Abstract
Cysteine string protein (Csp) is a J-domain-containing protein whose overexpression blocks the exit of cystic fibrosis transmembrane conductance regulator (CFTR) from the endoplasmic reticulum (ER). Another method of blocking ER exit, the overexpression of Sar1-GTP, however, yielded twice as much immature CFTR compared with Csp overexpression. This finding suggested that Csp not only inhibits CFTR ER exit but also facilitates the degradation of immature CFTR. This was confirmed by treatment with a proteasome inhibitor, which returned the level of immature CFTR to that found in cells expressing Sar1-GTP only. CspH43Q, which does not interact with Hsc70/Hsp70 efficiently, did not promote CFTR degradation, suggesting that the pro-degradative effect of Csp requires Hsc70/Hsp70 binding/activation. In agreement with this, Csp overexpression increased the amount of Hsc70/Hsp70 co-immunoprecipitated with CFTR, whereas overexpression of CspH43Q did not. The Hsc70/Hsp70 binding partner C terminus of Hsp70-interacting protein (CHIP) can target CFTR for proteasome-mediated degradation. Csp overexpression also increased the amount of CHIP co-immunoprecipitated with CFTR. In addition, CHIP interacted directly with Csp, which was confirmed by in vitro binding experiments. Csp overexpression also increased CFTR ubiquitylation and reduced the half-life of immature CFTR. These findings indicate that Csp not only regulates the exit of CFTR from the ER, but that this action is accompanied by Hsc70/Hsp70 and CHIP-mediated CFTR degradation.
Cysteine string protein (Csp, DnaJC5)2 is a member of the DnaJ/Hsp40 protein chaperone family (1, 2). Csp contains a short N-terminal sequence, followed by the J-domain, a linker region, and the central cysteine-rich region, which gives these proteins their name. The cysteine residues in this region are post-translationally modified by the palmitate groups that serve for the membrane attachment of Csp (3). This structure is followed by a C-terminal domain, which is the most variable region among the three Csp paralogs in the human genome.
Csp was originally discovered as an abundant protein in presynaptic junctions of Drosophila neurons (4). Csps are expressed at high levels in neurons and in cell types involved in regulated exocytosis (5–7), where they have been shown to govern exocytic secretory functions. For example, depolarization-induced synaptic vesicle exocytosis is impaired in neurons from Csp-deficient Drosophila (8), and Csp overexpression produced decreases in stimulated insulin release from β-cells and stimulated catecholamine release from chromaffin cells (9–11). Deletion of the Csp gene proved to be lethal both in Drosophila and in mice causing a progressive, fatal sensorimotor disorder characterized by developing neurodegenerative changes (12, 13).
As for other Hsp40 homologs, the J-domain is responsible for the ability of Csp to bind Hsc70/Hsp70 and stimulate its ATPase activity (14). Similar to other chaperone proteins, Csp can be found in many different protein complexes in the cell, and depending on the composition of these complexes, Csp has been linked to many different cellular processes, ranging from membrane fusion to protein folding and G-protein-mediated signaling.
Based on its direct interaction with the SNARE proteins syntaxin 1A and vesicle-associated membrane protein (synaptobrevin) Csp has been proposed to be a direct regulator of SNARE function (15, 16), a role further supported by Csp's phosphorylation-dependent, direct interaction with synaptotagmin, a Ca2+-sensing, SNARE-binding protein (17). As part of a chaperone complex with Hsp90, Hsp70, and α-guanine nucleotide dissociation inhibitor, Csp coordinates Ca2+-induced neurotransmitter release by regulating the retrieval of Rab3b from presynaptic membranes (18).
Csp forms a ternary complex with Hsc70/Hsp70 and small glutamine-rich tetratricopeptide repeat-containing protein. This complex is present on synaptic vesicles, and it can re-fold luciferase, leading to the proposal that Csp assists with the reactivation of unfolded proteins at the synapse (19). This same Csp-Hsc70-small glutamine-rich tetratricopeptide repeat-containing protein complex also interacts with heterotrimeric G-proteins. In this context Csp functions as a guanine-nucleotide exchange factor for GαS, which leads to stimulation of G-protein-dependent signaling and to G-protein-mediated inhibition of N-type Ca2+ channels (20, 21).
Our laboratory previously established a novel role for Csp at endoplasmic reticulum (ER) membranes in modulating the trafficking of the cystic fibrosis transmembrane conductance regulator (CFTR). The overexpression of Csp blocked the maturation of CFTR (22, 23), producing a dose-dependent reduction in mature (band C) CFTR and an increase in immature (band B) CFTR, which was localized to the ER. The Csp-induced block of CFTR maturation required Hsc70/Hsp70, because expression of a J-domain mutant of Csp (CspH43Q) that cannot stimulate Hsc70/Hsp70 ATPase activity allowed CFTR maturation. Conversely, the knock down of Csp promoted increased formation of mature CFTR. Together, these findings indicated that Csp negatively regulates CFTR progression to post-ER compartments.
In the present study, we provide evidence for another ER-based function of Csp: its involvement in proteasome-mediated CFTR degradation. Overexpression of Csp increased the association of CFTR with Hsp70/Hsc70 and with the E3 ubiquitin ligase, CHIP (C terminus of Hsp70-interacting protein). Using co-immunoprecipitation and in vitro binding assays, we demonstrated a direct interaction between Csp and CHIP. The overexpression of Csp also increased the ubiquitylation of CFTR, in agreement with its ability to reduce CFTR steadystate levels and its increased association with CHIP. Pairwise interactions between Csp, Hsp70/Hsc70, and CHIP suggest a model in which Csp coordinates the formation of a complex that facilitates the degradation of CFTR as it blocks CFTR exit from the ER.
EXPERIMENTAL PROCEDURES
Reagents, Plasmid Constructs, and Antibodies—DNA purification kits were obtained from Qiagen. DNA restriction endonucleases were from New England Biolabs. GC10 Competent Cells were purchased from Gene Choice. Protein G-agarose was from Genscript or Invitrogen. Reagents for SDS-PAGE were purchased from Bio-Rad and National Diagnostics (Atlanta, GA). Dithiothreitol and the Complete Protease Inhibitor Mixture was from Roche Applied Science; tissue culture reagents were from Invitrogen. Cycloheximide was from Calbiochem; Brefeldin A and other reagents were from Sigma.
The wild-type and H43Q Csp constructs were tagged with the myc epitope and inserted into the mammalian expression vector pcDNA3.1 (Invitrogen) as described (9). For expression of GST fusion proteins in Escherichia coli (BL21), the inserts were subcloned into pGEX6p-1 (GE Healthcare) using BamHI/EcoRI sites. FLAG-tagged Sar1-GTP (Sar1a H79G) and Sar1-GDP (Sar1a T39N) constructs in pcDNA3 were a kind gift from Dr. Serguei I. Bannykh (Yale University). The FLAG-tagged CHIP construct was made by PCR amplifying the full-length CHIP sequence and inserting it into pcDNA3-FLAG using KpnI/BamHI sites. All constructs were sequenced by Genewiz (South Plainfield, NJ) to verify fidelity.
Mouse monoclonal antibody against the second nucleotide binding domain of CFTR (clone M3A7) was purchased from Upstate Biotechnology, Lake Placid, NY. Mouse monoclonal antibody against the C terminus of CFTR (clone 24-1) was purified from hybridoma supernatant (HB-11947 from ATCC). Mouse monoclonal anti-CFTR antibody #217 (recognizes the C-terminal portion of the R domain of CFTR) was obtained from the Cystic Fibrosis Foundation Therapeutics (Bethesda, MD). The monoclonal antibody to human c-Myc (clone 9E10) developed by Dr. Michael Bishop was obtained from the Developmental Studies Hybridoma Bank, under the auspices of the NICHD, National Institutes of Health and maintained by the University of Iowa. Polyclonal anti-Sec23 and anti-Sec24 antibodies were raised in rabbits (24). The following antibodies were purchased from Sigma: mouse anti-Hsp70 (clone BRM-22, recognizes both Hsc70 and Hsp70), mouse anti-α-tubulin (clone DM1A), mouse anti-GST (clone GST-2), and rabbit anti-FLAG. Rabbit anti-ubiquitin was from Assay Designs (Ann Arbor, MI), rabbit anti-GFP was from Clontech, rabbit anti-calnexin was from Stressgen, and rabbit anti-CHIP was obtained from Calbiochem.
