Abstract
Recently, this laboratory identified a proton-coupled folate transporter (PCFT), with optimal activity at low pH. PCFT is critical to intestinal folate absorption and transport into the central nervous system because there are loss-of-function mutations in this gene in the autosomal recessive disorder, hereditary folate malabsorption. The current study addresses the role PCFT might play in another transport pathway, folate receptor (FR)-mediated endocytosis. FRα cDNA was transfected into novel PCFT+ and PCFT– HeLa sublines. FRα was shown to bind and trap folates in vesicles but with minimal export into the cytosol in PCFT– cells. Cotransfection of FRα and PCFT resulted in enhanced folate transport into cytosol as compared with transfection of FRα alone. Probenecid did not inhibit folate binding to FR, but inhibited PCFT-mediated transport at endosomal pH, and blocked FRα-mediated transport into the cytosol. FRα and PCFT co-localized to the endosomal compartment. These observations (i) indicate that PCFT plays a role in FRα-mediated endocytosis by serving as a route of export of folates from acidified endosomes and (ii) provide a functional role for PCFT in tissues in which it is expressed, such as the choroid plexus, where the extracellular milieu is at neutral pH.
Loss of function mutations of the proton-coupled folate transporter (PCFT),2 which functions optimally at low pH, are the molecular basis for the autosomal recessive disorder, hereditary folate malabsorption (HFM) (1–4). Infants present with this disorder several months after birth with marked folate deficiency anemia, hypogammaglobulinemia with immune deficiency and infections, neurological deficits, and often seizures (5). PCFT is highly expressed at the apical brush-border membrane of the duodenum and proximal jejunum (6–9) where the pH at the microclimate of the surface of this epithelium is low (pH 5.8–6.0), and folates are absorbed (1, 7, 10, 11). Hence, the failure to absorb folates in the absence of this transporter in HFM is expected. However, PCFT expression, and its associated folate transport activity at low pH, is observed in many tissues where the transport interface is presumed to be at pH 7.4 (12). Of particular interest is the mechanism by which PCFT mediates transport of folates into the central nervous system (CNS) where this transporter is expressed in brain and choroid plexus (1, 7, 13). Transport into the CNS is impaired in patients with HFM who have very low cerebrospinal fluid (CSF) folate levels and marked reversal of the blood:CSF folate gradient which is normally 2–3:1 (5).
Folates are also transported into cells by a receptor-mediated process. Folate receptor-α (FRα) is anchored to cell membranes via a glycosylphosphatidylinositol domain. Uptake begins with folate binding to receptor at the cell surface followed by invagination of the membrane and the formation of endosomes that traffic along microtubules to a perinuclear compartment before returning to the plasma membrane (14–16). During transit in the cytoplasm, endosomes acidify to a pH of ∼6.0–6.5 (17), folate is released from the receptor and exported from the intact endosome into the cytoplasm. This putative exporter was shown to require a trans-endosomal pH gradient (18–20).
The current report addresses the hypothesis that PCFT is an endosomal folate exporter and thereby plays a role in FRα-mediated endocytosis (1, 2, 21, 22), that the ubiquitous expression of PCFT in mammalian tissues may be related to this function, and that loss of this function may be a basis for the low CSF folate levels in HFM. The experimental approach utilized a series of HeLa sublines, developed in this laboratory, in which constitutive expression of FRα is negligible. HeLa R5 cells lack reduced folate carrier (RFC) function due to a genomic deletion of this gene (23). A derivative of R5 cells, HeLa R1-11 cells lack, in addition, PCFT expression, while an R1-11 revertant re-expresses PCFT (24). The impact of PCFT on FRα-mediated endocytosis, achieved by transfection of the receptor into these cell lines, was assessed under conditions in which there was negligible PCFT-mediated transport directly across the plasma membrane into cells.
EXPERIMENTAL PROCEDURES
Chemicals—[3′,5′,7,9-3H]folic acid, [3′,5′,7,9-3H](6S)-5-methylTHF (5-methyltetrahydrofolate), [3′,5′,7-3H(N)]MTX (methotrexate) and [3′,5′,7, 9-3H(N)](6S)-5-formylTHF (5-formyltetrahydrofolate) were purchased from Moravek Biochemicals (Brea, CA). Folic acid was purchased from Sigma Aldrich and MTX was obtained from the NCI, National Institutes of Health (Bethesda, MD). Both (6S)5-formylTHF and (6S)5-methylTHF were obtained from Schircks Laboratories (Jona, Switzerland). Tritiated compounds were purified before use by liquid chromatography.
Cells and Culture Conditions—The HeLa R5 cell line is a HeLa subline in which RFC was deleted from the genome under MTX selective pressure (23). HeLa R1 cells were derived from HeLa R5 cells under a second round of MTX selective pressure and lack, in addition, PCFT expression (24). R1-11 cells are a clonal derivative of R1 cells. R1-11R cells are revertants of R1-11 cells in which PCFT expression was regained progressively after culture in MTX-free medium over six months. While R1-11 and R1-11R cells are G418-resistant, R5 cells are G418-sensitive. All cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin.
Expression Vectors and Transfection—The coding region of C-terminal HA-tagged PCFT was cloned into pZeoSV2(+) (zeocin resistant) as described previously to obtain HA-PCFT-pZeoSV(+) and expression was driven by the SV40 promoter (25). Human FRα cDNA in pcDNA1 was a gift from Dr. Manohar Ratnam and was subcloned into pcDNA3.1 (hygromycin-resistant) with HindIII and XbaI to obtain FRα-pCDNA3.1. These expression vectors were transfected, alone or in combination, into HeLa R5, R1-11, R1-11R, or their clonal derivatives using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. The stable clones were selected in the presence of hygromycin (0.3 mg/ml) alone or in combination with zeocin (0.1 mg/ml).
