Abstract
HCV infection is well-known to be associated with autoimmune thyroiditis. However, the mechanisms by which HCV triggers thyroiditis are unknown. We hypothesized that HCV envelope proteins could induce thyroidal inflammation directly, thereby triggering thyroiditis by bystander activation mechanism. To test this hypothesis we examined whether the HCV receptor CD81 was expressed and functional on thyroid cells. We found significant levels of CD81 mRNA by QPCR analysis, as well as CD81 protein by flow cytometric (FACS) analysis. Incubation of thyroid cells with HCV envelope glycoprotein E2 resulted in E2 binding to thyroid cells and activation of IL-8, an important pro-inflammatory cytokine. Intriguingly, thyroid cells incubated with E2 continued to proliferate normally and did not undergo apoptosis, as was reported in hepatocytes. We conclude that: (1) HCV envelope glycoprotein E2 can bind to CD81 receptors which are expressed on thyroid cells and induce a cascade of signaling pathway leading to IL-8 release; (2) HCV may trigger thyroiditis in genetically susceptible individuals by bystander activation mechanisms.
Keywords: Hepatitis C virus, thyroiditis, autoimmunity, infection thyroid
INTRODUCTION
There is solid evidence demonstrating a role for infection in the etiology of autoimmune thyroid diseases (AITD) (1;2). Among the possible infectious agents, hepatitis C virus (HCV) has shown the strongest association with thyroid autoimmunity (3–11). However, the mechanisms by which HCV may trigger thyroid autoimmunity in susceptible individuals are still unknown. Nonetheless, HCV could trigger thyroid autoimmunity by altering immune responsiveness (12), by a direct effect on thyroid cells, or both. Indeed, several mechanisms have been proposed for induction of thyroid autoimmunity by viral agents such as HCV infection including: (1) viral induction of changes in self antigen expression, or exposure of cryptic epitopes; (2) induction of local inflammation (e.g. by cytokine release), resulting in activation of autoreactive T-cells (bystander mechanism); (3) molecular mimicry between viral antigens and thyroidal antigens; (4) induction of heat shock proteins in the thyroid; and (5) induction of aberrant expression of MHC class II molecules on thyroid cells (1).
One potentially attractive mechanism is by direct effects of the HCV on thyroid cells. Indeed, HCV infection is associated with a number of extrahepatic complications (13), and negative-strand HCV RNA has been amplified in the peripheral blood, granulocytes, monocytes/macrophages, dendritic cells, and in lymphocytes (14). Moreover, Laskus et al. detected negative-strand HCV RNA in the thyroid of 2 HCV-infected patients who died of acquired immunodeficiency syndrome (AIDS)-related complications (15). However, the immunologic, virologic, and genetic factors that regulate HCV replication in extrahepatic sites, including the thyroid, have not been explored, nor have the precise cell types supporting replication been fully characterized.
While infectious hepatitis C virions are responsible for productive infection, viral proteins that are shed from virions or that are part of non-infectious virions may also have important physiological consequences. For instance, it was recently found that HCV E2 proteins induce apoptosis in hepatocytes through STAT1 induction and upregulation of Fas ligand and the pro-apoptotic molecule Bid (16–18). The pro-inflammatory cytokine interleukin 8 (IL-8) was also upregulated by HCV E2 protein (19). Collectively, these data would suggest that HCV proteins themselves could significantly impact the thyroid environment and contribute directly to thyroid inflammation, as well. We hypothesized that exposure of thyrocytes to HCV proteins could trigger activation of regulatory cytokine genes, resulting in thyroidal inflammation. Therefore, we examined the effects of the HCV envelope protein E2 on human thyroid cells in primary cultures. Our results demonstrate that 1) human thyroid cells express the HCV receptor CD81; 2) HCV E2 protein binds to CD81 on human thyroid cells; and 3) E2 binding to thyroidal CD81 triggers IL-8 production.