Cell Culture, Transfections, Immunoblotting, and Co-immunoprecipitations—HEK293 and HeLa cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum (HyClone), 2 mm glutamine, 10 mm HEPES, and passaged every 3–4 days. CFBE41o– cells stably transduced with WT CFTR (CFBE-WT) (25, 26) were maintained in minimal essential medium supplemented with 10% fetal calf serum (HyClone), 50 units/ml penicillin, 50 μg/ml streptomycin, 2 mm l-glutamine, and 0.5 μg of puromycin and passaged every 3–4 days. CFBE-WT cells were transfected by electroporation; the contents of a nearly confluent 100-mm dish of CFBE-WT cells were used for each electroporation. Transfection efficiency was 20–30% as judged by expression of a GFP reporter plasmid.
For transient transfections of HEK293 cells, 0.5 × 106 cells were plated in each well of a 6-well plate and transfected using 2 μl of Lipofectamine2000 per microgram of DNA (Invitrogen) the following day. The amount of plasmid DNA used per well was: 1 μg for CFTR, 0.25 μg for Csp, and 0.25–0.5 μg for Sar1-GTP or Sar1-GDP. A plasmid encoding enhanced GFP was used to normalize DNA amounts between wells. Cells were washed twice the next day with Dulbecco's modified phosphate buffer (pH 7.2) and lysed in 0.5 ml of radioimmune precipitation assay buffer (Dulbecco's modified phosphate buffer, pH 7.2, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS) containing the Complete Protease Inhibitor Mixture, 2 mm phenylmethylsulfonyl fluoride, and 10 mm N-ethylmaleimide per well by rocking in the cold room for 30 min followed by six short bursts of sonication with a tip sonicator. For co-immunoprecipitations cells were lysed on the second day after transfection in 50 mm Tris-HCl (pH 7.2), 150 mm NaCl, 0.1% Triton X-100 plus inhibitors.
Cell lysates were cleared of debris by centrifugation at 16,000 × g for 20 min at 4 °C, and total protein concentrations were determined using the BCA Protein Assay kit from Pierce. Cell lysates were either co-immunoprecipitated (see below) or analyzed directly by SDS-PAGE and immunoblotting. Proteins separated in the SDS-PAGE gel were transferred overnight to a polyvinylidene difluoride membrane (PerkinElmer Life Sciences, pore size 0.45 μm); the membrane was incubated in blocking buffer (Dulbecco's modified phosphate buffer containing 5% skim milk powder, 2% bovine serum albumin, 0.5% Tween 20, and 0.1% thimerosal). Both primary and secondary antibodies were diluted in blocking buffer. Incubations with the primary antibody varied from 1 h at room temperature to overnight in the cold room depending on the protein assayed. Incubations with horseradish peroxidase-conjugated donkey anti-mouse immunoglobulin and donkey anti-rabbit immunoglobulin secondary antibodies (Jackson Immuno-Research Laboratories, West Grove, PA) were 45 min at room temperature. Blots were developed using the SuperSignal West Dura or SuperSignal Femto chemiluminescent substrate (Pierce) and standard autoradiography film.
Co-immunoprecipitations were done by incubating 0.5–1.0 mg of total cellular protein with specific antibodies and protein-G-agarose in the cold room for 3–5 h. Then the beads were washed four times with co-immunoprecipitation buffer, and the immunoprecipitated material was eluted from the beads by adding SDS-sample buffer and shaking at 95 °C for 5 min, or in the case of CFTR, at 37 °C for 20 min. Immunoprecipitated materials were analyzed by SDS-PAGE and immunoblotting as above.
In Vitro Binding Assay—Expression of GST, GST-CspWT, and GST-CspH43Q fusion proteins in BL21(E3) cells was induced with 1 mm isopropyl 1-thio-β-d-galactopyranoside at 25 °C for 3 h. Fusion proteins were purified from bacterial cell lysates using Glutathione Sepharose-4B beads (GE Healthcare) according to the manufacturer's instructions, dialyzed against Dulbecco's modified phosphate buffer and snap-frozen in liquid nitrogen in aliquots. His6-tagged full-length CHIP protein (CHIP FL, amino acids 1–303) was purified by Karl Devlin in the laboratory of Dr. Saurav Misra (Cleveland Clinic, Cleveland, OH). 10 μg of each purified fusion protein was incubated with 10 μl of pre-equilibrated glutathione Sepharose-4B beads (GE Healthcare) in 100 μl of co-immunoprecipitation buffer at 37 °C for 1 h. Unbound proteins were removed by two washes of co-immunoprecipitation buffer, and the beads were incubated with 10 μg of purified CHIP in a 100-μl volume for an additional 1 h at 37 °C or room temperature. After three washes with co-immunoprecipitation buffer, samples were resuspended in SDS-sample buffer, and the proteins were resolved by SDS-PAGE and proteins were visualized by staining the gel with Coomassie Brilliant Blue R-250. In the experiment shown in Fig. 4D only 5 μg of each protein was used, the incubations were done at 25 °C, and CHIP was detected by immunoblotting with rabbit anti-CHIP antibody.
FIGURE 4.
Csp binds CHIP. A, HEK293 cells were co-transfected with full-length FLAG-CHIP and GFP, CspWT, or CspH43Q plasmid DNA. Cell lysates were prepared on the second day after transfection, and Csp was co-immunoprecipitated with a mouse antibody recognizing the myc tag on Csp. The immunoprecipitated material (myc IPs) and the cell lysates were analyzed by SDS-PAGE and immunoblotted with rabbit antibody that recognizes the FLAG tag on CHIP (top panels) or with mouse anti-myc (recognizes Csp, middle panels) or with mouse antibody that recognizes both Hsc70 and Hsp70 (bottom panels). B, quantitation of CHIP and Hsc70/Hsp70 co-immunoprecipitated with CspH43Q. The amount of co-immunoprecipitating proteins was normalized to the amount of immunoprecipitated Csp and expressed as -fold change relative to the CspWT overexpressing control. Experiments were performed as in A (n = 4). C, overexpressed CHIP binds endogenous Csp in bronchial epithelial cells. CFBE-WT cells electroporated with FLAG-Sar1-GTP or FLAG-CHIP plasmid DNA, or left untransfected, were lysed on the third day after electroporation and co-immunoprecipitated with rabbit anti-FLAG antibody. The immunoprecipitated material (top panel) and cell lysates (three lower panels) were then assayed by immunoblotting with rabbit anti-Csp or rabbit anti-FLAG antibody, as indicated on the right. The black dots between the lanes in the top panel are probably due to a gel defect. D, purified GST, GST-CspWT, and GST-CspH43Q proteins were pre-bound to glutathione beads as indicated on the top and incubated with full-length CHIP (lanes 2–7). The supernatants of the beads were saved, and after washing the beads, the proteins were eluted. Equal aliquots of the supernatant (S; lanes 2, 4, and 6) and the material bound to the beads (P; lanes 3, 5, and 7) were separated by SDS-PAGE, and proteins were detected by staining with Coomassie Brilliant Blue R-250. Lane 1 contains a size marker; numbers refer to molecular mass in kilodaltons. The positions of GST, GST-CspWT, GST-CspH43Q, and CHIP in the gel are marked by arrows on the right. E, purified GST, GST-CspWT, or GST-CspH43Q were pre-bound to beads as indicated on the top and incubated with CHIP as indicated on the right. After washing the beads, the proteins were eluted from them, separated by SDS-PAGE, and immunoblotted with anti-CHIP. The panel on the left shows the CHIP input.