Folic Acid Surface Binding—Cells were seeded in 12-well plates, transient transfection was conducted on the second day, and cells were allowed to grow for two more days to reach near confluence. Cells were then washed twice with ice-cold acid buffer (10 mm sodium acetate, 150 mm NaCl, pH 3.5 with acetic acid) followed by a wash with ice-cold HBS (20 mm HEPES, 5 mm dextrose, 140 mm NaCl, 5 mm KCl, 2 mm MgCl2, pH 7.4). To assess FRα protein at the cell surface, cells were incubated for 15 min with 5 nm [3H]folic acid in ice-cold HBS in the presence or absence of 5 μm nonlabeled folic acid (1000-fold in excess of [3H]folic acid) as previously reported (13). After three ice-cold HBS washes, membrane-bound [3H]folic acid was released with the addition of the acid buffer (0.5 ml) and radioactivity in solution measured. The cells were dissolved in 0.5 ml of 0.2 n NaOH, and 10 μl of cell lysate were used for protein determination with the BCA protein assay (Pierce).
Assessment of PCFT Functional Activity—PCFT activity was determined by assessment of the initial rate of [3H]MTX uptake across the plasma membrane at a pH of 5.5, which is optimal for this transporter. In some experiments initial uptake was assessed at the higher endosomal pH. Cells grown in liquid scintillation vials were washed twice with HBS and incubated in the same buffer at 37 °C for 20 min. The incubation buffer was then aspirated, and transport was initiated by the addition of 0.5 ml of pre-warmed (37 °C) MBS (20 mm MES, 140 mm NaCl, 5 mm KCl, 2 mm MgCl2, pH 5.5) containing 0.5 μm [3H]MTX at 37 °C. After 2 min, uptake was quenched by the addition of 5 ml of ice-cold HBS. Cells were washed three times with ice-cold HBS and dissolved in 0.5 ml of 0.2 m NaOH at 65 °C for 40 min. Radioactivity in 0.4 ml of lysate was measured on a liquid scintillation spectrometer and normalized to the protein level.
FRα-mediated Transport and Uptake into Cytoplasm versus Membrane Vesicles—Cells grown in 12-well plates were washed twice with acid buffer (pH 3.5) and then once with ice-cold HBS (pH 8.0). The cells were then incubated with 0.5 ml of pre-warmed HBS (pH 8.0) containing 50 nm tritiated folate substrates in the presence or absence of 1 μm nonlabeled folic acid at 37 °C for 1 h. At this pH, transport directly across the plasma membrane mediated by PCFT is minimized and transport measured is due predominantly to FRα-mediated endocytosis.
Following cessation of uptake, the incubation buffers were removed and the cells were washed three times with ice-cold acid buffer (pH 3.5) and twice with ice-cold HBS (pH 8.0). For each wash, cells were incubated with the washing buffer for 5 min or longer. Total tritium accumulated in cells was determined as described above for the transport studies. To discriminate between uptake into vesicles and cytosol, cells were treated with 0.5 ml of hypotonic buffer (0.5 mm NaH2PO4, 1 mm EDTA, pH 7.0) containing 1 μm nonlabeled folic acid for 10 min at 4 °C. The cells were then detached from the plates by a cell lifter, and the suspension centrifuged at 14,000 rpm for 30 min at 4 °C. The supernatant was aspirated from the pellet, which was then dissolved in 0.2 ml of 0.2 m NaOH. Radioactivity and the amount of protein in both the supernatant and pellet were determined.
Immunohistochemical Localization of PCFT in the Choroid Plexus—Sections of C57BL/6 mouse brain were obtained by a protocol approved by the Institute of Animal Care and Use Committee (IACUC) at the Hahneman Medical College, Philadelphia, PA. Brain was prepared by standard procedures and 5-μm thickness cryosections were obtained from cerebrum to medulla oblongata. Sections were dried at room temperature, fixed in cold acetone for 10 min, then washed in phosphate-buffered saline (PBS, pH 7.4) for 15 min. Endogenous peroxidase activity was quenched by immersion of slides into 3% H2O2 for 15 min. After rinsing with PBS three times, slides were incubated with 5% goat serum at room temperature for 30 min before loading a polyclonal anti-mouse PCFT antibody (7) with incubation overnight at 4 °C. Control slides were treated in the same way without anti-serum. Each slide was then washed three times with PBS buffer, incubated with peroxidase-labeled goat anti-rabbit and anti-mouse IgG, then washed three times. The reaction was developed with 3,3′-diaminobenzidine tetrahydrochloride. Slides were counterstained with Mayer's hematoxylin and mounted in cytoseal™ 280. The anti-MmPCFT antibody, directed to a peptide ([C]EKVNPHPEFQQFPQSP) corresponding to amino acids 446–459 of the protein, were affinity-purified with the SulfolinkTrial Kit (Pierce) as previously reported (7).
Subcellular Localization of PCFT and FRα in R1-11 Transfectants—Stable clones of R1-11-FR2-PCFT and R1-11-FR2-Mock were generated by transfection of HA-PCFT-pZeoSV2(+) or pZeoSV2(+) into R1-11-FR2 cells. Cells grown on slides were washed with sterile PBS and incubated with folate-AlexaFluor 488 (26) for 60 min at 37 °C. After washing to remove unbound folate-AlexaFluor 488, cells were incubated in growth medium for 1, 2, or 4 h and fixed with 4% paraformaldehyde in PBS for 60 min at room temperature. Cells were then washed three times in PBS containing 0.1 m glycine and 0.05% sodium azide to quench unreacted aldehydes, and permeabilized with 0.1% Triton X-100 in PBS, 0.1 m glycine, 0.05% sodium azide for 5 min at room temperature. The fixed and permeabilized cells were washed three times in PBS containing 0.05 m glycine, 0.2% fish gelatin, and 0.05% sodium azide (blocking solution) and incubated in the same solution for a minimum of 60 min before exposure to anti-HA polyclonal antibody (Sigma) for 40 min. After washing with the blocking solution, cells were probed with anti-rabbit rhodamine red-x to label PCFT (Jackson Immunoresearch, West Grove, PA). Before observation, cells were coated with M800 Aqua Mount (Fisher Scientific Co, Pittsburgh, PA) and covered with a microscope coverslip before sealing with nail polish.
