Abstract
Tumor necrosis factor-α (TNFα)-induced hepatocyte death and liver injury can be mediated by multiple mechanisms, which could be evaluated by different animal models. Previous studies have defined the importance of Bid in mitochondrial apoptosis activation in adult mice treated with lipopolysaccharides in the presence of galactosamine (GalN), which suppresses NF-κB activation, but not in embryonic mice in which NF-κB activation is suppressed by genetic deletion of p65RelA. JNK has also been found important in TNFα-induced mitochondria activation and liver injury in the lipopolysaccharide/GalN and concanavalin A (ConA)/GalN models, but not in a ConA-only model in which NF-κB activation was not suppressed. To determine the mechanistic relationship of pathways mediated by Bid and JNK, we investigated these two molecules in TNFα injury models that had not been previously examined. Most importantly, we created and studied mice deficient in both Bid and JNK. We found that, like JNK, Bid was also required for TNFα-induced injury induced by concanavalin A/GalN but not by ConA alone. Furthermore, our results indicate that these two molecules function in a largely overlapped manner, with Bid being downstream of JNK in the adult livers. However, JNK, but not Bid, was able to contribute to the TNFα-induced liver apoptosis in RelA-deficient embryos. The Bid-independent role of JNK was also observed in the adult mice, mainly in the promotion of the lethal progression of the TNFα injury. This work defined both linear and parallel relationships of Bid and JNK in TNFα-induced hepatocyte apoptosis and liver injury.
TNFα3 could induce multiple prodeath mechanisms. The classic mechanism is the activation of the extrinsic apoptosis pathway, in which the engagement of TNF-R1 (tumor necrosis factor receptor 1) recruits the adaptor molecules, TRADD and FADD, which in turn recruit caspase-8. The activation of caspase-8 leads to the cleavage of a prodeath Bcl-2 family protein, Bid, which then triggers the mitochondria apoptosis pathway (1). Previous studies have established that Bid, a BH3-only prodeath Bcl-2 family protein, plays an important role in death receptor-mediated hepatocyte apoptosis and liver injury (2–7). Specifically, deletion of Bid leads to a complete protection against Fas-mediated liver injury. However, Bid-deficient mice are only partially protected from TNFα-induced liver injury in a model using LPS and GalN as the stimulation (5, 6).
TNFα-induced cell death is suppressed by concurrently activated NF-κB-mediated prosurvival events (8). The protection could be abolished by a general transcription inhibitor, such as actinomycin D or GalN, or by a specific deletion of a key element in the NF-κB pathway, such as p65RelA. RelA is the critical component of the p65-p50 NF-κB dimers that are responsible for the prosurvival mechanisms, including the suppression of caspase and sustained JNK activation (8–10). Deletion of RelA leads to defective NF-κB signaling, unrestrained TNFα toxicity, severe hepatocyte apoptosis, and embryonic lethality (11). The liver injury and lethality could be rescued by a concomitant deletion of TNFα (12), indicating that TNFα is the trigger of the demise. Deletion of Bid, however, was not able to suppress hepatocyte apoptosis and embryonic lethality caused by the deletion of p65RelA (6, 13).
There are several possibilities that may account for the failure of Bid deficiency to protect TNFα-induced liver injury. One may relate to the form of TNFα presented to the receptors. In the model of LPS/GalN, TNFα is mainly produced by macrophages and Kupffer's cells stimulated by LPS, whereas GalN sensitizes hepatocytes. Notably, TNFα generated in this manner is mainly secreted. It has been found that soluble TNFα primarily activates TNF-R1, whereas membrane TNFα activates both TNF-R1 and TNF-R2 (14). Since the two TNF receptors differ in their ability to activate the death pathway and the NF-κB pathway, it is possible that the membrane and soluble TNFα could induce cell death with different features, as manifested in the different models of TNFα injury (15–18). It is thus also possible that Bid-deficient mice might be more resistant to membrane TNFα in terms of liver injury (e.g. following the treatment with ConA or ConA/GalN).
Another possibility is that additional death mechanisms other than Bid are activated by TNFα receptors. Our earlier studies indicate that JNK, reactive oxygen species, and mitochondria permeability transition could contribute to the Bid-independent mechanisms in TNFα-induced cell death (6). JNKs have been long considered to be important for TNFα toxicity in both hepatocytes and nonhepatocytes (for reviews, see Refs. 9 and 10). Sustained JNK phosphorylation is correlated with TNFα toxicity and is observed when NF-κB activation is suppressed. Although there is a redundancy of JNK1 and JNK2, deletion of either could significantly block TNFα-induced death in hepatocytes in vitro (19, 20) and in vivo (18, 21–23).
Here we determined the relationship of the Bid-mediated pathway and the JNK-mediated pathway by examining mice deficient in Bid, JNK2, or both in several TNFα injury models where TNFα can be presented in soluble or membranous form.
EXPERIMENTAL PROCEDURES
Animals—Wild-type and Bid-deficient mice were maintained in C57BL/6 background as previously described (2). JNK1-deficient mice (B6.129S1-Mapk8tm1Flv/J) and JNK2-deficient mice (B6.129S2-Mapk9tm1Flv/J) maintained in C57BL/6 background were obtained from the Jackson Laboratory (Bar Harbor, ME). Bid/JNK2 doubly deficient mice were created by intercrossing the Bid-deficient mice with the JNK2-deficient mice. Mice heterozygous for the p65relA gene were maintained in a mixed background of C57BL/6 and 129SvJ as previously described (6, 11). These mice were further intercrossed to jnk1-/-, jnk2-/-, and/or bid-/- mice to create mice deficient or heterozygous for these genes as described under “Results.” Genotypes of the progenies were determined by PCR as previously described (11, 24, 25). Timed breeding was conducted, with the mornings when vaginal plugs were detected as day 0.5 (E0.5). Animal procedures were conducted according to the guidelines of the National Institutes of Health and protocols approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Reagents—The following antibodies were used: anti-caspase-8, anti-caspase-9, anti-caspase-3 (Cell Signaling, Boston, MA), anti-β-actin (Sigma), anti-cytochrome c, anti-Smac, anti-JNK (clone 666) (BD Biosciences, San Diego, CA), anti-phosphorylated JNK and c-Jun (Cell Signaling), anti-p65RelA (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and anti-Bid (6). GalN, LPS (Sigma L-2630, Escherichia coli 0111: B4), ConA, and phalloidin were obtained from Sigma.
