Abstract
Purpose
The Rpe65−/− mouse, used as a model for Leber congenital amaurosis, has slow rod degeneration and rapid cone loss, presumably due to the mistrafficking of cone opsins. This animal does not generate 11-cis retinal and both the loss of cones and the rod response are restored by 11-cis retinal administration. The Lrat−/− mouse likewise does not produce 11-cis retinal. We sought to determine if the same effects on rod and cone opsins in the Rpe65−/− mouse are also present in the Lrat−/− mouse, thereby establishing that these changes can be attributed to the lack of 11-cis retinal rather than some unknown function of RPE65.
Methods
Rod and cone opsins were localized by immunohistochemical methods. Functional opsin levels were determined by regeneration with 11-cis retinal. Isorhodopsin levels were determined from pigment extraction. Opsin phosphorylation was determined by mass spectrometry.
Results
Rods in both models degenerated slowly. Likewise, regenerable rod opsin levels were similar over the 6-month time course investigated, rod opsin was phosphorylated at a low level (~10%) and minimal 9-cis retinal was generated by a non-photic process giving a trace light response. In both models, both S-opsin and M/L-opsin failed to traffic to the cone outer segments appropriately, and rapid cone degeneration occurred. Cone opsin mistrafficking in both models was arrested upon 11-cis retinal administration.
Conclusions
Our data show that the Lrat−/− and Rpe65−/− mice are comparable models for studies of Leber congenital amaurosis and that the destructive cone opsin mistrafficking is due to the lack of 11-cis retinal.
Keywords: cones, opsin, retinal, rhodopsin, RPE65, LRAT, retinoid cycle, Leber congenital amaurosis
INTRODUCTION
Vision is initiated by the absorption of a photon by photosensitive proteins located in rod and cone photoreceptors. These proteins (opsins) are G-coupled protein receptors and have as their ligand 11-cis retinal, which isomerizes to the all-trans form with light, initiating the visual transduction cascade. The rod photoreceptors and their opsin rhodopsin have been extensively studied (for a recent review see1) but it is the cones which are critical for normal human daylight vision.
The generation of 11-cis retinal is critical for visual pigment regeneration and maintaining normal visual function. The 11-cis retinal is generated in the retinal pigment epithelium (RPE) from the all-trans retinyl esters and is then transported to the photoreceptors for the regeneration of the functional pigment.2 The process by which 11-cis retinal is generated (the retinoid cycle) has been the subject of study of many laboratories. The protein RPE65, a major protein of the RPE, has been shown to be critical for the production of 11-cis retinal3 and recently has been identified as the long sought isomerohydrolase,4–6 using the all-trans retinyl esters7 as the substrate. The possibility of a separate process for the generation of 11-cis retinal for cones has been raised,8–10 but this pathway does not appear to be a major pathway in rod dominant retinas.
The Rpe65 knockout mouse (Rpe65−/−) has been extensively studied.3 This animal has been shown to have no 11-cis retinal, but low levels of 9-cis retinal are generated, possibly by thermal isomerization, which leads to the formation of minimal levels of isorhodopsin (the photosensitive rod opsin/9-cis retinal pigment) and thus a minuscule visual response as measured by the electroretinogram (ERG).11 The level of pigmentation of the animal affects the quantities of this isomer, less being formed in the pigmented mouse.12 Large amounts of the all-trans esters accumulate as the conversion of the esters to the 11-cis retinal has been arrested.3 Rod photoreceptor degeneration is slow and the opsin itself is found to be phosphorylated.13,14 Several groups have shown that administration of 9- or 11-cis retinal can restore rod function, even in fully adult mice 18 months-old.13,15
However, unlike the rods, cone photoreceptors are found to degenerate rapidly in the Rpe65−/− mouse.16 The cone opsins (both the S-opsin and the M/L-opsin) are found to be mislocalized in Rpe65−/− mice, but the repeated administration of 11-cis retinal at an early age (~postnatal day 10 (P10)) for several days results in opsin moving to the cone outer segment (COS), which prevents the rapid cone degeneration.17 Recently, using adenovirus-expressing Rpe65 to efficiently deliver the Rpe65 gene to the RPE of Rpe65−/− mice, it has been shown that rod function can be restored as expected, but also that the cone opsin mislocalization can be corrected.18
However, it has been demonstrated that RPE65 is expressed at very low levels in cone outer segments in several different species19,20 (WB/JF preliminary unpublished observations, 2007). The function of RPE65 in cones is unknown. In order to confirm that the mislocalization of cone opsin was due to the lack of 11-cis retinal rather some unknown function of RPE65, it was necessary to examine another model in which the synthesis of 11-cis retinal is blocked.
