycerol and IP3 which would cause release of Ca2+ from the IP3-sensitive store, or Ca2+-induced Ca2+ release (CICR) from the intracellular stores.
IP3-sensitive stores.
Evidence that carbachol was acting through the IP3 signal transduction pathway to release Ca2+ was provided by experiments using drugs to interfere with various stages of the pathway. U-73122, an inhibitor of phospholipase C (21), consistently reduced the carbachol responses without affecting basal Ca2+. Xestospongin-C, used to block IP3 receptors (5), significantly reduced the Ca2+ signals and while 2-APB, used to block IP3-mediated Ca2+ release (for a review, see Refs. 2, 15), was less effective, it also caused a significant reduction. Taken together, our findings indicate that the cholinergic Ca2+ transients are at least partly mediated by release from IP3-sensitive stores. We interpret these results with a degree of caution, however, as both Xestospongin C and 2-APB have been shown to have other effects including inhibition of store-operated channels and blockade of the SERCA pump (2, 4, 6, 12, 19). In our experiments, the carbachol responses were not abolished but significantly reduced by 2-APB (30 and 100 μM), although there were occasions where 2-APB caused a steady increase in basal Ca2+, making it impossible to assess any effect on the carbachol response.
Ryanodine-sensitive Ca2+ stores.
Evidence for CICR from ryanodine-sensitive stores in response to carbachol stimulation came from experiments using ryanodine which causes depletion of the ryanodine-sensitive store, or tetracaine, a local anesthetic which blocks ryanodine receptors. In mouse bladder smooth muscle cells, carbachol transients were reduced but not blocked by ryanodine (22), whereas in the present study, ryanodine provided complete inhibition. Ryanodine or tetracaine inhibited the Ca2+ response to carbachol and on the majority of occasions, a small component due to Ca2+ influx was generally not evident. This could be explained by the influx component being inhibited as a result of the elevation of intracellular Ca2+ that occurred in the presence of ryanodine, leading to Ca2+-induced inactivation of Ca2+ current. Previous work on bladder ICC characterized voltage-dependent Ca2+ channels which undergo Ca2+-induced inactivation (18). Moreover, there is some evidence that in addition to blocking ryanodine receptors, tetracaine could directly block L-type channels (23), perhaps explaining why this drug blocked all of the carbachol response under conditions in which influx could occur. If the hypothesis that carbachol normally causes Ca2+ influx via L-type channels which in turn activates CICR from the ryanodine-sensitive store is correct, then application of tetracaine could initially prevent influx by blocking Ca2+ channels which would limit CICR as well as blocking the ryanodine receptors which are responsible for CICR.
In the presence of nifedipine or the absence of extracellular Ca2+, tetracaine blocked the carbachol responses, supporting the view that its main mode of action was via blockade of RyR (in nifedipine alone or in Ca2+-free, there would still be a carbachol-induced transient). However, it is worth noting that a study on guinea pig colonic smooth muscle cells (14) presents an important caveat when interpreting data relating to IP3-mediated Ca2+ release and CICR at ryanodine receptors. It was demonstrated that tetracaine and ryanodine could reduce IP3-mediated Ca2+ signals by directly affecting IP3 receptors or depleting the (shared) store of Ca2+, respectively. Ryanodine could block a carbachol response by opening the RyR and depleting the store therefore diminishing the Ca2+ available for release by IP3-mediated mechanisms. This might explain why ryanodine apparently blocked the carbachol response in bladder ICC, i.e., an indirect effect, from depletion of the “common” or “total” cellular SR. We addressed this by applying the Ca2+ ionophore, ionomycin, to a cell which had been pretreated with ryanodine, an approach used by White and McGeown (27). The results indicated that the store (combination of IP3 and RyR) had not been depleted by ryanodine as ionomycin could still evoke a large transient (in the absence of external Ca2+). This suggests that the blocking effect of ryanodine was not due to depletion of the total store content but that carbachol does release Ca2+ from ryanodine-sensitive stores.
Interaction of IP3- and ryanodine-sensitive stores.