Treatment of Mice—Mice were treated as previously described (3, 6). Briefly, male or female mice about 20–30 g in weight were intraperitoneally administered with GalN (20 mg/mice, for LPS study or 700 mg/kg for ConA study). Thirty minutes later, they were intraperitoneally given LPS at 50 μg/kg or intravenously given ConA at 25 mg/kg. All reagents were prepared in 0.9% (v/v) endotoxin-free sterile saline (Sigma). Animals were sacrificed at designated time points for analysis. Survival analysis was based on the time when mice became moribund.
Preparation of Liver Subfractions—Liver homogenates were prepared and fractionated as described previously (3, 6). Briefly, each liver was homogenized in 6 ml of Buffer A (200 mm mannitol, 70 mm sucrose, 1 mm EGTA, 10 mm HEPES, 0.1 mm phenylmethylsulfonyl fluoride, pH 7.2) with a Wheaten Dounce glass homogenizer (type B pestle) and centrifuged at 4 °C for 10 min at 700 × g. The supernatants were further centrifuged at 10,000 × g for 15 min. The supernatants were collected as the cytosol fraction. For Western blot and caspase activity measurement, liver lysates from individual mice within the same treatment group were pooled to avoid any bias caused by conditions in individual mouse.
Biochemical Assays—The serum level of ALT was measured using the assay kit purchased from Biotron Diagnostics (Hemet, CA) according to the manufacturer's instructions.
For immunoblot analysis, liver cytosol (80 μg) was separated by a 12% SDS-PAGE followed by transfer to polyvinylidene difluoride membranes and blotted with various antibodies as indicated. Blots were developed with SuperSignal West Pico chemiluminescent substrate (Pierce).
Caspase activities were measured as previously described (3, 6). Briefly, 15 μg of cytosol were incubated with 20 μm site-specific tetrapeptide substrates (Ac-DEVD-AFC for caspase-3, Ac-IETD-AFC for caspase-8, and Ac-LEHD-AFC for caspase-9; Calbiochem) in a caspase assay buffer (20 mm PIPES, 100 mm NaCl, 10 mm dithiothreitol, 1 mm EDTA, 0.1% (w/v) CHAPS, 10% (w/v) sucrose, pH 7.2) in a final volume of 200 μl. The fluorescence signals were detected by a fluorometer (Tecan GENios) at an excitation wavelength of 400 nm and emission wavelength of 510 nm.
Histology—Livers were dissected, fixed in 10% neutral buffered formalin, and paraffin-embedded. Sections were cut at 5 μm in thickness and stained with hematoxylin and eosin. In addition, frozen sections of livers from selected samples were stained with phalloidin and Hoechst 33342 to visualize the parenchymal structures and apoptotic nuclei. Images were obtained using a light microscope (Nikon Eclipse E200, Melville, CA) equipped with a digital camera (SPOT, Diagnostic Instruments, Sterling Heights, MI).
Statistical Analysis—All numerical results were expressed as the mean ± S.D. and represent data from a minimum of three independent experiments. The statistical analysis was performed with Sigma Stat 3.0 (SPSS Science, Chicago, IL) with a p value of less than 0.05 being considered as a significant difference. The survival rate was analyzed using the Kaplan-Meier method, and significance was determined by log rank analysis using SigmaStat 3.0 (SPSS Science).
RESULTS
Bid Is Important but Not Absolutely Required for the Mitochondria Apoptotic Response and Liver Injury Caused by ConA/GalN Treatment—To determine whether Bid-deficient mice would respond to membrane TNFα in a way different from that to soluble TNFα, mice were administrated with ConA, a T cell mitogen that leads to the increased expression of TNFα on the surface of T cells, which is the cytokine primarily responsible for the liver injury (26, 27). Unlike LPS, ConA alone could induce a reversible and mild liver injury without the sensitization with GalN (27, 28). However, when mice are sensitized with GalN, a significantly more severe injury can be induced with a rapid development of hepatocyte apoptosis, and the mortality is high (28). Interestingly, Bid-deficient mice seemed to be equally susceptible to ConA-only-induced liver injury as the wild type mice but were significantly protected from the more severe injury caused by ConA/GalN (Fig. 1, A and B).
FIGURE 1.
Bid-/- mice are significantly protected from liver injury induced by ConA/GalN, but not ConA alone. A, wild-type (Bid+/+) and Bid-deficient (Bid-/-) mice were treated with ConA or ConA/GalN for 8 h and sacrificed. The liver sections were stained with hematoxylin and eosin. Significantly less injury were observed in the Bid-deficient livers following ConA/GalN treatment. B, wild-type (Bid+/+) and Bid-deficient (Bid-/-) mice were treated with ConA/GalN for 6 h and sacrificed. The liver sections were stained with phalloidin (red) and Hoechst 33342 (blue). Disruption of the liver parenchymal and apoptotic nuclei could be clearly detected in the wild type mice but not in the Bid-deficient mice at this time. C, liver lysates from wild-type (Bid+/+) and Bid-deficient (Bid-/-) mice treated with ConA for 8 h were analyzed by immunoblot with an anti-caspase-3 antibody. Each line represents one mouse sample. C, nontreated; 1, 2, and 3, three treated samples; P, a positive control sample of wild type mice treated with LPS/GalN. D, liver lysates from wild-type (Bid+/+) and Bid-deficient (Bid-/-) mice treated with ConA/GalN and sacrificed at the designated time point were analyzed by immunoblot with the indicated antibodies. C-8, caspase-8; C-9, caspase-9; C-3, caspase-3.
Previous work had indicated that liver injury induced by ConA alone was not primarily caused by a caspase-mediated apoptotic process (23, 28). Consistently, caspase activation was not detected in either wild type or Bid-deficient livers 8 h after treatment when cellular injury was already apparent (Fig. 1C). However, in the presence of GalN, ConA could induce very robust caspase activation (Figs. 1D and 2A). Similar to the LPS/GalN regime, a ConA/GalN regime can induce a significant activation of the mitochondria apoptotic pathway with the release of cytochrome c and Smac around 4–6 h after the treatment (Fig. 1D). This kinetics was well correlated with the caspase activity arising at the same time frame (Fig. 2A). Notably, both the mitochondrial response and caspase activation were greatly diminished in Bid-deficient livers in terms of the kinetics and the severity (Fig. 1D), indicating that Bid, as in other cases of death receptor activation, is also the major molecule mediating the mitochondria activation in response to membrane TNFα in the ConA/GalN regime. Bid-independent activation of mitochondria and caspases occurred later (Fig. 1D), much like that observed in the LPS/GalN regime (6).
FIGURE 2.