MATERIALS AND METHODS
Materials
Dulbecco’s Modified Eagle’s Medium (DMEM) and penicillin-streptomycin were purchased from Fisher Scientific (Pittsburgh, PA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a tetrazole (MTT), Coon’s modification of Ham’s F12 media, thyroid-stimulating hormone, insulin, apotransferrin, and hydrocortisone were purchased from Sigma (St. Louis, MO). TRIzol solution and fetal bovine serum were purchased from Invitrogen (Carlsbad, CA). Human normal adult tissue total RNA, StrataScript QPCR cDNA Synthesis Kit and Brilliant SYBR Green QPCR Reagents were purchased from Stratagene (La Jolla, CA). Fluorescein isothiocyanate (FITC)-conjugated mouse anti-CD81 monoclonal antibody (clone JS-81) and FITC-conjugated nonspecific mouse immunoglobulin G1 (IgG1) control were purchased from BD Biosciences Pharmingen (San Jose, CA). Recombinant HCV E2 envelope protein and murine anti-E2 HCV monoclonal antibody were purchased from Immuno Diagnostics, Inc. (Woburn, MA). Phycoerythrin (PE)-conjugated goat anti-mouse IgG antibody, nomal mouse IgG1 and FCM wash buffer were purchased from Santa Cruz (Santa Cruz, CA).
Cell culture
The study was approved by the Institutional Review Board. Human thyroid primary cells were prepared from fresh, non-cancerous thyroid tissue adjacent to thyroid tumors that were removed at surgery. Tissue was minced and incubated in 200 U/ml of collagenase solution for 1 hour at 37°C. Cells were harvested and cultured in DMEM supplemented with 10% FBS, penicillin (100 U/ml), and streptomycin (100 μg/ml) (P0). Cells were passaged with 1:2 dilution and cultured until confluent (P1). P1 or P2 cells were used for experiments. We confirmed that the confluent cells were thyroid cells by Western Blot analysis for thyroglobulin. Huh7.5 cells were kindly provided by Apath LLC (St. Louis, MO) and maintained in DMEM high glucose medium supplemented with 10% FBS, penicillin (100 U/ml), streptomycin (100 μg/ml), and non-essential amino acids. The well-differentiated, nontransformed rat thyroid cell line, PCCL3 was kindly provided by Dr. James Fagin (Memorial Sloan-Kettering Cancer Center, NY) and propagated in H4 complete medium, which consisted of Coon’s modification of Ham’s F12 media containing 5% FBS, glutamine (286 μg/mL), apotransferrin (5 μg/mL), hydrocortisone (10 nmol/L), insulin (10 μg/mL), thyroid-stimulating hormone (10 mIU/mL), penicillin, and streptomycin. Cells were cultured at 37°C in 5% CO2; medium was replaced every 48 hours. Cells were counted with a Z1 Coulter Counter Cell and Particle Counter (Beckman Coulter, Inc. Fullerton, CA).
Quantitative real-time PCR for human CD81 mRNA
Total cellular RNA was extracted from human thyroid tissue using TRIzol reagent. Five μg of RNA extracted from thyroid tissues (see above), and from other human tissues (purchased from Stratagene) was used to synthesize cDNA by StrataScript QPCR cDNA Synthesis kit. mRNA levels of human CD81 were measured by quantitative real-time PCR (Q-PCR) as described (20) using the following CD81-specific primers: 5′-CGCTGCTGCTGCTGCTAAT-3′ and 5′-CCCTCATAGCCGGTCACATT-3′. CD81 levels were then normalized to β-actin levels using primers 5′-CATCAGCGAAGCAGAAGGC-3′ and 5′-GGCCAGCGTTTCTTGAGTCT-3′.
Binding of anti-CD81 monoclonal antibodies to cells
PCCL3 cells, Huh7.5 cells and thyroid cells were seeded into 6 cm dishes. Cells were harvested in phosphate-buffered saline (PBS), washed with PBS and resuspended in FCM wash buffer supplemented with 0.02% sodium azide. Cells (5 × 105) were incubated for 30 min at 4°C with 10 μl of FITC-conjugated mouse anti-CD81 monoclonal antibody (MAb) or FITC-conjugated nonspecific IgG1 control and washed twice. MAb binding was quantified by flow cytometry (mean fluorescence intensity [MFI]), using a COULTER EPICS XL-MCL Flow Cytometer (Beckman Coulter, Inc. Fullerton, CA).
E2 binding
Huh7.5 cells and human primary thyroid cells were seeded into 6 cm dishes and incubated until cells reached 80% confluency. Cells were harvested, washed with PBS and resuspended at 2 × 106/ml. Then 2 × 105 cells were incubated with or without 0.5 μg of HCV E2 protein for 1 h on ice in PBS and washed twice with FCM wash buffer. E2 binding to cells was detected by flow cytometry after labeling with anti-E2 MAb (1:200) followed by PE-conjugated goat anti-mouse IgG (1:200). For control, Cells were incubated with normal IgG1 followed by PE-conjugated goat anti-mouse IgG (1:200). Binding was measured by flow cytometry (mean fluorescence intensity [MFI]).
