Abstract
Regulation of intracellular calcium ([Ca2+]i) by erythropoietin (Epo) is an essential part of signaling pathways controlling proliferation and differentiation of erythroid progenitors, but regulatory mechanisms are largely unknown. TRPC3 and the homologous TRPC6 are two members of the transient receptor potential channel (TRPC) superfamily that are expressed on normal human erythroid precursors. Here we show that TRPC3 expression increases but TRPC6 decreases during erythroid differentiation. This is associated with a significantly greater increase in [Ca2+]i in response to Epo stimulation, suggesting that the ratio of TRPC3/TRPC6 is physiologically important. In HEK 293T cells heterologously expressing TRPC and erythropoietin receptor (Epo-R), Epo stimulated an increase in [Ca2+]i through TRPC3 but not TRPC6. Replacement of the C terminus of TRPC3 with the TRPC6 C terminus (TRPC3-C6C) resulted in loss of activation by Epo. In contrast, substitution of the C terminus of TRPC6 with that of TRPC3 (TRPC6-C3C) resulted in an increase in [Ca2+]i in response to Epo. Substitution of the N termini had no effect. Domains in the TRPC3 C terminus between amino acids 671 and 746 are critical for the response to Epo. Epo-R and phospholipase Cγ associated with TRPC3, and these interactions were significantly reduced with TRPC6 and TRPC3-C6C chimeras. TRPC3 and TRPC6 form heterotetramers. Coexpression of TRPC6 or C3/C6 chimeras with TRPC3 and Epo-R inhibited the Epo-stimulated increase in [Ca2+]i. In a heterologous expression system, Epo stimulation increased cell surface expression of TRPC3, which was inhibited by TRPC6. However, in primary erythroblasts, an increase in TRPC3 cell surface expression was not observed in erythroblasts in which Epo stimulated an increase in [Ca2+]i, demonstrating that increased membrane insertion of TRPC3 is not required. These data demonstrate that TRPC6 regulates TRPC3 activation by Epo. Endogenously, regulation of TRPC3 by TRPC6 may primarily be through modulation of signaling mechanisms, including reduced interaction of TRPC6 with phospholipase Cγ and Epo-R.
Regulation of intracellular calcium by erythropoietin is a signaling mechanism controlling the proliferation and differentiation of erythroid progenitors and precursors (1-4). Evidence implicating calcium in control of erythroid growth and differentiation include the following: (a) enhancement of Epo2-induced murine erythroid colony growth by the ionophore A23187 and inhibition by treatment with EGTA, a nonspecific chelator of Ca2+ (5); (b) demonstration that an increase in Ca2+ influx is an early and necessary step in the commitment to differentiation of murine erythroleukemia cells (6-8); and (c) the significant rise in [Ca2+]i stimulated by Epo at specific stages of normal human erythroid progenitor (burst-forming unit-erythroid; BFU-E) differentiation (2). We have characterized regulation of [Ca2+]i by Epo at the single cell level using BFU-E-derived cells at defined stages of differentiation and fluorescence microscopy coupled to digital video imaging (1, 2, 9-11), patch clamp (12), and microinjection (13). In addition to erythroid cells, erythropoietin receptors are expressed on megakaryocytes (14), endothelial cells (15, 16), placenta (17), myoblasts, myocytes (15, 18, 19), and neuronal cells (20), suggesting a function in nonhematopoietic cells. In keeping with the action of Epo in promoting survival of erythroid precursors, recent studies demonstrated that Epo has important tissue-protective effects on the brain, heart, and kidney (18-20). The ability of erythropoietin to activate Ca2+ influx and influence cell proliferation and viability via stimulation of its receptor has also been demonstrated in these cells. Myoblasts express Epo-R. Epo stimulated myoblast proliferation to expand the progenitor population during differentiation and an increase in [Ca2+]i that was dependent on extracellular Ca2+ influx (21). Epo-R has also been identified on neuronal cell lines (22, 23). Epo stimulated an increase in cell viability in serum and nerve growth factor-deprived cells and an increase in 45Ca2+ uptake and [Ca2+]i (22, 24). Recently, the role of Epo in stimulating tumor progression has become a major concern with relevance to its use in cancer patients (25). These data demonstrate the broad physiological importance of erythropoietin regulation of Ca2+ influx.
The transient receptor potential (TRP) channel TRPC3 was recently shown to be expressed on primary human erythroid cells and is an important calcium channel regulated by Epo (26). The TRP superfamily is a diverse group of calcium-permeable cation channels expressed on nonexcitable mammalian cells that are related to the archetypal Drosophila Trp and have been divided into six subfamilies (27-29). TRP channels are implicated in many physiological functions in eukaryotes and are now recognized to be involved in a number of diseases (28). Examples of regulatory mechanisms that are employed by TRP channel members are multiple isoform expressions through splicing and alternative transcription start sites (30, 31), channel trafficking and insertion into the plasma membrane (32-34), and formation of functionally different channels through association as homo- or heterotetramers (35, 36). Many members of the TRPC subfamily are receptor-activated and are regulated through phospholipase C (PLC)-mediated pathways. Activation of PLC results in hydrolysis of phosphatidylinositol 4,5-bisphosphate to inositol 1,4,5-trisphosphate (IP3) and diacylglycerol, and a number of modes of TRPC regulation by these transducers have been proposed (37-39). We recently demonstrated that the Epo-stimulated increase in [Ca2+]i through TRPC3 originated primarily from extracellular calcium influx, is mediated through PLCγ, and required interaction of PLCγ and IP3R with TRPC3 (26).
TRPC3 and TRPC6 share ∼73% homology in their amino acid sequence. TRPC proteins assemble based on structural similarities, and multimeric channel formation has been reported for TRPC3/6/7 (36, 40, 41). Here we determined that TRPC3 and TRPC6 are expressed on normal erythroid precursors. The ratio of TRPC3 to TRPC6 increases during erythroid differentiation and correlates functionally with the increase in the Epo-stimulated rise in [Ca2+]i. This suggests that the change in the TRPC3/TRPC6 ratio during erythroid differentiation may have physiological significance. Utilizing a model system of transfected HEK 293T cells to study responses of individual channels and mutants, we determined that Epo stimulates an increase in [Ca2+]i through TRPC3 but not TRPC6. Furthermore, coexpression of TRPC6 with TRPC3 inhibited the Epo-stimulated increase in [Ca2+]i. To identify domains responsible for the different channel responses, we prepared TRPC3-TRPC6 chimeras. These studies revealed that domains in the TRPC6 C terminus are responsible for the lack of response to Epo and that the TRPC6 C terminus has much weaker interactions with PLCγ and Epo-R than does TRPC3. These data demonstrate that TRPC6 has a role in regulating TRPC3 function in response to Epo. Although Epo stimulation enhanced heterologous TRPC3 insertion into the plasma membrane, and this was inhibited by TRPC6, a significant increase in Epo-stimulated cell surface expression of endogenous TRPC3 was not required for channel activation by Epo.
EXPERIMENTAL PROCEDURES
Tissues and Cell Lines—Human embryonic kidney (HEK) 293T cells were cultured in Dulbecco's modified Eagle's medium with 10% fetal calf serum. U937 cells were cultured in α-essential media with 10% fetal bovine serum. UT-7 cells were cultured in α-essential media with 10% fetal calf serum and 0.5 unit/ml erythropoietin (Amgen, Thousand Oaks, CA). TF-1 cells were cultured in RPMI 1640 medium with 10% fetal calf serum with 5 units/ml Epo (Amgen) or 1-2 ng/ml granulocyte-macrophage colony-stimulating factor (42). Peripheral blood from volunteer donors was obtained under protocols approved by the Institutional Review Board of this institution. Human BFU-E were cultured, and BFU-E-derived erythroblasts were harvested from methylcellulose culture at days 7, 10, and 14 as described previously (43). Human erythroid progenitors were also cultured using a two-phase liquid culture system (44, 45). Cells were harvested at day 8 of phase II and were predominantly proerythroblasts or basophilic normoblasts. CD34+ cells were purchased from AllCells, LLC, Emeryville, CA.
Transfection of hTRPC3, hTRPC6, and Epo-R into HEK 293T Cells—Human TRPC3 (hTRPC3) (gift of Dr. Lutz Birnbaumer) was subcloned into pQBI50 (QBiogene, Carlsbad, CA; BFP-TRPC3), pcDNA 3.1/V5-His TOPO (Invitrogen), or pQBI25 (QBiogene; GFP-TRPC3). Human TRPC6 (hTRPC6) (gift of Dr. Lutz Birnbaumer) was subcloned into pQBI50 or pCMV-Tag (Stratagene). Chimeric channels were constructed in which the N and the C termini of TRPC3 and TRPC6 were exchanged. TRPC6-C3C, TRPC6-C3N, TRPC3-C6C, TRPC3-C6C1, and TRPC3-C6C2 chimeras were generated as described below and cloned into vectors as noted. HEK 293T cells at 50-70% confluency were transfected with these vectors and/or pTracer-CMV expressing Epo-R using Lipofectamine Plus (Invitrogen) or Lipofectamine 2000 in accordance with the manufacturers' recommended protocols. HEK 293T cells were routinely studied 48 h after transfection.
Generation of Ext-V5-TRPC3 and TRPC3/TRPC6 Chimeras—TRPC3 with a V5 tag inserted between the first and second transmembrane domains (Ext-V5-TRPC3) was prepared by PCR-directed mutagenesis using the following primers: forward primer, 5′-GGCATCACCACGGGTAAGCCTATCCCTAACCCTCTCCTCGGTCTCGATTCTACGCTGCCCAATATCAC-3′, and reverse primer, 5′-GTGATATTGGGCAG-CGTAGAATCGAGACCGAGGAGAGGGTTAGGGATAGGCTTACCCGTGGTGATGCC-3′ (V5 tag is in boldface). The V5 tag was inserted between TRPC3 amino acids 413 and 414. The construct was then cloned into pcDNA 3.1/V5-His TOPO and pQBI50 vectors. For the construction of chimeras, the TRPC3 N terminus incorporated amino acids 1-381, and the TRPC3 C terminus included amino acids 671-848, and the TRPC6 C terminus included amino acids 728-931. Each DNA fragment was amplified in a total volume of 50 μl using 2.5 units of Pfu Ultra HF (Stratagene) polymerase and 0.4 μm of the following primers. To generate FLAG-TRPC6-C3C: 1) for the pCMV-Tag vector and TRPC6, forward, 5′-TGCGAAGACTTCCTTAGAAATTCA-3′, and reverse, 5′-GATCATGGCAATTAACATATTTAG-3′; 2) for the TRPC3 C-terminal insert, forward, 5′-AATAGCTCATATCAAgAAATTGAG-3′, and reverse, 5′-TCATTCACATCTCAGCATGCTG-3′. To generate FLAG-TRPC6-C3N: 1) for the pCMV-Tag vector and TRPC6, forward, 5′-CCATTCATGAAGTTTGTAGCACAC-3′, and reverse, 5′-CATGGCGGGAACGCCCGACTC-3′;2) for the TRPC3 N-terminal insert, forward, 5′-GAGGGAAGCCCATCCCTGAGACGC-3′, and reverse, 5′-GCTTCGCAGAATTTTCCCCAGC-3′. To generate V5-TRPC3-C6C: 1) for the pcDNA 3.1/V5-His TOPO vector and TRPC3, forward, 5′-GGAATTCTGCAGATATCCAGC-3′, and reverse, 5′-AATCATAGCAATTAGCATGTTG-3′; 2) for TRPC6 C-terminal insert, forward, 5′-AACAGTTCATTCCAGGAAATTG-3′, and reverse, 5′-TCTATTGGTTTCCTCTTGATTTGG-3′. Primers used to generate the amplification “insert” were phosphorylated at the 5′ end. Amplified fragments were gel-purified using Invitrogen SNAP columns, and appropriate fragments were ligated together using T4 ligase (Promega) according to the manufacturer's protocol. All constructs were sequenced.