Single or combined deletion of Bid and JNK2 confers significant protection against ConA/GalN-induced liver injury and mortality. A, wild-type (+/+; solid columns), Bid-deficient (Bid-/-; open columns), JNK2-deficient (JNK2-/-; crossed columns), and Bid/JNK2 doubly deficient (Bid/JNK2-/-; gray columns) mice were either untreated or treated with ConA/GalN and sacrificed at 2, 4, 6, and 8 h later. Mice were sacrificed, and the liver cytosol prepared, which was subjected to the measurement of caspase-3 activities (A) and immunoblot analysis with indicated antibodies (B; 6 h after treatment). Blood was also collected, and the serum level of ALT was determined (C). For A and C, results (mean ± S.D.) were from at least three mice for each group at the indicated time points. *, p < 0.05, compared with the wild type group. D, Kaplan-Meier survival analysis of wild-type (+/+, n = 7), Bid-/- (n = 15), JNK1-/- (n = 10), JNK2-/- (n = 13), and Bid/JNK2-/- (n = 11) mice following ConA/GalN treatment. p values indicated for each group were based on comparisons with the wild type group (log rank analysis). JNK1-/-, JNK2-/-, and Bid/JNK2-/- mice were significantly resistant to ConA/GalN.
The apoptotic response apparently dictated the fast progression of the liver injury in ConA/GalN treatment. Consistent with caspase activation, serum levels of ALT, a marker of liver injury, was increased at 4 and 6 h after treatment in wild-type and Bid-deficient mice, respectively (Fig. 2C). Thus, based on serum ALT and liver histology, deletion of Bid attenuated liver injury induced by ConA/GalN. All wild type mice died within 24 h, with a mean survival time of 7.45 h (Fig. 2D and Table 1). Deletion of Bid resulted in a noticeable but not significant prolonged survival time (8.14 h), and all mice still died within 24 h.
TABLE 1.
Survival analysis of mice with different Bid and JNK genotypes in TNF α-induced liver injury n is the number of mice used in each group. Survival refers to the percentage of mice that survived in the first 24-h period. Mean survival time and S.E. were determined by the Kaplan-Meier method.
|
Parameter
|
Genotype
|
||||
|---|---|---|---|---|---|
| +/+ | bid-/- | jnk1-/- | Jnk2-/- | bid/jnk2-/- | |
| ConA/GalN treatment | |||||
| n | 7 | 15 | 10 | 13 | 11 |
| Survival (%) | 0.0 | 0.0 | 10.0 | 15.4 | 27.3 |
| Mean survival time (h) | 7.453 | 8.144 | 10.717 | 13.154 | 13.263 |
| S.E. | 0.351 | 0.39 | 1.568 | 1.754 | 2.144 |
| p | vsa | 0.229 | 0.0026 | 0.0006 | 0.0002 |
| vs | 0.0437 | 0.0029 | 0.0071 | ||
| vs | 0.366 | 0.375 | |||
| vs | 0.843 | ||||
| LPS/GalN treatment | |||||
| n | 15 | 11 | 12 | 18 | 15 |
| Survival (%) | 0.0 | 0.0 | 8.3 | 16.7 | 40.0 |
| Mean survival time (h) | 6.4 | 7.783 | 9.616 | 10.857 | 15.007 |
| S.E. | 0.202 | 0.226 | 1.46 | 1.46 | 2.092 |
| p | vs | 0.0004 | 0.0023 | <0.0001 | <0.0001 |
| vs | 0.579 | 0.0414 | 0.0123 | ||
| vs | 0.345 | 0.035 | |||
| vs | 0.0123 | ||||
p values were determined by log rank analysis of the comparisons between the specific groups and the group indicated with “vs.” The values in bold have reached the significance level
These studies indicated that ConA alone initiated a non-caspase-dependent liver injury, as shown before (23, 28), which was not affected by the deletion of Bid. However, in the ConA/GalN scenario, deletion of Bid provided a significant protection against caspase activation and attenuated the injury and mortality. The failure of Bid-deficient mice to be completely protected from ConA/GalN treatment suggested that the Bid-independent mechanism could be activated regardless the form of TNFα, soluble or membranous.
Co-deletion of Bid and JNK2 Revealed both Overlapped and Nonoverlapped Functions of the Two Molecules—We thus went on to search whether additional prodeath molecules could be activated downstream of TNFα signaling that might compensate for the loss of Bid. In our earlier in vitro studies, we found that JNK and reactive oxygen species could contribute to the Bid-independent mechanisms (6). Interestingly, mice deficient in JNK2 were found to be significantly protected from LPS/GalN-induced (21) (Fig. S1) or ConA/GalN-induced (23) hepatocyte apoptosis and liver injury. We also found that JNK1-deficient mice had similar phenotypes, although less significant than JNK2-deficient mice (23) (Fig. S1). To further determine the relationship of the signaling pathways mediated by Bid and JNK, we established mice deficient in both Bid and JNK2 and compared their response with that of the singly deficient mice in the two models of liver injury.
The results suggested that the Bid-mediated pathway and the JNK2-mediated pathway were largely but not completely overlapped. Bid deletion had a more dramatic impact than JNK2 deletion on caspase activation and mitochondrial release of apoptogenic factors at the early time point (5–6 h) after ConA/GalN (Fig. 2, A and B) or LPS/GalN (Fig. 3, A and B) stimulation. Co-deletion of Bid and JNK2 did not result in a stronger inhibition than deletion of Bid alone, suggesting that most of the JNK2 effects on caspase activation were mediated by Bid. At the later time point (8 h after treatment), the Bid-independent activation of caspases became more evident, as revealed before (6). However, deletion of JNK2 did not seem to consistently or significantly confer any better protections than Bid deletion alone; nor did the double deletion of Bid and JNK2 (Figs. 2A and 3A). Large variations were observed among individual mice. Overall, the data seemed to indicate that JNK may not play a key role in Bid-independent caspase activation in these in vivo models.
FIGURE 3.
Single or combined deletion of Bid and JNK2 confers significant protection against LPS/GalN-induced liver injury and mortality. A and B, wild-type (+/+; black columns), Bid-deficient (Bid-/-; open columns), JNK2-deficient (JNK2-/-; crossed columns), and Bid/JNK2 doubly deficient (Bid/JNK2-/-; gray columns) mice were treated for the designated time (A) or for 5 h (B). Mice were sacrificed, and the liver cytosols were subjected to the measurement of caspase-3 activities (A) and to immunoblot analysis with indicated antibodies (B). Blood was also collected, and the serum level of ALT was determined (C). For A and C, results (mean ± S.D.) were from at least three mice for each group at the indicated time points. *, p < 0.05, compared with the wild type group. D, Kaplan-Meier survival analysis of wild-type (+/+; n = 15), Bid-/- (n = 11), JNK1-/- (n = 12), JNK2-/- (n = 18), and Bid/JNK2-/- (n = 15) mice following LPS/GalN treatment. p values indicated for each group were based on comparisons with the wild type group (log rank analysis). Bid-/-, JNK1-/-, JNK2-/-, and Bid/JNK2-/- mice were all significantly resistant to LPS/GalN. Similar results on Bid-/- mice had been published previously (6). The result was recapitulated here for comparison.