MTT assay
Huh7.5 and human thyroid primary cells were plated into ninety six-well plates at 3 × 103 or 1 × 104 cells per well and treated with various concentrations of E2 protein for 48 hours. Cell viability was measured by MTT assay as previously described (21).
IL-8 measurement
Cells grown to 70% confluence were treated with 5 μg/ml of E2 protein for 48 h. Supernatants were collected and assayed for IL-8 using an ELISA assay kit (BioLegend, Inc.; San Diego, CA) according to the manufacturer’s instructions. The minimum detectable concentration of IL-8 was 30 pg/ml.
Statistical analysis
The comparison of cell viability, and IL-8 levels before and after incubation with E2 protein were performed using the two-tailed unpaired Student’s t-test. P< 0.05 was considered significant.
RESULTS
CD81 expression
To determine the possible effects of HCV on thyroid cells, we first analyzed the expression of the major HCV receptor, CD81, in thyroid tissue. While human liver tissues showed the highest mRNA levels among the 10 tissues examined, a significant level of CD81 mRNA was demonstrated in thyroid tissue, (Figure 1A). Human CD81 protein levels were also measured by flow cytometry. Significant expression levels of CD81 protein were seen on human primary thyroid cells (Figure 1E) as well as human Huh7.5 hepatoma cells (Figure 1D). Human CD81 protein was not detected on rat PCCL3 thyroid cells (Figure 1C).
Figure 1. CD81 expression.
Q-PCR of CD81 mRNA in human tissues (peripheral blood mononuclear cells, brain, liver, lymph node, muscle, ovary, spleen, thyroid, thymus, and trachea). 5 μg of total RNA from 10 human tissues was used to synthesize cDNA and Q-PCR was performed. As expected, high levels of CD81 expression are seen in the liver; the thyroid tissue shows CD81 expression, albeit at low levels. Error bars show standard error (SE) (B) IgG1 control. Human thyroid primary cells were incubated with nonspecific IgG1 control and flow cytometry was performed for CD81 expression. (C) Cell negative control. Rat thyroid cells (PCCL3) were incubated with anti-human CD81 antibody and flow cytometry was performed. No human CD81 expression is observed. (D) Positive control. Human hepatoma cells (Huh7.5, known to express CD81) incubated with anti-human CD81 antibody. As expected high levels of CD81 expression are seen. (E) Primary human thyroid primary cells incubated with anti-human CD81 antibody. CD81 is expressed on primary human thyroid cells in culture.
E2 binding to thyroid cells
To establish the ability of E2 protein to bind to the thyroid cell surface, recombinant E2 protein was incubated with human primary thyroid cells or with Huh7.5 cells (2×105 cells per well), as a positive control. Cells were then incubated with anti-E2 antibody, followed by PE-conjugated secondary antibody incubation. Mouse IgG1 was used as a negative control. Huh7.5 cells and human primary thyroid cells showed E2 binding of 45% and 27%, respectively (Figures 2C and 2F, respectively).
Figure 2. E2 binding assay.
Huh7.5 cells or human primary thyroid cells were incubated with or without E2 protein for 60 min at room temperature. Cells were incubated with anti-E2 antibody or normal mouse IgG1, washed, treated with secondary antibody and subjected to flow cytometry to analyze E2 binding to cells. (A) Liver hepatoma cells or (D) primary human thyroid cells incubated with normal mouse IgG1; (B) Human hepatoma cells or (E) primary human thyroid cells incubated with anti-E2 antibody without E2 treatment; (C) Human hepatoma cells or (F) primary human thyroid cells incubated with anti-E2 antibody after E2 treatment; (G) Summarized data (error bars show SE).
E2 protein signaling in thyroid cells
1. Effect of E2 on cell growth
To study the effect of E2 on cell growth in thyroid cells, Huh7.5 cells or human primary thyroid cells were incubated with different concentrations of E2 protein for 48 hours and cell viability was measured by MTT assay. As previously reported, Huh7.5 cells showed growth inhibition after E2 treatment (22). In contrast, human primary cells continued to proliferate after E2 treatment (Figure 3). Therefore, E2 engagement of CD81 on thyroid cells did not induce thyroid cell apoptosis, as has been shown for hepatocyte cells (16;23). However, the data on induction of apoptosis by E2 in hepatocytes need to be confirmed, and therefore, currently it is unclear if this is a major mechanism of liver injury by HCV.