Lecithin-retinol acyltransferase (LRAT) catalyzes the esterification of all-trans-retinol to all-trans retinyl esters21 and is found in several tissues including the RPE.22 In the RPE, LRAT has a key role in the retinoid cycle23 as it is critical for the formation of the esters which is the essential substrate for the retinoid isomerase. The Lrat−/− mouse generated only trace levels of all-trans retinyl esters and no 11-cis retinal was found, indicating the retinoid visual cycle was blocked at the esterification step. Both the cone and rod visual functions were found to be attenuated,24 but the responsible chromophore for the residual visual function was not identified.
In this study, we have compared the rod and cone degeneration in both Rpe65−/− and Lrat−/− mouse models where the retinoid cycle is disrupted and no 11-cis retinal is synthesized. We conclude that the disruption of the retinoid cycle in these two models results in similar patterns of retinal degeneration: rapid cone degeneration with cone opsin mislocalization to the inner segment and relatively slow rod degeneration with correct rod opsin localization.
METHODS
Animals
Lrat−/− mice were genotyped as described.24 Rpe65−/− mice3 were provided by T. Michael Redmond (Laboratory of Retinal Cell and Molecular Biology, National Eye Institute). Unless otherwise noted, all experiments were conducted on cyclic light-reared, age-matched animals. For isorhodopsin accumulation measurements, animals 2 months-of-age were dark-reared for 8 weeks. Animal experiments were performed in accordance with the policy for the Use of Animals in Neuroscience research and conformed to the recommendations of the Association of Research for Vision and Ophthalmology. The procedures were approved by the Medical University of South Carolina Animal Care and Use Committee, and the Institutional Animal Care and Use Committee at the University of Utah. Lrat−/− mice were housed in the dark starting at day P10. Experimental animals were injected intraperitoneally with 11-cis retinal (0.5 mg per dose) in 100 μL vehicle (1.0% ethanol, 10% bovine serum albumin, and 0.9% NaCl) on days P10, P13 and P17. Animals were sacrificed at age P20 for the cone opsin localization experiments. Littermate control mice were injected on the same schedule with vehicle alone.
Pigment Measurements
Tissue isolation and all subsequent steps were conducted under dim red light (Kodak GBX-2). The retina was dissected and homogenized in 1% dodecylmaltoside (buffered with 100 mM sodium phosphate buffer, pH 7.4). The sample was shaken at 4°C for 2 h, centrifuged (88,000g for 10 min), and measured in a Cary 300 spectrophotometer (Varian, Walnut Creek, CA). Difference spectra were determined from measurements before and after bleaching with white light in the presence of freshly prepared 20 mM hydroxylamine, pH 7.0. The isorhodopsin concentration was calculated based on the absorption at λmax = 487 nm using the extinction coefficient: ε (isorhodopsin) = 43,000 M−1cm−1.25 To determine the regenerable opsin present, homogenized retinas of age-matched animals were supplemented with 11-cis retinal (80 μM) for 2 h prior to obtaining difference spectra.14 Samples were washed extensively (8X) with 100 mM phosphate buffer after the incubation with retinal which resulted in lower levels of pigment than reported from direct extraction techniques. Rhodopsin concentrations were calculated using the extinction coefficient of 40,000 M−1 cm−1.26
Opsin Phosphorylation Measurements
Opsin phosphorylation was determined using mass spectrometry as described.14,27 In brief, retinas were homogenized in 8 M urea and digested overnight with Asp-N (5 ng/200 μL, Sigma, St. Louis, MO) in 10 mM Tris buffer, pH 7.6 at 37°C. Supernatants were collected by centrifugation (120,000g) and analyzed online with a Finnigan LTQ ion-trap mass spectrometer (Thermo-Finnigan Instrument Systems, Inc., San Jose, CA). The values are slightly different than those previously published14 due to improved instrumentation.
Immunohistochemistry
Age-matched mouse eyes were immersion-fixed for 2 h using freshly prepared 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) and cryoprotected. Eyes were embedded, frozen and sectioned at 12–14 μm thickness prior to incubations for immunocytochemistry (described in17,28). Affinity-purified primary antibodies were applied to each group of 2 to 4 sections in a humidified chamber overnight at 4°C. Propidium iodide (Invitrogen Corporation, Carlsbad, CA, 1:3000 dilution) was added to the solution containing FITC- or Alexa-488 (Molecular Probe, Eugene, OR) -conjugated secondary antibody. The sections were viewed using either a Zeiss LSM 510 inverted Laser Scan confocal microscope with a 40X-1.3 NA oil objective lens and optical slit setting of <0.9 μm, or a Zeiss fluorescence microscope. The following antibodies were used in this study: rhodopsin (1:1000; generously provided by Robert Molday, University of British Columbia), anti-S-opsin and anti-M/L-opsin (1:500; Chemicon/Millipore, Temecula, CA) and anti-S-opsin and anti-M/L-opsin (1:500; generously provided by Jeannie Chen; University of Southern California).
