Abstract
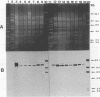
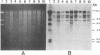
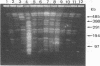
Preliminary results suggested that the diffusion in France of the SHV-4 extended-spectrum beta-lactamase was probably due to the spread of one single epidemic strain of Klebsiella pneumoniae. In this study, we tested various phenotypic and genotypic markers to compare K. pneumoniae strains producing this enzyme isolated in 14 French hospitals between 1987 and 1989. All of the strains were of the same capsule serotype, K25. Twelve of them were of the same biotype: weak urease activity and no sucrose fermentation. Among the six plasmid profiles observed, one accounted for eight strains. Large plasmids of 170 kb encoding SHV-4 beta-lactamase were present in all strains of K. pneumoniae and could be transferred by conjugation with high frequency to Escherichia coli J53-2 or HB101 from all except one strain. Plasmid EcoRI restriction patterns suggested that these plasmids were closely related and similar to pUD18 encoding SHV-3 beta-lactamase, originally described in France and differing from SHV-4 by one amino acid substitution. Ribotyping with EcoRI and HindIII and genomic fingerprinting with XbaI by pulsed-field gel electrophoresis were concordant and suggested that 12 of the isolates recovered from the 14 hospitals were probably the same strain. Dissemination in France of the SHV-4 extended-spectrum beta-lactamase was thus essentially due to the diffusion of a single K. pneumoniae clone.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arlet G., Philippon A. Construction by polymerase chain reaction and use of intragenic DNA probes for three main types of transferable beta-lactamases (TEM, SHV, CARB) [corrected]. FEMS Microbiol Lett. 1991 Jul 15;66(1):19–25. doi: 10.1016/0378-1097(91)90414-6. [DOI] [PubMed] [Google Scholar]
- Arlet G., Sanson-le Pors M. J., Rouveau M., Fournier G., Marie O., Schlemmer B., Philippon A. Outbreak of nosocomial infections due to Klebsiella pneumoniae producing SHV-4 beta-lactamase. Eur J Clin Microbiol Infect Dis. 1990 Nov;9(11):797–803. doi: 10.1007/BF01967377. [DOI] [PubMed] [Google Scholar]
- Bauernfeind A., Rosenthal E., Eberlein E., Holley M., Schweighart S. Spread of Klebsiella pneumoniae producing SHV-5 beta-lactamase among hospitalized patients. Infection. 1993 Jan-Feb;21(1):18–22. doi: 10.1007/BF01739303. [DOI] [PubMed] [Google Scholar]
- Billot-Klein D., Gutmann L., Collatz E. Nucleotide sequence of the SHV-5 beta-lactamase gene of a Klebsiella pneumoniae plasmid. Antimicrob Agents Chemother. 1990 Dec;34(12):2439–2441. doi: 10.1128/aac.34.12.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingen E. H., Desjardins P., Arlet G., Bourgeois F., Mariani-Kurkdjian P., Lambert-Zechovsky N. Y., Denamur E., Philippon A., Elion J. Molecular epidemiology of plasmid spread among extended broad-spectrum beta-lactamase-producing Klebsiella pneumoniae isolates in a pediatric hospital. J Clin Microbiol. 1993 Feb;31(2):179–184. doi: 10.1128/jcm.31.2.179-184.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun-Buisson C., Legrand P., Philippon A., Montravers F., Ansquer M., Duval J. Transferable enzymatic resistance to third-generation cephalosporins during nosocomial outbreak of multiresistant Klebsiella pneumoniae. Lancet. 1987 Aug 8;2(8554):302–306. doi: 10.1016/s0140-6736(87)90891-9. [DOI] [PubMed] [Google Scholar]
- Buré A., Legrand P., Arlet G., Jarlier V., Paul G., Philippon A. Dissemination in five French hospitals of Klebsiella pneumoniae serotype K25 harbouring a new transferable enzymatic resistance to third generation cephalosporins and aztreonam. Eur J Clin Microbiol Infect Dis. 1988 Dec;7(6):780–782. doi: 10.1007/BF01975048. [DOI] [PubMed] [Google Scholar]
- Coovadia Y. M., Johnson A. P., Bhana R. H., Hutchinson G. R., George R. C., Hafferjee I. E. Multiresistant Klebsiella pneumoniae in a neonatal nursery: the importance of maintenance of infection control policies and procedures in the prevention of outbreaks. J Hosp Infect. 1992 Nov;22(3):197–205. doi: 10.1016/0195-6701(92)90044-m. [DOI] [PubMed] [Google Scholar]