Figure 3. E2 effect on cell viability in human primary thyroid cells.
Both human primary thyroid cells and Huh7.5 cells were incubated with E2 protein (0, 1, 3, 5, 10 μg/ml) for 48 hours. Cell viability was analyzed by MTT assay (error bars show SE). * p<0.05; ** p<0.01.
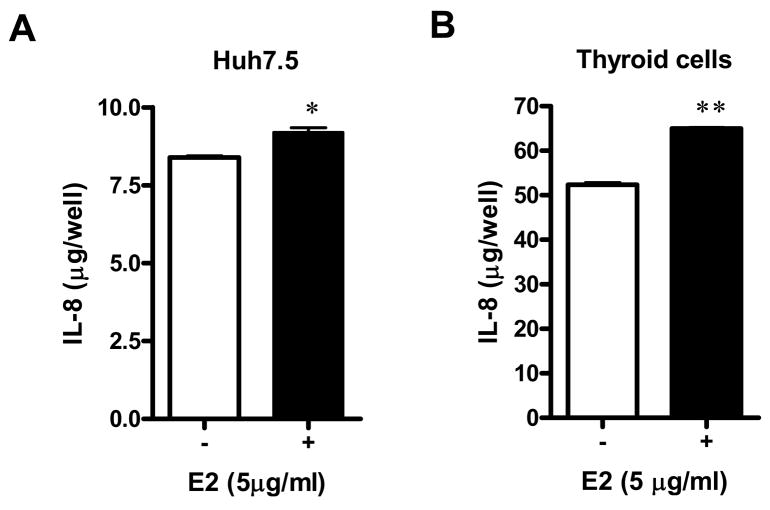
2. Effect of E2 on IL-8 production
Previous studies have demonstrated that E2 protein stimulates IL-8 production in hepatocyte-derived cell lines (19). To examine whether E2 binding to CD81 can also results in IL-8 induction in thyroid cells, human primary thyroid cells or Huh7.5 cells were incubated with 5 μg/ml of E2 for 48 hours. IL-8 protein secretion was then quantified in the culture medium. Both cell types showed a significant increase of IL-8 after E2 treatment (Figures 4A and B). IL-8 levels increased only 10% in Huh7.5 cells in the presence of HCV E2 protein (Figure 4A). Surprisingly, an even higher increase in IL-8 secretion was seen when human primary thyroid cells were incubated with E2. The concentration of IL-8 in the medium of thyroid cells not treated with E2 was 52.4 ug/well, and after E2 treatment it increased by 24% to 65.0 ug/well (p=0.002) [Figure 4B].
Figure 4. IL-8 induction by E2 treatment.
Huh7.5 cells (A) or human primary thyroid cells (B) were incubated with 5 μg/ml of E2 for 48 hours. Supernatant IL-8 levels were then quantified by ELISA (error bars show SE). * p<0.05; ** p<0.01.
DISCUSSION
Globally, hepatitis C virus infects more than 170 million people, with over 4.1 million persons exposed to HCV in the United States alone (24). Although some individuals may spontaneously resolve acute HCV infection, the majority of infected individuals will develop chronic HCV infection characterized by HCV antibody seropositivity and persistent viremia. In addition to the well-known hepatic complications caused by chronic HCV infection, chronic infection is also associated with a multitude of extrahepatic complications including autoimmune thyroiditis (4). The development of autoimmune thyroiditis in patients with chronic HCV infection is intriguing, as it may suggest an additional extrahepatic reservoir of HCV replication, as well as potential mechanisms by which viruses can trigger autoimmunity in general.
Two main theories have been proposed for the induction of autoimmunity by viral agents (25): (1) the molecular mimicry theory suggests that sequence similarities between viral proteins and self proteins can induce a cross-over immune response to self antigens (1); (2) the bystander activation theory proposes that viral infection of a certain tissue can induce local inflammation (e.g. by cytokine release), resulting in activation of autoreactive T-cells that were dormant or suppressed by peripheral regulatory mechanisms (26). Our results, showing that HCV envelope protein E2 can bind to CD81 receptors expressed on thyroid cells and trigger IL-8 release, support the bystander activation hypothesis. Indeed, recent data favor the bystander activation as the predominant mechanism by which viral agents trigger autoimmunity both in autoimmune diabetes (27), as well as in autoimmune thyroiditis (28). Hence, it is likely that the association between HCV infection and thyroid autoimmunity (4;5) (6–10) is due to HCV infection of the thyroid resulting in release of pro-inflammatory mediators such as IL-8, and induction of thyroid autoimmunity by bystander activation mechanisms.