The effects of these two molecules on blood liver enzyme levels were similar. Deletion of Bid, but not JNK2, had noticeable inhibitory effects on blood ALT levels, and combined deletion of Bid and JNK2 did not further reduce the level (Figs. 2C and 3C), suggesting that Bid was mainly responsible for the liver damage and that most of the injury was probably caused by Bid-mediated caspase activation, which could overlap with that mediated by JNK.
Paradoxically, deletion of JNK1 or JNK2 conferred a greater protection against mortality than deletion of Bid, with JNK2 deletion being more significant (Figs. 2D and 3D and Table 1). However, co-deletion of JNK2 and Bid resulted in the best protection, particularly in the LPS/GalN models. In the ConA/GalN model, although the mean survival times were similar between the JNK2-deficient and Bid/JNK2-deficient mice (13.15 and 13.26 h, respectively), the survival rate was 15.4 and 27.3% (Table 1). These differences were even larger in the LPS/GalN model with 40% of Bid/JNK2-deficient mice surviving and a mean survival time of 15.01 h, whereas 16.7% of JNK2-deficient mice survived, with a mean survival time of 10.86 h. Thus, it seems that although JNK had less impact on caspase activation and blood ALT levels, it had a significantly greater impact on the mortality, suggesting that mortality is not necessarily all caused by Bid-mediated caspase activation and that JNK might have other major effects on the animal survival. From this aspect, JNK and Bid had apparently nonoverlapped functions.
Deletion of JNK1 or Deletion of JNK2 Alone or in Combination with Bid Could Not Fully Rescue TNFα-dependent p65RelA Deficiency-induced Embryonic Lethality and Apoptosis in the Liver—p65RelA is the critical component of the p65-p50 NF-κB complex, which mediates the canonical NF-κB pathway following TNFα stimulation (8, 11). p65RelA-deficient mice suffer liver injury and die during embryonic development, which can be rescued by co-deletion of TNFα, implicating the importance of TNFα-induced hepatocyte apoptosis in embryonic lethality (11, 12). Previous studies indicated that deletion of bid could not suppress RelA deficiency-induced lethality and apoptosis activation in the liver (6, 13). Considering the apparent importance of JNK in TNFα-induced cell death and, in particular, the animal mortality, we explored the role of JNK1 and JNK2 in RelA deficiency-induced lethality by deleting JNK either alone or in combination with Bid.
JNK1- and JNK2-deficient mice were crossed to the heterozygous RelA-deficient mice to obtain progenies that were homozygous for JNK1 or JNK2 deletion but heterozygous for RelA deletion. These mice were further intercrossed, and the progenies were screened for the presence of double mutants. We had not observed a single JNK1/RelA or JNK2/RelA double mutant in the progenies (n = 61 and 96, respectively), suggesting that JNK1 or JNK2 deficiency could not rescue the RelA deficiency-induced embryonic lethality. We then examined the embryos that resulted from such intercross at the gestation ages of E14.5–E16.5.
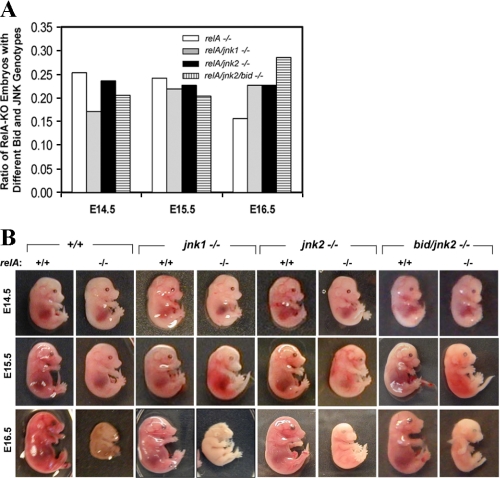
A birth ratio close to the expected 0.25 for RelA-deficient embryos could be observed up to E15.5, which was then greatly reduced by E16.5 (Fig. 4A). We could recover some RelA-deficient embryos by E16.5, although they had apparently died by then (Fig. 4B). There were almost no RelA-deficient embryos recovered beyond E16.5 (data not shown). Deletion of JNK1 or JNK2 resulted in an increased recovery of RelA-deficient embryos at E16.5, so that the percentage of RelA-deficient embryos recovered increased from around 16% to around 23% (Fig. 4A).
FIGURE 4.
Limited suppression of RelA deficiency-induced lethality by JNK1 or JNK2 deletion in the presence or absence of Bid. A, heterozygous RelA-deficient mice carrying no additional genetic defect, or homozygous jnk1 deletion, or homozygous jnk2 deletion, or homozygous bid and jnk2 deletion were intercrossed. The ratio of relA-deficient embryos at E14.5, E15.5, or E16.5 versus all embryos were calculated on the background of relA-/- only (open column), relA/jnk1-/- (gray column), relA/jnk2-/- (solid column), or relA/jnk2/bid-/- (lined column). The number of embryos analyzed ranges from 22 to 99 per group. The expected percentage of RelA-deficient embryos is around 25% from such breeding if there is no loss of the embryos. The observed percentage is noticeably lower for RelA-deficient embryos at E16.5, which seemed to be improved with the deletion of JNK1 or JNK2 with or without concomitant deletion of Bid. B, representative embryos harvested at E14.5, E15.5, and E16.5 from the above mentioned breeding were shown. E14.5 and E15.5 RelA-deficient (relA-/-) embryos looked paler than their RelA wild type (relA+/+) siblings, and their livers looked hemorrhagic. All E14.5 and E15.5 RelA-deficient embryos shown in this panel had increased caspase activities in their livers. In addition, all recovered E16.5 RelA-deficient embryos seemed to have died with reduced size and deteriorating morphology. Note the apparently better preserved E16.5 RelA-/- dead embryos on the JNK1- or JNK2-deficient background with or without Bid, suggesting a delayed death.