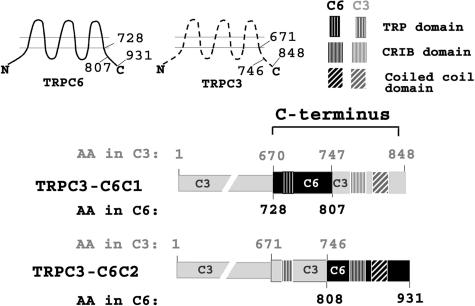
Generation of TRPC3-C6C1 and TRPC3-C6C2 Chimeras—TRPC3-C6C1 included TRPC3 amino acids 1-670 and 747-848 and TRPC6 amino acids 728-807; TRPC3-C6C2 included TRPC3 amino acids 1-746 and TRPC6 amino acids 808-931 (C6C2) (Fig. 5). The 5′ (C1) and 3′ (C2) parts of the C termini were exchanged between TRPC3 and TRPC6 using megaprimer-mediated domain swapping PCR technique. For TRPC3-C6C1, a 1162-bp megaprimer was amplified using the BFP-TRPC3-C6C chimera as a template and the following set of primers: forward primer, 5′-CCTTCCATTCCTGGCCATTG-3′, and reverse primer, 5′-TTTAACCTGGATTCTTCATTTATCTTG-3′. For TRPC3-C6C2, the 331-bp megaprimer was amplified using FLAG-TRPC6 as a template and the following sets of primers: forward primer, 5′-ATGGGTAACTCAAAGAAGAAACTTGGAA-3′, and as a reverse primer either 5′-TATCTGCAGAATTCCTCTATTGGTTTCCTC-3′ (with no stop codon for C terminus V5-tagged TRPC3-C6C2) or 5′-CAGGTTGCTGCATCATCTATTGGTTTCCTC-3′ (with stop codon for N-terminal BFP-tagged TRPC3-C6C2). The 50-μl PCR contained 50 ng of a template, 0.4 μm of each primer, 0.2 mm dNTPs, and 2.5 units of Pfu Ultra HF (Stratagene) polymerase in a 1× manufacturer buffer. After initial denaturation at 95 °C for 2 min, DNA fragments were amplified through 35 cycles (95 °C, 30 s; 50 °C 30 s; 72 °C 60 s for 331 bp or 90 s for 1162 bp)] followed by elongation for 5 min at 72 °C. The amplified megaprimers were subjected to electrophoresis (1% agarose gel), purified using Qiagen Minelute extraction kit, and used as primers in the second round of PCR. The 50-μl PCR contained 100 ng of the megaprimer, 10 ng of a template (either V5-TRPC3 or BFP-TRPC3), 50 μm dNTPs, and 2.5 units of Pfu Ultra HF (Stratagene) polymerase in a 1× manufacturer buffer. After initial denaturation 95 °C for 90 s, DNA plasmids were amplified through 18 cycles (94 °C, 60 s; 55 °C 60 s; 68 °C 18 min) followed by elongation for 5 min at 72 °C. The amplified DNA was then digested with DpnI (Promega) for 1 h at 37 °C. 1-2 μl of digested PCR was used for transformation of competent DH5α (Invitrogen). Clones were verified by digestion of DNA with restriction enzymes and by sequencing.
FIGURE 5.
Schema of chimeras of subdivided TRPC3 and TRPC3 C termini. Amino acid (AA) composition of TRPC3 and TRPC6 chimeras and localization of TRP, CRIB, and coiled-coil domains are shown.
Generation of TRPC6 F731Y—Substitution of phenylalanine at amino acid 731 of TRPC6 with tyrosine was performed with PCR-based site-directed mutagenesis using the following PAGE purified primers: forward primer, 5′-GCCATGATCAACAGTTCATATCAGGAAATTGAGGATGAC-3′, and reverse primer, 5′-GTCATCCTCAATTTCCTGATATGAACTGTTGATCATGGC-3′. The tyrosine codon is shown in boldface. The 50-μl PCR contained 50 ng of a template (either BFP-TRPC6 or FLAG-TRPC6), 100 ng of each primer, 0.2 mm dNTPs, and 2.5 units of Pfu Ultra HF (Stratagene) polymerase in a 1× manufacturer buffer. After initial denaturation 95 °C for 2 min, DNA fragments were amplified through 18 cycles (95 °C, 30 s; 50 °C 30 s; 72 °C 18 min) followed by elongation for 10 min at 72 °C. The amplified DNA was then digested with DpnI (Promega) for 1 h at 37 °C. 1-2 μl of digested PCR was used for transformation of competent DH5α (Invitrogen). Clones were verified by digestion of DNA with restriction enzymes and by sequencing. TRPC3 Y674F was prepared previously (26).
Measurement of [Ca2+]i with Digital Video Imaging—HEK 293T cells were transfected with empty pQBI50 vector, pQBI50 vector expressing wild type TRPC3, TRPC6, and/or chimeric TRPC3/TRPC6, and with pTracer-CMV expressing Epo-R. pTracer-CMV contains a CMV promoter that drives expression of Epo-R and an SV40 promoter that drives expression of GFP. The pQBI50 vector used a CMV promoter to drive expression of BFP fused to TRPC3, TRPC6, or chimeras. Successful transfection of individual HEK 293T cells with pQBI50 vectors was verified by detection of BFP (excitation, 380 nm; emission, 435 nm) and transfection of pTracer-CMV by detection of GFP (excitation, 478 nm; emission, 535 nm) with our fluorescence microscopy-coupled digital video imaging system (11, 12, 46). To study changes in [Ca2+]i in transfected cells, we were not able to use Fura-2 as the detection fluorophore because its excitation and emission wavelengths overlap with GFP. Instead, we used the fluorescent indicator Fura Red (excitation, 440 and 490 nm; emission, 600 nm long pass), a dual wavelength excitation probe (47, 48). At 48 h post-transfection, HEK 293T cells were loaded with 5 μm Fura Red-AM (Molecular Probes, Eugene, OR) for 20-25 min at 37 °C in the presence of Pluronic F-127. The extracellular buffer routinely contained 0.68 mm CaCl2. HEK 293T cells were then treated with 0-40 units/ml recombinant Epo or vehicle (PBS). [Ca2+]i was measured in individual cells at base line and at 1-5-min intervals for 20 min by determining the fluorescence intensity ratio R (F440/F490). The constants Sf2, Sb2, and the K′D of Fura Red were calibrated, and Rmin and Rmax were measured for Fura Red as described previously (3). [Ca2+]i was calculated using the formula [Ca2+]i = K′D((R - Rmin)/(Rmax - R)) (Sf2/Sb2). Primary human erythroid precursors were removed from methylcellulose culture of peripheral blood BFU-E at days 7 and 10, adhered to fibronectin-coated glass coverslips, and loaded with Fura-Red for experiments to measure [Ca2+]i.
Immunoblotting and Immunoprecipitation—For Western blotting, whole cell lysates or immunoprecipitates were separated on 8% polyacrylamide gels, followed by transfer to Hybond-C Extra membranes (Amersham Biosciences). Western blotting was performed as described previously (3). Blots were incubated with anti-TRPC3 (1:400, Alomone Labs, Jerusalem, Israel), anti-TRPC3 (C) targeted to the human C-terminal sequence RRRRLQDIEMGMGNSKSRLN (1:1000, Bethyl Laboratories, Inc., Montgomery, TX) (41), anti-TRPC3 (N) targeted to the murine N-terminal sequence LNGDLESAEPLERHGHKASL (1:1000, Bethyl Laboratories, Inc.) (49), anti-TRPC6 (1:250, Alomone Labs; 1:500, Abcam, Inc., Cambridge, MA), anti-V5-HRP (1:10,000, Invitrogen), anti-FLAG (1:1000, Sigma), anti-PLCγ (1:1000, SC-81, Santa Cruz Biotechnology, Inc., Santa Cruz, CA), anti-Epo-R (1:1000, SC697, Santa Cruz Biotechnology, Inc.), anti-actin (1:10,000, Sigma), anti-tubulin (1:10,000, Sigma) antibodies, or streptavidin-HRP (Pierce). Blots were washed and incubated with the appropriate horseradish peroxidase (HRP)-conjugated antibodies (1:2000). Enhanced chemiluminescence (ECL) was used for detection of signal.
To examine the interaction of TRPC3 with TRPC6, PLCγ, or Epo-R, HEK 293T cells were transfected with hTRPC3 (in pcDNA3.1/V5-His TOPO), hTRPC6 (in pCMV tag), TRPC3/TRPC6 chimeras in either vector, hEpo-R (in pcDNA3), rat PLCγ1 (in pcDNA3), or combinations of these vectors. Cells were washed in ice-cold Hanks' balanced salt solution and lysed in buffer (50 mm Tris, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1% Triton X-100) supplemented with Complete Protease Inhibitor Mixture (Roche Applied Science). Protein lysates were incubated with protein A-Sepharose for 1 h at 4 °C. Precleared lysates were collected and incubated with preimmune rabbit serum, anti-V5 (Invitrogen), anti-Epo-R, or anti-PLCγ1 antibodies and protein G-Sepharose or anti-FLAG-agarose (Sigma) for 2 h at 4 °C. Immunoprecipitates were washed three times, and sample buffer (2×) was added to the pellets. The samples were heated at 60 °C for 30 min with the exception of FLAG immunoprecipitates, which were eluted using FLAG peptide (Sigma). Western blotting was performed as described above, and blots were probed with anti-V5-HRP, or anti-FLAG, anti-Epo-R, anti-PLCγ1, or anti-actin antibodies, followed by the appropriate HRP-conjugated secondary antibodies and ECL. Band intensity on Western blots was quantified with the use of a calibrated densitometer (model GS800, Bio-Rad) using Quantity One software.
Immunolocalization of TRPC3, Epo-R, and TRPC6 in HEK 293T Cells—HEK 293T cells were plated on Lab-Tek chamber slides (Nalge Nunc International, Rochester, NY). After 24 h, cells were transfected with Epo-R and Ext-V5-TRPC3 with or without FLAG-TRPC6. After transfection for 24 h, cells were placed on ice and washed three times with ice-cold PBS. For permeabilized cells, cells were fixed with 3% paraformaldehyde and permeabilized with 0.05% Triton X-100 in PBS for 2 min on ice. Cells were labeled with primary antibody (1:50; rabbit anti-mouse Epo-R, mouse anti-V5, or rabbit anti-mouse TRPC6 antibodies). Slides were then labeled with a mixture of Alexa Fluor 594 goat anti-rabbit IgG (1:200; Molecular Probes, Eugene, OR) and Alexa Fluor 488 goat anti-mouse IgG (1:200; Molecular Probes). Nonpermeabilized cells were first labeled with primary antibody and then fixed with 3% paraformaldehyde followed by labeling with secondary antibody. Coverslips were mounted using Vectashield mounting medium with 4′,6-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA). Images were acquired with the Leica TCS SP2 AOBS Confocal Microscope in the Penn State College of Medicine Imaging Core Facility.
Fluorescence Recovery after Photobleaching (FRAP)—HEK 293T cells grown in 35-mm glass bottom dishes were transfected using Lipofectamine 2000 with plasmid DNAs expressing Epo-R and GFP-TRPC3, with or without FLAG-TRPC6, and cultured for 24 h. Cells were treated with Epo (40 units/ml) for 1-5 min at the time of confocal microscopy. FRAP experiments were performed using a Leica TCS SP2 AOBS confocal microscope with lbd.BL 63.0 × 1.40 OIL UV objective (50). A region of cell membrane was photobleached to ∼10% of its initial fluorescence, using five 2-s pulses of 405 mm and 488 lasers. Recovery of fluorescence within the bleached region was recorded using 30 frames acquired at 0.829-s intervals followed by 30 frames at 2-s intervals, finally followed by 30 frames at 10-s intervals. Data were corrected for the background bleaching using the reference area and fitted to a single exponential using the IGOR Pro 6.01 program. Half-time fluorescence recovery (t½) and mobile fraction were analyzed statistically using one-way analysis of variance (51-53).
Biotinylation of Cell Surface Proteins—HEK 293T cells were transfected for 48 h with V5-tagged wild type TRPC3, FLAG-tagged wild type TRPC6 or chimeras, and Epo-R. Cells were stimulated by 40 units/ml Epo for 0, 1, 5, 10, or 20 min. After washing three times with ice-cold PBS, pH 8.0, cells were then incubated for 30 min at 4 °C with 1 mm Sulfo-NHS-Biotin (Pierce) (54). The biotinylation reaction was terminated by washing cells three times with PBS containing 100 mm glycine to quench and remove excess biotin. Cells were then lysed, and immunoprecipitation was performed with anti-V5 antibody as described previously (3). Western blotting was performed with immunoprecipitation pellets, and blots were probed with streptavidin-HRP, anti-V5-HRP antibody, or anti-FLAG antibody followed by HRP-goat anti-mouse antibody. ECL was used for detection of signal. In some experiments, BFU-E-derived erythroblasts were removed from methylcellulose culture at day 10 or from liquid culture of primary progenitors at phase II day 8 for cell surface expression studies.
RESULTS
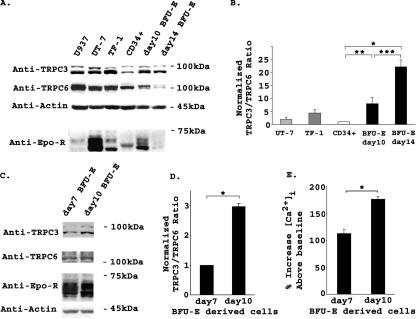
TRPC3 and TRPC6 Are Differentially Expressed during Normal Erythroid Differentiation—TRPC3 is activated by Epo (26). Because TRPC3 and TRPC6 can form functional heterotetramers (36), and TRPC6 inhibited calcium influx through TRPC2 (3), we examined expression of these TRPC on human erythroid cells. Lysates were prepared from the human Epo-responsive cell lines UT-7 and TF-1 and from the monocytic leukemia cell line U937. Primary human erythroid cells were first studied at different stages of differentiation, including CD34+ cells (immature hematopoietic progenitor cells), day 10 BFU-E derived erythroblasts removed from methylcellulose culture (predominantly proerythroblasts and basophilic normoblasts), and day 14 BFU-E derived erythroblasts (polychromatic and orthochromatic normoblasts). Western blotting demonstrated expression of TRPC3 and TRPC6 in all of these hematopoietic cells (Fig. 1A). Although expression of TRPC3 increased during differentiation of primary erythroid cells, expression of TRPC6 decreased. TRPC3 and TRPC6 expression in three experiments was quantitated with densitometry at different stages of normal human erythroid differentiation (CD34+, day 10 and day 14 BFU-E-derived erythroblasts). Results showed a significant increase in TRPC3 expression relative to TRPC6 as erythroid cells matured (Fig. 1B). Epo-R expression peaked at day 10 of culture and was dramatically reduced by day 14 (Fig. 1A).