Whether HCV replicate in thyroid cells and whether such replication in thyroid cells is a pre-requisite for the induction of thyroidal inflammation is not currently known. However, one study has suggested that HCV is indeed present in the thyroid (15).
The two HCV envelope glycoproteins, E1 and E2, bind to certain surface molecules, including CD81, that mediate viral attachment and entry (29). Moreover, it has been demonstrated that binding of E1 and E2 to the CD81 receptor - without productive HCV infection - results in a cascade of intracellular signals, both in hepatocytes (30), as well as in other cells (31;32). In particular, cross-linking of CD81 on T-cells by HCV E2 protein lowered the threshold for IL-2 release, increased secretion of IL-4 and interferon gamma, and provided strong costimulatory signals for T cells resulting in proliferation of T-cells (31;32). Similarly, engagement of CD81 on B cells by E2 protein triggers the JNK pathway and leads to proliferation of naïve B lymphocytes (33;34). Thus, it appears that multiple cell types that express CD81 receptor, including thyrocytes, may engage HCV glycoproteins, even in the absence of productive HCV infection. Moreover, CD81 engagement by E1 and E2 can then activate intracellular signaling pathways triggering a tissue inflammatory response. Thus, our data demonstrating that CD81 is expressed on thyrocytes and that E2 can bind CD81 resulting in IL-8 release strongly support the hypothesis that HCV can trigger thyroiditis even without infecting thyroid cells.
Interestingly, incubation of human thyroid cells with E2 did not inhibit cell growth, while hepatocytes incubated with E2 showed increased cell death (Figure 3). These data suggest that HCV proteins do not induce thyroid inflammation by inducing thyrocyte apoptosis directly, but more likely do so by induction of cytokine release. However, it is possible that HCV E2 proteins may bind to and activate local T lymphocytes (32), thereby resulting in indirect induction of thyrocyte apoptosis by activated T-cells in vivo. Finally, it is possible that HCV could directly infect thyrocytes and thereby induce apoptosis.
It is likely that genetic susceptibility also plays a role in the induction of thyroiditis by HCV. Indeed, there is solid evidence for a genetic predisposition to thyroid autoimmunity. Studies have identified several susceptibility genes for autoimmune thyroid disease, including thyroglobulin (35), CD40 (36), CTLA-4 (37–40), PTPN22 (41), TSH receptor (42), and HLA-DR sequence variants (43;44) (reviewed in (45)). Additional putative AITD genes were also mapped (e.g. FOXP3 (46)). One attractive mechanism is that in genetically susceptible individuals certain self-reactive T-cell clones escape deletion. HCV could then trigger local thyroidal inflammation, either by direct thyrocyte infection, or through engagement of circulating E1 and E2 proteins, resulting in activation of these autoreactive T-cell clones leading to autoimmune thyroiditis. Interestingly, therapy for HCV with interferon alpha is a risk factor for thyroiditis independent of HCV infection (5;47). Here, the mechanism of induction of thyroiditis may also involve bystander activation by interferon alpha inducing local thyroidal inflammation.
In summary, we have shown that CD81 is expressed in thyroid cells and can bind HCV envelope protein E2 leading to IL-8 production. These data suggest that local effects of HCV proteins can induce thyroiditis by bystander activation mechanisms.