Confocal images were acquired using a Radiance 2100 MP Rainbow (Bio-Rad, Hemel Hempstead, England) on a TE2000 (Nikon, Tokyo, Japan) inverted microscope using a 60× oil 1.4 NA lens. The rhodamine red X was excited at 543 nm using the green HeNe laser and fluorescence emission greater than 570 nm was collected. The AlexaFluor 488 signal was excited using the 488-nm line of the 4-line argon laser and emission between 500 and 540 nm was collected.
RESULTS
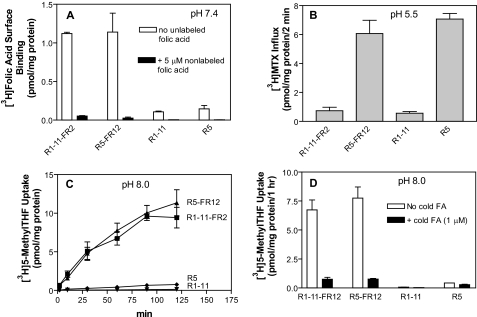
Effects of Stable Transfection of FRα into PCFT-competent R5 and PCFT-deficient R1-11 Cells on Surface Binding and Folate Transport—FRα was stably expressed in PCFT-deficient R1-11 cells and PCFT-competent R5 cells, both of which lack genomic RFC. One clone from each transfected cell line (R1-11-FR2 and R5-FR12) was selected for further study because they had a similar level of folate receptor expression as assessed by folic acid binding to the cell surface (panel A, Fig. 1). PCFT functional expression, assessed by [3H]MTX influx at pH 5.5, was comparable in R5 and R5-FR12 cells but negligible in R1-11 and R1-11-FR2 cells (panel B, Fig. 1).
FIGURE 1.
Comparison of folate transport in HeLa R1-11 (PCFT–) and HeLa R5 (PCFT+) cells in the presence and absence of FRα expression. Panel A, FRα expression as determined by the difference in [3H]folic acid (5 nm) surface binding at pH 7.4 in the presence and absence of 5 μm (1000-fold in excess) nonlabeled folic acid. Panel B, level of PCFT-functional expression as assessed by [3H]MTX (0.5 μm) influx directly across the plasma membrane over 2 min at pH 5.5. Panel C, FRα-mediated uptake of 5-[3H]methylTHF (50 nm) at pH 8.0. Under these conditions transport directly across the plasma membrane mediated by PCFT is minimized; uptake occurs almost entirely by receptor-mediated endocytosis. Panel D, specificity of 5-methylTHF transport mediated by FRα at pH 8.0 was verified by co-addition of 1 μm nonlabeled folic acid that blocked FRα-mediated endocytosis. Data for all panels are the mean ± S.E. from three independent experiments.
FRα-mediated transport was then determined with 50 nm 5-[3H]methylTHF at pH 8.0. As indicated in panel C, Fig. 1, uptake was increased as a function of incubation time and was markedly higher in both FRα transfectants than in their recipient cells; there was no significant difference in uptake between R5-FR12 and R1-11-FR2 cells. At this pH, transport directly across the plasma membrane mediated by PCFT, as assessed in FRα-negative cells, was negligible. 5-[3H]MethylTHF transport in the transfectants was almost completely abolished by the co-addition of 1 μm nonlabeled folic acid, indicating that transport was mediated solely by FRα under these conditions (panel D of Fig. 1). While the total levels of 5-[3H]methylTHF accumulated in R5-FR12 and R1-11-FR2 cells were the same, the distribution between the vesicle and cytoplasm compartments were different as indicated in the next section.
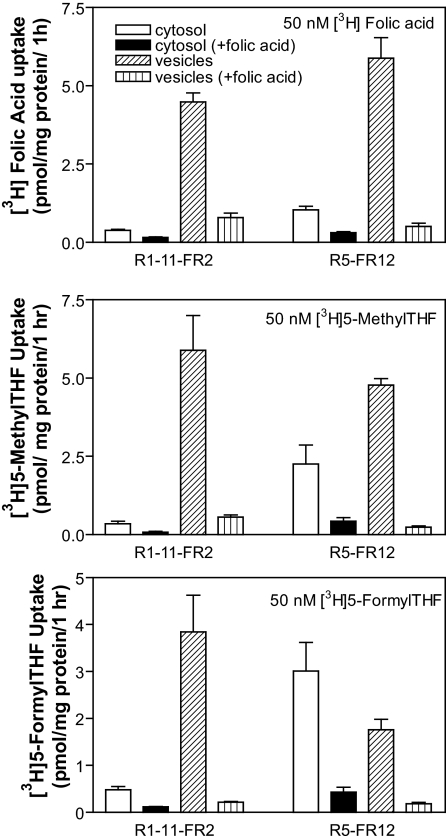
Assessment of Folate Uptake into Cytosolic and Vesicular Compartments—FRα-mediated transport involves a sequence of events in which the folate is first bound to receptor, following which the receptor-folate-plasma membrane complex invaginates to form an endosome that traffics within the cytoplasm. During the latter phase, the endosome acidifies, folate dissociates from the receptor, and is exported from the intact endosome into the cytoplasm. Studies were conducted to discriminate between folates localized to membrane vesicles and folates in the cytosol. Uptake was assessed with 50 nm [3H]folic acid at pH 8.0 to minimize direct transport across the plasma membrane mediated by PCFT. Folic acid has the highest affinity for FRα among the folates. In fact, the affinity of folic acid for the receptor is so high, that folic acid does not significantly dissociate from the receptor at the pH achieved in the acidified endosome (15). After completion of the uptake interval, cells were ruptured by hypotonic buffer and vesicles were separated from the cytosol by centrifugation. As indicated in the upper panel of Fig. 2, uptake of folic acid in the cytosol of R1-11-FR2 and R5-FR12 cells was small in comparison to the component in the vesicles, consistent with vesicular trapping of this substrate (18). Hence, these fractions were well separated under these conditions. Consistent with transport mediated primarily by FRα, uptake into vesicles was markedly suppressed by the co-addition of 1 μm nonlabeled folic acid. After subtracting the FRα-independent fraction, folic acid trapped in vesicles was 30% greater in R5-FR12 cells than in R1-11-FR2 cells (p = 0.04). There was also a small increase (p = 0.02) in cytosolic uptake of folic acid in the R5-FR12 cells compared with R1-11-FR2 cells.