FIGURE 1.
Endogenous expression of TRPC3 and TRPC6 in human hematopoietic cells. A, Western blotting was performed on lysates from U937 cells, UT-7 and TF-1 Epo-responsive cell lines, CD34+ cells, and day 10 and 14 BFU-E-derived erythroblasts. Equivalent amounts of protein were loaded in each lane. Immunoblotting with anti-TRPC3 antibody demonstrated increased expression of TRPC3 during erythroid differentiation, whereas blotting with anti-TRPC6 showed that TRPC6 expression decreased. Blots were probed with anti-actin antibody to compare loading of lanes. Blots were also probed with anti-Epo-R antibody. Representative results of three experiments are shown. B, densitometry was used to quantitate TRPC3 and TRPC6 bands from three experiments of lysates from CD34+ cells and day 10 and day 14 BFU-E-derived erythroblasts. The TRPC3/TRPC6 ratio was calculated and normalized to CD34+ cells to allow comparison between experiments. The mean normalized ratio ± S.E. is shown. The TRPC3/TRPC6 ratio was significantly less in CD34+ cells compared with day 10 (**, p ≤ 0.05) and day 14 erythroblasts (*, p ≤ 0.002). TRPC3/TRPC6 ratio was significantly greater in day 14 compared with day 10 cells (***, p ≤ 0.02). C, Western blotting was performed on lysates from day 7 and 10 BFU-E-derived erythroblasts with antibodies to TRPC3, TRPC6, Epo-R, and actin. Equivalent amounts of protein were loaded in each lane. Representative results of three experiments are shown. D, densitometry was used to quantitate TRPC3 and TRPC6 bands from three experiments with day 7 and 10 BFU-E-derived erythroblasts. The TRPC3/TRPC6 ratio was calculated, and the mean ratio ± S.E. normalized to day 7 cells is shown. The TRPC3/TRPC6 ratio was significantly less at day 7 compared with day 10 (*, p ≤ 0.01). E, [Ca2+]i was measured in day 7 (n = 30) and 10 (n = 33) BFU-E derived erythroblasts at base line and over 20 min of Epo stimulation. The mean % increase in [Ca2+]i above base line was calculated (% increase = peak [Ca2+]i divided by base line [Ca2+]i × 100%, -100% (baseline); *, p ≤ 0.001.
We previously demonstrated that the Epo-stimulated increase in [Ca2+]i is stage of differentiation-specific (2). To further characterize endogenous expression of TRPC3 and TRPC6, we performed Western blotting on lysates from human erythroblasts removed from methylcellulose culture at days 7 (predominantly late erythroid progenitors and proerythroblasts) and 10 of differentiation. We did not study day 14 cells further because of their low expression of Epo-R. Blots were probed with antibodies to TRPC3, TRPC6, Epo-R, and actin. Representative results of studies from one of three different donors are shown in Fig. 1C. TRPC3 and TRPC6 expression was quantitated with densitometry and results confirmed a significant increase in TRPC3 expression relative to TRPC6 as cells matured (Fig. 1D). [Ca2+]i was measured at base line and over 20 min of Epo stimulation in day 7 and 10 BFU-E-derived cells loaded with Fura Red. The Epo-stimulated rise in [Ca2+]i increased significantly from day 7 (less mature cells, low TRPC3/TRPC6 ratio) compared with day 10 (more mature cells, higher TRPC3/TRPC6 ratio; Fig. 1E). These studies demonstrate that the increase in the TRPC3/TRPC6 ratio observed during erythroid differentiation is associated with an increase in [Ca2+]i in response to Epo.
Epo Modulates Calcium Influx through TRPC3 but Not through TRPC6—To compare Epo-modulated calcium influx through TRPC3 with that of TRPC6, we utilized a model system in which TRPC3 and TRPC6 expression could be controlled. HEK 293T cells were transfected with Epo-R subcloned into pTracer-CMV and human TRPC3 or TRPC6 subcloned into pQB150. Endogenous TRPC3 and TRPC6 are expressed at low levels in HEK 293T cells. Utilizing fluorescence microscopy of single cells, successful transfection of Epo-R was verified by detection of GFP, and successful transfection of TRPC3 or TRPC6 was confirmed by detection of BFP in the same cells. As shown previously (26), in cells cotransfected with Epo-R and BFP-TRPC3, Epo stimulated a large and sustained increase in [Ca2+]i above base line (236 ± 7%, Table 1), which peaked at 10-20 min. This was significantly greater than that observed in cells transfected with Epo-R and BFP-TRPC6 (74 ± 5%, p ≤ 0.0001), confirming previous data that Epo-R modulates [Ca2+]i through TRPC3 but not TRPC6. The increase in [Ca2+]i in Epo-treated cells coexpressing TRPC6 and Epo-R was similar to that observed in cells expressing Epo-R alone (26). This is probably because of Epo-R activation of endogenous channels that include low levels of TRPC3.
TABLE 1.
Epo stimulation of [Ca2+]i in HEK 293T cells transfected with Epo-R and TRPC3, TRPC6, or TRPC3/TRPC6 chimeras
HEK 293T cells were transfected with BFP-TRPC3, BFP-TRPC6, or BFP TRPC3/TRPC6 chimeras and Epo-R. Fura-Red loaded cells were treated with 40 units/ml Epo. [Ca2+]i (mean ± S.E., in nm) was measured at base line and by monitoring over 20 min of Epo stimulation. % increase above base line (mean ± S.E. % increase) = peak [Ca2+]i divided by base line [Ca2+]i × 100%, − 100% (base line). n = number of individual cells studied.
|
Transfection
|
Stimulation
|
[Ca2+]i, nm
|
% increase
|
n
|
|
|---|---|---|---|---|---|
| Base line | Peak | ||||
| BFP-TRPC3 + Epo-R | PBS | 39 ± 3 | 51 ± 3 | 35 ± 3 | 23 |
| Epo | 42 ± 3 | 137 ± 8 | 236 ± 7a | 26 | |
| BFP-TRPC6 + Epo-R | PBS | 31 ± 1 | 42 ± 1 | 37 ± 2 | 9 |
| Epo | 37 ± 2 | 64 ± 3 | 74 ± 5b | 31 | |
| BFP-TRPC6-C3N + Epo-R | PBS | 41 ± 4 | 57 ± 4 | 40 ± 4 | 18 |
| Epo | 50 ± 5 | 74 ± 8 | 46 ± 4b | 19 | |
| BFP-TRPC6-C3C + Epo-R | PBS | 41 ± 3 | 55 ± 4 | 37 ± 4 | 20 |
| Epo | 54 ± 5 | 148 ± 10 | 183 ± 10a,b | 14 | |
| BFP-TRPC3-C6C + Epo-R | PBS | 32 ± 1 | 45 ± 1 | 40 ± 3 | 9 |
| Epo | 32 ± 1 | 63 ± 1 | 98 ± 2a,b | 18 | |
Data indicate significantly greater % increase than Epo-stimulated cells expressing wild type TRPC6 (p ≤ 0.001).
Data indicate % increase in Epo-stimulated cells, which is significantly different from cells expressing wild type TRPC3 (p < 0.0001).
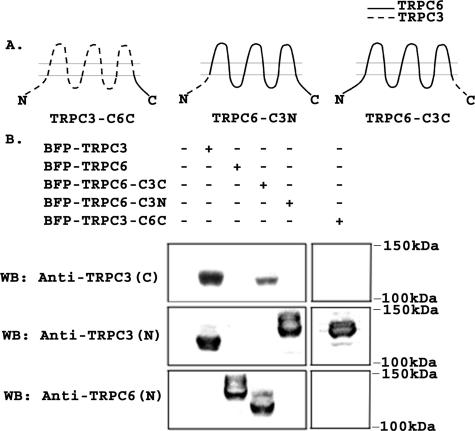
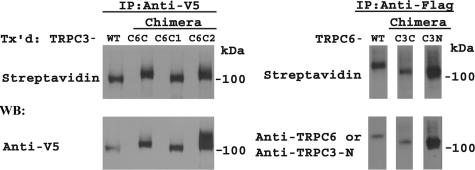
Domains Required for TRPC3 Activation by Epo Are Located in the TRPC3 C Terminus—TRPC3 and TRPC6 share ∼73% homology in their amino acid sequence, but TRPC3 is activated by Epo stimulation, whereas TRPC6 is not. To identify domains responsible for the different responses to Epo, chimeric channels were constructed in which the C terminus of TRPC3 was substituted with that of TRPC6 (TRPC3-C6C), or the C terminus of TRPC6 was substituted with that of TRPC3 (TRPC6-C3C). In addition, the N terminus of TRPC6 was substituted with that of TRPC3 (TRPC6-C3N). A schema of these channels is shown in Fig. 2A. The identity of chimeras was confirmed with sequencing and with Western blotting. HEK 293T cells were transfected with chimeras expressed in pQBI50, and lysates were prepared. As shown in Fig. 2B, anti-TRPC3 (C) directed to the TRPC3 C terminus recognized wild type TRPC3 and TRPC6-C3C, but not TRPC6, TRPC6-C3N, or TRPC3-C6C. Anti-TRPC3 (N) directed to the TRPC3 N terminus recognized wild type TRPC3, TRPC3-C6C, and TRPC6-C3N but not TRPC6 or TRPC6-C3C. Anti-TRPC6 recognized wild type TRPC6 and TRPC6-C3C but not TRPC3, TRPC3-C6C, or TRPC6-C3N. The higher molecular weights observed are because of linkage of channels to BFP and to the different molecular weights of chimeric channels compared with wild type.
FIGURE 2.
Schema of TRPC3/TRPC6 chimeras. A, schematic model of TRPC3-C6C, TRPC6-C3N, and TRPC6-C3C chimeras. B, Western blot (WB) of lysates from HEK 293T cells transfected with BFP-TRPC3, BFP-TRPC6, BFP-TRPC6-C3C, BFP-TRPC6-C3N, or BFP-TRPC3-C6C. Blots were probed with antibodies that recognize the C terminus of human TRPC3 (anti-TRPC3 (C)), the N terminus of murine and human TRPC3 (anti-TRPC3 (N)), or the N terminus of TRPC6 (anti-TRPC6 (N)). Representative results of two experiments are shown.
To identify the domains that are important for TRPC3 activation by Epo, HEK 293T cells were transfected with Epo-R and TRPC6-C3N, TRPC6-C3C, or TRPC3-C6C in pQBI50. TRPC6 or TRPC3 was transfected with Epo-R as controls. Substitution of the N terminus of TRPC6 with that of TRPC3 (TRPC6-C3N) did not result in a significantly greater increase in [Ca2+]i after Epo stimulation (46 ± 4%) compared with TRPC6 (74 ± 5%; Table 1). In contrast, substitution of the C terminus of TRPC6 with the C terminus of TRPC3 (TRPC6-C3C) resulted in a significantly greater increase in [Ca2+]i in response to Epo (183 ± 10%, p ≤ 0.0001) than observed with wild type TRPC6 (Table 1). Substitution of the C terminus of TRPC3 with the C terminus of TRPC6 (TRPC3-C6C) resulted in a significant reduction in [Ca2+]i after Epo stimulation (98 ± 2%, p ≤ 0.0001) compared with wild type TRPC3 (236 ± 7%, Table 1). These results suggest that domains in the C termini of TRPC3 and TRPC6 are largely responsible for their different responses to Epo.