Acknowledgments
This work was supported in part by grants DK61659, DK067555, and DK073681 from NIDDK (to YT) and DA022148 from NIDA (to JTB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tomer Y, Davies TF. Infection, Thyroid Disease and Autoimmunity. Endocr Rev. 1993;14:107–120. doi: 10.1210/edrv-14-1-107. [DOI] [PubMed] [Google Scholar]
- 2.Tomer Y, Davies TF. Infections and autoimmune endocrine disease. Baillière’s Clin Endocrinol Metab. 1995;9:47–70. doi: 10.1016/s0950-351x(95)80819-1. [DOI] [PubMed] [Google Scholar]
- 3.Mandac JC, Chaudhry S, Sherman KE, Tomer Y. The clinical and physiological spectrum of interferon-alpha induced thyroiditis: Toward a new classification. Hepatology. 2006;43(4):661–672. doi: 10.1002/hep.21146. [DOI] [PubMed] [Google Scholar]
- 4.Tomer Y, Villanueva R. Hepatitis C and thyroid autoimmunity: is there a link? Am J Med. 2004;117(1):60–61. doi: 10.1016/j.amjmed.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Tomer Y, Blackard JT, Akeno N. Interferon alpha treatment and thyroid dysfunction. Endocrinol Metab Clin North Am. 2007;36(4):1051–1066. doi: 10.1016/j.ecl.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tran A, Quaranta JF, Benzaken S, Thiers V, Chau HT, Hastier P, et al. High prevalence of thyroid autoantibodies in a prospective series of patients with chronic hepatitis C before interferon therapy. Hepatology. 1993;18(2):253–257. [PubMed] [Google Scholar]
- 7.Ganne-Carrie N, Medini A, Coderc E, Seror O, Christidis C, Grimbert S, et al. Latent autoimmune thyroiditis in untreated patients with HCV chronic hepatitis: a case-control study. J Autoimmun. 2000;14(2):189–193. doi: 10.1006/jaut.1999.0360. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Soto L, Gonzalez A, Escobar-Jimenez F, Vazquez R, Ocete E, Olea N, et al. Increased risk of autoimmune thyroid disease in hepatitis C vs hepatitis B before, during, and after discontinuing interferon therapy. Arch Intern Med. 1998;158(13):1445–1448. doi: 10.1001/archinte.158.13.1445. [DOI] [PubMed] [Google Scholar]
- 9.Preziati D, La Rosa L, Covini G, Marcelli R, Rescalli S, Persani L, et al. Autoimmunity and thyroid function in patients with chronic active hepatitis treated with recombinant interferon alpha-2a. Eur J Endocrinol. 1995;132(5):587–593. doi: 10.1530/eje.0.1320587. [DOI] [PubMed] [Google Scholar]
- 10.Antonelli A, Ferri C, Pampana A, Fallahi P, Nesti C, Pasquini M, et al. Thyroid disorders in chronic hepatitis C. Am J Med. 2004;117(1):10–13. doi: 10.1016/j.amjmed.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 11.Obermayer-Straub P, Manns MP. Hepatitis C and D, retroviruses and autoimmune manifestations. J Autoimmun. 2001;16(3):275–285. doi: 10.1006/jaut.2000.0488. [DOI] [PubMed] [Google Scholar]
- 12.Toubi E, Gordon S, Kessel A, Rosner I, Rozenbaum M, Shoenfeld Y, et al. Elevated serum B-Lymphocyte activating factor (BAFF) in chronic hepatitis C virus infection: association with autoimmunity. J Autoimmun. 2006;27(2):134–139. doi: 10.1016/j.jaut.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Strassburg CP, Vogel A, Manns MP. Autoimmunity and hepatitis C. Autoimmun Rev. 2003;2(6):322–331. doi: 10.1016/s1568-9972(03)00036-3. [DOI] [PubMed] [Google Scholar]
- 14.Blackard JT, Kemmer N, Sherman KE. Extrahepatic replication of HCV: insights into clinical manifestations and biological consequences. Hepatology. 2006;44(1):15–22. doi: 10.1002/hep.21283. [DOI] [PubMed] [Google Scholar]
- 15.Laskus T, Radkowski M, Wang LF, Vargas H, Rakela J. Search for hepatitis C virus extrahepatic replication sites in patients with acquired immunodeficiency syndrome: specific detection of negative-strand viral RNA in various tissues. Hepatology. 1998;28(5):1398–1401. doi: 10.1002/hep.510280531. [DOI] [PubMed] [Google Scholar]
- 16.Munshi N, Balasubramanian A, Koziel M, Ganju RK, Groopman JE. Hepatitis C and human immunodeficiency virus envelope proteins cooperatively induce hepatocytic apoptosis via an innocent bystander mechanism. J Infect Dis. 2003;188(8):1192–1204. doi: 10.1086/378643. [DOI] [PubMed] [Google Scholar]