Morphological examination (Fig. 4B) of the recovered embryos indicated that some of the E14.5 and all of the E15.5 RelA-deficient embryos looked paler than their wild type and heterozygous counterparts, and their livers looked more hemorrhagic, suggesting the onset of liver injury. The internal organs of these embryos were still intact, and the liver could be recovered for further analysis. By E16.5, all RelA-deficient embryos were apparently dead, with reduced, partially absorbed bodies and degrading internal organs. No livers could be recovered. Although concomitant deletion of JNK1 or JNK2 did not change the type of pathologies observed in E14.5 and E15.5 RelA-deficient embryos, the progression to death in these embryos seemed to be somewhat delayed, based on the better preserved morphology of the recovered embryos at E16.5 (Fig. 4B). This was particularly noticeable for JNK2/RelA doubly deficient mutants. These data seemed to be consistent with the increased number of such embryos recovered at E16.5 (Fig. 4A) and suggested that JNK could play a role in RelA deficiency-induced early embryonic lethality.
Consistent with the morphological finding, only a portion of E14.5 RelA-deficient embryos underwent apoptosis with increased caspase activities in the liver. Using the caspase activation as a more objective criterion, we found that about 57.1% (12 of 21) of single RelA-deficient embryos, 62.5% (5 of 8) of doubly RelA/JNK1-deficient embryos, and 50% (6 of 12) of doubly RelA/JNK2-deficient embryos had developed the TNFα-induced liver injury. However, RelA-deficient embryos lacking JNK1 or JNK2 tended to have a lower caspase activity, although a large variation could be observed among individual embryos (Fig. 5A). By E15.5, apoptosis progressed significantly in all RelA-deficient embryos no matter whether they expressed JNK or not (n = 7–11/group). Correspondingly, the caspase activities were further elevated, but the difference between wild type and JNK1- or JNK2-deficient embryos remained observable although not statistically significant (Fig. 5A). Activation of the effector caspase-3 was confirmed by immunoblot analysis (Fig. 5, B and C), indicating the cleavage of the pro-form to the activated p19 form. No caspase-3 cleavage was found in the RelA wild type or heterozygous embryos or E14.5 RelA-deficient embryos that had not yet developed the caspase activity. The extent of the caspase-3 and caspase-9 cleavage correlated with the measured activities.
FIGURE 5.
Limited inhibition of RelA deficiency-induced caspase activation by JNK1 or JNK2 deletion in the presence or absence of Bid. A, livers harvested from E14.5 and E15.5 RelA-positive and RelA-deficient embryos with different JNK and Bid genotypes were lysed. Caspase-3, -8, and -9 activities in the liver lysates were measured. Increased caspase activities were only observed in RelA-deficient livers, which were expressed as the -fold changes over the RelA wild type controls (mean ± S.D.). Note that only the positive data were averaged and plotted. The number of embryos analyzed for each genotype at E14.5 and E15.5 is indicated at the bottom. A significant portion of E14.5 RelA-deficient livers did not show any caspase activation (see “Results” for more details). Reduced caspase activities could be observed in RelA/JNK1 or RelA/JNK2-deficient embryos in the presence or absence of Bid, compared with the RelA-deficient only embryos, although not statistically significant (t test, p > 0.05). B and C, lysates of livers harvested from E14.5 (B) or E15.5 (C) embryos with different RelA and JNK genotypes were analyzed by immunoblot assay with the antibodies indicated. Cleavage of caspase-3 from the p32 pro form to the activated p19 form could be observed in some RelA-deficient (relA-/-) livers but not in other livers. JNK2 is predominantly in the p54 form, whereas JNK1 is predominantly in the p46 form. D, lysates of livers harvested from E15.5 embryos with different RelA and JNK genotypes were analyzed by immunoblot with anti-c-Jun antibodies (total and phosphorylated forms). Phosphorylated c-Jun could be detected in RelA-deficient embryos.
Phosphorylation of JNK could not be observed in either wild type or RelA-deficient embryos at E14.5 or E15.5 (data not shown). We were unable to perform in vitro kinase assays using anti-JNK immunoprecipitates because of the limited amount of liver tissue that could be harvested from the individual embryos at E14.5 or E15.5. We thus examined the phosphorylation status of c-Jun, a common substrate of JNK, to assess the activity of JNK. Direct detection of phosphorylated c-Jun in the liver extracts from E14.5 embryos was not successful, regardless of the status of RelA expression or the caspase activation (data not shown). However, we were able to observe c-Jun phosphorylation in E15.5 embryos in a RelA-suppressible manner (Fig. 5D). Thus, phosphorylated c-Jun could be detected in E15.5 RelA-deficient embryos but not in RelA-positive embryos. Deletion of either JNK1 or JNK2 alone was not sufficient to eliminate the phosphorylation of c-Jun, suggesting that both JNK isoforms could be involved in the process. This seemed to be different from what was observed in the adult mice, in which JNK1 was the major c-Jun kinase following TNFα stimulation (Fig. S1B) (6, 21). It must be pointed out that c-Jun phosphorylation by JNK does not necessarily indicate that c-Jun is the target of JNK for its death-promoting effect.
These findings suggested that deletion of JNK1 or JNK2 alone had only limited impact on RelA deficiency and other mechanisms had to be involved. We thus explored whether additional deletion of Bid would improve the pathology of the RelA-deficient embryos. Early studies indicated that deletion of Bid alone did not seem to have any impact on RelA deficiency-induced caspase activation and lethality (6, 13). However, that might be due to the compensatory effects of JNK. Thus, mice deficient in Bid and JNK2 but heterozygous for RelA were created. Intercross between these mice led to the generation of embryos deficient in all three genes. We found that like JNK2/RelA doubly deficient embryos, Bid/JNK2/RelA triply deficient embryos died around E16.5, and the correct ratio was maintained at least by E16.5 (Fig. 4A). These embryos had a morphological presentation similar to those of the JNK2 and JNK1 single deletion embryos (Fig. 4B). Thus, they survived better than RelA singly deficient embryos (Fig. 4) and Bid/RelA doubly deficient embryos (6). Consistently, although caspase activation could be detected in all of the triple knock-out livers by E15.5, the activity was greatly reduced to the same level as that in the JNK2 single deleted embryos (Fig. 5A). However, it was no lower than that in the JNK2/RelA doubly deficient livers. Thus the rescue of RelA-deficient embryos was very much like that of ConA/GalN-treated adult mice, in which JNK2 but not Bid played a more important role in mortality. In addition, in this RelA-deficient model, JNK2 also seemed more important than Bid in controlling caspase activation (Fig. 5) (6).