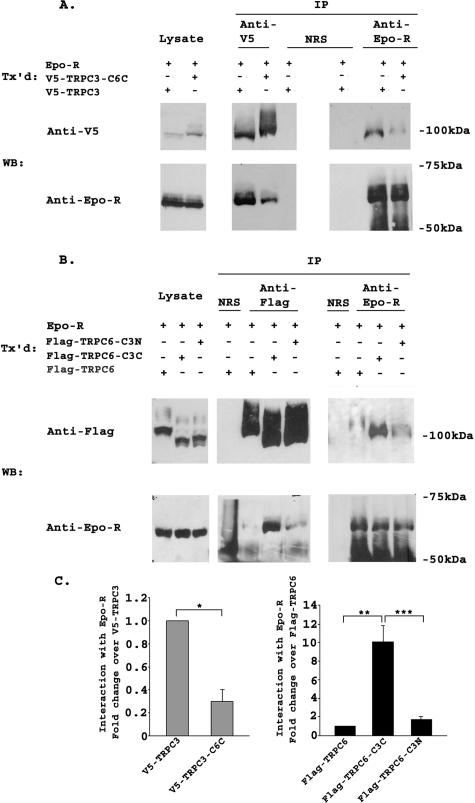
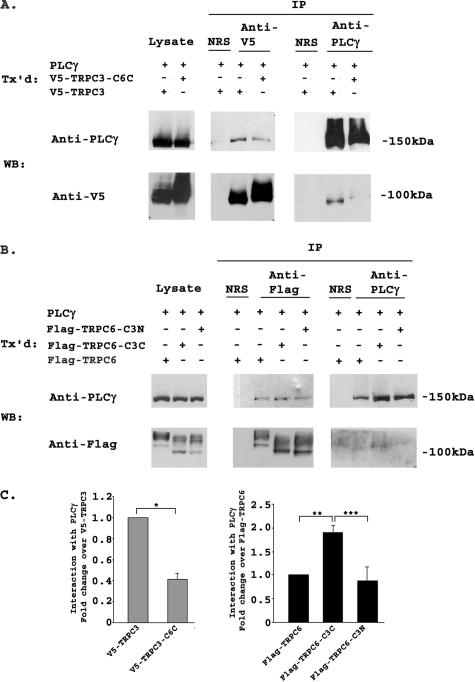
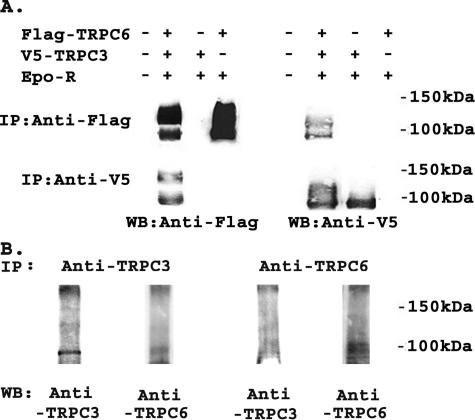
TRPC3 and TRPC6 C Termini Differ in Their Interaction with Epo-R and PLCγ—TRPC3 has been shown previously to interact with Epo-R and PLCγ (26). To examine the role of the C terminus of TRPC3 in this association, we performed immunoprecipitation with lysates from cells transfected with Epo-R and V5-TRPC3, V5-TRPC3-C6C, FLAG-TRPC6, FLAG-TRPC6-C3C, or FLAG-TRPC6-C3N. Representative results of three experiments are shown in Fig. 3, A and B. The intensity of bands precipitated with each antibody was quantitated by densitometry, and the relative ratio of Epo-R associated with each channel, normalized to V5-TRPC3 or FLAG-TRPC6, was calculated (Fig. 3C). Following immunoprecipitation with anti-V5 or anti-Epo-R antibodies, results of three experiments showed that the interaction of V5-TRPC3-C6C with Epo-R was significantly reduced compared with that of wild type TRPC3 with Epo-R (Fig. 3, A and C; p ≤ 0.01). In lysates from cells that coexpressed Epo-R and FLAG-TRPC6, FLAG-TRPC6-C3C, or FLAG-TRPC6-C3N, precipitation with anti-FLAG-agarose or anti-Epo antibody demonstrated much greater association of Epo-R with FLAG-TRPC6-C3C than with FLAG-TRPC6 or FLAG-TRPC6-C3N (Fig. 3, B and C; p ≤ 0.001). The interaction of TRPC6 with Epo-R was very weak compared with the interaction of Epo-R with TRPC3. Because no interaction was observed following precipitation with normal rabbit serum, this weak association did not appear to be nonspecific. These data suggest that domains in the C terminus of TRPC3 are responsible for the strong association of TRPC3 with Epo-R, and that differences in these domains are responsible for the weaker association of TRPC6 with Epo-R.
FIGURE 3.
Interaction of TRPC3/TRPC6 chimeras with Epo-R. A, HEK 293T cells were transfected (Tx'd) with Epo-R and V5-TRPC3 or V5-TRPC3-C6C. Lysates were immunoprecipitated (IP) with anti-V5, anti-Epo-R antibodies, or normal rabbit serum (NRS). Western blots (WB) of lysates and immunoprecipitates were probed with anti-V5-HRP or anti-Epo-R and appropriate secondary antibody. Representative results of three similar experiments are shown. B, HEK 293T cells were transfected with Epo-R and FLAG-TRPC6, FLAG-TRPC6-C3C, or FLAG-TRPC6-C3N. Lysates were immunoprecipitated with anti-FLAG-agarose, anti-Epo-R, or normal rabbit serum. Western blots of lysates and immunoprecipitates were probed with anti-FLAG or anti-Epo-R antibodies. Representative results of three experiments are shown. C, densitometry was used to quantitate Epo-R, V5-TRPC3, FLAG-TRPC6, and chimeric channel bands from three experiments using transfected HEK 293T cells. The Epo-R to V5-TRPC3 or V5-TRPC3-C6C ratio or Epo-R to FLAG-TRPC6, FLAG-TRPC6-C3C, or FLAG-TRPC6-C3N ratio was calculated and normalized to V5-TRPC3 or FLAG-TRPC6 to allow comparison between experiments. The mean normalized ratio ± S.E. was determined for three separate experiments. The Epo-R/V5-TRPC3-C6C ratio was significantly less than the Epo-R/V5-TRPC3 ratio (*, p ≤ 0.01), and the Epo-R/FLAG-TRPC6-C3C ratio was significantly greater than the Epo-R/FLAG-TRPC6 or FLAG-TRPC6-C3N ratio (**, ***, p ≤ 0.001).
Interaction of the C-terminal domains of TRPC3 and TRPC6 with PLCγ was then examined. Cells were transfected with PLCγ and V5-TRPC3, V5-TRPC3-C6C, FLAG-TRPC6, FLAG-TRPC6-C3C, or FLAG-TRPC6-C3N, and immunoprecipitation was performed with anti-V5, anti-PLC antibody, or anti-FLAG-agarose (Fig. 4, A and B). The intensity of precipitated bands was quantitated, and the relative ratio of PLCγ associated with each channel was calculated for three experiments as described above for Epo-R (Fig. 4C). The amount of PLCγ that associated with V5-TRPC3-C6C was significantly reduced compared with that which associated with wild type TRPC3 (Fig. 4, A and C; p ≤ 0.01). In cells that coexpressed PLCγ and FLAG-TRPC6, FLAG-TRPC6-C3C, or FLAG-TRPC6-C3N, association of PLCγ with FLAG-TRPC6-C3C was significantly greater than with FLAG-TRPC6 (p ≤ 0.01) or FLAG-TRPC6-C3N (p ≤ 0.001) (Fig. 4, B and C). Similar results were observed in three experiments. Although all TRPC6 constructs immunoprecipitated poorly with anti-PLCγ antibody, the greatest coprecipitation was observed between FLAG-TRPC6-C3C and PLCγ. These results suggest that domains in the C terminus of TRPC3 are involved in the strong association of TRPC3 with PLCγ, and when the C terminus of TRPC3 is substituted with the C terminus of TRPC6, the association is greatly reduced. This association is not eliminated, which was expected because other binding sites for PLCγ exist on TRPC3 (55). The lack of strong interaction of TRPC6 with Epo-R and PLCγ may contribute to the absence of response to Epo of cells expressing TRPC6 and Epo-R.
FIGURE 4.
Interaction of TRPC3/TRPC6 chimeras with PLCγ. A, HEK 293T cells were transfected (Tx'd) with PLCγ and V5-TRPC3 or V5-TRPC3-C6C. Lysates were immunoprecipitated (IP) with anti-V5, anti-PLCγ antibodies, or normal rabbit serum (NRS). Western blots (WB) of lysates and immunoprecipitates were probed with anti-V5-HRP or anti-PLCγ and appropriate secondary. Representative results of three similar experiments are shown. B, HEK 293T cells were transfected with PLCγ and FLAG-TRPC6, FLAG-TRPC6-C3C, or FLAG-TRPC6-C3N. Lysates were immunoprecipitated with anti-FLAG-agarose, anti-PLCγ, or normal rabbit serum. Western blots of lysates and immunoprecipitates were probed with anti-FLAG or anti-PLCγ antibodies. Representative results of three experiments are shown. C, densitometry was used to quantitate PLCγ, V5-TRPC3, FLAG-TRPC6, and chimeric channel bands from three experiments using transfected HEK 293T cells. The PLCγ to V5-TRPC3 or V5-TRPC3-C6C ratio or PLCγ to FLAG-TRPC6, FLAG-TRPC6-C3C, or FLAG-TRPC6-C3N ratio was calculated for three separate experiments and normalized to V5-TRPC3 or FLAG-TRPC6 to allow comparison between experiments. The mean normalized ratio ± S.E. is shown. The PLCγ/V5-TRPC3-C6C ratio was significantly less than the PLCγ/V5-TRPC3 ratio (*, p ≤ 0.01) and the PLCγ/FLAG-TRPC6-C3C ratio was significantly greater than the PLCγ/FLAG-TRPC6 (**, p ≤ 0.01) or FLAG-TRPC6-C3N ratio (***, p ≤ 0.001).
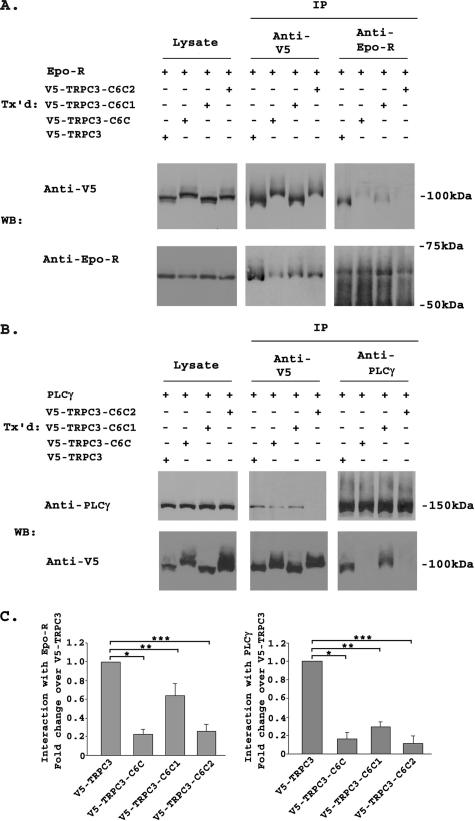
A Region in the TRPC3 C Terminus Including the TRP Domain Is Required for the Response of TRPC3 to Epo—To further determine the domains involved in the response of TRPC3 but not TRPC6 to Epo, chimeras were made after subdividing regions in the TRPC3 and TRPC6 C termini (Fig. 5). Two chimeras were prepared that retained amino acids 1-670 of TRPC3 but had different C-terminal ends. One, named TRPC3-C6C1, contained amino acids 728-807 of TRPC6 and amino acids 747-848 of TRPC3 (38). The other, called TRPC3-C6C2, contained amino acids 671-746 of TRPC3 and amino acids 808-931 of TRPC6. The locations of the “TRP domain,” the calmodulin-binding/inositol 1,4,5-triphosphate receptor-binding site called the “CRIB domain,” and the “coiled-coil” domain for each of these chimeras are shown schematically on Fig. 5. HEK 293T cells were transfected with BFP-TRPC3-C6C1 or BFP-TRPC3-C6C2 and Epo-R. BFP-TRPC3 or BFP-TRPC6 and Epo-R were transfected as controls. The increase in [Ca2+]i in response to Epo stimulation for cells expressing TRPC3-C6C2 (186 ± 4% increase above base line, see Table 2) was marginally but significantly reduced compared with wild type TRPC3-expressing cells (220 ± 5%, p ≤ 0.0001). In contrast, the increase in [Ca2+]i in response to Epo in cells expressing TRPC3-C6C1 (109 ± 6%, Table 2) was dramatically reduced compared with cells expressing TRPC3 (p ≤ 0.0001). These data suggest that domains in the proximal TRPC3 C terminus between amino acids 671 and 746 are of critical importance for the response of TRPC3 to Epo, and that domains between amino acids 747 and 848 have a minor role.
TABLE 2.
Response to Epo of cells transfected with Epo-R and TRPC3/C6 chimeras and mutants
HEK 293T cells were transfected with BFP-TRPC3, BFP-TRPC6, BFP-TRPC3/TRPC6 chimeras, BFP-TRPC3 Y674F, or BFP-TRPC3 F731Y and Epo-R. Fura-Red loaded cells were treated with 40 units/ml Epo. [Ca2+]i (mean ± S.E., in nm) was measured at base line and by monitoring over 20 min of Epo stimulation. % increase above base line (mean ± S.E. % increase) = peak [Ca2+]i divided by base line [Ca2+]i × 100%, - 100% (base line). n = number of individual cells studied.
|
Transfection
|
Stimulation
|
[Ca2+]i, nm
|
% increase
|
n
|
|
|---|---|---|---|---|---|
| Base line | Peak | ||||
| BFP-TRPC3 + Epo-R | PBS | 36 ± 1 | 49 ± 1 | 36 ± 2 | 16 |
| Epo | 35 ± 1 | 114 ± 1 | 220 ± 5 | 26 | |
| BFP-TRPC6 + Epo-R | PBS | 36 ± 1 | 50 ± 1 | 38 ± 2 | 17 |
| Epo | 37 ± 1 | 75 ± 1 | 104 ± 3a | 26 | |
| BFP-TRPC3-C6C1 + Epo-R | PBS | 35 ± 1 | 48 ± 1 | 35 ± 2 | 18 |
| Epo | 35 ± 1 | 73 ± 2 | 109 ± 6a | 19b | |
| BFP-TRPC3-C6C2 + Epo-R | PBS | 35 ± 1 | 49 ± 1 | 38 ± 1 | 20 |
| Epo | 35 ± 1 | 101 ± 1 | 186 ± 4a | 26b | |
| BFP-TRPC3 Y674F + Epo-R | PBS | 35 ± 1 | 49 ± 1 | 38 ± 1 | 16 |
| Epo | 35 ± 1 | 115 ± 3 | 212 ± 8 | 21 | |
| BFP-TRPC6 F731Y + Epo-R | PBS | 35 ± 1 | 49 ± 1 | 38 ± 1 | 17 |
| Epo | 35 ± 1 | 74 ± 2 | 109 ± 3a | 20 | |
Data indicate significantly reduced % increase compared with Epo-stimulated cells expressing wild type TRPC3 (p ≤ 0.0001).