FIGURE 2.
Discrimination of tritiated folates in cytosol and vesicles following transport mediated by FRα. Cells were exposed to 50 nm folic acid (upper panel), 5-methylTHF (middle panel), or 5-formylTHF (lower panel) for 1 h at pH 8.0. After lysis of cells in hypotonic buffer, the vesicle and cytosolic fractions were separated by centrifugation. The symbols for the figures, indicated in the upper panel, is the same for all panels. Co-addition of 1 μm folic acid was utilized to suppress FRα-mediated transport. Data for all panels are the mean ± S.E. from three independent experiments.
The partition of tritiated 5-methylTHF or 5-formylTHF between cytosol and vesicles was also determined after the same cell lines were exposed to 50 nm levels of these substrates for 1 h at pH 8.0. These folates have a relatively lower affinity for FRα than folic acid and dissociate from FRα at endosomal pH (15). As indicated in the middle panel of Fig. 2, cytosolic 5-methylTHF in R5-FR12 cells was 6.7-fold greater than that in R1-11-FR2 cells (p = 0.04). While vesicular 5-MTHF was less in the R5-FR12 line, this difference was not statistically significant (p = 0.24). The same pattern was also seen with 5-formylTHF (bottom panel of Fig. 2). Cytosolic 5-formylTHF in R5-FR12 cells was 7-fold greater than in R1-11-FR2 cells (p = 0.02), whereas vesicular 5-formylTHF in R5-FR12 cells was about half that in R1-11-FR2 cells; in this case, a statistically significant difference (p = 0.04). In R5-FR12 cells, the 5-formylTHF level in the cytosol exceeded that in vesicles. Hence, FRα-mediated uptake of 5-formylTHF and 5-methylTHF into the cytosol was far greater in the PCFT+ than PCFT– cells. Based upon these findings, 5-formylTHF appeared to be the best substrate for assessment of FRα-mediated transport and was chosen for subsequent studies.
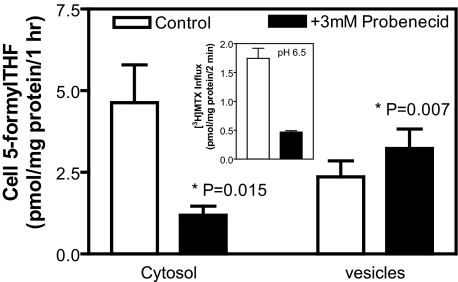
Effect of Probenecid on FRα-mediated Transport—Probenecid has been reported to inhibit FRα-mediated transport without affecting folate binding to the receptor (20), suggesting that probenecid suppresses export of folates from endosomes. The effect of probenecid (3 mm) on FRα-mediated transport was determined with 50 nm 5-[3H]formylTHF in R5-FR12 cells at pH 8.0 over 1 h. As shown in Fig. 3, probenecid inhibited uptake of 5-formylTHF into the cytosol by 75% (p = 0.015) while increasing the level in vesicles by 36% (p = 0.007), consistent with suppression of export from endosomes. Next, the effect of 3 mm probenecid on PCFT-mediated transport across the plasma membrane was determined in R5-FR12 cells at pH 6.5, which approximates the intravesicular pH of FRα-containing endosomes (17). As indicated in the inset of Fig. 3, PCFT-mediated transport, assayed with [3H]MTX was suppressed by 75%.
FIGURE 3.
Effect of 3 mm probenecid on partition of transported 5-formylTHF between cytosol and vesicles in R5-FR12 cells. Cells were exposed to 50 nm 5-[3H]formylTHF for 1 h at pH8.0 in the presence or absence of 3 mm probenecid. The data represent transport mediated specifically by FRα; the component not inhibited by 1 μm nonlabeled folic acid was subtracted, as indicated in Fig. 2. The inset illustrates the effect of 3 mm probenecid on PCFT-mediated transport across the plasma membrane. This was assessed with 0.5 μm [3H]MTX at pH 6.5 over 2 min to simulate the impact of probenecid on PCFT-mediated export from endosomes. Data are the mean ± S.E. from five independent experiments.
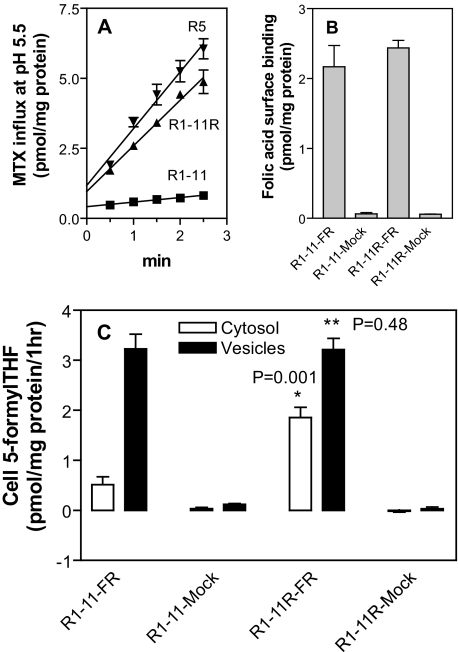
Effect of Transient Expression of FRα in R1-11 and R1-11R Cells on 5-FormylTHF Transport—To avoid the clonal variability associated with stable transfectants, transient transfection was performed to further assess the impact of PCFT on FRα-mediated transport. The transfection recipients were the PCFT-deficient R1-11 line and the R1-11R revertant; in the latter, constitutive PCFT expression had been restored. As indicated in panel A of Fig. 4, the level of PCFT activity (assessed with [3H]MTX at pH 5.5) regained in R1-11R cells was close to that of R5 cells. FRα expression, as assessed by surface binding, was similar in the R1-11 and R1-11R FRα transfectants (panel B, Fig. 4). 5-[3H]FormylTHF transport mediated by FRα is illustrated in panel C of Fig. 4. There was no significant transport in the mock transfectants, R1-11-Mock and R1-11R-Mock. 5-FormylTHF transport into cytosol was 3.5-fold greater (p = 0.001) in R1-11R-FR cells than in R1-11-FR cells; vesicular 5-formylTHF levels were comparable. Hence, the presence of PCFT augmented FRα-mediated 5-formylTHF transport into cytosol in R1-11R revertants in which near constitutive levels of PCFT function were restored.