Peanut Agglutinin (PNA) Lectin Labeling
The RPE-choroid layer was separated from the retina-lens complex and fixed in 4% formaldehyde in phosphate buffered saline for 4 h at 4°C. After washing with buffer (30 min × 3, at 4°C), retinas were incubated with the 0.2 mg/mL PNA lectin FITC-conjugated (lectin Arachi hypogaea; Sigma, St. Louis, MO) overnight at 4°C. Retinas were washed (20 min × 3, at 4°C), mounted on a slide, and cover-slipped after application of an anti-fade solution (Prolong; Molecular Probes, Eugene, OR).20 Samples were viewed with a Zeiss fluorescence microscope (Axioplan II; Carl Zeiss Inc., Jena, Germany) using a 100 W mercury light source and FITC filters. Cones were counted from the ventral field (n = 6 eyes).
RESULTS
Rod Degeneration Slow in Both Rpe65−/− and the Lrat−/− Mice
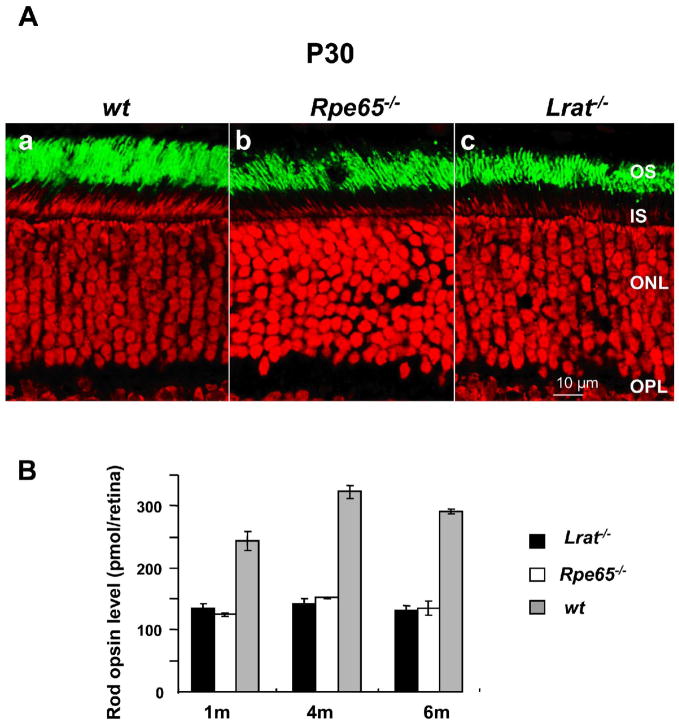
The rod photoreceptors in both the Rpe65−/− and the Lrat−/− mouse are reported to degenerate slowly.3,24 A comparison of the two models with the wt mouse at P30 (Fig. 1A) shows the Lrat−/− mouse and the Rpe65−/−mouse to be comparable with rod outer segments decreased in length and abundance of rod nuclei when compared with wt mice. Rod opsin is present in the rod outer segments with no evidence of mislocalization. To determine that the opsin in the two models can form rhodopsin if the ligand 11-cis retinal is available, in vitro experiments were conducted, adding 11-cis retinal to retina homogenates and assaying for the formation of rhodopsin. Measurements at several ages demonstrate that the levels are very similar (Fig. 1B). Although the regenerated rhodopsin levels are certainly decreased from the wt animals, there was no significant variation between these two models and no significant decrease between 1 and 6 months-of-age in either model.
Figure 1. Comparison of rod opsin in 1-month-old wt, Rpe65−/− and Lrat−/− retinae.
(A) Immunocytochemistry Sections probed using antibody directed against rhodopsin (green). Nuclei were counterstained with propdium iodide (red); scale bar represents 10 μm. Abbreviations: OS, outer segments; IS, inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer. (B) Relative regenerable opsin levels in Lrat−/− and Rpe65−/− retinae.Levels of available opsin were determined by the incubation of homogenized retinae of age-matched 1-month, 4-month and 6-month-old animals reared in cyclic light with 11-cis retinal (80 μM) for 2 hours. Difference spectra were determined from measurements before and after bleaching in the presence of hydroxylamine (20 mM). Available opsin levels were calculated from regenerated rhodopsin concentrations using ε = 40,000 M−1cm−1 at λmax = 500 nm. Data are presented as means ±SEM, n = 3. White bars: Rpe65−/− mice; black bars: Lrat−/− mice, gray bars: wt mice.