Biosynthetic Labeling and Cycloheximide Chases—HEK293 cells were plated and transfected as described above, except the 6-well plates were pre-treated with 0.01% poly-l-lysine (Sigma) for better adherence of cells to the plastic. The following day the transfected cells were washed and preincubated in methionine- and cysteine-free Dulbecco's modified Eagle's medium for 30 min, and then metabolically labeled with the same medium containing 100 μCi/ml EasyTag EXPRE35S35S Protein Labeling Mix (PerkinElmer Life Sciences) for 25–30 min at 37 °C. When MG132 was used, it was present during both the preincubation and the labeling periods. Cells were then washed again, lysed in radioimmune precipitation assay buffer, and lysates were cleared from insoluble material by centrifugation. 1 μl of mouse anti-CFTR#217 was pre-bound to 30 μl of packed protein G-agarose beads (Invitrogen) for each immunoprecipitation, and the washed beads were incubated with 70 μg of total cellular protein for 4–6 h. The beads were then washed three times with ice-cold radioimmune precipitation assay buffer. Immunoprecipitated proteins were eluted from the beads by adding 50 μl of 2× SDS-sample buffer (containing 200 mm dithiothreitol) and shaking at 37 °C for 60 min and were analyzed using SDS-PAGE and autoradiography. Cycloheximide chases were performed on the day following transfection by changing the medium on the cells to medium containing 100 μg/ml cycloheximide (freshly diluted from a 100 mg/ml stock in DMSO) and 10 μg/ml Brefeldin A (freshly diluted from a 10 mg/ml stock in ethanol), lysing the cells at indicated time points and analyzing the cell lysates by immunoblotting.
Csp Knockdown—shRNA constructs were made with the GeneClip U1 Hairpin Cloning System (Promega, Madison, WI). shRNA1 targets nucleotide 278–296 of DnaJC5 mRNA and was constructed using the following oligonucleotides: 5′-TCTCGAAGCTTGCCTTGAAATATAAGTTCTCTATATTTCAAGGCAAGCTTCCT-3′ and 5′-CTGCAGGAAGCTTGCCTTGAAATATAGAGAACTTATATTTCAAGGCAAGCTTC-3′. The scrambled control of shRNA1 was constructed using the following oligonucleotides: 5′-TCTCGTCTAGACCAGAGTTATTAAAGTTCTCTTAATAACTCTGGTCTAGACCT-3′ and 5′-CTGCAGGTCTAGACCAGAGTTATTAAGAGAACTTTAATAACTCTGGTCTAGAC-3′. shRNA2 targets nucleotide 267–285 of DnaJC5 mRNA and was constructed using the following oligonucleotides: 5′-TCTCAAGTCCTATCGGAAGCTTGTTCAAGAGACAAGCTTCCGATAGGACTTCT-3′ and 5′-CTGCAGAAGTCCTATCGGAAGCTTGTCTCTTGAACAAGCTTCCGATAGGACTT-3′. The scrambled control of shRNA2 was constructed using the following oligonucleotides: 5′-TCTCGCACATGCGTATGCATGTATTCAAGAGATACATGCATACGCATGTGCCT-3′ and 5′-CTGCAGGCACATGCGTATGCATGTATCTCTTGAATACATGCATACGCATGTGC-3′. 2 × 105 HeLa cells were plated in wells of 6-well plates and transfected with 1 μg of shRNA plasmid DNA using 2.5 μl of Lipofectamine2000 per microgram of DNA (Invitrogen) the following day. The cells were transfected on the second day after the first transfection with 1 μg of CFTR WT plasmid DNA per well and lysed as described above.
Data Analysis—Bands in images of digitized films were quantitated using ImageJ.3
All results are expressed as means ± S.E. Statistical calculations were done with Microsoft Excel using Student's two-tailed t test; results with a p value ≤0.05 were considered statistically significant.
RESULTS
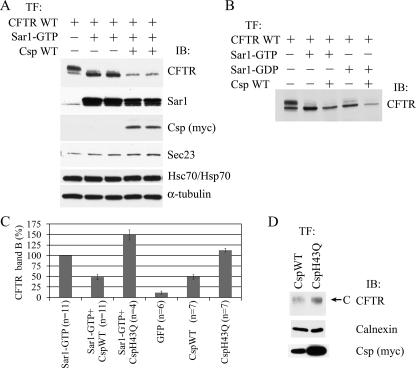
Csp Affects the Level of CFTR Expression—Our laboratory has reported earlier that overexpression of Csp produced a dose-dependent reduction in mature (band C) CFTR and an increase in immature (band B) CFTR, which accumulated in the endoplasmic reticulum (ER) (22, 23). To determine whether Csp was acting on CFTR present in vesicular carriers on their way from the endoplasmic reticulum to the Golgi apparatus or on CFTR still in the ER, we employed overexpression of Sar1-GTP (H79G), a constitutively active mutant of Sar1, as an independent means of blocking CFTR exit from the ER. Sar1 activation is essential for recruitment of the Sec23/24 complex to the ER membrane and to initiate COPII coat assembly. However, Sar1-GTP cannot hydrolyze GTP, and it therefore blocks ER export by interfering with the fission and uncoating of COPII vesicles (27). As expected, overexpression of Sar1-GTP blocked CFTR progression, as evident by the absence of mature CFTR (Fig. 1A) (28). Combined overexpression of CspWT with Sar1-GTP decreased the level of immature (band B) CFTR to 48 ± 6% of that observed with Sar1-GTP alone (Fig. 1, A and C), indicating that the effect of Csp goes beyond simply blocking the transport of CFTR to the Golgi. Taken together with our prior finding that knockdown of Csp produced marked increases in CFTR levels (22), the data of Fig. 1A led us to the hypothesis that Csp may regulate the degradation of CFTR.
FIGURE 1.
A, overexpression of Csp leads to a decrease in CFTR level. HEK293 cells were transfected with CFTR and/or Sar1-GTP and CspWT plasmid DNA as indicated at the top of the figure. The next day cell lysates were prepared and analyzed by SDS-PAGE and immunoblotting for the proteins indicated on the right of each panel. B, the effect of Csp does not depend on formation of COPII vesicles. Transfections were done as indicated at the top of the figure, and results were analyzed as in A. C, summary of Sar1-GTP and Csp overexpression experiments. HEK293 cells were co-transfected with CFTR plasmid DNA and the plasmid constructs indicated at the bottom of each bar. The next day cell lysates were prepared, and results were analyzed as in A. The amount of CFTR band B is expressed relative to that observed for co-expression of Sar1-GTP. The data indicate that the effect of Csp on CFTR expression depends on Hsc70/Hsp70. D, overexpression of CspWT decreases CFTR level in bronchial epithelial cells. CFBE-WT cells were electroporated with CspWT or CspH43Q plasmid DNA (6 μg) and assayed on the 4th day as in A. Band C CFTR is indicated by arrow.
To discern whether Csp is acting on CFTR sequestered in COPII vesicles, we employed a different mutant of Sar1, Sar1-GDP (T39N). Sar1-GDP is restricted to the GDP-bound state and functions as a competitive inhibitor of wild-type Sar1 recruitment, thereby preventing Sec23/24 attachment to the ER membrane and coat assembly. Therefore, similar to Sar1-GTP, Sar1-GDP also blocks CFTR maturation (28), but without the formation of COPII intermediates (27). When overexpressed with Sar1-GDP, Csp still reduced the level of immature CFTR below that observed with Sar1-GDP alone, indicating that the action of Csp on CFTR degradation does not require that CFTR be in COPII vesicles (Fig. 1B). This idea was further corroborated by co-immunoprecipitation experiments showing a decrease in the interaction of members of the inner layer of the COPII coat (Sec23 and Sec24) with CFTR when Csp was overexpressed (supplemental Fig. S1).
Given the above, it was not surprising that the overexpression of CspWT alone, without co-expression of Sar1-GTP, produced CFTR band B levels similar (49 ± 5%) to those observed with CspWT+Sar1-GTP co-expression (Fig. 1C). Because the overexpression of Sar1-GTP seemed to stabilize immature CFTR in the ER, it was postulated that the recruitment of CFTR to COPII vesicles can prevent ERAD in BHK cells (28). The observation that CspWT+Sar1-GTP co-expression results in higher CFTR band B levels than CspWT+Sar1-GDP co-expression (Fig. 1B) may indicate that CFTR sequestered in COPII vesicles is protected from ERAD to some extent in HEK293 cells, as well. That overexpression of Csp had the same negative effect on CFTR expression, independent of whether COPII intermediates could be formed, indicates that either Csp can overcome the postulated protective effect of Sar1-GTP or that Csp acts on CFTR before it is captured in COPII vesicles (27) (see also Fig. 6B, below).
FIGURE 6.