Data indicate significant differences in % increase of Epo-stimulated cells, between these two groups (p ≤ 0.001).
TRPC3 Tyr-674 is located within this critical C-terminal domain and is a predicted binding site for the Src homology 2 domains of a number of signaling molecules. The cognate tyrosine in TRPC6 is a phenylalanine at amino acid position 731. To determine whether this site is responsible for differential responsiveness of TRPC3 and TRPC6 to Epo, we prepared two mutants, TRPC3 with tyrosine 674 substituted with phenylalanine (TRPC3 Y674F) and TRPC6 with phenylalanine at amino acid 731 mutated to tyrosine (TRPC6 F731Y). As shown in Table 2, BFP-TRPC3 Y674F responded to Epo with an increase in [Ca2+]i (212 ± 8%) similar to that of TRPC3 (p = 0.4), whereas TRPC6 F731Y demonstrated an increase in [Ca2+]i (109 ± 3%) similar in magnitude to TRPC6 (p = 0.3). Therefore, this specific tyrosine site was not responsible for the differential response between the two channels.
To confirm that these chimeric channels (TRPC3-C6C, TRPC3-C6C1, TRPC3-C6C2, TRPC6-C3C, and TRPC6-C3N) are all inserted in the plasma membrane, HEK 293T cells were transfected with these channels and Epo-R. Externalization was assessed by biotinylation of cell surface proteins. All of these mutants were expressed at the cell surface (Fig. 6).
FIGURE 6.
Plasma membrane insertion of TRPC3/TRPC3 chimeras detected with cell surface biotinylation. Cell surface biotinylation was performed with HEK 293T cells expressing V5-TRPC3, V5-TRPC3-C6C, V5-TRPC3-C6C1, V5-TRPC3-C6C2, FLAG-TRPC6, FLAG-TRPC6-C3C, or FLAG-TRPC6-C3N and Epo-R. Lysates were prepared, and immunoprecipitation (IP) performed with anti-V5 antibody or anti-FLAG-agarose. Western blotting (WB) was performed on immunoprecipitation pellets with streptavidin-HRP to detect biotinylation and either anti-V5-HRP to detect total TRPC3 chimeras or anti-TRPC6 or anti-TRPC3-N antibodies to detect total TRPC6 chimeras. Representative results of two experiments are shown. Tx'd, transfected.
To identify the TRPC3 domains with which Epo-R and PLCγ interact, cells were transfected with V5-TRPC3, V5-TRPC3-C6C, V5-TRPC3-C6C1, or V5-TRPC3-C6C2 and Epo-R or PLCγ. Immunoprecipitation and Western blotting was performed with anti-V5, anti-Epo-R, or anti-PLCγ antibodies (Fig. 7, A and B). The intensity of precipitated bands was quantitated, and the relative ratio of Epo-R (six experiments) or PLCγ (four experiments) associated with each channel was calculated (Fig. 7C). The associations of Epo-R and PLCγ with both TRPC3-C6C1 and TRPC3-C6C2 were significantly reduced compared with TRPC3, but not eliminated (Fig. 7, A-C; p ≤ 0.01, p ≤ 0.001). However, TRPC3-C6C1 had a significantly reduced rise in [Ca2+]i in response to Epo compared with TRPC3-C6C2 (Table 2; p ≤ 0.0001), suggesting that factors other that Epo-R and PLCγ binding are responsible for a component of the different responses.
FIGURE 7.
Interaction of TRPC3-C6C1 and TRPC3C6C2 with Epo-R and PLCγ. A, HEK 293T cells were transfected (Tx'd) with Epo-R and V5-TRPC3, V5-TRPC3-C6C, V5-TRPC3-C6C1, or V5-TRPC3-C6C2. Lysates were immunoprecipitated (IP) with anti-V5 or anti-Epo-R antibodies. Western blots (WB) of lysates and immunoprecipitates were probed with anti-V5-HRP or anti-Epo-R and appropriate secondary antibodies. Representative results of six similar experiments are shown. B, HEK 293T cells were transfected with PLCγ and V5-TRPC3, V5-TRPC3-C6C, V5-TRPC3-C6C1, or V5-TRPC3-C6C2. Lysates were immunoprecipitated with anti-V5 or anti-PLCγ antibodies. Western blots (WB) of lysates and immunoprecipitates were probed with anti-V5-HRP or anti-PLCγ and appropriate secondary antibody. Representative results of four similar experiments are shown. C, densitometry was used to quantitate Epo-R, PLCγ, V5-TRPC3, V5-TRPC3-C6C, V5-TRPC3-C6C1, and V5-TRPC3-C6C2 bands from six (Epo-R) or four (PLCγ) experiments. The Epo-R or PLCγ to V5-TRPC3, V5-TRPC3-C6C, V5-TRPC3-C6C1, or V5-TRPC3-C6C2 ratio was calculated and normalized to that of V5-TRPC3 to allow comparison between experiments. The mean normalized ratio ± S.E. was determined. The Epo-R/V5-TRPC3 ratio was significantly greater than the Epo-R/V5-TRPC3-C6C ratio (*, p ≤ 0.001), Epo-R/V5-TRPC3-C6C1 ratio (**, p ≤ 0.01), or Epo-R/V5-TRPC3-C6C2 ratio (***, p ≤ 0.001). The PLCγ/V5-TRPC3 ratio was significantly greater than the PLCγ/V5-TRPC3-C6C ratio (*, p ≤ 0.001), PLCγ/V5-TRPC3-C6C1 ratio (**, p ≤ 0.001), or PLCγ/V5-TRPC3-C6C2 ratio (***, p ≤ 0.001).
TRPC6 Inhibits the Epo-stimulated Rise in [Ca2+]i through TRPC3—To determine whether the change in the ratio of TRPC3 to TRPC6 during erythroid differentiation has functional significance, the ability of TRPC6 to modulate Epo-stimulated calcium influx through TRPC3 was investigated. HEK 293T cells transfected with Epo-R, BFP-TRPC3, and empty pQBI50 vector or BFP-TRPC6 were stimulated with Epo. The increase in [Ca2+]i in cells expressing Epo-R, BFP-TRPC3, and BFP-TRPC6 (125 ± 4% above base line, p ≤ 0.0001; Table 3) was significantly less than that observed in cells expressing Epo-R, BFP-TRPC3, and empty pQBI50 vector (230 ± 4%; Table 3). These data suggest that TRPC6 inhibits calcium influx though TRPC3. To confirm that the inhibition of calcium influx in cells expressing TRPC6 did not result from decreased expression of TRPC3, Western blotting was performed on lysates from HEK 293T cells expressing Epo-R, and BFP-TRPC3, BFP-TRPC6, or both. The amount of TRPC3 expressed in cells cotransfected with Epo-R, which responded to Epo with a significant increase in [Ca2+]i, was similar to that expressed in cells that were cotransfected with Epo-R and TRPC6 (Fig. 8). The higher molecular weights of TRPC3 and TRPC6 shown here compared with primary erythroid cells (Fig. 1) are secondary to linkage of channels to BFP.
TABLE 3.
Response to Epo of HEK 293T cells transfected with Epo-R and multiple TRPCs
HEK 293T cells were transfected with BFP-TRPC3, and BFP-TRPC6, BFP-TRPC3/TRPC6 chimeras or empty pQBI50 vector, and Epo-R. Fura-Red loaded cells were treated with 40 units/ml Epo. [Ca2+]i (mean ± S.E., in nm) was measured at base line and by monitoring over 20 min of Epo stimulation. % increase above base line (mean ± S.E. % increase) = peak [Ca2+]i divided by base line [Ca2+]i × 100%, - 100% (baseline). n = number of individual cells studied.
|
Transfection
|
Stimulation
|
[Ca2+]i, nm
|
% increase
|
n
|
|
|---|---|---|---|---|---|
| Base line | Peak | ||||
| BFP-TRPC3 + EmptyVec + Epo-R | PBS | 35 ± 1 | 48 ± 1 | 37 ± 2 | 24 |
| Epo | 36 ± 1 | 119 ± 3 | 230 ± 4 | 25 | |
| BFP-TRPC3 + BFP-TRPC6 + Epo-R | PBS | 35 ± 1 | 48 ± 1 | 37 ± 1 | 24 |
| Epo | 35 ± 1 | 78 ± 3 | 125 ± 4a | 22 | |
| BFP-TRPC3 + BFP-TRPC6-C3C + Epo-R | PBS | 31 ± 1 | 44 ± 1 | 40 ± 2 | 12 |
| Epo | 31 ± 1 | 98 ± 3 | 217 ± 9 | 14 | |
| BFP-TRPC3 + BFP-TRPC6-C3N + Epo-R | PBS | 31 ± 1 | 43 ± 1 | 38 ± 2 | 12 |
| Epo | 32 ± 1 | 70 ± 2 | 118 ± 4a | 14 | |
| BFP-TRPC3 + BFP-TRPC3-C6C + Epo-R | PBS | 36 ± 1 | 48 ± 1 | 34 ± 2 | 10 |
| Epo | 36 ± 1 | 78 ± 2 | 117 ± 5a | 11 | |
| BFP-TRPC3 + BFP-TRPC3-C6C1 + Epo-R | PBS | 41 ± 1 | 55 ± 1 | 34 ± 1 | 26 |
| Epo | 39 ± 1 | 96 ± 3 | 147 ± 5* | 27 | |
| BFP-TRPC3 + BFP-TRPC3-C6C2 + Epo-R | PBS | 39 ± 1 | 54 ± 1 | 35 ± 1 | 26 |
| Epo | 38 ± 1 | 119 ± 4 | 209 ± 6a | 26 | |
Data indicate % increase in Epo-stimulated cells which is significantly different from cells expressing wild type TRPC3 and empty vector (p ≤ 0.01).
FIGURE 8.
Western blot (WB) of HEK 293T cells transfected (Tx'd) with Epo-R, BFP-TRPC3, and BFP-TRPC6. HEK 293T cells were cotransfected with Epo-R, and BFP-TRPC3, BFP-TRPC6, or both. Equivalent amounts of protein lysates were loaded in each lane, and Western blotting was performed with anti-TRC3, anti-TRPC6, or anti-Epo-R antibodies, followed by ECL.
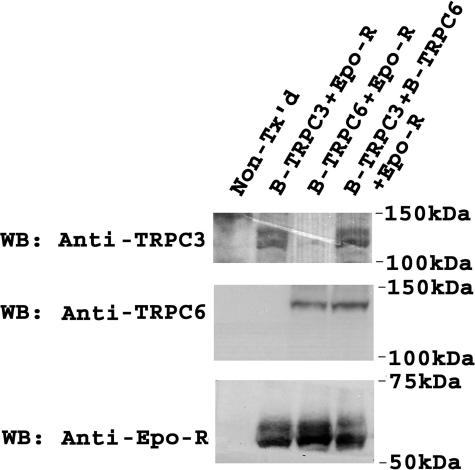
As an initial step to explore the mechanisms through which TRPC6 inhibits Epo-stimulated calcium influx through TRPC3, we reexamined whether these two channels coassociate. Immunoprecipitation was performed on lysates from HEK 293T cells transfected with Epo-R, V5-TRPC3, FLAG-TRPC6, or combinations with anti-V5 antibody or anti-FLAG-agarose (Fig. 9A). We also examined endogenous interaction in the erythroid cell line TF-1 (Fig. 9B). Western blotting demonstrated that TRPC3 and TRPC6 reciprocally immunoprecipitated (Fig. 9), confirming that these channels interact. This suggests that TRPC6 may inhibit TRPC3 through direct interaction.
FIGURE 9.
Interaction of transfected and endogenous TRPC3 and TRPC6. A, V5-TRPC3 and/or FLAG-TRPC6 were expressed in HEK 293T cells with Epo-R. Immunoprecipitation (IP) was performed on lysates with anti-V5 antibody or anti-FLAG-agarose. Western blotting (WB) was performed after immunoprecipitation with anti-V5 or anti-FLAG antibodies. Representative results of five experiments are shown. B, immunoprecipitation was performed on lysates from TF-1 erythroid cells with anti-TRPC3 or anti-TRPC6 antibody to detect endogenous interactions. Western blotting was performed after immunoprecipitation with anti-TRPC3 or anti-TRPC3 antibodies. Representative results of two experiments are shown.