Isorhodopsin Formed in Lrat−/− Mouse
In both the Rpe65−/− and the Lrat−/− mouse, a small light response is generated as measured by the ERG.3,24 However, no 11-cis retinal has been detected in either animal. In the Rpe65−/− mouse, it has been found that a small amount of 9-cis retinal is generated leading to the formation of the rod pigment, isorhodopsin. On dark-rearing over a number of weeks, this pigment accumulates to a significant level, indicating that the generation of the 9-cis retinal is by a non-photic process.11 As it had been shown that 11-cis retinal is also lacking in the Lrat−/− mouse,24 we investigated if these animals also generated 9-cis retinal. Care was taken that the animals had similar coat pigmentation as this has been shown to have an effect on the levels of 9-cis retinal.12 After 8 weeks of dark-rearing, isorhodopsin levels were found to be 12.6 ±0.8 pmol in the Lrat−/− mouse retina, as compared with 27.3 ±0.6 pmol/retina in the age-matched Rpe65−/− animals dark-reared for the same period (Fig. 2). Therefore we attribute the small light-induced ERG response in the Lrat−/− mouse to this 9-cis retinal. The finding that less 9-cis retinal is generated in the Lrat−/− animals than the Rpe65−/− mice suggests that the 9-cis retinal may be arising from the ester form, which is elevated in the Rpe65−/− animals, but the exact source of this isomer remains unknown.
Figure 2. Relative isorhodopsin levels in Lrat−/− and Rpe65−/− retinae.
Animals of 2-months-of-age were dark-reared for 8 weeks. Pigment levels were determined from difference absorption spectra. Data are presented as means ±SEM (n = 3) and analyzed by two-tailed Student t test, accepting a significant value of P <0.05. White bars: Rpe65−/− mice; black bars: Lrat−/− mice.
Rod Opsin Phosphorylated in the Lrat−/− Mouse
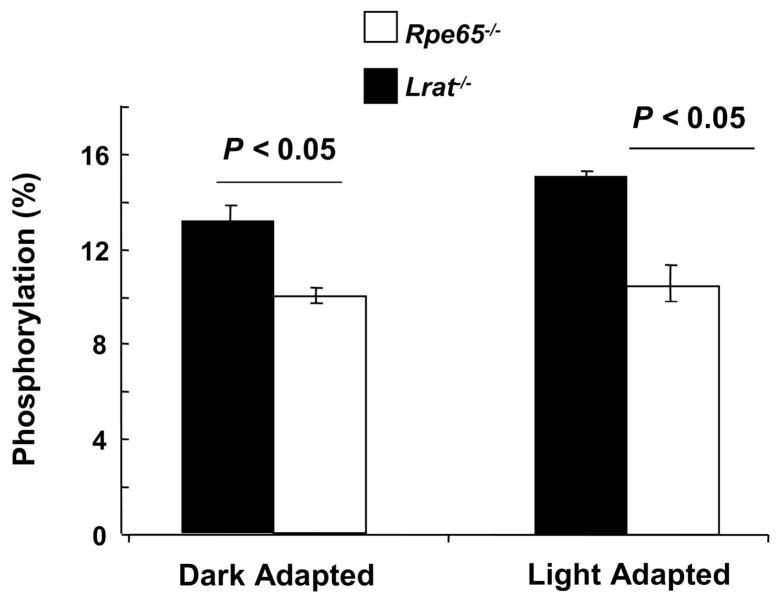
The rod opsin in the Rpe65−/− mouse is known to be phosphorylated.13,14 In order to determine if the opsin in the Lrat−/− mouse is likewise phosphorylated, analysis of the opsin from the animals was performed by mass spectrometry using known methodology.14 As shown for the Rpe65−/− mouse, Lrat−/− opsin was phosphorylated, independent of light exposure. The opsin phosphorylation level in the light-adapted Lrat−/− mouse (13.1 ±0.7%) was slightly increased over that found in the light-adapted Rpe65−/− mouse (10.4 ±0.7%, P <0.05). As expected, there was no difference in the phosphorylation levels between dark- and light-adapted animals in either model, as there is only a minimal light response (Fig. 3). These results support the hypothesis that this low level of constitutive opsin phosphorylation may have a role in slowing rod degeneration.
Figure 3. Opsin phosphorylation levels in Lrat−/− and Rpe65−/− mice.
Retinae of 2-month-old Rpe65−/− and Lrat−/− mice were homogenized in 8 M urea and the pellet digested with Asp-N to cleave the opsin C-terminus. The peptides were analyzed by HPLC/MS/MS using a LTQ mass spectrometer. Data are presented as percentages of phosphorylated peptides (means ±SEM (n = 3)) and analyzed by the two-tailed Student t test, accepting a significant value of P <0.05. White bars: Rpe65−/− mice; black bars: Lrat−/− mice.