DISCUSSION
TNFα-induced liver injury could be evaluated in several models, including those of LPS/GalN, ConA, ConA/GalN, and RelA deletion. Deletion of RelA leads to embryonic lethality; thus, it is also a model for studying TNFα-induced injury in embryonic livers. The other three models are applied to adult mice only. Previous studies from this laboratory have defined the important role of Bid in the liver injury caused by LPS/GalN but not by RelA deletion (3, 5, 6, 29). We and others have also defined that JNK, particularly JNK2, is important for liver injury caused by LPS/GalN (21) (Fig. 3 and Fig. S1) and by ConA/GalN (23) but not by ConA alone (23), although others found that JNK participated in ConA alone-induced liver injury (18). The present study evaluated the contribution of Bid to TNFα-induced liver injury in the ConA and ConA/GalN models and the contribution of JNK1 and JNK2 to TNFα-induced liver injury in the RelA deletion model. Furthermore, we explored the relationship of Bid and JNK-mediated pathways in mice deficient in both Bid and JNK2. By comparing the effects of Bid and JNK in all of the four models of TNFα injury, we aimed to define the mechanistic relationship of Bid and JNK using various parameters.
As in the LPS/GalN treatment, Bid-deficient mice demonstrated significant resistance to ConA/GalN-induced mitochondria apoptotic response and liver injury. As defined in earlier studies (16–18), a major difference between the two injury paradigms is the cellular origin of TNFα and hence the form of TNFα that binds to the TNFα receptors on the target cells. ConA-stimulated TNFα is from T cells (12, 16, 18, 30) and is mainly in the membrane form, which could bind to both TNF-R1 and TNF-R2, whereas LPS-stimulated TNFα is from macrophages and Kupffer's cells and is mainly in the soluble form, which primarily binds to TNF-R1 (16–18). Despite the fact that membrane and soluble TNFα may activate certain intracellular signaling pathways to different extents because of the differential involvement of TNF-R2 (18), we have not observed any significant differences in the impact of Bid on mitochondria activation between the LPS/GalN and the ConA/GalN regimes. Deletion of Bid resulted in the arrest of mitochondria apoptotic activation at the early time points (4–6 h), and in both cases a Bid-independent mitochondria and caspase activation arose around 8 h. There were no mice that could survive beyond 24 h. Thus, it does not seem that the Bid-independent cell death is related to the source or the form of TNFα that binds to the hepatocytes.
As shown in early studies, ConA alone mainly induces a non-apoptotic and non-caspase-mediated liver injury, which relies on TNFα and other cytokines, such as INFγ (30–32). Consistent with the known molecular targets, deletion of Bid did not affect this process. This finding was also consistent with our observations that suppression of neither FADD nor JNK conferred protections against this type of injury (23).
When the effects of Bid and JNK are examined in the apoptotic context in the LPS/GalN or ConA/GalN model, it seems that JNK largely works upstream of Bid. In addition, the proapoptotic effects of JNK may not be related to its c-Jun kinase activity. On one hand, c-Jun phosphorylation had not been found to be associated with hepatocyte apoptosis (19). On the other hand, although JNK1 possesses the majority of the c-Jun kinase activity, it is JNK2 that seems to play a more important role in TNFα-induced liver injury. A recent study had found that another JNK kinase target, Itch, is important for JNK1-mediated apoptosis (22). Itch is an E3 ligase that can target cFLIP for ubiquitin-dependent proteasomal degradation. It can be phosphorylated and activated by both JNK1 and JNK2 (33). Thus, JNK2 could activate Itch in the liver following TNFα stimulation, although other possibilities that JNK2 works through a different target could not be ruled out. cFLIP suppresses caspase-8 activation by competitively binding to FADD, and its degradation would thus allow more caspase-8 activation (34). Indeed, we found that FLIP degradation was inhibited in the absence of JNK1 or JNK2 (23). Consistently, caspase-8 activation and Bid cleavage were all reduced (21, 23). In addition, in both ConA/GalN and LPS/GalN regimes, deletion of Bid seemed to have a more significant impact on mitochondria and caspase activation than JNK deficiency at the early time point (Figs. 2A and 3A), indicating that Bid works downstream of JNK. As the result, simultaneous deletion of both Bid and JNK2 did not lead to further suppression, compared with Bid deletion alone. Taken together, these data suggest that JNK and Bid could work in a linear pathway, and Bid activation can be regulated by the JNK activity (Fig. 6).
FIGURE 6.
A working model for the interactions between Bid and JNK-mediated pathways in TNFα-induced liver injury and mortality. TNFα-induced apoptosis is mediated by caspase-8, which cleaves both downstream effector caspase-3 and Bid. In the Type II cells, such as the hepatocytes, cleavage of Bid is important for effective caspase-3 activation via the mitochondria pathway. JNK, particularly JNK2, is also important for TNFα-induced apoptosis. Its effect may be primarily mediated by its promotion of FLIP degradation, which in turn allows effective caspase-8, and therefore Bid, activation. Thus, the proapoptosis activity of JNK2 is mainly mediated by Bid. Studies on mice deficient in Bid or in JNK2 or in both support the notion that Bid works downstream of JNK2 but also suggest that JNK could contribute to some Bid-independent mitochondrial activation (6). Other Bid-independent mechanisms of mitochondria activation could include reactive oxygen species (6). In addition, JNK2 and JNK1 apparently have a greater role in regulating the mortality of injured mice, which can be independent of Bid. The relative contribution of Bid versus JNK to mortality may also be affected by how the liver injury is induced in different model systems, with Bid playing minimal roles in the embryonic model of RelA deficiency but some roles in the adult models. Finally, a major determinant of mortality could be the necrotic activity of TNFα.
Nevertheless, JNK could also activate mitochondria in a Bid-independent manner, as shown in TNFα-induced hepatocyte apoptosis in vitro (6). JNK could work through direct activation of Bax and other means to exert its Bid-independent effects (35). The evidence that this could also occur in the in vivo models of TNFα injury would have to be present at the later time of treatment (around 8 h), when the Bid-independent activation of caspase arose (6) (Fig. 3A). Indeed, it seems that JNK2-deficient mice demonstrated overall lower caspase activation than the Bid-deficient mice, and deletion of both Bid and JNK2 could lead to a stronger suppression at the later time point in the LPS/GalN regime (Fig. 3A). However, large variations did exist among individual mice, and we thus do not consider that this Bid-independent effect of JNK is significant or is the major mechanism by which JNK affects mitochondria and caspase activation (Fig. 6).