FIGURE 4.
Transport in R1-11 and the PCFT-reverted R1-11R cells transiently transfected with FRα. Panel A, PCFT functional expression was assessed in R1-11, R1-11R, and R5 cells by measurement of [3H]MTX (0.5 μm) influx at pH 5.5. Panel B, FRα levels assayed by [3H]folic acid surface binding in cells transiently transfected with FRα cDNA or vector only (Mock). Panel C, FRα-mediated 5-[3H]formylTHF transport into vesicles and cytosol in transient transfectants. *, Student's t test between cytosol values. **, Student's t test between vesicle values. Data for all panels are the mean ± S.E. from three independent experiments.
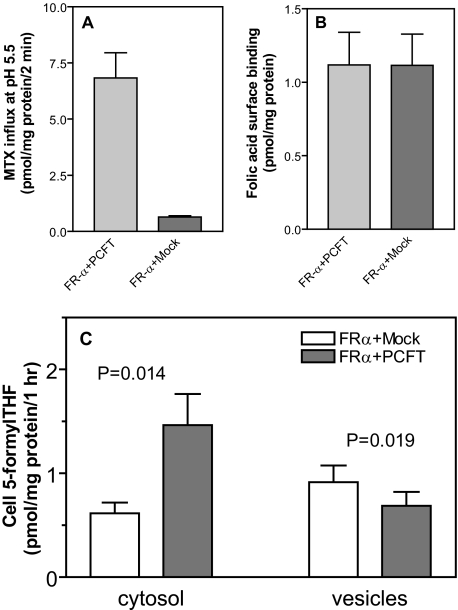
Effect of Transient Co-expression of PCFT and FRα in R1-11 Cells—Using another approach to assess the impact of PCFT on FRα-mediated transport activity, uptake of 5-[3H]formylTHF was assessed in R1-11 cells transfected with FRα and co-transfected with either PCFT or the vector alone (Mock). PCFT-mediated transport across the plasma membrane, measured at pH 6.5, was 10-fold higher in the FR-α, PCFT+ transfectant than in the FR-α mock transfectant (panel A, Fig. 5). While FRα expression was similar in the two transfectants (panel B, Fig. 5), PCFT increased FRα-mediated transport of 5-[3H]formylTHF at pH 8.0 into the cytosol by a factor of 2.4 (p = 0.014) and decreased the 5-formylTHF level in vesicles by 25% (p = 0.019) compared with the mock-transfected, PCFT– cells (panel C, Fig. 5).
FIGURE 5.
Comparison of FRα-mediated transport in R1-11 cells transiently transfected with FRα cDNA, alone, or co-transfected with PCFT cDNA. Panel A, PCFT functional expression as assessed by [3H]MTX (0.5 μm) influx at pH 5.5. Panel B, FRα expression as assessed by [3H]folic acid surface binding. Panel C, FRα-mediated 5-[3H]formylTHF transport into vesicles and cytosol. Data for all panels are the mean ± S.E. from four independent experiments.
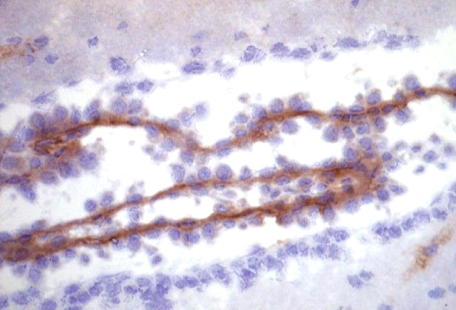
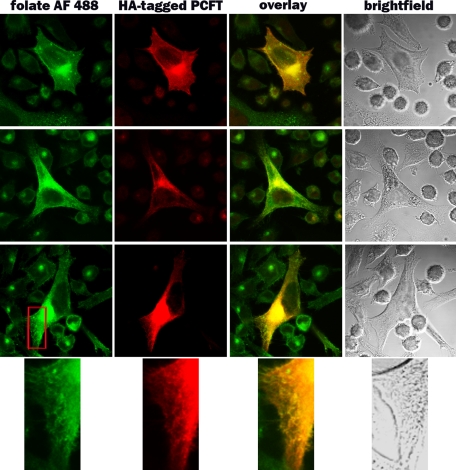
Localization of PCFT in Murine Choroid Plexus; Co-localization of FRα and PCFT within HeLa R1-11 Cells Stably Transfected with PCFT—Localization of PCFT in murine choroid plexus was examined by immunohistochemistry using a polyclonal anti-MmPCFT antibody. As indicated in Fig. 6, PCFT was localized to the basolateral membrane of choroid plexus. Localization at the subcellular level was assessed using folic acid linked to Alexa Fluor 488, which binds to FRα at the cell surface, following which the complex enters cells by endocytosis, and is retained within endosomes. Endogenous PCFT was visualized using an anti-HA antibody. These studies employed the R1-11-FR2 cells stably transfected with PCFT containing an HA insert in the C terminus. Three different cells are portrayed in Fig. 7. The folic acid-AlexaFluor488 (green) is visualized in dense clusters within the cytoplasm, particularly in the perinuclear endosomal compartment, and as punctuate staining within the plasma membrane. PCFT (green) is similarly located. Co-localization is indicated by the yellow coloration when the two images were overlaid. Of particular note is the co-localization in the plasma membrane, the perinuclear endosomal compartment, and within endosomes as seen in the higher magnification.
FIGURE 6.
Localization of endogenous PCFT in murine choroid plexus. PCFT was visualized with a polyclonal antibody to a peptide derived from the C terminus of the murine protein. Slides were counterstained with Mayer's hematoxylin. The immunohistochemical procedures are described in detail under “Experimental Procedures.”
FIGURE 7.