Cone Photoreceptors Rapidly Degenerate
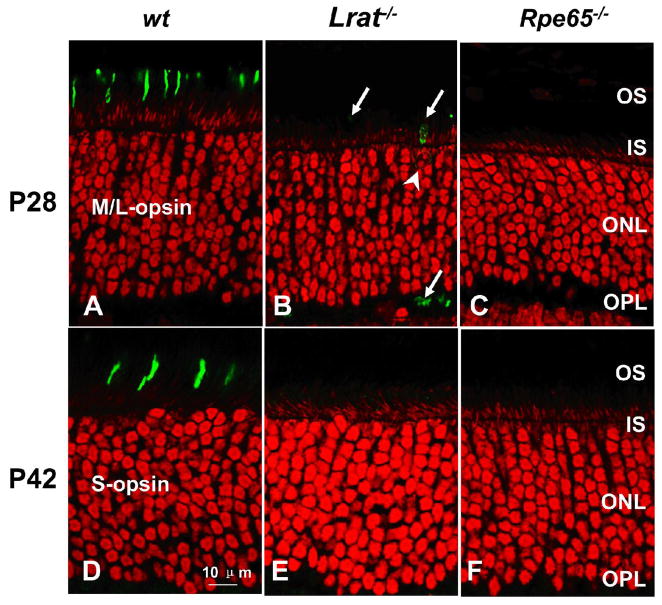
The cones of the Rpe65−/−mice are known to degenerate rapidly.16 In the Lrat−/− mouse, the cones likewise degenerate very quickly. Figure 4 demonstrates that the M/L-opsin is almost totally absent at P28 for both models and S-opsin is totally gone at P42, demonstrating that the cones are rapidly lost in both models and the two phenotypes appear to be very comparable. When cones are counted in flat-mount PNA lectin preparations from P30 animals (ventral sections as shown in Fig. 4), the cones were found to be <5% of wt cones for both the Rpe65−/− and the Lrat−/− mice (Lrat−/−: 2.1 ±0.7; Rpe65−/−: 4.6 ±2.7; P =0.02).
Figure 4. Confocal immunolocalization of cone opsins in wt, Lrat−/− and Rpe65−/− retinae.
(A–C) P28 wt and mutant retinae are stained for M/L-opsin. The M/L-opsin is nearly absent at this stage. A perinuclear ring (B; arrowhead) represents the endoplasmic reticulum-region where M/L-opsin (arrow) is synthesized. (D–F) P42 wt and mutant retinae are stained for S-opsin. The S-opsin is completely missing in the Lrat−/− and Rpe65−/− retinae at this stage, whereas the wt cones appear healthy with the opsin in the outer segments. Nuclei are contrasted with propidium iodide (red); scale bar represents 10 μm. All retinae sections passed through the optic nerve, and photoreceptors were imaged ventral (inferior) to the nerve where the degeneration was most advanced. Abbreviations as in Figure 1A.
Cone Opsin Mislocalizes
Mislocalization of both the S-opsin and the M/L-opsin has been shown to occur in the Rpe65−/− mouse. When studied in a mouse model lacking the rod opsin pool, the Rpe65−/− Rho−/− mouse, both the S-opsin and the M/L-opsin were observed to be distributed throughout the cone inner segment (CIS), cone cell body, axon and synaptic pedicle, while essentially absent in the COS.17 At P28, COS disintegration appeared to be far advanced in the Lrat−/− mouse retina and M/L-opsin mislocalized to the inner segment, perinuclear region, axon, and synaptic pedicle (arrows, Figs. 4B and 5B, E).
Figure 5. Effect of 11-cis retinal on cone opsin distribution.
Retina sections (14 μm thickness, dorsal of the optic nerve) of P20 mice were stained with antibodies against S-opsin (A–C) and M/L-opsin (D–F) with and without 11-cis retinal injections: (A, D) C57BL/6 wt mice; (B, E) Lrat−/− mice; (C, F) Lrat−/− mice with11-cis injections. For the untreated knockout mice, the opsin was distributed throughout the entire cell (cone inner and outer segment, cell body, axon and synaptic pedicle). Upon 11-cis retinal administration, less cone opsin was localized inappropriately to the synaptic pedicle, whereas more cone opsin was appropriately localized to the outer segment. Abbreviations: OS, outer segment; IS, inner segment; CB, cell body; Ax, axon; SP, synaptic pedicle. Scale bar = 25 μm.
Cone Opsin Mislocalization Corrected with Administration of 11-cis Retinal
Upon repeated administration of 11-cis retinal to very young Rpe65−/− Rho−/− animals, both opsins were targeted to the cone outer segment.17 To study the effect of 11-cis retinal treatments in the Lrat−/− retinas, littermates were randomly assigned to the experimental or the control groups and kept in the dark starting at P10. The animals were injected intraperitoneally with 11-cis retinal [0.5 mg per dose in 100 μL vehicle (10% ethanol, 10% bovine serum albumin, and 0.9% NaCl)] or vehicle alone on days P10, P13 and P17. The animals were sacrificed at age P20. As shown in Figure 5, the mislocalization of cone opsins (both S-opsin and M/L-opsin) was partially corrected after the administration of 11-cis retinal. Compared with untreated retinae, the synaptic pedicles are less intensely stained, whereas more cone outer segments stained heavily for the cone opsins, indicating more cone opsin migrated properly into the outer segment after 11-cis retinal injections. The results in the Lrat−/− mouse are less dramatic than in the Rpe65−/−Rho−/− mouse due to the large pool of rod opsin taking up the available 11-cis retinal. These results confirmed that the 11-cis retinal is critical to normal cone opsin trafficking.