Csp accelerates turnover of CFTR. A, HEK293 cells were transfected with CFTR and Sar1-GTP, or CFTR and CspWT, or CFTR and CspH43Q plasmids and the next day treated with 100 μg/ml cycloheximide and 10 μg/ml brefeldin A for 0, 1, 2, or 4 h. The relative CFTR content of cell lysates was determined by immunoblotting (top panels); calnexin served as loading control (lower panels). B, quantitation of band B CFTR at each time point. Data points represent mean ± S.E. from three experiments performed as in A. C, Csp overexpression promotes CFTR ubiquitylation early in CFTR biogenesis. HEK293 cells were transfected with CFTR, CFTR and CspH43Q, or CFTR and CspWT plasmids and the next day radiolabeled for 25 min. CFTR was immunoprecipitated from cell lysates and biosynthetically labeled CFTR was detected by SDS-PAGE and autoradiography. Cells treated to inhibit the proteasome received 50 μm MG132 for 30 min before and during the radiolabeling period. The overexposed autoradiograph shows the high molecular weight CFTR smear above the CFTR band that is especially prominent in CspWT-overexpressing samples.
The Csp-induced reduction in immature CFTR depended on its ability to activate Hsc70/Hsp70 ATPase. CspH43Q bears a mutation in its HPD motif that has been shown to be important for stimulation of the ATPase domain of Hsp70 when the two proteins interact (29). In contrast to CspWT, combined overexpression of CspH43Q with Sar1-GTP increased the level of immature CFTR to 149 ± 12% of that observed in cells overexpressing Sar1-GTP only (p < 0.01). This is consistent with previously described findings (22), showing that Csp has a direct chaperone function that does not require the cooperation of Hsp70: it promotes the biogenesis of CFTR band B, and it retards the aggregation of purified CFTR NBD1.
Overexpression of CspWT also decreased the level of CFTR in CFBE41o– bronchial epithelial cells stably expressing WT CFTR (CFBE-WT cells) as compared with overexpression of CspH43Q (Fig. 1D), demonstrating that this action of Csp is not HEK293 cell-specific.
Whereas the effect of CspWT on the level of CFTR was not dependent on Sar1, the effect of CspH43Q on CFTR band B level varied depending on whether Sar1-GTP was also overexpressed or not. The average amount of band B dropped from 149 ± 12% to an average of 112 ± 4% (p < 0.05 H43Q alone versus Sar1-GTP alone) when Sar1-GTP was omitted, most probably reflecting transport of CFTR to the Golgi, which is not blocked by the CspH43Q mutant (Fig. 1C).
Because the blocking of ER exit was not necessary for the reduction of CFTR level by Csp, in the subsequent experiments (except Fig. 5C) Csp was not expressed in combination with Sar1-GTP or Sar1-GDP to make interpretation of the results easier. For consistency, however, we maintained a sample in which CFTR was co-expressed with Sar1-GTP, to serve as control.
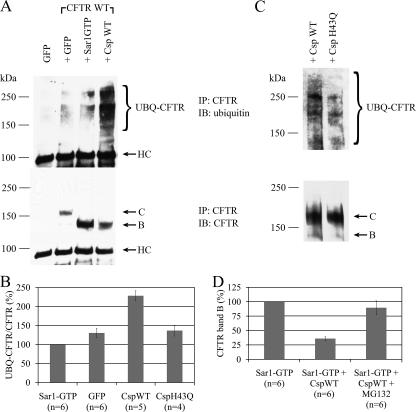
FIGURE 5.
Csp overexpression increases the level of ubiquitylated CFTR. A, HEK293 cells were co-transfected with plasmid DNA of GFP alone or with that of CFTR plus GFP, Sar1-GTP, or CspWT. Cell lysates were prepared on the second day after transfection, and CFTR was immunoprecipitated (IP) using mouse anti-CFTR antibodies. The immunoprecipitated material was analyzed by SDS-PAGE and immunoblotting (IB) with rabbit anti-ubiquitin (upper panel), and subsequently with mouse anti-CFTR (lower panel). HC indicates the heavy chain of the immunoglobulin used for immunoprecipitation. B, quantitation of experiments performed as described in A. The amount of ubiquitylated CFTR was normalized to the amount of total immunoprecipitated CFTR and plotted in comparison to the level of ubiquitylated CFTR observed for Sar1-GTP overexpression (n = 6 for GFP, n = 5 for CspWT and n = 4 for CspH43Q). C, overexpression of CspWT causes an increase in CFTR ubiquitylation in bronchial epithelial cells. CFBE WT cells were electroporated with CspWT or CspH43Q plasmid DNA (3 μg), cell lysates were prepared on the third day after transfection and assayed as in A. D, HEK293 cells were co-transfected with CFTR plasmid DNA and the plasmid constructs indicated at the bottom of each bar. The next day cell lysates were prepared and results were analyzed as in Fig. 1A. Proteasome inhibition was 5 h using 25 μm MG132. The amount of CFTR band B is expressed relative to that observed with co-expression of Sar1-GTP (n = 6).
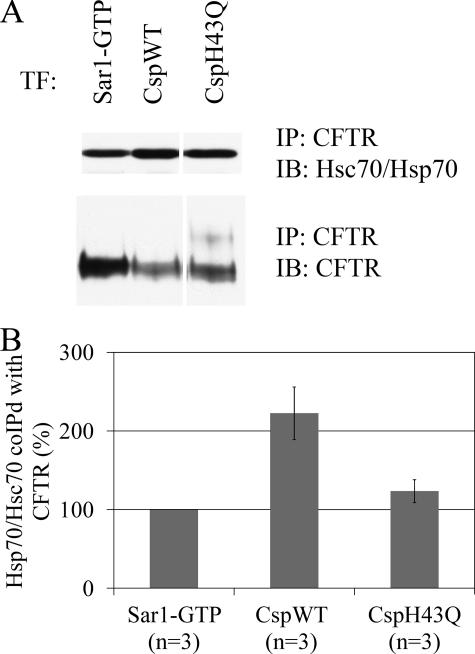
Csp Overexpression Increases the Interaction of Hsc70/Hsp70 and CHIP with CFTR—The failure of the Csp variant (CspH43Q) bearing a mutation in its HPD motif to mediate the degradation of CFTR implicated Hsc70/Hsp70 as an important component of Csp-mediated CFTR degradation. Because Hsp40s increase the binding of Hsc70/Hsp70 to substrates (30), and Csp binds both Hsc70/Hsp70 and CFTR (22), we hypothesized that overexpression of Csp is likely to increase the amount of Hsc70/Hsp70 bound to CFTR. This was of interest because Hsc70 and Hsp70 are known to play important roles in the degradation of CFTR (31, 32). Indeed, overexpression of CspWT increased the amount of Hsc70/Hsp70 that co-immunoprecipitated with CFTR as compared with the overexpression of Sar1-GTP or CspH43Q (Fig. 2, A and B). Overexpression of CspWT increased the amount of Hsc70/Hsp70 co-immunoprecipitating with CFTR by 2.2- ± 0.3-fold as compared with overexpression of Sar1-GTP (p = 0.02), whereas overexpression of CspH43Q did not have a significant effect.
FIGURE 2.
CspWT overexpression increases the association of Hsc70/Hsp70 with CFTR. A, HEK293 cells were co-transfected with CFTR and CspWT, CspH43Q, or Sar1-GTP plasmid DNA. Cell lysates were prepared on the second day after transfection, and CFTR was co-immunoprecipitated. The immunoprecipitated material was analyzed by SDS-PAGE and immunoblotting with mouse antibody that recognizes both Hsc70 and Hsp70 (upper panel) or with mouse anti-CFTR (lower panel). B, quantitation of Hsc70/Hsp70 co-immunoprecipitated with CFTR when, normalized to the amount of immunoprecipitated CFTR and expressed as -fold change relative to cells co-expressing Sar1-GTP (n = 3).
In general, the conserved HPD motif of J-domain-containing proteins is necessary for both binding Hsp70 and stimulating its ATPase activity (33). Csp variants bearing a mutation in their HPD motif are defective in stimulating the ATPase activity of and in binding to Hsc70 (14, 23, 29). Therefore, it was not surprising that we also found that the amount of Hsc70/Hsp70 co-immunoprecipitating with CspH43Q was only 31 ± 11% of that co-immunoprecipitating with CspWT (p < 0.01, supplemental Fig. S2, A and B). These findings confirm in vivo the results of the previous in vitro experiments on Csp-Hsp70 interactions. Taken together, the results indicate that overexpression of CspWT elicits the degradation of CFTR through a mechanism that involves Hsc70/Hsp70, whereas CspH43Q does not trigger the same mechanism, because it binds Hsc70/Hsp70 poorly.