Subcellular co-localization of folate-AlexaFluor 488 and PCFT. R1-11-FR2 cells stably transfected with human PCFT were incubated with 100 nm folate-AlexaFluor 488 for 60 min at 37 °C. After washing to remove unbound folate-AlexaFluor 488, cells were incubated in fresh medium for 1 h before fixation and examination by confocal microscopy (see under “Experimental Procedures”). Three representative cells (rows 1–3) are shown. Column 1 indicates the localization of the internalized folate-AlexaFluor 488 (green). Column 2 indicates the localization of HA-tagged endogenous PCFT visualized with an anti-HA antibody (red). Co-localization of the two markers is seen in the overlay (yellow) in column 3. A magnification of a portion of each cell (marked by the red box in row 3) is indicated in row 4.
DISCUSSION
This study provides evidence that supports a role for PCFT in folate receptor-mediated endocytosis by serving as a route of export of folates from acidified endosomes into the cytoplasm. This provides an explanation, at the molecular level, for earlier predictions that such a mechanism is present in mammalian cells (19, 20). This is based upon both functional and co-localization data: At the functional level: (i) FRα-mediated transport into the cytosol was greater in PCFT-competent R5 than PCFT-deficient R1-11 cells, (ii) FRα-mediated transport was greater in R1-11R cells, which have regained PCFT function than in HeLa R1-11 cells, and (iii) FRα-mediated transport was greater in R1-11 cells co-transfected with FRα and PCFT cDNA than with receptor cDNA alone. Finally, probenecid markedly inhibited PCFT transport function at endosomal pH, produced a comparable suppression of FRα-mediated transport into the cytosol while increasing folate trapped in endosomes, all consistent with probenecid inhibition of PCFT-mediated endosomal folate export. These functional studies are supported by imaging of the subcellular localization of these transporters. Folic acid linked to Alexa488Fluor is transported into these cells via FRα-mediated endocytosis and remains within endosomes because it is essentially irreversibly bound to FRα at endosomal pH. Endogenously synthesized PCFT detected by immunohistochemistry was co-localized to the endosomal perinuclear compartment and within endosomes. This is consistent with a study from another laboratory which co-localized PCFT with the early endosomal marker, transferrin, within macrophages (27). PCFT is extensively glycosylated and while the extent of glycosylation of the protein localized to endosomes is not known, the deglycosylated form has been shown to be active (28).
It is unclear, at this point, whether PCFT is the sole route of endosomal export since some FRα-mediated transport into the cytoplasm was detected in PCFT– cells. This might have been due to nonspecific leakage from damaged endosomes, an explanation supported by the observation that folate conjugates of membrane-impermeable molecules also leak slowly into the cytosol (29, 30). However, the possibility cannot be excluded that there is, in addition another, or other, endosomal folate export pathways. RFC is excluded since this gene is not present in the genome of the HeLa cell lines studied.
There is at least one example in which FRα can function physiologically in the absence of PCFT. Hence, while inactivation of FRα is embryonic lethal in mice (31), there are no developmental defects associated with folate deficiency at birth in individuals with HFM even when PCFT function is absent (1–5). However, during early embryonic development it is possible that the requirement for FRα is more related to the capacity of receptor to bind and store folates than to transport folates. Signs and symptoms of HFM appear two or more months after birth when maternal-provided folate is depleted. At this time, there is macrocytic anemia and other signs associated with systemic folate deficiency. Often seizures develop reflecting very low or absent folate levels in the CSF resulting in CNS folate deficiency (5).
These data also provide one explanation for the observation that patients with HFM, who have loss-of-function mutations in PCFT, fail to transport folates into the CSF. In normal individuals there is a high (2–3-fold) CSF:blood folate gradient consistent with the active transport of folates across the choroid plexus. Studies on transport of folates into the choroid plexus in vivo and in vitro further substantiate a role for this organ in the transport of folates (32–34). FRα and PCFT mRNA are both expressed in choroid plexus (13, 35). PCFT, as shown in this report, localizes to the basolateral membrane at the capillary interface. FRα is detected primarily at the apical membrane, but appears to be present at the basolateral membrane of ependymal cells as well (34–38). RFC was shown earlier to be expressed at the apical membrane at the CSF interface (39). Both interfaces might be expected to be at neutral pH. While Na+/H+ antiporters are expressed at the basolateral membrane (40) and could produce an acid microclimate at this site that facilitates PCFT-mediated transport, as occurs in the small intestine; there is no evidence, as yet, that this is the case.
One possible model that encompasses both FRα and PCFT in this process envisions FRα-mediated transport across the basolateral membrane generating a gradient across ependymal cells, followed by the downhill flow of folates across the apical membrane into the CSF mediated by RFC. While RFC produces small folate gradients directed into individual cells, it mediates rapid bidirectional fluxes of folates (41). The expression of both PCFT and FRα in the choroid plexus raises the possibility that the defect in folate transport into the CSF in HFM may be related to the role PCFT plays in FRα function. Further evidence supporting a role for FRα in folate transport into the CSF comes from studies of patients with cerebral folate deficiency. This is an acquired disorder in which intestinal folate absorption is normal as are blood folate blood levels, but CSF folate levels are reduced (42). In cerebral folate deficiency there are no mutations in FRα, PCFT, or RFC (43); rather, the defect has been attributed to the presence of auto-antibodies that bind to and block FRα function (44–46).
The divalent metal transporter-1 (DMT1, SLC11A2, NRAMP2) is another example of a proton-coupled transporter that plays a role in both intestinal absorption and endosomal export (47, 48). A spontaneous mutation (G185R) in this protein in the mk mouse and Belgrade rat results in impaired intestinal iron absorption that is only partially responsive to parenteral iron because of an intrinsic defect in iron export from endosomes within reticulocytes that results in impaired iron utilization in hematopoiesis (49–51). The phenotype of the murine DMT1-null mouse obtained by targeted inactivation of this gene is consistent with the role of this carrier in these two distinct transport processes (52). Hence, an inherited loss of DMT1 in rat or mouse share, in common with an inherited loss of PCFT in man, a defect in the intestinal absorption of their substrates and neither is completely corrected by the parenteral administration of their substrates. This is due to accompanying transport defects involving specific tissues that require these proteins for normal function; in the former, hematopoietic cells, in the latter, the central nervous system.