It is interesting to note that JNK-deficient mice were more resistant to LPS/GalN- or ConA/GalN-induced mortality than Bid-deficient mice (Table 1 and Figs. 2D and 3D). Combined deletion of Bid and JNK2 resulted in an even better protection in the LPS/GalN model, with up to 40% mice surviving beyond 24 h. Mortality in these injury models is thought to be due to retention of large amounts of blood in the injured liver, which causes circulation failure (hypovolemic shock) (36). Blood infiltration into the liver parenchymal is obvious in these cases (Fig. 1A) (3, 23) and is probably caused by the destruction of the endothelial cells as part of the inflammation and cytokine response (37). These effects could be more dependent on JNK than on Bid and may not be related to caspase activation (Fig. 6). Thus, JNK-deficient mice could be better protected. Another major mortality factor that would become important in the absence of Bid and/or JNK is the necrotic and/or autophagic liver injury that might account for some or all of the Bid/JNK2-independent death. This possibility needs to be further addressed in future studies. Finally, we cannot rule out that ConA/GalN or LPS/GalN treatment could have unknown impact on other organ systems, where Bid and/or JNK is also deleted. The individual contribution of these factors to mortality could be different in the LPS/GalN and ConA/GalN stimulations, due to factors such as the different cytokine profiles under the two conditions (30–32).
The better protection against the mortality by JNK in TNFα-induced injury is also evident in the RelA deficiency model. Co-deletion of JNK1 or JNK2 together with RelA notably delayed the death and/or the absorption of the embryos, although the rescue seems to prolong the survival by less than a day. Although most RelA-deficient embryos would die and become absorbed at E15.5–E16.5, more JNK1/RelA or JNK2/RelA-deficient embryos were preserved at E16.5 with a better morphology. On the other hand, co-deletion of Bid did not seem to have any positive impact in improving embryo survival (6) (data not shown). Biochemically, co-deletion of JNK, particularly JNK2, was also more effective than co-deletion of Bid in reducing caspase activation in the RelA-deficient liver, and combined deletion of Bid and JNK2 did not yield more protection than JNK2 deletion alone. From this point of view, the rescue of embryonic lethality by JNK deletion is correlated with the modulation of caspase activation, and for some yet to be defined reasons, Bid does not seem to be involved in these processes at all.
The deletion of JNK1 or JNK2 did not alter the onset of caspase activation and apoptosis in RelA-deficient livers but only the severity of the process. Deletion of JNK1 also moderately retarded the death of IKKβ-deficient embryos to a similar extent (22). The failure of a complete and effective rescue may be due to the overlapping function of JNK1 and JNK2. This assumption could be tested theoretically by a concomitant deletion of both JNK isoforms. However, JNK1/JNK2 doubly deficient embryos prematurely die at about the same gestation time as the RelA-deficient embryos (38). Thus, although it is possible to create a JNK1/JNK2/RelA triple deficient strain, the embryos may still die even if the RelA deficiency could be corrected by JNK1/2 deficiency, due to other defects caused by the latter at the same critical time point. Incidentally, this role of JNK in embryo development and adult mice reflects its dual functions in cell death and cell survival and the complex interactions with other signaling pathways.
Although these studies suggest that TNFα-induced apoptosis in adult livers and in RelA-deficient embryonic livers could share some common mechanisms, in which JNK, particularly JNK2, plays important roles, the apparently insufficient suppression of apoptosis in the RelA-deficient embryos by single JNK deletion suggests that there could be important differences between the two systems, which may not be simply attributed to the redundant functions of JNK1 and JNK2. This consideration is supported by the observations that neither deletion of Bid (6, 13) nor overexpression of Bcl-2 (39) could suppress hepatocyte apoptosis in the RelA-deficient embryos, whereas both maneuvers could lead to a significant protection against TNFα-induced liver injury (6, 40) (this study).
In summary (Fig. 6), using a genetic approach, we studied and compared the role of Bid and JNK in various models of TNFα-induced apoptosis and liver injury. Although these two molecules function in a largely overlapped manner with Bid being downstream of JNK in the adult livers, JNK, but not Bid, seems to contribute more to the TNFα-induced apoptosis in RelA-deficient embryonic livers. The Bid-independent role of JNK could also be observed in adult mice, mainly in the promotion of the irreversible and lethal progression of the injury.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant R01CA 83817 (to X.-M. Y.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
Footnotes
The abbreviations used are: TNFα, tumor necrosis factor-α; ConA, concanavalin A; GalN, d-galactosamine; JNK, c-Jun N-terminal kinase; LPS, lipopolysaccharide(s); PIPES, 1,4-piperazinediethanesulfonic acid; CHAPS, 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid; En, embryonic day n; AFC, 7-amino-4-trifluoromethyl coumarin.
References
- 1.Gross, A., Yin, X. M., Wang, K., Wei, M. C., Jockel, J., Milliman, C., Erdjument-Bromage, H., Tempst, P., and Korsmeyer, S. J. (1999) J. Biol. Chem. 274 1156-1163 [DOI] [PubMed] [Google Scholar]
- 2.Yin, X. M., Wang, K., Gross, A., Zhao, Y., Zinkel, S., Klocke, B., Roth, K. A., and Korsmeyer, S. J. (1999) Nature 400 886-891 [DOI] [PubMed] [Google Scholar]
- 3.Zhao, Y., Li, S., Childs, E. E., Kuharsky, D. K., and Yin, X.-M. (2001) J. Biol. Chem. 276 27432-27440 [DOI] [PubMed] [Google Scholar]