- 17.Balasubramanian A, Ganju RK, Groopman JE. Signal transducer and activator of transcription factor 1 mediates apoptosis induced by hepatitis C virus and HIV envelope proteins in hepatocytes. J Infect Dis. 2006;194(5):670–681. doi: 10.1086/505708. [DOI] [PubMed] [Google Scholar]
- 18.Balasubramanian A, Koziel M, Groopman JE, Ganju RK. Molecular mechanism of hepatic injury in coinfection with hepatitis C virus and HIV. Clin Infect Dis. 2005;41(Suppl 1):S32–S37. doi: 10.1086/429493. [DOI] [PubMed] [Google Scholar]
- 19.Balasubramanian A, Ganju RK, Groopman JE. Hepatitis C virus and HIV envelope proteins collaboratively mediate interleukin-8 secretion through activation of p38 MAP kinase and SHP2 in hepatocytes. J Biol Chem. 2003;278(37):35755–35766. doi: 10.1074/jbc.M302889200. [DOI] [PubMed] [Google Scholar]
- 20.Jacobson EM, Huber AK, Akeno N, Sivak M, Li CW, Concepcion E, et al. A CD40 Kozak sequence polymorphism and susceptibility to antibody-mediated autoimmune conditions: The role of CD40 tissue-specific expression. Genes Immun. 2007;8:205–214. doi: 10.1038/sj.gene.6364375. [DOI] [PubMed] [Google Scholar]
- 21.Knauf JA, Elisei R, Mochly-Rosen D, Liron T, Chen XN, Gonsky R, et al. Involvement of protein kinase Cepsilon (PKCepsilon) in thyroid cell death. A truncated chimeric PKCepsilon cloned from a thyroid cancer cell line protects thyroid cells from apoptosis. J Biol Chem. 1999;274(33):23414–23425. doi: 10.1074/jbc.274.33.23414. [DOI] [PubMed] [Google Scholar]
- 22.Flint M, Maidens C, Loomis-Price LD, Shotton C, Dubuisson J, Monk P, et al. Characterization of hepatitis C virus E2 glycoprotein interaction with a putative cellular receptor, CD81. J Virol. 1999;73(8):6235–6244. doi: 10.1128/jvi.73.8.6235-6244.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu LX, Liu J, Xie YH, Kong YY, Ye Y, Wang CL, et al. Expression of hepatitis C virus envelope protein 2 induces apoptosis in cultured mammalian cells. World J Gastroenterol. 2004;10(20):2972–2978. doi: 10.3748/wjg.v10.i20.2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 25.Benoist C, Mathis D. Autoimmunity. The pathogen connection Nature. 1998;394(6690):227–228. doi: 10.1038/28282. [DOI] [PubMed] [Google Scholar]
- 26.Fournie GJ, Mas M, Cautain B, Savignac M, Subra JF, Pelletier L, et al. Induction of autoimmunity through bystander effects. Lessons from immunological disorders induced by heavy metals. J Autoimmun. 2001;16(3):319–326. doi: 10.1006/jaut.2000.0482. [DOI] [PubMed] [Google Scholar]
- 27.Horwitz MS, Bradley LM, Harbertson J, Krahl T, Lee J, Sarvetnick N. Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nat Med. 1998;4(7):781–785. doi: 10.1038/nm0798-781. [DOI] [PubMed] [Google Scholar]
- 28.Arata N, Ando T, Unger P, Davies TF. By-stander activation in autoimmune thyroiditis: studies on experimental autoimmune thyroiditis in the GFP+ fluorescent mouse. Clin Immunol. 2006;121(1):108–117. doi: 10.1016/j.clim.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 29.Barth H, Liang TJ, Baumert TF. Hepatitis C virus entry: molecular biology and clinical implications. Hepatology. 2006;44(3):527–535. doi: 10.1002/hep.21321. [DOI] [PubMed] [Google Scholar]
- 30.Fang X, Zeisel MB, Wilpert J, Gissler B, Thimme R, Kreutz C, et al. Host cell responses induced by hepatitis C virus binding. Hepatology. 2006;43(6):1326–1336. doi: 10.1002/hep.21191. [DOI] [PubMed] [Google Scholar]