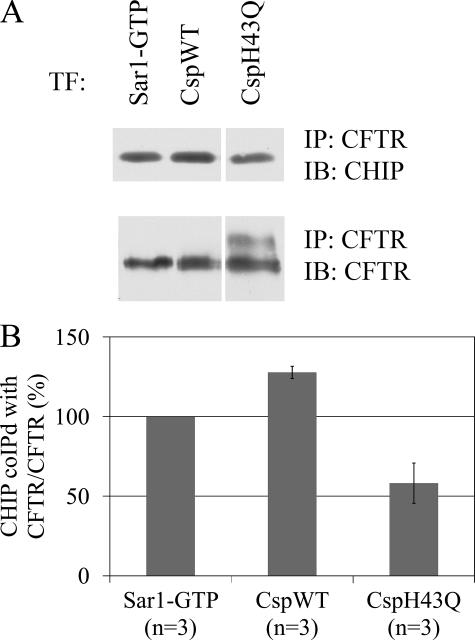
The C terminus of Hsp70-Interacting Protein (CHIP) is a ubiquitin ligase (E3) that has been shown to target immature CFTR for proteasomal degradation (34). This effect is dependent on its tetratricopeptide repeat motif (mediating its interaction with Hsp70) and U-box (necessary for polyubiquitylation of proteins). The increased association of Hsc70/Hsp70 with CFTR was also accompanied by an increase in the amount of CHIP co-immunoprecipitating with CFTR (Fig. 3A). Quantitation of the results revealed that 28 ± 4% more CHIP co-immunoprecipitated with CFTR when CspWT was overexpressed as compared with Sar1-GTP (Fig. 3B, p < 0.05). Overexpression of CspH43Q, however, failed to increase the amount of CHIP coimmunoprecipitating with CFTR.
FIGURE 3.
CspWT overexpression increases the association of CHIP with CFTR. A, HEK293 cells were transfected and immunoprecipitated as in Fig. 2. The immunoprecipitated material was analyzed by SDS-PAGE and immunoblotting with rabbit anti-CHIP (upper panel) or mouse anti-CFTR (lower panel). B, quantitation of the CHIP co-immunoprecipitated with CFTR normalized to the amount of immunoprecipitated CFTR and expressed as the -fold change relative to Sar1-GTP overexpression (n = 3).
Csp Binds CHIP Directly—A ternary complex consisting of Csp, Hsc70, and small glutamine-rich tetratricopeptide repeat-containing protein has been described earlier, in which pairwise interactions existed between all three components (19). Because both Csp and CHIP interact with Hsc70/Hsp70, we analyzed whether there was a direct interaction between Csp and CHIP. Co-immunoprecipitation experiments demonstrated that both CspWT and CspH43Q co-immunoprecipitated CHIP (Fig. 4A). We noticed, however, a lack of correlation between the amounts of CHIP and Hsc70/Hsp70 in complex with CspWT and CspH43Q. Whereas CspH43Q and CspWT co-immunoprecipitated similar amounts of CHIP, significantly less Hsc70/Hsp70 co-immunoprecipitated with CspH43Q as compared with CspWT (Fig. 4B, p < 0.05), in agreement with data presented in supplemental Fig. S2, A and B. This suggested that Hsc70/Hsp70 may not be required to serve as a bridge between Csp and CHIP; rather, there might be a direct interaction between the two proteins. The interaction of CHIP with Csp was further corroborated in bronchial epithelial cells: overexpressed FLAG-tagged CHIP protein co-immunoprecipitated the endogenous Csp protein in CFBE-WT cells (Fig. 4C).
To test for direct binding between Csp and CHIP, we performed pulldown experiments using purified recombinant CHIP, CspWT, and CspH43Q proteins. Both GST-CspWT and GST-CspH43Q, pre-bound to glutathione beads, pulled down similar amounts of purified CHIP protein, whereas the GST control did not bind detectable CHIP (Fig. 4D). The identity of the CHIP protein was also confirmed by immunoblotting in an independent experiment (Fig. 4E).
Csp Promotes CFTR Ubiquitylation—Our protein interaction analysis and the newly characterized binding between Csp and the E3 ligase CHIP (Fig. 4, A–D), suggested that Csp promoted proteasome-mediated degradation of CFTR by increasing ubiquitylation of CFTR. We therefore asked whether the overexpression of Csp affected CFTR ubiquitylation. Indeed, we found that overexpression of CspWT dramatically increased CFTR ubiquitylation (Fig. 5A). On average, more than twice as much ubiquitin was conjugated to CFTR in cells that were overexpressing CspWT, as compared with cells overexpressing Sar1-GTP (Fig. 5B). This level of CFTR ubiquitylation was also significantly more than that observed when either GFP or CspH43Q was overexpressed (Fig. 5B, p < 0.05 for all). Formally, the increased pool of ubiquitylated CFTR could be either due to an increase in the conjugation of ubiquitin to CFTR or to slower clearance of already ubiquitylated CFTR. Because CspWT reduces the level of CFTR band B relative to Sar1-GTP, it is unlikely that it slows the degradation of already ubiquitylated CFTR; therefore, we concluded that Csp increases the conjugation of ubiquitin to CFTR.
The effect of wild-type Csp on CFTR ubiquitylation is not limited to HEK293 cells. Overexpression of CspWT also mediated increased ubiquitylation of CFTR as compared with overexpression of CspH43Q in CFBE-WT bronchial epithelial cells (Fig. 5C).
If Csp indeed lowers CFTR level by promoting the degradation of CFTR by the proteasome, inhibiting the proteasome should attenuate the effect of Csp. Therefore, we treated transfected cells with MG132, an inhibitor of the proteasome, at 12.5 μm, 25 μm, or 50 μm dose for 5 h. Already at 12.5 μm, we saw an increase in CFTR level that was even greater at 25 μm dose of MG132; 50 μm MG132 did not result in a further increase. Treatment of cells overexpressing Sar1-GTP+CspWT with 25 μm MG132 returned CFTR band B levels to 90 ± 12% of that observed in Sar1-GTP-overexpressing control cells (Fig. 5D) indicating that the activity of the proteasome is required for Csp to lower the level of CFTR.
Csp Accelerates CFTR Turnover—The data presented so far are consistent with the model that Csp promotes the proteasomal degradation of CFTR by increasing its ubiquitylation by increasing the interaction of CFTR with CHIP, with Csp apparently serving as a direct link between CFTR and CHIP. If our model is correct, the differences seen in steady-state levels of CFTR (Fig. 1C) should be reflected in a shorter half-life of CFTR when CspWT is overexpressed as opposed to overexpression of Sar1-GTP or CspH43Q. Therefore, we transfected HEK293 cells with plasmid DNA encoding CFTR plus that of Sar1-GTP or CspWT or CspH43Q. The next day cells were treated with cycloheximide and brefeldin A for 0, 1, 2, or 4 h to block both the synthesis of new polypeptides and the maturation of band B CFTR to band C CFTR. This treatment was necessary, because the exit of CFTR from the ER when CspH43Q is overexpressed would make invalid any comparison of the half-lives of band B between Sar1-GTP, CspWT, and CspH43Q overexpression. In agreement with our model, the results showed that band B CFTR disappeared from the cells at a much faster rate when CspWT was overexpressed as compared with overexpression of Sar1-GTP or CspH43Q: at 2 h of chase, on average only 48% of the initial amount of band B remained in the cells when CspWT was overexpressed, whereas 89% or 97% was still present when CspH43Q, or Sar1-GTP was overexpressed, respectively (Fig. 6, A and B). It is of note that band B CFTR disappeared from the cells at a similar rate whether CspH43Q or Sar-1GTP was overexpressed. Therefore overexpression Sar1-GTP does not seem have a major effect on ERAD of CFTR in HEK293 cells, unlike in BHK cells (28).