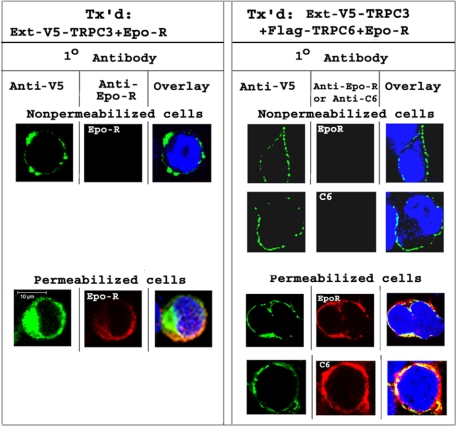
Subcellular Localization of TRPC3 Is Modulated by TRPC6—To confirm that TRPC3 is expressed on the cell membrane, immunolocalization studies were performed with HEK 293T cells expressing TRPC3 with a V5 tag inserted between the first and second transmembrane domains, a site that is externally accessible if TRPC3 is inserted in the plasma membrane (Ext-V5-TRPC3). Nonpermeabilized cells expressing Ext-V5-TRPC3 and Epo-R were stained with anti-V5 or anti-Epo-R and appropriate secondary antibodies and studied with confocal microscopy. Representative results, shown in Fig. 10, demonstrate that TRPC3 is inserted in the cell membrane. Epo-R was not visualized in nonpermeabilized cells because anti-Epo-R antibody recognizes an intracellular site. In contrast, in permeabilized cells, expression of V5-TRPC3 and Epo-R was observed at or near the plasma membrane as well as in the cytoplasm, and colocalization was observed in merged images. In cells cotransfected with Ext-V5-TRPC3, FLAG-TRPC6, and Epo-R, TRPC6 was not observed in nonpermeabilized cells because its antibodies were directed to an intracellular N-terminal domain. Ext-V5-TRPC3 was observed at the cell surface. In permeabilized cells, expression of TRPC3, TRPC6, and Epo-R was observed at or near the plasma membrane and in the cytoplasm.
FIGURE 10.
Immunolocalization of TRPC3 and Epo-R. HEK 293T cells were transfected (Tx'd) with Ext-V5-TRPC3, Epo-R, with or without TRPC6. Cells were permeabilized or not. Cells were stained with anti-V5, anti-Epo-R, or anti-TRPC6 antibodies, and then with Alexa Fluor 488 goat anti-mouse and Alexa Fluor 594 goat anti-rabbit antibodies. Nuclei were identified by 4′,6-diamidino-2-phenylindole staining. Representative results of images obtained in the midplane of the cell with confocal microscopy are shown.
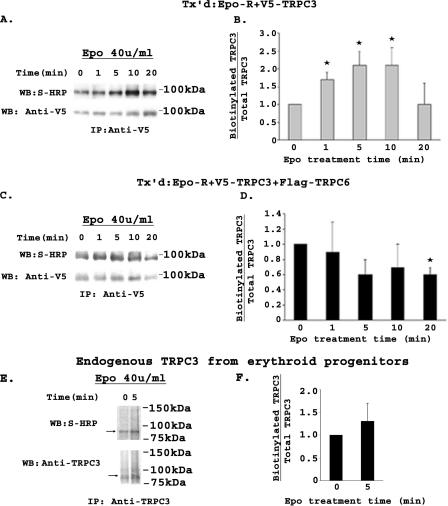
To determine whether Epo modulates externalization of TRPC3 and the role of TRPC6, HEK 293T cells were transfected with Epo-R and V5-TRPC3, with or without FLAG-TRPC6. At 48 h, cells were stimulated with Epo for 0, 1, 5, 10, and 20 min. Externalization of TRPC3 was assessed by biotinylation of cell surface proteins, followed by immunoprecipitation with anti-V5 antibody (32, 54). Total V5-TRPC3 was determined by probing Western blots with anti-V5 antibody, and biotinylated TRPC3, representing the fraction inserted in the cell membrane, was identified by probing blots with streptavidin-HRP. Representative results of Western blots from four experiments are shown in Fig. 11, A and C. Biotinylated and total TRPC3 bands were quantitated with densitometry. The ratios of biotinylated TRPC3/total TRPC3 at each time point were standardized to time 0 to allow statistical comparison between four individual experiments. TRPC3 cell surface expression significantly increased 2-fold after 1-10 min of Epo stimulation (Fig. 11B, p ≤ 0.02). The increase in TRPC3 cell membrane insertion observed in response to Epo was not found in cells that coexpressed FLAG-TRPC6 (Fig. 11D).
FIGURE 11.
Modulation of membrane insertion of TRPC3 by Epo detected by cell surface biotinylation. HEK 293T cells transfected (Tx'd) with Epo-R and V5-TRPC3 without (A and B) or with FLAG-TRPC6 (C and D) were stimulated with 40 units/ml Epo. Biotinylation of cell surface proteins was performed, and V5-TRPC3 immunoprecipitated (IP) from lysates with anti-V5 antibody. Western blots (WB) were probed with streptavidin-HRP to detect biotinylated TRPC3 and anti-V5-HRP to detect total V5-TRPC3. Representative results of Western blots from four experiments are shown in A and C. Biotinylated and total TRPC3 bands were quantitated with densitometry, and the ratio was normalized to time 0. The mean ± S.E. of the biotinylated/total TRPC3 ratios at 0, 1, 5, 10, and 20 min from four experiments are shown (B and D). * indicates a significant difference in the ratio compared with time 0 (p ≤ 0.02). E, Epo-stimulated cell surface expression of endogenous TRPC3 was examined using BFU-E-derived erythroblasts at day 10 of methylcellulose culture (two experiments) or erythroblasts from phase II day 8 of liquid culture (one experiment). Cells were stimulated with 40 units/ml Epo for 0 or 5 min and biotinylated, and TRPC3 was immunoprecipitated from lysates with anti-TRPC3 antibody. Western blots were probed with streptavidin-HRP to detect biotinylated TRPC3 and anti-TRPC3 to detect total TRPC3. A representative result of three Western blots is shown. F, biotinylated and total TRPC3 bands were quantitated with densitometry, and the ratio was normalized to time 0. The mean ± S.E. of the biotinylated/total TRPC3 ratios at 0 and 5 min from the three experiments are shown. No significant difference in the ratio at 5 min compared with time 0 was detected.
The ability of Epo to increase cell surface expression of TRPC3 was also studied in primary erythroid cells. BFU-E-derived human erythroblasts at day 10 of methylcellulose culture (two experiments) or erythroblasts harvested from phase II at day 8 of liquid culture (predominantly proerythroblasts and basophilic normoblasts; one experiment) were stimulated with Epo for 5 min. Externalization of TRPC3 was assessed by cell surface biotinylation as described above, except that TRPC3 was immunoprecipitated and detected with anti-TRPC3 antibody (Fig. 11E). The ratio of biotinylated to total TRPC3 did not significantly increase after Epo stimulation (Fig. 11F). These data demonstrate that an increase in membrane insertion of TRPC3 is not required endogenously for a significant increase in [Ca2+]i (Fig. 1E). These experiments did not distinguish whether the amount of TRPC6 expressed in day 10 cells was sufficient to inhibit TRPC3 cell surface insertion but too low to inhibit function, or if other factors are involved so that endogenous trafficking of TRPC3 does not occur.
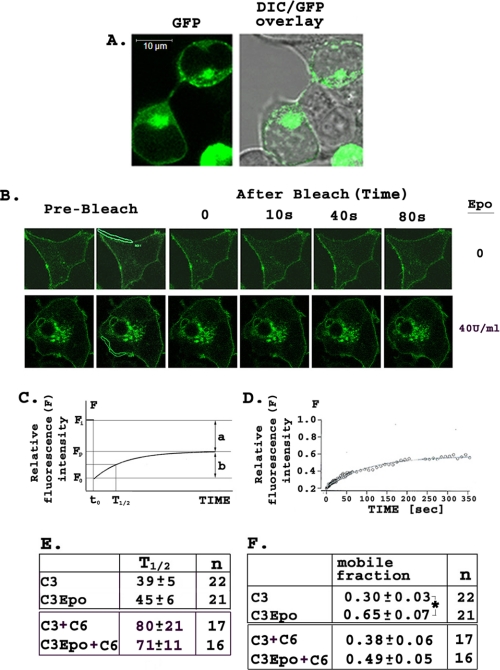
FRAP—The ability of Epo to regulate cell surface expression of TRPC3 was also studied with FRAP. HEK 293T cells were transfected with GFP-TRPC3 and Epo-R, or GFP-TRPC3, FLAG-TRPC6, and Epo-R. FRAP images were obtained before and after photobleaching a section of the cell membrane, and the time course and magnitude of recovery of fluorescence in the presence and absence of Epo treatment was quantitated. Differential interference contrast (Fig. 12A) showed that TRPC3 is localized at or near the cell membrane. Fig. 12B shows representative cells before photobleaching and then at 0, 10, 40, and 80 s after photobleaching. The cell in the lower panel of Fig. 12B was photobleached in the presence of Epo. Approximately 80-90% of fluorescence was lost during photobleaching. For analysis of data, the half-time (t½) is defined as the time required for recovery of one-half of the final fluorescence level (Fp; Fig. 12C), and the mobile fraction is defined as the fraction of fluorescent protein that can move into the bleached region during the time course of the experiment. Mobile fraction is calculated as the ratio of fluorescence recovered between bleach and plateau (Fp - Fo; Fig. 12C) over initial fluorescence minus fluorescence after photobleaching (Fi - Fo). Fluorescence recovery was monitored for 6 min. A representative graph showing the time course of fluorescence recovery of a single cell over 6 min is shown in Fig. 12D. The mean ± S.E. t½ value for control cells expressing TRPC3 was 39 ± 5 s, and there was no statistically significant difference in t½ between untreated and Epo (40 units/ml)-treated cells (45 ± 6 s; Fig. 12E) transfected with GFP-TRPC3 and Epo-R. This suggests that the rate of localization of TRPC3 to the cell membrane does not change after Epo stimulation. There was also no difference in t½ of fluorescence recovery between Epo-treated and untreated cells expressing GFP-TRPC3, Epo-R, and FLAG-TRPC6. Although only a percentage of initial fluorescence was recovered after photobleaching, a greater mobile fraction of TRPC3 was detected in Epo-treated (0.65 ± 0.07, p ≤ 0.001; Fig. 12F) compared with untreated cells (0.30 ± 0.03%) transfected with GFP-TRPC3 and Epo-R. There was no statistically significant increase in the mobile fraction of untreated and Epo-treated cells cotransfected with GFP-TRPC3, FLAG-TRPC6, and Epo-R. These data suggest that the increase of TRPC3 expressed at the cell surface is not because of the increased rate of insertion of TRPC3 protein at the cell membrane but rather to an increase in the mobile fraction of TRPC3 in response to Epo stimulation, which was not observed in the presence of TRPC6.
FIGURE 12.
FRAP. A, localization of GFP-TRPC3 in HEK 293T cells transfected with GFP-TRPC3 and Epo-R examined with confocal microscopy and differential interference contrast (DIC). B, representative FRAP experiment in HEK 293T expressing Epo-R and GFP-TRPC3. Upper panels show untreated cells before photobleaching and then at 10, 40, and 80 s after photobleaching. Lower panels show recovery of cells photobleached in the presence of Epo (40 units/ml). Area of membrane bleached is shown in prominent white lines. C, schema showing calculation of half-time (t½) and the mobile fraction. When the region is bleached, fluorescence decreases from Fi, the initial fluorescence value, to Fo at time to immediately after photobleaching. Fluorescence recovers over time until it reaches plateau value Fp. t½ is the time to recovery of half of plateau fluorescence (Fp). a = the immobile fraction, b = the mobile fraction, calculated from Fii, Fp, and Fo. D, graph from a representative FRAP experiment showing relative fluorescence recovery after photobleaching (IgorPro analysis). E, t½ (in seconds) of fluorescence recovery with or without Epo stimulation (n = number of cells). F, mobile fraction with and without Epo stimulation (n = number of cells). Mean ± S.E. calculated using one-way analysis of variance (*, p ≤ 0.001).