- 4.Li, S., Zhao, Y., He, X., Kim, T.-H., Kuharsky, D. K., Rabinowich, H., Chen, J., Du, C., and Yin, X.-M. (2002) J. Biol. Chem. 277 26912-26920 [DOI] [PubMed] [Google Scholar]
- 5.Zhao, Y., Ding, W. X., Qian, T., Watkins, S., Lemasters, J. J., and Yin, X. M. (2003) Gastroenterology 125 854-867 [DOI] [PubMed] [Google Scholar]
- 6.Chen, X., Ding, W. X., Ni, H. M., Gao, W., Shi, Y. H., Gambotto, A. A., Fan, J., Beg, A. A., and Yin, X. M. (2007) Mol. Cell. Biol. 27 541-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaufmann, T., Tai, L., Ekert, P. G., Huang, D. C., Norris, F., Lindemann, R. K., Johnstone, R. W., Dixit, V. M., and Strasser, A. (2007) Cell 129 423-433 [DOI] [PubMed] [Google Scholar]
- 8.Kucharczak, J., Simmons, M. J., Fan, Y., and Gelinas, C. (2003) Oncogene 22 8961-8982 [DOI] [PubMed] [Google Scholar]
- 9.Lin, A. (2003) BioEssays 25 17-24 [DOI] [PubMed] [Google Scholar]
- 10.Schwabe, R. F., and Brenner, D. A. (2006) Am. J. Physiol. 290 G583-G589 [DOI] [PubMed] [Google Scholar]
- 11.Beg, A. A., Sha, W. C., Bronson, R. T., Ghosh, S., and Baltimore, D. (1995) Nature 376 167-170 [DOI] [PubMed] [Google Scholar]
- 12.Doi, T. S., Marino, M. W., Takahashi, T., Yoshida, T., Sakakura, T., Old, L. J., and Obata, Y. (1999) Proc. Natl. Acad. Sci. U. S. A. 96 2994-2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufmann, T., Gugasyan, R., Gerondakis, S., Dixit, V. M., and Strasser, A. (2007) Cell Death Differ. 14 637-639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grell, M., Douni, E., Wajant, H., Lohden, M., Clauss, M., Maxeiner, B., Georgopoulos, S., Lesslauer, W., Kollias, G., Pfizenmaier, K., and Scheurich, P. (1995) Cell 83 793-802 [DOI] [PubMed] [Google Scholar]
- 15.Gantner, F., Kusters, S., Wendel, A., Hatzelmann, A., Schudt, C., and Tiegs, G. (1997) J. Pharmacol. Exp. Ther. 280 53-60 [PubMed] [Google Scholar]
- 16.Kusters, S., Tiegs, G., Alexopoulou, L., Pasparakis, M., Douni, E., Kunstle, G., Bluethmann, H., Wendel, A., Pfizenmaier, K., Kollias, G., and Grell, M. (1997) Eur. J. Immunol. 27 2870-2875 [DOI] [PubMed] [Google Scholar]
- 17.Nowak, M., Gaines, G. C., Rosenberg, J., Minter, R., Bahjat, F. R., Rectenwald, J., MacKay, S. L., Edwards, C. K., III, and Moldawer, L. L. (2000) Am. J. Physiol. 278 R1202-R1209 [DOI] [PubMed] [Google Scholar]
- 18.Maeda, S., Chang, L., Li, Z. W., Luo, J. L., Leffert, H., and Karin, M. (2003) Immunity 19 725-737 [DOI] [PubMed] [Google Scholar]
- 19.Schwabe, R. F., Uchinami, H., Qian, T., Bennett, B. L., Lemasters, J. J., and Brenner, D. A. (2004) FASEB J. 18 720-722 [DOI] [PubMed] [Google Scholar]
- 20.Liu, H., Lo, C. R., and Czaja, M. J. (2002) Hepatology 35 772-778 [DOI] [PubMed] [Google Scholar]
- 21.Wang, Y., Singh, R., Lefkowitch, J. H., Rigoli, R. M., and Czaja, M. J. (2006) J. Biol. Chem. 281 15258-15267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang, L., Kamata, H., Solinas, G., Luo, J. L., Maeda, S., Venuprasad, K., Liu, Y. C., and Karin, M. (2006) Cell 124 601-613 [DOI] [PubMed] [Google Scholar]
- 23.Ni, H. M., Chen, X., Ding, W. X., Schuchmann, M., and Yin, X. M. (2008) Am. J. Pathol. 173 962-972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong, C., Yang, D. D., Wysk, M., Whitmarsh, A. J., Davis, R. J., and Flavell, R. A. (1998) Science 282 2092. [DOI] [PubMed] [Google Scholar]
- 25.Yang, D. D., Conze, D., Whitmarsh, A. J., Barrett, T., Davis, R. J., Rincon, M., and Flavell, R. A. (1998) Immunity 9 575-585 [DOI] [PubMed] [Google Scholar]
- 26.Gantner, F., Leist, M., Jilg, S., Germann, P. G., Freudenberg, M. A., and Tiegs, G. (1995) Gastroenterology 109 166-176 [DOI] [PubMed] [Google Scholar]
- 27.Trautwein, C., Rakemann, T., Brenner, D. A., Streetz, K., Licato, L., Manns, M. P., and Tiegs, G. (1998) Gastroenterology 114 1035-1045 [DOI] [PubMed] [Google Scholar]
- 28.Kunstle, G., Hentze, H., Germann, P. G., Tiegs, G., Meergans, T., and Wendel, A. (1999) Hepatology 30 1241-1251 [DOI] [PubMed] [Google Scholar]
- 29.Ding, W. X., Ni, H. M., DiFrancesca, D., Stolz, D. B., and Yin, X. M. (2004) Hepatology 40 403-413 [DOI] [PubMed] [Google Scholar]
- 30.Gantner, F., Leist, M., Lohse, A. W., Germann, P. G., and Tiegs, G. (1995) Hepatology 21 190-198 [DOI] [PubMed] [Google Scholar]
- 31.Hong, F., Jaruga, B., Kim, W. H., Radaeva, S., El-Assal, O. N., Tian, Z., Nguyen, V. A., and Gao, B. (2002) J. Clin. Invest. 110 1503-1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Streetz, K., Fregien, B., Plumpe, J., Korber, K., Kubicka, S., Sass, G., Bischoff, S. C., Manns, M. P., Tiegs, G., and Trautwein, C. (2001) J. Immunol. 167 514-523 [DOI] [PubMed] [Google Scholar]
- 33.Gao, M., Labuda, T., Xia, Y., Gallagher, E., Fang, D., Liu, Y. C., and Karin, M. (2004) Science 306 271-275 [DOI] [PubMed] [Google Scholar]
- 34.Irmler, M., Thome, M., Hahne, M., Schneider, P., Hofmann, K., Steiner, V., Bodmer, J. L., Schroter, M., Burns, K., Mattmann, C., Rimoldi, D., French, L. E., and Tschopp, J. (1997) Nature 388 190-195 [DOI] [PubMed] [Google Scholar]
- 35.Tsuruta, F., Sunayama, J., Mori, Y., Hattori, S., Shimizu, S., Tsujimoto, Y., Yoshioka, K., Masuyama, N., and Gotoh, Y. (2004) EMBO J. 23 1889-1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yin, X. M., and Ding, W. X. (2003) Curr. Mol. Med. 3 491-508 [DOI] [PubMed] [Google Scholar]
- 37.Lawson, J. A., Fisher, M. A., Simmons, C. A., Farhood, A., and Jaeschke, H. (1998) Hepatology 28 761-767 [DOI] [PubMed] [Google Scholar]
- 38.Kuan, C.-Y., Yang, D. D., Roy, D. R. S., Davis, R. J., Rakic, P., and Flavell, R. A. (1999) Neuron 22 667-676 [DOI] [PubMed] [Google Scholar]
- 39.Gugasyan, R., Christou, A., O'Reilly, L. A., Strasser, A., and Gerondakis, S. (2006) Cell Death Differ. 13 1235-1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Molle, W., Denecker, G., Rodriguez, I., Brouckaert, P., Vandenabeele, P., and Libert, C. (1999) J. Immunol. 163 5235-5241 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.