Next, we transfected HEK293 cells with plasmid DNA constructs for the expression of either CFTR alone, or that of CFTR and CspWT, or CFTR and CspH43Q. The next day we biosynthetically labeled the cells with radioactive methionine and cysteine for 30 min, and then immunoprecipitated CFTR from the cell lysates (supplemental Fig. S3A). Quantitation of the results showed that co-expressing CspWT with CFTR reduced the amount of labeled CFTR present at the end of the pulse period on average by 21% as compared with cells expressing CFTR only (supplemental Fig. S3B, compare first and third columns; n = 3, p < 0.05). Co-expression of CspH43Q with CFTR had no effect on the amount of labeled CFTR present at the end of the pulse period, indicating that the decrease produced by CspWT was not due to translational competition or reduced transfection efficiency of the CFTR plasmid. This was further corroborated by the result that in the presence of the proteasomal inhibitor MG132 (50 μm) there was no difference in the amount of labeled CFTR present at the end of the labeling period from cells co-expressing CspWT as compared with cells expressing CFTR alone or CFTR plus CspH43Q (supplemental Fig. S3B). From these results we concluded that an extended stay of CFTR in the ER is not required for the action of Csp on CFTR.
Inhibition of the activity of the proteasome leads to accumulation of ubiquitylated proteins and treatment with MG132 is frequently used to capture fast degrading ubiquitylated intermediates of proteins that show up as a ladder, or much more frequently, as a smear above the full-length polypeptide. To see whether we can demonstrate that the Csp-mediated ubiquitylation of CFTR occurs early during CFTR biogenesis, we transfected HEK293 cells with plasmid DNA of CFTR alone, or that of CFTR and CspWT, or CFTR and CspH43Q, and biosynthetically labeled the cells for 25 min in either the presence or absence of 50 μm MG132, and then immunoprecipitated CFTR from the cell lysates. We used stringent detergent conditions for cell lysis and immunoprecipitation to minimize protein-protein interactions to ensure that the signal that we detect is derived from CFTR polypeptides and not from other proteins co-immunoprecipitating with CFTR. Examining the high molecular weight CFTR-smear from cells radiolabeled for 25 min in the absence or in the presence of 50 μm MG132, showed enhanced high molecular mass signals in MG132-treated cells that were more prominent in cells overexpressing CspWT (Fig. 6C), indicating a CspWT-induced increase in ubiquitylated CFTR early during CFTR biogenesis, before the appearance of band C CFTR. Although CspWT mediated a substantial increase in the amount of ubiquitylated CFTR within the first 25 min of translation, we saw only a 21% decrease in the amount of CFTR translated during a similar time period (supplemental Fig. S3B), probably indicating that degradation by the proteasomal machinery may be the rate-limiting.
Csp Knockdown Increases CFTR Levels—Our results strongly suggest that overexpression of CspWT leads to rapid ubiquitylation and degradation of CFTR. Next, we wanted to further analyze whether Csp plays a role in CFTR turnover when it is not overexpressed. If endogenously expressed Csp also promotes CFTR degradation, a reduction in Csp levels should result in higher CFTR levels. To modulate endogenous Csp levels, we designed two new shRNA constructs targeting Csp. The newly designed shRNA constructs target only DnaJC5 alpha from the three Csp genes present in humans and in mice. DnaJC5 alpha is, however, the paralog generally expressed in human tissues (35); the other two Csp paralogs (DnaJC5B and DnaJC5G) are testis-specific, at least in mice (12).
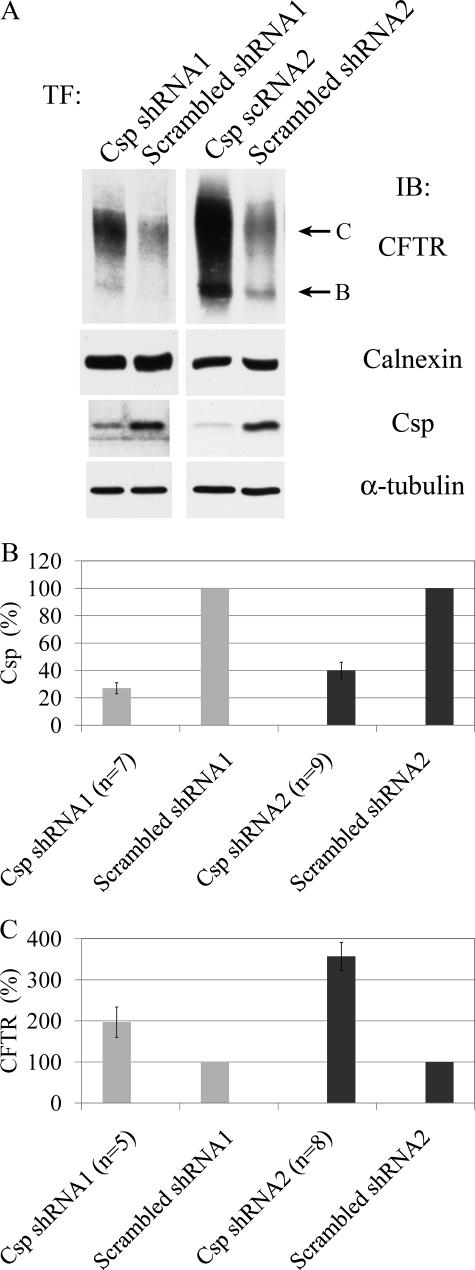
Transfection of HeLa cells with Csp shRNA1 or Csp shRNA2 caused a specific decrease in Csp level with a corresponding increase in the level of CFTR (Fig. 7A). Csp levels were reduced by 60–70% as compared with cells transfected with the respective scrambled shRNA control (Fig. 7B). In agreement with our previous results obtained in 3T3 cells using RNA interference transfection (22), the decrease in Csp expression caused a 2- to 3.5-fold increase in CFTR levels (Fig. 7C), demonstrating that Csp promotes degradation of CFTR not only when it is overexpressed, but at endogenous levels, as well.
FIGURE 7.
Csp knockdown increases the level of CFTR. A, HeLa cells were transfected with Csp shRNA constructs as well as with their respective scrambled controls and 2 days later transfected with CFTR. The next day cell lysates were prepared and assayed for their CFTR and Csp content by immunoblotting as before. Calnexin was used as loading control for CFTR blots andα-tubulin as loading control for Csp blots. B, quantitation of Csp results of experiments performed as described in A. C, quantitation of CFTR results of experiments performed as described in A.
DISCUSSION
In this study, we provide evidence for a novel function of Csp and its involvement in proteasome-mediated protein degradation in the ER. Csp promotes CFTR degradation regardless of whether CFTR exit from the ER is blocked by the action of Sar1-GDP, Sar1-GTP, or Csp itself. The pro-degradative effect of Csp requires its physical interaction with Hsc70/Hsp70; Csp also increases the interaction of CFTR with Hsc70/Hsp70 and with the E3 ubiquitin ligase, CHIP. Csp overexpression promotes a marked increase in CFTR ubiquitylation, providing an explanation for the ability of Csp to decrease immature CFTR expression levels via proteasome-mediated degradation. Pulse labeling studies showed that Csp impacts CFTR degradation early during its biosynthesis. Moreover, we demonstrated that endogenously expressed Csp also promotes CFTR degradation, because knocking down Csp expression leads to an increase in CFTR levels.
Csp plays several roles in neurons, where it modulates protein interactions that function in neurotransmitter release. These include the G-protein modulation of N-type Ca2+ channels that transduce synaptic exocytic events, as well as the chaperoning of SNARE and Rab protein interactions that contribute to synaptic vesicle fusion. This and prior studies from our laboratory indicate that Csp also plays varied roles in the ER, where it modulates the biogenesis of CFTR. Csp can act both as a chaperone of CFTR biosynthesis and as an inhibitor of CFTR progression from ER to Golgi. Both of these actions of Csp increase immature CFTR levels, clouding its degradation-promoting activity. We recognized the impact of Csp on CFTR degradation when we compared the level of immature CFTR during Csp overexpression with that obtained when exit of CFTR from the ER was blocked by interfering with the function of the COPII coat (i.e. overexpression of the GTP- or GDP-Sar1 mutants).
We previously showed that overexpression of Csp does not produce an increase in Grp78 (Bip) levels, so that the stimulation of an unfolded protein response is not responsible for Csp-induced CFTR degradation. The Csp-mediated regulation of ER exit and its pro-degradative action support the concept that Csp plays a physiological function as a negative regulator of the biogenesis of mature CFTR. This conclusion is supported by Csp knockdown studies, which produced a severalfold increase in steady-state CFTR expression levels.