Acknowledgments
We thank Dr. Derek Doorneweerd for synthesis of folate AlexaFluor 488 and for culturing cells and Eugenia Tsai for excellent technical assistance. Microscopy data were acquired in the Purdue Cancer Center Analytical Cytometry Shared Resource supported by Cancer Center Support Grant 2P30CA23168 from the NCI, National Institutes of Health. DNA sequencing was performed in the Genomics Shared Resource of the Albert Einstein Cancer Center supported, in part, by Cancer Center Support Grant CA-13330 from the NCI, National Institutes of Health.
This work was supported, in whole or in part, by National Institutes of Health Grant CA-82621 from the NCI. This work was also supported by a grant from the Mesothelioma Applied Research Foundation. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: PCFT, proton-coupled folate transporter; FR, folate receptor; HFM, hereditary folate malabsorption; RFC, reduced folate carrier; MES, 4-morpholineethanesulfonic acid; PBS, phosphate-buffered saline; THF, tetrahydrofolate; MTX, methotrexate.
References
- 1.Qiu, A., Jansen, M., Sakaris, A., Min, S. H., Chattopadhyay, S., Tsai, E., Sandoval, C., Zhao, R., Akabas, M. H., and Goldman, I. D. (2006) Cell 127 917–928 [DOI] [PubMed] [Google Scholar]
- 2.Zhao, R., Min, S. H., Qiu, A., Sakaris, A., Goldberg, G. L., Sandoval, C., Malatack, J. J., Rosenblatt, D. S., and Goldman, I. D. (2007) Blood 110 1147–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Min, S. H., OH, S. Y., Karp, G. I., Poncz, M., Zhao, R., and Goldman, I. D. (2008) J. Pediatr. 153 435–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lasry, I., Berman, B., Straussberg, R., Sofer, Y., Bessler, H., Sharkia, M., Glaser, F., Jansen, G., Drori, S., and Assaraf, Y. G. (2008) Blood 112 2055–2061 [DOI] [PubMed] [Google Scholar]
- 5.Geller, J., Kronn, D., Jayabose, S., and Sandoval, C. (2002) Medicine (Baltimore) 81 51–68 [DOI] [PubMed] [Google Scholar]
- 6.Shayeghi, M., Latunde-Dada, G. O., Oakhill, J. S., Laftah, A. H., Takeuchi, K., Halliday, N., Khan, Y., Warley, A., McCann, F. E., Hider, R. C., Frazer, D. M., Anderson, G. J., Vulpe, C. D., Simpson, R. J., and McKie, A. T. (2005) Cell 122 789–801 [DOI] [PubMed] [Google Scholar]
- 7.Qiu, A., Min, S. H., Jansen, M., Malhotra, U., Tsai, E., Cabelof, D. C., Matherly, L. H., Zhao, R., Akabas, M. H., and Goldman, I. D. (2007) Am. J. Physiol. Cell Physiol. 293 C1669–C1678 [DOI] [PubMed] [Google Scholar]
- 8.Inoue, K., Nakai, Y., Ueda, S., Kamigaso, S., Ohta, K. Y., Hatakeyama, M., Hayashi, Y., Otagiri, M., and Yuasa, H. (2008) Am. J. Physiol. Gastrointest. Liver Physiol. 294 G660–G668 [DOI] [PubMed] [Google Scholar]
- 9.Subramanian, V. S., Reidling, J. C., and Said, H. M. (2008) Am. J. Physiol. Cell Physiol. 295 C828–C835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McEwan, G. T., Lucas, M. L., Denvir, M., Raj, M., McColl, K. E., Russell, R. I., and Mathan, V. I. (1990) J. Med. Eng. Technol. 14 16–20 [DOI] [PubMed] [Google Scholar]
- 11.Mason, J. B., and Rosenberg, I. H. (1994) in Physiology of the Gastrointestinal Tract (Johnson, L. R., ed) Raven Press, New York
- 12.Zhao, R., and Goldman, I. D. (2007) Cancer Metastasis Rev. 26 129–139 [DOI] [PubMed] [Google Scholar]
- 13.Wollack, J. B., Makori, B., Ahlawat, S., Koneru, R., Picinich, S. C., Smith, A., Goldman, I. D., Qiu, A., Cole, P. D., Glod, J., and Kamen, B. (2008) J. Neurochem. 104 1494–1503 [DOI] [PubMed] [Google Scholar]
- 14.Anderson, R. G. W., Kamen, B. A., Rothberg, K. G., and Lacey, S. W. (1992) Science 255 410–411 [DOI] [PubMed] [Google Scholar]
- 15.Kamen, B. A., and Smith, A. K. (2004) Adv. Drug Deliv. Rev. 56 1085–1097 [DOI] [PubMed] [Google Scholar]
- 16.Salazar, M. D., and Ratnam, M. (2007) Cancer Metastasis Rev. 26 141–152 [DOI] [PubMed] [Google Scholar]
- 17.Yang, J., Chen, H., Vlahov, I. R., Cheng, J. X., and Low, P. S. (2007) J. Pharmacol. Exp. Ther. 321 462–468 [DOI] [PubMed] [Google Scholar]
- 18.Kamen, B. A., Wang, M.-T., Streckfuss, A. J., Peryea, X., and Anderson, R. G. W. (1988) J. Biol. Chem. 263 13602–13609 [PubMed] [Google Scholar]
- 19.Prasad, P. D., Mahesh, V. B., Leibach, F. H., and Ganapathy, V. (1994) Biochim. Biophys. Acta Mol. Cell Res. 1222 309–314 [DOI] [PubMed] [Google Scholar]
- 20.Kamen, B. A., Smith, A. K., and Anderson, R. G. W. (1991) J. Clin. Investig. 87 1442–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Umapathy, N. S., Gnana-Prakasam, J. P., Martin, P. M., Mysona, B., Dun, Y., Smith, S. B., Ganapathy, V., and Prasad, P. D. (2007) Investig. Ophthalmol. Vis. Sci. 48 5299–5305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrews, N. C. (2007) Cell Metab. 5 5–6 [DOI] [PubMed] [Google Scholar]