DISCUSSION
The purpose of this study was to determine if the two mouse models lacking 11-cis retinal have similar patterns of rod and cone degeneration and are therefore both appropriate models for LCA. Even though both the Lrat and Rpe65 genes are candidate genes for LCA (e.g.,29,30), the rationale for this comparison was the concern that the unexpected cone opsin mistrafficking observed in the Rpe65−/− mouse might be related to some unknown function of RPE65, possibly within the cone photoreceptors. We therefore examined the Lrat−/− mouse which likewise does not produce 11-cis retinal, but has normal RPE65 production and localization. The rod opsin levels in these two models is essentially identical, with some opsin phosphorylation which is not observed in wt animals. Both models show a minuscule level of 9-cis retinal production. The rods in both these models degenerate slowly and have no evidence of opsin mislocalization.
The cones of both the Lrat−/− and the Rpe65−/− mice degenerate rapidly, with eventual complete loss of cone function. Immunohistochemistry indicates that the cone opsins in both models are not trafficking to the outer segments appropriately and the remaining outer segments appear stunted. The degeneration and cone opsin mistrafficking can be arrested with administration of 11-cis retinal at an early age. We have confirmed functional cone recovery in the Rpe65−/− Rho−/− mouse, but were unable to confirm recovery of cone function in the Lrat−/− mouse due to the interference of rods. We proposed that the lack of trafficking to the outer segments is the cause of cone degeneration. The cone opsin localization patterns look very similar in these two models lacking 11-cis retinal, with opsin found throughout the inner segment down to the pedicle. This mistrafficking appears to be a post-Golgi targeting disorder, as the opsin is exported from the endoplasmic reticulum, but cannot be transported to the outer segment.
In general, rod pigments are more stable than cone pigments, such as in their lability to hydroxylamine.31 Another distinction between the rod and cone opsins are that cone opsins are known to be more highly phosphorylated than the rod opsins on light activation, having more serines and theonines in the C-terminus.32 Also, the two mouse cone opsins lack the posttranslational modification of cysteine palmitylation, proposed to form the eighth “helix” on rod opsins.33 Our data may suggest that the 11-cis retinal induces a conformational change that is necessary for recognition by a chaperone or transporting complex. Without this conformational change, the cone opsins cannot be transported to the outer segment, where it is required for disk (and therefore outer segment) stability, similar to the requirement for rhodopsin in rod outer segment formation.34,35
The trafficking of rod opsin has been studied in several species (for a recent review, see36). Our results indicate that rod opsin traffics normally in the absence of 11-cis retinal. There is some in vitro evidence that 11-cis retinal improves protein stability of certain rhodopsin mutants as well as movement from the endoplasmic reticulum to the cell membrane,37 but there are no reports of 11-cis retinal availability affecting normal rhodopsin trafficking in vivo. The C-terminal amino acids of rhodopsin have been implicated in being essential for proper targeting,38 and a general VXPX motif for targeting has been postulated36 to be involved in sorting into post-Golgi carriers through binding with ARF-4.39 Sorting mechanism for cone pigments are unknown, however, both cone pigment primary sequences carry a C-terminal sequence VSPA (M/L-opsin) or VGPH (S-opsin), each of which could qualify as a targeting sequence. Lack of correct targeting of cone pigments in the absence of 11-cis retinal could be explained by masking of the C-terminal targeting sequence or other targeting signals in the C-terminal region (or elsewhere in the cytoplasmic domain of cone pigments). It is conceivable that presence of 11-cis retinal enables cone pigments to assume a conformation in which the targeting signal(s) are exposed.
Acknowledgments
The authors thank Michael Redmond, National Eye Institute at National Institutes of Health, for the gift of the Rpe65−/− mice and Robert Molday University of Vancouver, and Jeannie Chen, University of Southern California, for the gift of rhodopsin and cone opsin antibodies. We also thank Dusanka Deretic, University of New Mexico, and Kris Palczewski, Case Western Reserve University for helpful discussions.
Grant information: National Institutes of Health (NIH), National Eye Institute grants EY04939 (RKC), EY13520 (BR), EY14793 (MUSC vision core), EY08123 (WB), EY014800-039003 (University of Utah core grant), CO6 RR015455 from the NIH National Center for Research Resources to MUSC; Foundation Fighting Blindness, Inc., grants to RKC and Center Grant to the University of Utah; and Research to Prevent Blindness (RPB), New York, unrestricted grants to Ophthalmology at MUSC and the Univ. of Utah, RPB Senior Scientific Investigator Award (RKC) and RPB Olga Keith Weiss Scholar Award (BR).