Identification of TRPC6 Domains Required for Inhibition of TRPC3 Activation by Epo—TRPC function as tetramers, and we demonstrate here that TRPC6 interacts with TRPC3 and inhibits its activation by Epo. To further examine the mechanisms, HEK 293T cells were cotransfected with Epo-R, TRPC3, and either empty pQBI50 vector, BFP-TRPC6, or BFP-TRPC3/TRPC6 chimeras. Cells were loaded with Fura Red and stimulated with Epo, and [Ca2+]i was measured. Cotransfection of TRPC6 with Epo-R and TRPC3 resulted in significant inhibition in the rise in [Ca2+]i in response to Epo (125 ± 4%, p ≤ 0.0001; Table 3) compared with cells transfected with TRPC3 and empty vector (230 ± 4%). TRPC3-expressing cells cotransfected with TRPC6-C3C had a rise in [Ca2+]i (217 ± 9%) equivalent to that observed in cells expressing wild type TRPC3. In contrast, TRPC3-expressing cells coexpressing TRPC6-C3N had a rise in [Ca2+]i (118 ± 4%) equivalent to that of cells coexpressing TRPC6 (125 ± 4%; Table 3). These data demonstrate that inhibition of Epo-stimulated calcium influx through TRPC3 by TRPC6 is mediated through C-terminal domains. To further identify the domains, cells were transfected with BFP-TRPC3 and Epo-R, and BFP-TRPC3-C6C1 or BFP-TRPC3-C6C2. Both TRPC3-C6C1 and TRPC3-C6C2 inhibited Epo-stimulated calcium influx through TRPC3, demonstrating that domains in both the proximal and distal C termini of TRPC6 are involved. However, the proximal TRPC6 C-terminal domains present in TRPC3-C6C1 were far more effective in blocking the response of TRPC3 to Epo compared with TRPC3-C6C2 (Table 3).
DISCUSSION
TRPC3 and TRPC6 are two calcium-permeable channels that are differentially expressed on human erythroid precursors. Although TRPC3 is the first human TRP channel shown to be regulated by a hematopoietic growth factor, erythropoietin (26), the homologous TRPC6 does not respond to Epo stimulation (3). In addition, TRPC6 inhibited Epo activation of TRPC3 when coexpressed, suggesting that it may be part of the regulatory pathway of TRPC3. Here we examined the mechanisms responsible for the different responses of TRPC3 and TRPC6 to Epo stimulation, and we identified channel domains in the TRPC C termini that may be involved.
Our first finding is that TRPC3 expression increases and TRPC6 expression decreases during erythroid differentiation as cells mature from CD34+ cells to erythrocytes, resulting in a significant increase in the TRPC3/TRPC6 ratio (Fig. 1). [Ca2+]i increased in response to Epo stimulation as the TRPC3/TRPC6 ratio increased, until the late stages of erythroid differentiation when Epo-R expression was reduced. These data suggest that the change in the TRPC3/TRPC6 ratio may have a role in regulating differentiation of erythroid cells. This expression pattern was also observed in studies of developmental expression of TRPC in rat cerebellum. TRPC3 expression increased during the first 6 weeks after birth, whereas TRPC6 as well as TRPC4 expression were significantly down-regulated (56). Demonstration that TRPC3/TRPC6 expression is modulated in erythroid differentiation, together with the changes in TRPC levels during cerebellar development, suggests that differential TRPC3/TRPC6 expression may have a broader role in tissue differentiation. Another example of differential regulation of TRPC3 and TRPC6 is found in essential hypertension. Experiments with monocytes from patients with essential hypertension showed that TRPC3 but not TRPC6 expression was increased compared with normotensive controls (57).
The second finding of our report is that TRPC3 but not TRPC6 is gated by Epo, supporting the conclusion that although highly homologous, TRPC3 and TRPC6 are nonredundant (58, 59). Although in some experimental systems TRPC3 and TRPC6 have similar expression and responses to agonist stimulation (60), as observed here they have also been shown to have different gating characteristics. For example, the cation channel blocker flufenamic acid inhibited TRPC3 but potentiated the activity of TRPC6 (61, 62). Activation of TRPC6 by vasopressin was not inhibited by genistein or mutation of the cognate of TRPC3 Tyr-226 to phenylalanine, as was the case for TRPC3 (63).
Epo-R modulates TRPC3 activation utilizing PLCγ through a mechanism involving complex formation between TRPC3, PLCγ and Epo-R, and TRPC3 and IP3R (26). To identify sequences that explain the absence of TRPC6 gating following Epo stimulation, chimeras were generated in which the N- and C-terminal domains of TRPC3 and TRPC6 were exchanged. The third finding of this study is that the TRPC3 C terminus is essential for TRPC3 gating in response to Epo stimulation, because substitution of the TRPC3 C terminus with that of TRPC6 eliminated the Epo-stimulated increase in [Ca2+]i through TRPC3. Reciprocally, substitution of the TRPC6 C terminus with that of TRPC3 resulted in a TRPC6 channel which was activated by Epo. We examined the interaction of TRPC6 with Epo-R and PLCγ. Immunoprecipitation experiments demonstrated that interaction of both Epo-R and PLCγ with TRPC6 was much weaker than that with TRPC3. Experiments with TRPC3/TRPC6 chimeras confirmed that the TRPC3 C terminus is important for TRPC3 interaction with Epo-R and PLCγ, and suggested that one explanation for the poor response of TRPC6 to Epo is reduced interaction of the TRPC6 C terminus with these key signal transducers.
Our initial studies with transfected HEK 293T cells allowed us to manipulate TRPC3 and TRPC6 expression in cells that expressed Epo-R. These studies showed that when TRPC3 is expressed with Epo-R, providing a situation in which TRPC3 monomeric channels predominantly form, Epo-R activation promotes calcium influx through TRPC3. In contrast, when only TRPC6 is expressed in these cells with Epo-R, resulting in predominantly TRPC6 monomeric channels, no significant increase in [Ca2+]i through TRPC6 is observed after Epo stimulation. When TRPC3 and TRPC6 are both coexpressed with Epo-R, TRPC3/TRPC6 heteromeric channels form, as shown by immunoprecipitation. A fourth finding of this study is that the increase in [Ca2+]i stimulated by Epo through TRPC3 is significantly reduced in the presence of TRPC6, suggesting that the TRPC6 in heteromeric TRPC3/TRPC6 channels inhibits opening of TRPC3 in response to Epo. These studies with transfected cells suggest the following model for Epo activation of TRPC3. TRPC3 monomeric channels are gated following activation of Epo-R by Epo, but TRPC6 monomeric channels are not. When the stoichiometry of TRPC3 and TRPC6 expression results in heteromeric channel formation, Epo-R regulation of TRPC3 is inhibited. This model is consistent with our observations in primary erythroid cells. Our data suggest that TRPC6 modulates TRPC3 activation by Epo through direct interaction. We confirmed that TRPC3 and TRPC6 associate endogenously (36, 41).
With two different approaches, confocal microscopy and cell surface biotinylation, we demonstrated using HEK 293T cells transfected with TRPC3 with or without TRPC6 that TRPC3 is expressed at the cell membrane. Cell surface biotinylation experiments demonstrated that Epo stimulation increased membrane insertion of TRPC3. This increase in agonist-stimulated cell surface expression of TRPC3 was observed previously with carbachol (50), epidermal growth factor (34), and arginine-vasopressin (64) but not with all TRPC3 agonists (34). We also investigated TRPC3 mobility and translocation to the cell membrane in response to Epo in transfected cells using FRAP. We did not observe a significant increase in t½, half-time to plateau fluorescence recovery, for TRPC3 after Epo stimulation. The t½ for TRPC3 we determined was in agreement with that previously published in control cells (50). However, we did observe a significant increase in TRPC3 mobility in Epo-treated cells, suggesting that Epo stimulation may increase cell surface expression of TRPC3 by increasing TRPC3 membrane recycling. TRPC6 coexpression blocked both the Epo-stimulated increase in TRPC3 cell surface expression measured with biotinylation and the increase in TRPC3 mobility detected with FRAP. These data suggest that a mechanism through which TRPC6 could modulate TRPC3 activity is through inhibition of the Epo-stimulated increase in cell surface expression of TRPC3. However, in the primary erythroid cells we studied, Epo stimulated a significant increase in [Ca2+]i but did not affect endogenous TRPC3 cell surface localization, suggesting that this mechanism is not physiologically important at the stage of erythroid differentiation we studied. This is consistent with previous reports that channel trafficking and activation are not necessarily associated (34) and that directed translocation of TRPC3 is not critical in channel activation.
The function of several domains in the C terminus of TRPC channels has been explored previously. A short hydrophobic sequence following the sixth transmembrane domain called the TRP domain was found to be unimportant in channel assembly and localization, but it is required for the response to agonists for channels, including TRPM8 (65) and TRPV1 (66). A common calmodulin binding/inositol 1,4,5-triphosphate receptor-binding site, called the CRIB domain, is further downstream in the C terminus of all TRPC proteins (38). For TRPC3, calmodulin binding to this site inhibits channel activation, and IP3R binding activates it (38, 67). For TRPC6, agonist-stimulated calcium entry has been reported to be dependent on calmodulin binding in some studies (68), whereas in other studies calmodulin binding was inhibitory (69). Another region called the “coiled-coil ” domain 3′ to TRP and CRIB domains is involved in channel activation and may have a role in subunit association (65, 70). To identify the domains in the TRPC3 C terminus that are required for Epo activation, we generated two chimeras, both of which retained amino acids 1-670 of TRPC3. TRPC3-C6C1 expressed the TRPC6 TRP domain and the TRPC3 CRIB and coiled-coil domains (38). TRPC3-C6C2 expressed the TRPC3 TRP domain and the TRPC6 CRIB and coiled-coil domains (38). Substitution of the CRIB domain and the coiled-coil domain of TRPC3 with that of TRPC6 (TRPC3-C6C2) marginally reduced the Epo-stimulated increase in [Ca2+]i. This is not unexpected because the amino acid sequences of these TRPC6 domains are very similar to those of TRPC3. In contrast, TRPC3-C6C1 did not respond to Epo, demonstrating that substitution of 76 amino acids of TRPC3 following the last transmembrane domain is sufficient to eliminate Epo-stimulated calcium influx. Whether this resulted from deletion of a positive regulatory domain in TRPC3 or introduction of an inhibitory region present in TRPC6 is uncertain at present. This region includes the TRPC3 TRP domain, which may be responsible for the failure of response to Epo because this region is reported to be important in agonist activation. Alternatively, other motif differences between TRPC3 and TRPC6 in this region may be responsible for the different responsiveness to Epo. One of these is the tyrosine at TRPC3 674, which is phenylalanine in the cognate amino acid 731 in TRPC6. Our studies with TRPC3 Y674F and TRPC6 F674Y suggest this site is not of key importance. Differences in the sequences of TRPC3 and TRPC6 in this region include serine phosphorylation sites, glycosylation sites, and nonoverlapping sequences. Identification of the critical amino acids involved in the different responses and understanding their function in TRPC3 activation by Epo will require additional mutagenesis and functional studies. Recent three-dimensional reconstruction of TRPC3 (71) and TRPM2 (72) demonstrated the complexities involved in channel gating and elucidated how the intricate structure of the C terminus provides multiple docking ports to integrate a number of signals that influence channel opening.
In summary, we demonstrate here that the activity of TRPC3 but not TRPC6 is regulated by Epo. This difference may be functionally important in erythropoiesis in view of modulation in expression during erythroid differentiation. Exogenously expressed TRPC6 inhibited Epo-stimulated TRPC3 activity. Reduced interaction of TRPC6 with Epo-R and PLCγ may partially explain the lack of response of TRPC6 to Epo. The ability of TRPC6 to inhibit Epo-stimulated cell surface expression of TRPC3 does not appear to be physiologically important, because Epo did not increase endogenous TRPC3 surface location while stimulating an increase in [Ca2+]i in primary erythroid cells. A small sequence in the TRPC3 C terminus is a key domain in differential responsiveness of these two channels and may have relevance for the selectiveness of TRPC channel activation by other agonists.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 DK46778 (to B. A. M.) and R01 HL58672 and R01 HL74854 (to J. Y. C.). This work was also supported by the Four Diamonds Fund of the Pennsylvania State University College of Medicine and by the Canadian Institute for Health Research (to D. L. B.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: Epo, erythropoietin; Epo-R, erythropoietin receptor; PLCγ, phospholipase Cγ; [Ca2+]i, intracellular calcium; TRP, transient receptor potential; TRPC, transient receptor potential channel; BFU-E, burst-forming unit-erythroid; HRP, horseradish peroxidase; IP3R, inositol 1,4,5-trisphosphate receptor; FRAP, fluorescence recovery after photobleaching; PBS, phosphate-buffered saline; h, human; CMV, cytomegalovirus; GFP, green fluorescent protein; ECL, enhanced chemiluminescence; BFP, blue fluorescent protein.