Direct Chaperone Function of Csp—Earlier, we showed that Csp binds directly to the R-domain and the N terminus of CFTR (23). Consistent also with a direct chaperone action, both CspWT and CspH43Q prevented the in vitro aggregation of the first nucleotide binding domain 1 (NBD1) of CFTR with the same efficiency (23), and similar data were provided for Hdj-2, another Hsp40 homologue, and its HPD mutant (36). In this study, we show that the overexpression of CspH43Q led to an increase in the level of immature CFTR (band B), exceeding that obtained with Sar1 mutants alone. We interpret this finding as evidence of the chaperone effect of CspH43Q on CFTR biosynthesis. We previously demonstrated that the co-expression of Csp or Hdj-2 elicited increases in immature CFTR, that their effects on immature CFTR level were additive and did not depend on interactions with Hsp70: equivalent increases were observed with the HPD mutants of both Csp or Hdj-2 (23). Therefore, these Hsp40 homologues interact directly with CFTR to facilitate early steps in its biogenesis. However, similar to other chaperone interactions, they also promote protein degradation when their association with CFTR is prolonged.
Indirect Pro-degradative Function of Csp—As summarized above, the degradative action of CspWT was revealed by a comparison of the CFTR levels attained when the ER exit of CFTR was blocked by overexpression of CspWT versus the Sar1 mutants. In co-immunoprecipitation experiments, we demonstrated that Csp increases the interaction of CFTR with Hsc70/Hsp70 as well as with CHIP, and that Csp interacts physically with both CHIP and Hsc70/Hsp70. We also provided evidence that Csp can bind CHIP directly, without Hsc70/Hsp70 serving as a bridge between the two proteins. Findings supporting this conclusion include: (a) CspH43Q, a mutant of Csp that binds Hsc70/Hsp70 poorly, retained its ability to co-immunoprecipitate CHIP, and (b) purified CHIP and CspWT or CspH43Q proteins interacted in an in vitro binding assay. This novel interaction of Csp with CHIP suggests that Csp links CFTR to the ubiquitin-proteasome system in a manner similar to the pro-degradative function of Hdj-2 (37).
Requirement for Hsc70/Hsp70—The pro-degradative action of Csp required a functional J-domain: the Hsc70/Hsp70 binding and activation mutant, CspH43Q, did not promote CFTR degradation. Considering that CspH43Q retains its ability to bind CHIP (Fig. 4), it was somewhat surprising that CspH43Q did not share the ability of CspWT to promote CFTR ubiquitylation. Rather, CspH43Q produced a decrease in the amount of CHIP co-immunoprecipitating with CFTR. The requirement for Csp's HPD motif in CFTR degradation argues for an active role of Hsc70/Hsp70 in promoting the E3-mediated interaction with, and ubiquitylation of, substrate. That is, in order for Csp to promote the degradation of CFTR via its association with CHIP, Hsc70/Hsp70 must enter the complex.
This conclusion is consistent with two previous analyses of CHIP activity, both of which showed that the presence of both Hsp40 and Hsp70 was required for efficient multiubiquitylation of CFTR NBD1 or denatured luciferase by CHIP in in vitro ubiquitylation reactions (37, 38). Similarly, the binding of CHIP to CFTR through the H43Q mutant of Csp does not mediate efficient in vivo ubiquitylation of CFTR due to the inability of CspH43Q to bring Hsc70/Hsp70 into the complex. In addition, overexpression of CspH43Q apparently decreases the binding of CHIP to CFTR, quite possibly by sequestering CHIP away from the membrane, because ∼50% of overexpressed Csp is localized in the cytosol (data not shown).
Csp Action Depends on Its Binding Partners—This study adds the Csp-Hsc70/Hsp70-CHIP complex, which mediates increased ubiquitylation and proteasome-dependent degradation of CFTR, to the varied cellular processes affected by Csp. The involvement of a single protein, depending on its binding partners, in such widely different cellular processes as proteasomal degradation and vesicle fusion has been previously documented for the AAA-ATPase p97. Whereas the adaptor molecules, Ufd1 and Npl4, link this protein to ERAD pathways, p97 also mediates membrane fusion events, including post-mitotic reassembly of the Golgi, in complex with p47, and constitutive maintenance of the ER and Golgi in association with p37 (39–42). Therefore, depending on its interaction partners, p97 mediates a diverse set of functions. A similar pattern is emerging for Csp.
Csp Is a Novel Hsp40 Regulator of CHIP—Considering the potent pro-degradative effect of CHIP, it is not surprising that its activity is highly regulated. Two negative regulators of CHIP activity have already been described, HsBP1 and BAG-2 (43, 44). The overexpression of either CHIP inhibitor produces a substantial increase in the steady-state levels of CFTR. Both proteins interact with the ATPase domain of Hsc70/Hsp70 and form ternary complexes with Hsc70/Hsp70 and CHIP. Similar to Csp, HsBP1 also binds CHIP directly, whereas BAG-2 does not.
As discussed above, Hdj-2 (DnaJA1), another membrane-associated Hsp40, has been shown to be necessary for the efficient in vitro ubiquitylation of NBD1 of CFTR by CHIP (37). In this way, Hdj-2 could be interpreted as a positive regulator of CHIP function. However, the overexpression of Hdj-2 with CFTR in vivo increases both immature and mature CFTR levels rather than promoting CFTR degradation (23). Therefore, the chaperone function of Hdj-2 outweighs its pro-degradative effect. HSJ1 (DnaJB2) is another J-domain-containing protein that increases CHIP-mediated substrate ubiquitylation in vitro (45), and it decreased CFTR levels via proteasome-mediated degradation. It is not known, however, whether HSJ1 can interact directly with CHIP. In demonstrating the direct interaction between Csp and CHIP, this study provides the first evidence for protein degradation facilitated by a direct interaction of a J-domain protein with the E3 ligase, CHIP. In this manner, while Csp is blocking the progression of CFTR to the Golgi, it is also promoting the degradation of CFTR via a complex with Hsc70/Hsp70 and CHIP.
Supplementary Material
Acknowledgments
We thank Dr. Saurav Misra and Karl Devlin (Cleveland Clinic, Cleveland, OH) for purified CHIP protein. We are grateful to Mark Silvis (University of Pittsburgh, Pittsburgh, PA) for providing purified mouse anti-CFTR, Dr. Kathryn Peters for help with electroporations and to Dr. Linton Traub, Dr. Kuntala Shome, Amie Steinhauser, and Matt B. O'Brian (University of Pittsburgh, Pittsburgh, PA) for reagents and advice. B. Z. S. greatly acknowledges the contributions to his work by Béla Schmidt Sr., Dr. Harvey R. Colten, and Trufa Schmidt.
This work was supported, in whole or in part, by National Institutes of Health Grants DK68196, DK72506, and DK62318. This work was also supported by the Cystic Fibrosis Foundation. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
Footnotes
The abbreviations used are: Csp, cysteine string protein; BHK, baby hamster kidney; CFTR, cystic fibrosis transmembrane conductance regulator; CHIP, C terminus of Hsp70-interacting protein; CHO, Chinese hamster ovary; ER, endoplasmic reticulum; ERAD, ER-associated degradation; GFP, green fluorescent protein; GST, glutathione S-transferase; Hsc70, 70-kDa heat shock cognate protein; Hsp70, 70-kDa heat shock protein; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptor; NMD1, nucleotide binding domain 1; WT, wild type; E3, ubiquitin-protein isopeptide ligase; shRNA, short hairpin RNA.