Abbreviations
- CIS
cone inner segment
- COS
cone outer segment
- ERG
electroretinogram
- LRAT
lecithin:retinol acyl transferase
- S-opsin
short wave cone opsin
- M/L-opsin
middle/long wave cone opsin
- P
postnatal
- PNA
peanut agglutinin
- RPE
retinal pigment epithelium
- RPE65
65 kDa RPE-specific protein (retinoid isomerase)
References
- 1.Ridge KD, Palczewski K. Visual rhodopsin sees the light: structure and mechanism of G protein signaling. J Biol Chem. 2007;282:9297–9301. doi: 10.1074/jbc.R600032200. [DOI] [PubMed] [Google Scholar]
- 2.Wald G. The molecular basis of visual excitation. Nature. 1968;219:800–807. doi: 10.1038/219800a0. [DOI] [PubMed] [Google Scholar]
- 3.Redmond TM, Yu S, Lee E, et al. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 4.Moiseyev G, Chen Y, Takahashi Y, Wu BX, Ma JX. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proc Natl Acad Sci USA. 2005;102:12413–12418. doi: 10.1073/pnas.0503460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin M, Li S, Moghrabi WN, Sun H, Travis GH. Rpe65 is the retinoid isomerase in bovine retinal pigment epithelium. Cell. 2005;122:449–459. doi: 10.1016/j.cell.2005.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redmond TM, Poliakov E, Yu S, Tsai JY, Lu Z, Gentleman S. Mutation of key residues of RPE65 abolishes its enzymatic role as isomerohydrolase in the visual cycle. Proc Natl Acad Sci USA. 2005;102:13658–13663. doi: 10.1073/pnas.0504167102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moiseyev G, Crouch RK, Goletz P, Oatis J, Jr, Redmond TM, Ma JX. Retinyl esters are the substrate for isomerohydrolase. Biochemistry. 2003;42:2229–2238. doi: 10.1021/bi026911y. [DOI] [PubMed] [Google Scholar]
- 8.Mata NL, Ruiz A, Radu RA, Bui TV, Travis GH. Chicken retinas contain a retinoid isomerase activity that catalyzes the direct conversion of all-trans-retinol to 11-cis-retinol. Biochemistry. 2005;44:11715–11721. doi: 10.1021/bi050942m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schonthaler HB, Lampert JM, Isken A, et al. Evidence for RPE65-independent vision in the cone-dominated zebrafish retina. Eur J Neurosci. 2007;26:1940–1949. doi: 10.1111/j.1460-9568.2007.05801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muniz A, Villazana-Espinoza ET, Hatch AL, Trevino SG, Allen DM, Tsin AT. A novel cone visual cycle in the cone-dominated retina. Exp Eye Res. 2007;85:175–184. doi: 10.1016/j.exer.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fan J, Rohrer B, Moiseyev G, Ma JX, Crouch RK. Isorhodopsin rather than rhodopsin mediates rod function in RPE65 knock-out mice. Proc Natl Acad Sci USA. 2003;100:13662–13667. doi: 10.1073/pnas.2234461100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan J, Wu BX, Sarna T, Rohrer B, Redmond TM, Crouch RK. 9-cis Retinal increased in retina of Rpe65 knockout mice with decrease in coat pigmentation. Photochem Photobiol. 2006;82:1461–1467. doi: 10.1562/2006-02-02-RA-793. [DOI] [PubMed] [Google Scholar]
- 13.Van Hooser JP, Liang Y, Maeda T, et al. Recovery of visual functions in a mouse model of Leber congenital amaurosis. J Biol Chem. 2002;277:19173–19182. doi: 10.1074/jbc.M112384200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ablonczy Z, Crouch R, Goletz P, et al. 11-cis Retinal reduces constitutive opsin phosphorylation and improves quantum catch in retinoid-deficient mouse rod photoreceptors. J Biol Chem. 2002;277:40491–40498. doi: 10.1074/jbc.M205507200. [DOI] [PubMed] [Google Scholar]
- 15.Rohrer B, Goletz P, Znoiko S, et al. Correlation of regenerable opsin with rod ERG signal in Rpe65−/− mice during development and aging. Invest Ophthalmol Vis Sci. 2003;44:310–315. doi: 10.1167/iovs.02-0567. [DOI] [PubMed] [Google Scholar]
- 16.Znoiko SL, Rohrer B, Lu K, Lohr HR, Crouch RK, Ma JX. Downregulation of cone-specific gene expression and degeneration of cone photoreceptors in the Rpe65−/− mouse at early ages. Invest Ophthalmol Vis Sci. 2005;46:1473–1479. doi: 10.1167/iovs.04-0653. [DOI] [PubMed] [Google Scholar]