- 23.Zhao, R., Gao, F., Hanscom, M., and Goldman, I. D. (2004) Clin. Cancer Res. 10 718–727 [DOI] [PubMed] [Google Scholar]
- 24.Zhao, R., Chattopadhyay, S., Hanscom, M., and Goldman, I. D. (2004) Clin. Cancer Res. 10 8735–8742 [DOI] [PubMed] [Google Scholar]
- 25.Zhao, R., Qiu, A., Tsai, E., Jansen, M., Akabas, M. H., and Goldman, I. D. (2008) Mol. Pharmacol. 74 854–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He, W., Wang, H., Hartmann, L. C., Cheng, J. X., and Low, P. S. (2007) Proc. Natl. Acad. Sci. U. S. A. 104 11760–11765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schaer, C. A., Vallelian, F., Imhof, A., Schoedon, G., and Schaer, D. J. (2008) J. Leukoc. Biol. 83 325–333 [DOI] [PubMed] [Google Scholar]
- 28.Unal, E. S., Zhao, R., Qiu, A., and Goldman, I. D. (2008) Biochim. Biophys. Acta 1178 1407–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leamon, C. P., and Low, P. S. (1992) J. Biol. Chem. 267 24966–24971 [PubMed] [Google Scholar]
- 30.Leamon, C. P., Pastan, I., and Low, P. S. (1993) J. Biol. Chem. 268 24847–24854 [PubMed] [Google Scholar]
- 31.Piedrahita, J. A., Oetama, B., Bennett, G. D., van Waes, J., Kamen, B. A., Richardson, J., Lacey, S. W., Anderson, R. G., and Finnell, R. H. (1999) Nat. Genet. 23 228–232 [DOI] [PubMed] [Google Scholar]
- 32.Spector, R., and Lorenzo, A. V. (1975) Science 187 540–542 [DOI] [PubMed] [Google Scholar]
- 33.Spector, R., and Lorenzo, A. V. (1975) Am. J. Physiol. 229 777–782 [DOI] [PubMed] [Google Scholar]
- 34.Kennedy, M. D., Jallad, K. N., Lu, J., Low, P. S., and Ben Amotz, D. (2003) Pharm. Res. 20 714–719 [DOI] [PubMed] [Google Scholar]
- 35.Weitman, S. D., Lark, R. H., Coney, L. R., Fort, D. W., Frasca, V., Zurawski, V. R., Jr., and Kamen, B. A. (1992) Cancer Res. 52 3396–3401 [PubMed] [Google Scholar]
- 36.Weitman, S. D., Weinberg, A. G., Coney, L. R., Zurawski, V. R., Jennings, D. S., and Kamen, B. A. (1992) Cancer Res. 52 6708–6711 [PubMed] [Google Scholar]
- 37.Selhub, J., and Franklin, W. A. (1984) J. Biol. Chem. 259 6601–6606 [PubMed] [Google Scholar]
- 38.Patrick, T. A., Kranz, D. M., van Dyke, T. A., and Roy, E. J. (1997) J. Neurooncol. 32 111–123 [DOI] [PubMed] [Google Scholar]
- 39.Wang, Y., Zhao, R., Russell, R. G., and Goldman, I. D. (2001) Biochim. Biophys. Acta 1513 49–54 [DOI] [PubMed] [Google Scholar]
- 40.Segal, M. B. (2000) Cell Mol. Neurobiol. 20 183–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao, R., Seither, R., Brigle, K. E., Sharina, I. G., Wang, P. J., and Goldman, I. D. (1997) J. Biol. Chem. 272 21207–21212 [DOI] [PubMed] [Google Scholar]
- 42.Ramaekers, V. T., and Blau, N. (2004) Dev. Med. Child Neurol. 46 843–851 [DOI] [PubMed] [Google Scholar]
- 43.Moretti, P., Peters, S. U., del Gaudio, D., Sahoo, T., Hyland, K., Bottiglieri, T., Hopkin, R. J., Peach, E., Min, S. H., Goldman, D., Roa, B., Bacino, C. A., and Scaglia, F. (2008) J. Autism Dev. Disord. 38 1170–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramaekers, V. T., Rothenberg, S. P., Sequeira, J. M., Opladen, T., Blau, N., Quadros, E. V., and Selhub, J. (2005) N. Engl. J. Med. 352 1985–1991 [DOI] [PubMed] [Google Scholar]
- 45.Ramaekers, V. T., Blau, N., Sequeira, J. M., Nassogne, M. C., and Quadros, E. V. (2007) Neuropediatrics 38 276–281 [DOI] [PubMed] [Google Scholar]
- 46.Schwartz, R. S. (2005) N. Engl. J. Med. 352 1948–1950 [DOI] [PubMed] [Google Scholar]
- 47.Gunshin, H., Mackenzie, B., Berger, U. V., Gunshin, Y., Romero, M. F., Boron, W. F., Nussberger, S., Gollan, J. L., and Hediger, M. A. (1997) Nature 388 482–488 [DOI] [PubMed] [Google Scholar]
- 48.Andrews, N. C., and Schmidt, P. J. (2007) Annu. Rev. Physiol. 69 69–85 [DOI] [PubMed] [Google Scholar]
- 49.Fleming, M. D., Trenor, C. C., III, Su, M. A., Foernzler, D., Beier, D. R., Dietrich, W. F., and Andrews, N. C. (1997) Nat. Genet. 16 383–386 [DOI] [PubMed] [Google Scholar]
- 50.Fleming, M. D., Romano, M. A., Su, M. A., Garrick, L. M., Garrick, M. D., and Andrews, N. C. (1998) Proc. Natl. Acad. Sci. U. S. A. 95 1148–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Garrick, M. D., Gniecko, K., Liu, Y., Cohan, D. S., and Garrick, L. M. (1993) J. Biol. Chem. 268 14867–14874 [PubMed] [Google Scholar]
- 52.Gunshin, H., Fujiwara, Y., Custodio, A. O., Direnzo, C., Robine, S., and Andrews, N. C. (2005) J. Clin. Investig. 115 1258–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]