The involvement of both ryanodine and IP3-sensitive pathways raises the possibility that IP3 and ryanodine stores in bladder ICC are linked, or alternatively, that there is one store which can be released by either receptor type. If it is the case that the ICC contain one store, it seems that it is not emptied by the carbachol exposure, because application of carbachol immediately followed by caffeine results in Ca2+ release by both agents. This experiment could also be explained by there being two discrete stores and each agent acting on a different store. However, the reverse experiment showed that application of caffeine, followed by carbachol, gave rise to release from the caffeine, but not then by carbachol. If a single store scenario is correct, perhaps the caffeine challenge was sufficient to empty the store so that carbachol could not cause further release. This experiment would argue against there being two discrete Ca2+ stores. While this specific question cannot be satisfied in the present investigation, it is clear that there is interaction of IP3- and ryanodine-sensitive Ca2+ release mechanisms.
Cholinergic stimulation of ICC in intact detrusor preparations.
Intact tissue preparations loaded with Ca2+ indicators revealed that ICC and smooth muscle cells with distinctively different spontaneous Ca2+-signaling patterns both responded to carbachol by increasing the frequency and amplitude of their signals in situ. The physiological significance of the smooth muscle response to carbachol is clear–the major excitatory neurotransmission to the bladder is parasympathetic and one would therefore expect cholinergic agonists to increase intracellular Ca2+ and cause contraction. The situation is less clear in the ICC and future work will reveal whether the Ca2+ transients are perhaps associated with the release of a factor which influences the contractility of neighbouring smooth muscle cells. A possible scenario may be that detrusor ICC normally send inhibitory signals to the smooth muscle during bladder filling to prevent unwanted coordinated contractions and the ICC Ca2+ response to cholinergic stimulation (at the start of micturition) may override this inhibitory signal. The finding that detrusor ICC respond to cholinergic stimulation by firing Ca2+ transients is markedly different than the situation in ICC from the suburothelial region of the guinea pig bladder which have been reported to exhibit no response to exogenously applied carbachol and which therefore are unlikely to contain cholinergic receptors (30). This difference may indicate an important division of labor in these subpopulations of ICC in the guinea pig bladder with the suburothelial ICC currently understood to have a regulatory role in the sensation of bladder fullness (30) and the detrusor ICC modulating the smooth muscle activity.
In conclusion, c-Kit-positive ICC in the bladder have close structural relationships with cholinergic nerves. Freshly dispersed detrusor ICC and ICC in situ respond to cholinergic stimulation by firing Ca2+ transients. The source of Ca2+ underlying these events is partially due to influx but mainly attributable to release from intracellular stores via IP3- and ryanodine-sensitive pathways.
GRANTS
Financial assistance from Biotechnology and Biological Sciences Research Council Queen's University Belfast, The Wellcome Trust, and Tenovus-Scotland is gratefully acknowledged. A. D. Lyons is supported by a studentship from School of Medicine and Dentistry, QUB. C. Carson is supported by a studentship from Department of Education and Learning, Northern Ireland.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Andersson KE, Arner A. Urinary bladder contraction and relaxation: physiology and pathophysiology. Physiol Rev 84: 935–986, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-Aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J 10: 1145–1150, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Brading AF, McCloskey KD. Mechanisms of disease: specialized interstitial cells of the urinary tract–an assessment of current knowledge. Nat Clin Pract Urol 2: 546–554, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Castonguay A, Robitaille R. Xestospongin C is a potent inhibitor of SERCA at a vertebrate synapse. Cell Calcium 32: 39–47, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Gafni J, Munsch JA, Lam TH, Catlin MC, Costa LG, Molinski TF, Pessah IN. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-trisphosphate receptor. Neuron 19: 723–733, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Gregory RB, Rychkov G, Barritt GJ. Evidence that 2-aminoethoxydiphenyl borate is a novel inhibitor of store-operated Ca2+ channels in liver cells, and acts through a mechanism which does not involve inositol trisphosphate receptors. Biochem J 354: 285–290, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashitani H, Yanai Y, Suzuki H. Role of interstitial cells and gap junctions in the transmission of spontaneous Ca2+ signals in detrusor smooth muscles of the guinea pig urinary bladder. J Physiol 559: 567–581, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hegde SS, Choppin A, Bonhaus D, Briaud S, Loeb M, Moy TM, Loury D, Eglen RM. Functional role of M2 and M3 muscarinic receptors in the urinary bladder of rats in vitro and in vivo. Br J Pharmacol 120: 1409–1418, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hegde SS, Eglen RM. Muscarinic receptor subtypes modulating smooth muscle contractility in the urinary bladder. Life Sci 64: 419–428, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Horowitz B, Ward SM, Sanders KM. Cellular and molecular basis for electrical rhythmicity in gastrointestinal muscles. Annu Rev Physiol 61: 19–43, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Kim TW, Koh SD, Ordog T, Ward SM, Sanders KM. Muscarinic regulation of pacemaker frequency in murine gastric interstitial cells of Cajal. J Physiol 546: 415–425, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma HT, Patterson RL, van Rossum DB, Birnbaumer L, Mikoshiba K, Gill DL. Requirement of the inositol trisphosphate receptor for activation of store-operated Ca2+ channels. Science 287: 1647–1651, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Ma HT, Venkatachalam K, Li HS, Montell C, Kurosaki T, Patterson RL, Gill DL. Assessment of the role of the inositol 1,4,5-trisphosphate receptor in the activation of transient receptor potential channels and store-operated Ca2+ entry. J Biol Chem 276: 18888–18896, 2001. [DOI] [PubMed] [Google Scholar]
- 14.MacMillan D, Chalmers S, Muir TC, McCarron JG. IP3-mediated Ca2+ increases do not involve the ryanodine receptor, but ryanodine receptor antagonists reduce IP3-mediated Ca2+ increases in guinea pig colonic smooth muscle cells. J Physiol 569: 533–544, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P-3-induced Ca2+ release. Jpn J Biochem 122: 498–505, 1997. [DOI] [PubMed] [Google Scholar]
- 16.McCloskey KD, Gurney AM. Kit-positive cells in the guinea pig bladder. J Urol 168: 832–836, 2002. [PubMed] [Google Scholar]
- 17.McCloskey KD Characterization of outward currents in interstitial cells from the guinea pig bladder. J Urol 173: 296–301, 2005. [DOI] [PubMed] [Google Scholar]
- 18.McCloskey KD Calcium currents in interstitial cells from the guinea pig bladder. BJU Int 97: 1338–1343, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Prakriya M, Lewis RS. Potentiation and inhibition of Ca2+ release-activated Ca2+ channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP3 receptors. J Physiol 536: 3–19, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanders KM A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology 111: 492–515, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Smith RJ, Sam LM, Justen JM, Bundy GL, Bala GA, Bleasdale JE. Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J Pharmacol Exp Ther 253: 688–697, 1990. [PubMed] [Google Scholar]
- 22.Sugita M, Tokutomi N, Tokutomi Y, Terasaki H, Nishi K. The properties of caffeine- and carbachol-induced intracellular Ca2+ release in mouse bladder smooth muscle cells. Eur J Pharmacol 348: 61–70, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Sugiyama K, Muteki T. Local anesthetics depress the calcium current of rat sensory neurons in culture. Anesthesiology 80: 1369–1378, 1994. [DOI] [PubMed] [Google Scholar]
- 24.Sui GP, Rothery S, Dupont E, Fry CH, Severs NJ. Gap junctions and connexin expression in human suburothelial interstitial cells. BJU Int 90: 118–129, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Sui GP, Wu C, Fry CH. Electrical characteristics of suburothelial cells isolated from the human bladder. J Urol 171: 938–943, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Wang P, Luthin GR, Ruggieri MR. Muscarinic acetylcholine receptor subtypes mediating urinary bladder contractility and coupling to GTP binding proteins. J Pharmacol Exp Ther 273: 959–966, 1995. [PMC free article] [PubMed] [Google Scholar]
- 27.White C, McGeown JG. Carbachol triggers RyR-dependent Ca2+ release via activation of IP3 receptors in isolated rat gastric myocytes. J Physiol 542: 725–733, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu C, Sui GP, Fry CH. Purinergic regulation of guinea pig suburothelial myofibroblasts. J Physiol 559: 231–243, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu C, Bayliss M, Newgreen D, Mundy AR, Fry CH. A comparison of the mode of action of ATP and carbachol on isolated human detrusor smooth muscle. J Urol 162: 1840–1847, 1999. [PubMed] [Google Scholar]
- 30.Wu C, Sui G, Fry CH. The role of the L-type Ca2+ channel in refilling functional intracellular Ca2+ stores in guinea pig detrusor smooth muscle. J Physiol 538: 357–369, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamanishi T, Chapple CR, Chess-Williams R. Which muscarinic receptor is important in the bladder? World J Urol 19: 299–306, 2001. [DOI] [PubMed] [Google Scholar]