- Grimont F., Grimont P. A. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986 Sep-Oct;137B(2):165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- Gutmann L., Ferré B., Goldstein F. W., Rizk N., Pinto-Schuster E., Acar J. F., Collatz E. SHV-5, a novel SHV-type beta-lactamase that hydrolyzes broad-spectrum cephalosporins and monobactams. Antimicrob Agents Chemother. 1989 Jun;33(6):951–956. doi: 10.1128/aac.33.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann L., Williamson R., Moreau N., Kitzis M. D., Collatz E., Acar J. F., Goldstein F. W. Cross-resistance to nalidixic acid, trimethoprim, and chloramphenicol associated with alterations in outer membrane proteins of Klebsiella, Enterobacter, and Serratia. J Infect Dis. 1985 Mar;151(3):501–507. doi: 10.1093/infdis/151.3.501. [DOI] [PubMed] [Google Scholar]
- Hammami A., Arlet G., Ben Redjeb S., Grimont F., Ben Hassen A., Rekik A., Philippon A. Nosocomial outbreak of acute gastroenteritis in a neonatal intensive care unit in Tunisia caused by multiply drug resistant Salmonella wien producing SHV-2 beta-lactamase. Eur J Clin Microbiol Infect Dis. 1991 Aug;10(8):641–646. doi: 10.1007/BF01975816. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A., Medeiros A. A. More extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 1991 Sep;35(9):1697–1704. doi: 10.1128/aac.35.9.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby G. A., Sutton L. Properties of plasmids responsible for production of extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 1991 Jan;35(1):164–169. doi: 10.1128/aac.35.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarlier V., Nicolas M. H., Fournier G., Philippon A. Extended broad-spectrum beta-lactamases conferring transferable resistance to newer beta-lactam agents in Enterobacteriaceae: hospital prevalence and susceptibility patterns. Rev Infect Dis. 1988 Jul-Aug;10(4):867–878. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- Johnson A. P., Weinbren M. J., Ayling-Smith B., Du Bois S. K., Amyes S. G., George R. C. Outbreak of infection in two UK hospitals caused by a strain of Klebsiella pneumoniae resistant to cefotaxime and ceftazidime. J Hosp Infect. 1992 Feb;20(2):97–103. doi: 10.1016/0195-6701(92)90111-x. [DOI] [PubMed] [Google Scholar]
- Kitzis M. D., Billot-Klein D., Goldstein F. W., Williamson R., Tran Van Nhieu G., Carlet J., Acar J. F., Gutmann L. Dissemination of the novel plasmid-mediated beta-lactamase CTX-1, which confers resistance to broad-spectrum cephalosporins, and its inhibition by beta-lactamase inhibitors. Antimicrob Agents Chemother. 1988 Jan;32(1):9–14. doi: 10.1128/aac.32.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knothe H., Shah P., Krcmery V., Antal M., Mitsuhashi S. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection. 1983 Nov-Dec;11(6):315–317. doi: 10.1007/BF01641355. [DOI] [PubMed] [Google Scholar]
- Legrand P., Fournier G., Buré A., Jarlier V., Nicolas M. H., Decré D., Duval J., Philippon A. Detection of extended broad-spectrum beta-lactamases in Enterobacteriaceae in four French hospitals. Eur J Clin Microbiol Infect Dis. 1989 Jun;8(6):527–529. doi: 10.1007/BF01967473. [DOI] [PubMed] [Google Scholar]
- Maslow J. N., Brecher S. M., Adams K. S., Durbin A., Loring S., Arbeit R. D. Relationship between indole production and differentiation of Klebsiella species: indole-positive and -negative isolates of Klebsiella determined to be clonal. J Clin Microbiol. 1993 Aug;31(8):2000–2003. doi: 10.1128/jcm.31.8.2000-2003.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K. S., Urban C., Eagan J. A., Berger B. J., Rahal J. J. Nosocomial outbreak of Klebsiella infection resistant to late-generation cephalosporins. Ann Intern Med. 1993 Sep 1;119(5):353–358. doi: 10.7326/0003-4819-119-5-199309010-00001. [DOI] [PubMed] [Google Scholar]