References
- 1.Miller, B. A., Scaduto, R. C., Jr., Tillotson, D. L., Botti, J. J., and Cheung, J. Y. (1988) J. Clin. Investig. 82 309-315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller, B. A., Cheung, J. Y., Tillotson, D. L., Hope, S. M., and Scaduto, R. C., Jr. (1989) Blood 73 1188-1194 [PubMed] [Google Scholar]
- 3.Chu, X., Tong, Q., Cheung, J. Y., Wozney, J., Conrad, K., Mazack, V., Zhang, W., Stahl, R., Barber, D. L., and Miller, B. A. (2004) J. Biol. Chem. 279 10514-10522 [DOI] [PubMed] [Google Scholar]
- 4.Richmond, T. D., Chohan, M., and Barber, D. L. (2005) Trends Cell Biol. 15 146-155 [DOI] [PubMed] [Google Scholar]
- 5.Misiti, J., and Spivak, J. L. (1979) J. Clin. Investig. 64 1573-1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillo, B., Ma, Y. S., and Marks, A. R. (1993) Blood 81 783-792 [PubMed] [Google Scholar]
- 7.Hensold, J. O., Dubyak, G., and Housman, D. E. (1991) Blood 77 1362-1370 [PubMed] [Google Scholar]
- 8.Levenson, R., Housman, D., and Cantley, L. (1980) Proc. Natl. Acad. Sci. U. S. A. 77 5948-5952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yelamarty, R. V., Miller, B. A., Scaduto, R. C., Jr., Yu, F. T., Tillotson, D. L., and Cheung, J. Y. (1990) J. Clin. Investig. 85 1799-1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung, J. Y., Elensky, M. B., Brauneis, U., Scaduto, R. C., Jr., Bell, L. L., Tillotson, D. L., and Miller, B. A. (1992) J. Clin. Investig. 90 1850-1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller, B. A., Barber, D. L., Bell, L. L., Beattie, B. K., Zhang, M. Y., Neel, B. G., Yoakim, M., Rothblum, L. I., and Cheung, J. Y. (1999) J. Biol. Chem. 274 20465-20472 [DOI] [PubMed] [Google Scholar]
- 12.Cheung, J. Y., Zhang, X. Q., Bokvist, K., Tillotson, D. L., and Miller, B. A. (1997) Blood 89 92-100 [PubMed] [Google Scholar]
- 13.Miller, B. A., Bell, L., Hansen, C. A., Robishaw, J. D., Linder, M. E., and Cheung, J. Y. (1996) J. Clin. Investig. 98 1728-1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fraser, J. K., Tan, A. S., Lin, F. K., and Berridge, M. V. (1989) Exp. Hematol. 17 10-16 [PubMed] [Google Scholar]
- 15.Manolis, A. S., Tzeis, S., Triantafyllou, K., Michaelidis, J., Pyrros, I., Sakellaris, N., Kranidis, A., and Melita, H. (2005) Curr. Drug Targets Cardiovasc. Haematol. Disord. 5 355-375 [DOI] [PubMed] [Google Scholar]
- 16.Anagnostou, A., Lee, E. S., Kessimian, N., Levinson, R., and Steiner, M. (1990) Proc. Natl. Acad. Sci. U. S. A. 87 5978-5982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawyer, S. T., Krantz, S. B., and Sawada, K. (1989) Blood 74 103-109 [PubMed] [Google Scholar]
- 18.Bogoyevitch, M. A. (2004) Cardiovasc. Res. 63 208-216 [DOI] [PubMed] [Google Scholar]
- 19.Rossert, J., and Eckardt, K. U. (2005) Nephrol. Dial. Transplant. 20 1025-1028 [DOI] [PubMed] [Google Scholar]
- 20.Hasselblatt, M., Ehrenreich, H., and Siren, A. L. (2006) J. Neurosurg. Anesthesiol. 18 132-138 [DOI] [PubMed] [Google Scholar]
- 21.Ogilvie, M., Yu, X., Nicolas-Metral, V., Pulido, S. M., Liu, C., Ruegg, U. T., and Noguchi, C. T. (2000) J. Biol. Chem. 275 39754-39761 [DOI] [PubMed] [Google Scholar]
- 22.Masuda, S., Nagao, M., Takahata, K., Konishi, Y., Gallyas, F., Jr., Tabira, T., and Sasaki, R. (1993) J. Biol. Chem. 268 11208-11216 [PubMed] [Google Scholar]
- 23.Siren, A. L., Fratelli, M., Brines, M., Goemans, C., Casagrande, S., Lewczuk, P., Keenan, S., Gleiter, C., Pasquali, C., Capobianco, A., Mennini, T., Heumann, R., Cerami, A., Ehrenreich, H., and Ghezzi, P. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 4044-4049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh, A., and Greenberg, M. E. (1995) Science 268 239-247 [DOI] [PubMed] [Google Scholar]
- 25.May, K. A., and Khuri, F. R. (2008) Cancer Res. 68 4013-4017 [DOI] [PubMed] [Google Scholar]
- 26.Tong, Q., Hirschler-Laszkiewicz, I., Zhang, W., Conrad, K., Neagley, D. W., Barber, D. L., Cheung, J. Y., and Miller, B. A. (2008) J. Biol. Chem. 283 10385-10395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Venkatachalam, K., and Montell, C. (2007) Annu. Rev. Biochem. 76 387-417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nilius, B., Owsianik, G., Voets, T., and Peters, J. A. (2007) Physiol. Rev. 87 165-217 [DOI] [PubMed] [Google Scholar]
- 29.Reaves, B. J., and Wolstenholme, A. J. (2007) Biochem. Soc. Trans. 35 77-80 [DOI] [PubMed] [Google Scholar]
- 30.Vazquez, E., and Valverde, M. A. (2006) Semin. Cell Dev. Biol. 17 607-617 [DOI] [PubMed] [Google Scholar]
- 31.Uemura, T., Kudoh, J., Noda, S., Kanba, S., and Shimizu, N. (2005) Biochem. Biophys. Res. Commun. 328 1232-1243 [DOI] [PubMed] [Google Scholar]
- 32.Cayouette, S., and Boulay, G. (2007) Cell Calcium 42 225-232 [DOI] [PubMed] [Google Scholar]
- 33.Bezzerides, V. J., Ramsey, I. S., Kotecha, S., Greka, A., and Clapham, D. E. (2004) Nat. Cell Biol. 6 709-720 [DOI] [PubMed] [Google Scholar]
- 34.Smyth, J. T., Lemonnier, L., Vazquez, G., Bird, G. S., and Putney, J. W., Jr. (2006) J. Biol. Chem. 281 11712-11720 [DOI] [PubMed] [Google Scholar]
- 35.Strubing, C., Krapivinsky, G., Krapivinsky, L., and Clapham, D. E. (2001) Neuron 29 645-655 [DOI] [PubMed] [Google Scholar]
- 36.Hofmann, T., Schaefer, M., Schultz, G., and Gudermann, T. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 7461-7466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hofmann, T., Obukhov, A. G., Schaefer, M., Harteneck, C., Gudermann, T., and Schultz, G. (1999) Nature 397 259-263 [DOI] [PubMed] [Google Scholar]
- 38.Tang, J., Lin, Y., Zhang, Z., Tikunova, S., Birnbaumer, L., and Zhu, M. X. (2001) J. Biol. Chem. 276 21303-21310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vazquez, G., Wedel, B. J., Trebak, M., St John Bird, G., and Putney, J. W., Jr. (2003) J. Biol. Chem. 278 21649-21654 [DOI] [PubMed] [Google Scholar]
- 40.Eder, P., Poteser, M., and Groschner, K. (2007) Handb. Exp. Pharmacol. 179 77-92 [DOI] [PubMed] [Google Scholar]
- 41.Goel, M., Sinkins, W. G., and Schilling, W. P. (2002) J. Biol. Chem. 277 48303-48310 [DOI] [PubMed] [Google Scholar]
- 42.Wakao, H., Harada, N., Kitamura, T., Mui, A. L., and Miyajima, A. (1995) EMBO J. 14 2527-2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang, M. Y., Harhaj, E. W., Bell, L., Sun, S. C., and Miller, B. A. (1998) Blood 92 1225-1234 [PubMed] [Google Scholar]
- 44.Pope, S. H., Fibach, E., Sun, J., Chin, K., and Rodgers, G. P. (2000) Eur. J. Haematol. 64 292-303 [DOI] [PubMed] [Google Scholar]
- 45.Bony, V., Gane, P., Bailly, P., and Cartron, J. P. (1999) Br. J. Haematol. 107 263-274 [DOI] [PubMed] [Google Scholar]
- 46.Chu, X., Cheung, J. Y., Barber, D. L., Birnbaumer, L., Rothblum, L. I., Conrad, K., Abrasonis, V., Chan, Y. M., Stahl, R., Carey, D. J., and Miller, B. A. (2002) J. Biol. Chem. 277 34375-34382 [DOI] [PubMed] [Google Scholar]
- 47.Kurebayashi, N., Harkins, A. B., and Baylor, S. M. (1993) Biophys. J. 64 1934-1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu, Y., and Clusin, W. T. (1997) Am. J. Physiol. 273 H2161-H2169 [DOI] [PubMed] [Google Scholar]
- 49.Goel, M., Sinkins, W. G., Zuo, C. D., Estacion, M., and Schilling, W. P. (2006) Am. J. Physiol. 290 F1241-F1252 [DOI] [PubMed] [Google Scholar]
- 50.Singh, B. B., Lockwich, T. P., Bandyopadhyay, B. C., Liu, X., Bollimuntha, S., Brazer, S. C., Combs, C., Das, S., Leenders, A. G., Sheng, Z. H., Knepper, M. A., Ambudkar, S. V., and Ambudkar, I. S. (2004) Mol. Cell 15 635-646 [DOI] [PubMed] [Google Scholar]
- 51.Reits, E. A., and Neefjes, J. J. (2001) Nat. Cell Biol. 3 E145-E147 [DOI] [PubMed] [Google Scholar]
- 52.Gomez, C. Y., and Hope, T. J. (2006) J. Virol. 80 8796-8806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Axelrod, D., Koppel, D. E., Schlessinger, J., Elson, E., and Webb, W. W. (1976) Biophys. J. 16 1055-1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cayouette, S., Lussier, M. P., Mathieu, E. L., Bousquet, S. M., and Boulay, G. (2004) J. Biol. Chem. 279 7241-7246 [DOI] [PubMed] [Google Scholar]
- 55.van Rossum, D. B., Patterson, R. L., Sharma, S., Barrow, R. K., Kornberg, M., Gill, D. L., and Snyder, S. H. (2005) Nature 434 99-104 [DOI] [PubMed] [Google Scholar]
- 56.Huang, W. C., Young, J. S., and Glitsch, M. D. (2007) Cell Calcium 42 1-10 [DOI] [PubMed] [Google Scholar]
- 57.Liu, D. Y., Thilo, F., Scholze, A., Wittstock, A., Zhao, Z. G., Harteneck, C., Zidek, W., Zhu, Z. M., and Tepel, M. (2007) J. Hypertens. 25 799-808 [DOI] [PubMed] [Google Scholar]
- 58.Dietrich, A., Kalwa, H., Rost, B. R., and Gudermann, T. (2005) Pfluegers Arch. 451 72-80 [DOI] [PubMed] [Google Scholar]
- 59.Dietrich, A., Mederos y Schnitzler, M., Kalwa, H., Storch, U., and Gudermann, T. (2005) Naunyn-Schmiedeberg's Arch. Pharmacol. 371 257-265 [DOI] [PubMed] [Google Scholar]
- 60.Jia, Y., Zhou, J., Tai, Y., and Wang, Y. (2007) Nat. Neurosci. 10 559-567 [DOI] [PubMed] [Google Scholar]
- 61.Inoue, R., Okada, T., Onoue, H., Hara, Y., Shimizu, S., Naitoh, S., Ito, Y., and Mori, Y. (2001) Circ. Res. 88 325-332 [DOI] [PubMed] [Google Scholar]
- 62.Peppiatt-Wildman, C. M., Albert, A. P., Saleh, S. N., and Large, W. A. (2007) J. Physiol. (Lond.) 580 755-764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kawasaki, B. T., Liao, Y., and Birnbaumer, L. (2006) Proc. Natl. Acad. Sci. U. S. A. 103 335-340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goel, M., Sinkins, W. G., Zuo, C. D., Hopfer, U., and Schilling, W. P. (2007) Am. J. Physiol. 293 F1476-F1488 [DOI] [PubMed] [Google Scholar]
- 65.Phelps, C. B., and Gaudet, R. (2007) J. Biol. Chem. 282 36474-36480 [DOI] [PubMed] [Google Scholar]
- 66.Garcia-Sanz, N., Valente, P., Gomis, A., Fernandez-Carvajal, A., Fernandez-Ballester, G., Viana, F., Belmonte, C., and Ferrer-Montiel, A. (2007) J. Neurosci. 27 11641-11650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang, Z., Tang, J., Tikunova, S., Johnson, J. D., Chen, Z., Qin, N., Dietrich, A., Stefani, E., Birnbaumer, L., and Zhu, M. X. (2001) Proc. Natl. Acad. Sci. U. S. A. 98 3168-3173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boulay, G. (2002) Cell Calcium 32 201-207 [DOI] [PubMed] [Google Scholar]
- 69.Kwon, Y., Hofmann, T., and Montell, C. (2007) Mol. Cell 25 491-503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mei, Z. Z., Xia, R., Beech, D. J., and Jiang, L. H. (2006) J. Biol. Chem. 281 38748-38756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mio, K., Ogura, T., Kiyonaka, S., Hiroaki, Y., Tanimura, Y., Fujiyoshi, Y., Mori, Y., and Sato, C. (2007) J. Mol. Biol. 367 373-383 [DOI] [PubMed] [Google Scholar]
- 72.Maruyama, Y., Ogura, T., Mio, K., Kiyonaka, S., Kato, K., Mori, Y., and Sato, C. (2007) J. Biol. Chem. 282 36961-36970 [DOI] [PubMed] [Google Scholar]