- 31.Crotta S, Stilla A, Wack A, D’Andrea A, Nuti S, D’Oro U, et al. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J Exp Med. 2002;195(1):35–41. doi: 10.1084/jem.20011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wack A, Soldaini E, Tseng C, Nuti S, Klimpel G, Abrignani S. Binding of the hepatitis C virus envelope protein E2 to CD81 provides a co-stimulatory signal for human T cells. Eur J Immunol. 2001;31(1):166–175. doi: 10.1002/1521-4141(200101)31:1<166::aid-immu166>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 33.Rosa D, Saletti G, De Gregorio E, Zorat F, Comar C, D’Oro U, et al. Activation of naive B lymphocytes via CD81, a pathogenetic mechanism for hepatitis C virus-associated B lymphocyte disorders. Proc Natl Acad Sci U S A. 2005;102(51):18544–18549. doi: 10.1073/pnas.0509402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Landau DA, Saadoun D, Calabrese LH, Cacoub P. The pathophysiology of HCV induced B-cell clonal disorders. Autoimmun Rev. 2007;6(8):581–587. doi: 10.1016/j.autrev.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Ban Y, Greenberg DA, Concepcion E, Skrabanek L, Villanueva R, Tomer Y. Amino acid substitutions in the thyroglobulin gene are associated with susceptibility to human and murine autoimmune thyroid disease. Proc Natl Acad Sci USA. 2003;100:15119–15124. doi: 10.1073/pnas.2434175100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobson EM, Concepcion E, Oashi T, Tomer Y. A Graves’ disease-associated Kozak sequence single-nucleotide polymorphism enhances the efficiency of CD40 gene translation: a case for translational pathophysiology. Endocrinology. 2005;146(6):2684–2691. doi: 10.1210/en.2004-1617. [DOI] [PubMed] [Google Scholar]
- 37.Kouki T, Sawai Y, Gardine CA, Fisfalen ME, Alegre ML, DeGroot LJ. CTLA-4 Gene Polymorphism at Position 49 in Exon 1 Reduces the Inhibitory Function of CTLA-4 and Contributes to the Pathogenesis of Graves’ Disease. J Immunol. 2000;165(11):6606–6611. doi: 10.4049/jimmunol.165.11.6606. [DOI] [PubMed] [Google Scholar]
- 38.Vaidya B, Imrie H, Perros P, Young ET, Kelly WF, Carr D, et al. The cytotoxic T lymphocyte antigen-4 is a major Graves’ disease locus. Hum Mol Genet. 1999;8(7):1195–1199. doi: 10.1093/hmg/8.7.1195. [DOI] [PubMed] [Google Scholar]
- 39.Ban Y, Davies TF, Greenberg DA, Kissin A, Marder B, Murphy B, et al. Analysis of the CTLA-4, CD28 and inducible co-stimulator (ICOS) genes in autoimmune thyroid disease. Genes Immun. 2003;4:586–593. doi: 10.1038/sj.gene.6364018. [DOI] [PubMed] [Google Scholar]
- 40.Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423(6939):506–511. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 41.Velaga MR, Wilson V, Jennings CE, Owen CJ, Herington S, Donaldson PT, et al. The codon 620 tryptophan allele of the lymphoid tyrosine phosphatase (LYP) gene is a major determinant of Graves’ disease. J Clin Endocrinol Metab. 2004;89(11):5862–5865. doi: 10.1210/jc.2004-1108. [DOI] [PubMed] [Google Scholar]
- 42.Dechairo BM, Zabaneh D, Collins J, Brand O, Dawson GJ, Green AP, et al. Association of the TSHR gene with Graves’ disease: the first disease specific locus. Eur J Hum Genet. 2005;13(11):1223–1230. doi: 10.1038/sj.ejhg.5201485. [DOI] [PubMed] [Google Scholar]
- 43.Jacobson EM, Huber A, Tomer Y. The HLA gene complex in thyroid autoimmunity: From epidemiology to etiology. J Autoimmun. 2008;30(1–2):58–62. doi: 10.1016/j.jaut.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ban Y, Davies TF, Greenberg DA, Concepcion ES, Osman R, Oashi T, et al. Arginine at position 74 of the HLA-DRb1 chain is associated with Graves’ disease. Genes Immun. 2004;5:203–208. doi: 10.1038/sj.gene.6364059. [DOI] [PubMed] [Google Scholar]
- 45.Jacobson EM, Tomer Y. The CD40, CTLA-4, thyroglobulin, TSH receptor, and PTPN22 gene quintet and its contribution to thyroid autoimmunity: Back to the future. J Autoimmun. 2007;28:85–98. doi: 10.1016/j.jaut.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ban Y, Tozaki T, Tobe T, Ban Y, Jacobson EM, Concepcion ES, et al. The regulatory T cell gene FOXP3 and genetic susceptibility to thyroid autoimmunity: An association analysis in Caucasian and Japanese cohorts. J Autoimmun. 2007;28:201–207. doi: 10.1016/j.jaut.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 47.Oppenheim Y, Ban Y, Tomer Y. Interferon induced Autoimmune Thyroid Disease (AITD): a model for human autoimmunity. Autoimmun Rev. 2004;3(5):388–393. doi: 10.1016/j.autrev.2004.03.003. [DOI] [PubMed] [Google Scholar]