- Naumovski L., Quinn J. P., Miyashiro D., Patel M., Bush K., Singer S. B., Graves D., Palzkill T., Arvin A. M. Outbreak of ceftazidime resistance due to a novel extended-spectrum beta-lactamase in isolates from cancer patients. Antimicrob Agents Chemother. 1992 Sep;36(9):1991–1996. doi: 10.1128/aac.36.9.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas M. H., Jarlier V., Honore N., Philippon A., Cole S. T. Molecular characterization of the gene encoding SHV-3 beta-lactamase responsible for transferable cefotaxime resistance in clinical isolates of Klebsiella pneumoniae. Antimicrob Agents Chemother. 1989 Dec;33(12):2096–2100. doi: 10.1128/aac.33.12.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangon B., Bizet C., Buré A., Pichon F., Philippon A., Regnier B., Gutmann L. In vivo selection of a cephamycin-resistant, porin-deficient mutant of Klebsiella pneumoniae producing a TEM-3 beta-lactamase. J Infect Dis. 1989 May;159(5):1005–1006. doi: 10.1093/infdis/159.5.1005. [DOI] [PubMed] [Google Scholar]
- Petit A., Gerbaud G., Sirot D., Courvalin P., Sirot J. Molecular epidemiology of TEM-3 (CTX-1) beta-lactamase. Antimicrob Agents Chemother. 1990 Feb;34(2):219–224. doi: 10.1128/aac.34.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippon A., Labia R., Jacoby G. Extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 1989 Aug;33(8):1131–1136. doi: 10.1128/aac.33.8.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péduzzi J., Barthélémy M., Tiwari K., Mattioni D., Labia R. Structural features related to hydrolytic activity against ceftazidime of plasmid-mediated SHV-type CAZ-5 beta-lactamase. Antimicrob Agents Chemother. 1989 Dec;33(12):2160–2163. doi: 10.1128/aac.33.12.2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice L. B., Willey S. H., Papanicolaou G. A., Medeiros A. A., Eliopoulos G. M., Moellering R. C., Jr, Jacoby G. A. Outbreak of ceftazidime resistance caused by extended-spectrum beta-lactamases at a Massachusetts chronic-care facility. Antimicrob Agents Chemother. 1990 Nov;34(11):2193–2199. doi: 10.1128/aac.34.11.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon K. P., King A., Phillips I., Nicolas M. H., Philippon A. Importance of organisms producing broad-spectrum SHV-group beta-lactamases into the United Kingdom. J Antimicrob Chemother. 1990 Mar;25(3):343–351. doi: 10.1093/jac/25.3.343. [DOI] [PubMed] [Google Scholar]
- Sirot J., Chanal C., Petit A., Sirot D., Labia R., Gerbaud G. Klebsiella pneumoniae and other Enterobacteriaceae producing novel plasmid-mediated beta-lactamases markedly active against third-generation cephalosporins: epidemiologic studies. Rev Infect Dis. 1988 Jul-Aug;10(4):850–859. doi: 10.1093/clinids/10.4.850. [DOI] [PubMed] [Google Scholar]
- Smith C. L., Cantor C. R. Purification, specific fragmentation, and separation of large DNA molecules. Methods Enzymol. 1987;155:449–467. doi: 10.1016/0076-6879(87)55030-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Nagano Y. Rapid procedure for isolation of plasmid DNA and application to epidemiological analysis. J Clin Microbiol. 1984 Oct;20(4):608–613. doi: 10.1128/jcm.20.4.608-613.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tompkins L. S. The use of molecular methods in infectious diseases. N Engl J Med. 1992 Oct 29;327(18):1290–1297. doi: 10.1056/NEJM199210293271808. [DOI] [PubMed] [Google Scholar]
- Vernet V., Madoulet C., Chippaux C., Philippon A. Incidence of two virulence factors (aerobactin and mucoid phenotype) among 190 clinical isolates of Klebsiella pneumoniae producing extended-spectrum beta-lactamase. FEMS Microbiol Lett. 1992 Sep 1;75(1):1–5. doi: 10.1016/0378-1097(92)90447-v. [DOI] [PubMed] [Google Scholar]
- Wheatcroft R., Williams P. A. Rapid methods for the study of both stable and unstable plasmids in Pseudomonas. J Gen Microbiol. 1981 Jun;124(2):433–437. doi: 10.1099/00221287-124-2-433. [DOI] [PubMed] [Google Scholar]
- de Champs C., Sirot D., Chanal C., Poupart M. C., Dumas M. P., Sirot J. Concomitant dissemination of three extended-spectrum beta-lactamases among different Enterobacteriaceae isolated in a French hospital. J Antimicrob Chemother. 1991 Apr;27(4):441–457. doi: 10.1093/jac/27.4.441. [DOI] [PubMed] [Google Scholar]