W. S. Rasband, ImageJ, National Institutes of Health, Bethesda, MD, 1997–2007.
References
- 1.Qiu, X. B., Shao, Y. M., Miao, S., and Wang, L. (2006) Cell. Mol. Life Sci. 63 2560–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chamberlain, L. H., and Burgoyne, R. D. (2000) J. Neurochem. 74 1781–1789 [DOI] [PubMed] [Google Scholar]
- 3.Gundersen, C. B., Mastrogiacomo, A., Faull, K., and Umbach, J. A. (1994) J. Biol. Chem. 269 19197–19199 [PubMed] [Google Scholar]
- 4.Zinsmaier, K. E., Hofbauer, A., Heimbeck, G., Pflugfelder, G. O., Buchner, S., and Buchner, E. (1990) J. Neurogenet. 7 15–29 [DOI] [PubMed] [Google Scholar]
- 5.Mastrogiacomo, A., Parsons, S. M., Zampighi, G. A., Jenden, D. J., Umbach, J. A., and Gundersen, C. B. (1994) Science 263 981–982 [DOI] [PubMed] [Google Scholar]
- 6.Takamori, S., Holt, M., Stenius, K., Lemke, E. A., Gronborg, M., Riedel, D., Urlaub, H., Schenck, S., Brugger, B., Ringler, P., Muller, S. A., Rammner, B., Grater, F., Hub, J. S., De Groot, B. L., Mieskes, G., Moriyama, Y., Klingauf, J., Grubmuller, H., Heuser, J., Wieland, F., and Jahn, R. (2006) Cell 127 831–846 [DOI] [PubMed] [Google Scholar]
- 7.Chamberlain, L. H., Henry, J., and Burgoyne, R. D. (1996) J. Biol. Chem. 271 19514–19517 [DOI] [PubMed] [Google Scholar]
- 8.Ranjan, R., Bronk, P., and Zinsmaier, K. E. (1998) J. Neurosci. 18 956–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang, H., Kelley, W. L., Chamberlain, L. H., Burgoyne, R. D., and Lang, J. (1999) J. Cell Sci. 112 1345–1351 [DOI] [PubMed] [Google Scholar]
- 10.Brown, H., Larsson, O., Branstrom, R., Yang, S.-N., Leibiger, B., Leibiger, I., Fried, G., Moede, T., Deeney, J. T., Brown, G. R., Jacobsson, G., Rhodes, C. J., Braun, J. E. A., Scheller, R. H., Corkey, B. E., Berggren, P.-O., and Meister, B. (1998) EMBO J. 17 5048–5058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham, M. E., and Burgoyne, R. D. (2000) J. Neurosci. 20 1281–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-Chacon, R., Wolfel, M., Nishimune, H., Tabares, L., Schmitz, F., Castellano-Munoz, M., Rosenmund, C., Montesinos, M. L., Sanes, J. R., Schneggenburger, R., and Sudhof, T. C. (2004) Neuron 42 237–251 [DOI] [PubMed] [Google Scholar]
- 13.Zinsmaier, K. E., Eberle, K. K., Buchner, E., Walter, N., and Benzer, S. (1994) Science 263 977–980 [DOI] [PubMed] [Google Scholar]
- 14.Chamberlain, L. H., and Burgoyne, R. D. (1997) Biochem. J. 322 853–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nie, Z., Ranjan, R., Wenniger, J. J., Hong, S. N., Bronk, P., and Zinsmaier, K. E. (1999) J. Neurosci. 19 10270–10279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leveque, C., Pupier, S., Marqueze, B., Geslin, L., Kataoka, M., Takahashi, M., De Waard, M., and Seagar, M. (1998) J. Biol. Chem. 273 13488–13492 [DOI] [PubMed] [Google Scholar]
- 17.Evans, G. J., and Morgan, A. (2002) Biochem. J. 364 343–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakisaka, T., Meerlo, T., Matteson, J., Plutner, H., and Balch, W. E. (2002) EMBO J. 21 6125–6135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tobaben, S., Thakur, P., Fernandez-Chacon, R., Sudhof, T. C., Rettig, J., and Stahl, B. (2001) Neuron 31 987–999 [DOI] [PubMed] [Google Scholar]
- 20.Natochin, M., Campbell, T. N., Barren, B., Miller, L. C., Hameed, S., Artemyev, N. O., and Braun, J. E. A. (2005) J. Biol. Chem. 280 30236–30241 [DOI] [PubMed] [Google Scholar]
- 21.Magga, J. M., Jarvis, S. E., Arnot, M. I., Zamponi, G. W., and Braun, J. E. A. (2000) Neuron 28 195–204 [DOI] [PubMed] [Google Scholar]
- 22.Zhang, H., Peters, K. W., Sun, F., Marino, C. R., Lang, J., Burgoyne, R. D., and Frizzell, R. A. (2002) J. Biol. Chem. 277 28948–28958 [DOI] [PubMed] [Google Scholar]
- 23.Zhang, H., Schmidt, B. Z., Sun, F., Condliffe, S. B., Butterworth, M. B., Youker, R. T., Brodsky, J. L., Aridor, M., and Frizzell, R. A. (2006) J. Biol. Chem. 281 11312–11321 [DOI] [PubMed] [Google Scholar]
- 24.Aridor, M., Weissman, J., Bannykh, S., Nuoffer, C., and Balch, W. E. (1998) J. Cell Biol. 141 61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei, D. C., Kunzelmann, K., Koslowsky, T., Yezzi, M. J., Escobar, L. C., Xu, Z., Ellison, A. R., Rommens, J. M., Tsui, L. C., Tykocinski, M., and Gruenert, D. C. (1996) Gene Ther. 3 427–436 [PubMed] [Google Scholar]
- 26.Swiatecka-Urban, A., Brown, A., Moreau-Marquis, S., Renuka, J., Coutermarsh, B., Barnaby, R., Karlson, K. H., Flotte, T. R., Fukuda, M., Langford, G. M., and Stanton, B. A. (2005) J. Biol. Chem. 280 36762–36772 [DOI] [PubMed] [Google Scholar]
- 27.Bielli, A., Haney, C. J., Gabreski, G., Watkins, S. C., Bannykh, S. I., and Aridor, M. (2005) J. Cell Biol. 919–924 [DOI] [PMC free article] [PubMed]
- 28.Wang, X., Matteson, J., An, Y., Moyer, B., Yoo, J.-S., Bannykh, S., Wilson, I. A., Riordan, J. R., and Balch, W. E. (2004) J. Cell Biol. 167 65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chamberlain, L. H., and Burgoyne, R. D. (1997) J. Biol. Chem. 272 31420–31426 [DOI] [PubMed] [Google Scholar]
- 30.Bukau, B., and Horwich, A. L. (1998) Cell 92 351–366 [DOI] [PubMed] [Google Scholar]
- 31.Yang, Y., Janich, S., Cohn, J. A., and Wilson, J. M. (1993) Proc. Natl. Acad. Sci. U. S. A. 90 9480–9484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang, Y., Nijbroek, G., Sullivan, M. L., McCracken, A. A., Watkins, S. C., Michaelis, S., and Brodsky, J. L. (2001) Mol. Biol. Cell 12 1303–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsai, J., and Douglas, M. G. (1996) J. Biol. Chem. 271 9347–9354 [DOI] [PubMed] [Google Scholar]
- 34.Meacham, G. C., Patterson, C., Zhang, W., Younger, J. M., and Cyr, D. M. (2001) Nat. Cell Biol. 3 100–105 [DOI] [PubMed] [Google Scholar]
- 35.Chamberlain, L. H., and Burgoyne, R. D. (1996) J. Biol. Chem. 271 7320–7323 [DOI] [PubMed] [Google Scholar]
- 36.Meacham, G. C., Lu, Z., King, S., Sorscher, E., Tousson, A., and Cyr, D. M. (1999) EMBO J. 18 1492–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Younger, J. M., Ren, H.-Y., Chen, L., Fan, C.-Y., Fields, A., Patterson, C., and Cyr, D. M. (2004) J. Cell Biol. 167 1075–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murata, S., Minami, Y., Minami, M., Chiba, T., and Tanaka, K. (2001) EMBO Rep. 2 1133–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jentsch, S., and Rumpf, S. (2007) Trends Biochem. Sci. 32 6–11 [DOI] [PubMed] [Google Scholar]
- 40.Halawani, D., and Latterich, M. (2006) Mol. Cell 22 713–777 [DOI] [PubMed] [Google Scholar]
- 41.Uchiyama, K., Totsukawa, G., Puhka, M., Kaneko, Y., Jokitalo, E., Dreveny, I., Beuron, F., Zhang, X., Freemont, P., and Kondo, H. (2006) Dev. Cell 11 803–816 [DOI] [PubMed] [Google Scholar]
- 42.Meyer, H. H., Wang, Y., and Warren, G. (2002) EMBO J. 21 5645–5652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alberti, S., Bohse, K., Arndt, V., Schmitz, A., and Hohfeld, J. (2004) Mol. Biol. Cell 15 4003–4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arndt, V., Daniel, C., Nastainczyk, W., Alberti, S., and Hohfeld, J. (2005) Mol. Biol. Cell 16 5891–5900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Westhoff, B., Chapple, J. P., van der Spuy, J., Hohfeld, J., and Cheetham, M. E. (2005) Curr. Biol. 15 1058–1064 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.