- 17.Rohrer B, Lohr HR, Humphries P, Redmond TM, Seeliger MW, Crouch RK. Cone opsin mislocalization in Rpe65−/− mice: a defect that can be corrected by 11-cis retinal. Invest Ophthalmol Vis Sci. 2005;46:3876–3882. doi: 10.1167/iovs.05-0533. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Moiseyev G, Takahashi Y, Ma JX. RPE65 gene delivery restores isomerohydrolase activity and prevents early cone loss in Rpe65−/− mice. Invest Ophthalmol Vis Sci. 2006;47:1177–1184. doi: 10.1167/iovs.05-0965. [DOI] [PubMed] [Google Scholar]
- 19.Ma J, Xu L, Othersen DK, Redmond TM, Crouch RK. Cloning and localization of RPE65 mRNA in salamander cone photoreceptor cells. Biochim Biophys Acta. 1998;1443:255–261. doi: 10.1016/s0167-4781(98)00221-8. [DOI] [PubMed] [Google Scholar]
- 20.Znoiko SL, Crouch RK, Moiseyev G, Ma JX. Identification of the RPE65 protein in mammalian cone photoreceptors. Invest Ophthalmol Vis Sci. 2002;43:1604–1609. [PubMed] [Google Scholar]
- 21.Ong DE, MacDonald PN, Gubitosi AM. Esterification of retinol in rat liver. Possible participation by cellular retinol-binding protein and cellular retinol-binding protein II. J Biol Chem. 1988;263:5789–5796. [PubMed] [Google Scholar]
- 22.Saari JC, Bredberg DL. Lecithin:retinol acyltransferase in retinal pigment epithelial microsomes. J Biol Chem. 1989;264:8636–8640. [PubMed] [Google Scholar]
- 23.Saari JC, Bredberg DL, Farrell DF. Retinol esterification in bovine retinal pigment epithelium: reversibility of lecithin:retinol acyltransferase. Biochem J. 1993;291(Pt 3):697–700. doi: 10.1042/bj2910697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Batten ML, Imanishi Y, Maeda T, et al. Lecithin-retinol acyltransferase is essential for accumulation of all-trans-retinyl esters in the eye and in the liver. J Biol Chem. 2004;279:10422–10432. doi: 10.1074/jbc.M312410200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshizawa T, Wald GB. Pre-lumirhodopsin and the bleaching of visual pigments. Nature. 1963;197:1279–1286. doi: 10.1038/1971279a0. [DOI] [PubMed] [Google Scholar]
- 26.Wald GB, Brown PK. The molar extinction of rhodopsin. J Gen Physiol. 1953;37:189–200. doi: 10.1085/jgp.37.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ablonczy Z, Crouch RK, Knapp DR. Mass spectrometric analysis of integral membrane proteins at the subpicomolar level: application to rhodopsin. J Chromatogr B. 2005;825:169–175. doi: 10.1016/j.jchromb.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 28.Baehr W, Karan S, Maeda T, et al. The function of Guanylate Cyclase 1 (GC1) and Guanylate Cyclase 2 (GC2) in rod and cone photoreceptors. J Biol Chem. 2007;282:8837–8847. doi: 10.1074/jbc.M610369200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson DA, Li Y, McHenry CL, et al. Mutations in the gene encoding lecithin retinol acyltransferase are associated with early-onset severe retinal dystrophy. Nat Genet. 2001;28:123–124. doi: 10.1038/88828. [DOI] [PubMed] [Google Scholar]
- 30.Marlhens F, Bareil C, Griffoin JM, et al. Mutations in RPE65 cause Leber’s congenital amaurosis. Nat Genet. 1997;17:139–141. doi: 10.1038/ng1097-139. [DOI] [PubMed] [Google Scholar]
- 31.Wald G, Brown PK, Smith PH. Iodopsin. J Gen Physiol. 1955;38:623–681. doi: 10.1085/jgp.38.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kennedy MJ, Dunn FA, Hurley JB. Visual pigment phosphorylation but not transducin translocation can contribute to light adaptation in zebrafish cones. Neuron. 2004;41:915–928. doi: 10.1016/s0896-6273(04)00086-8. [DOI] [PubMed] [Google Scholar]
- 33.Ablonczy Z, Kono M, Knapp DR, Crouch RK. Palmitylation of cone opsins. Vis Res. 2006;46:4493–4501. doi: 10.1016/j.visres.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Humphries MM, Rancourt D, Farrar GJ, et al. Retinopathy induced in mice by targeted disruption of the rhodopsin gene. Nat Genet. 1997;15:216–219. doi: 10.1038/ng0297-216. [DOI] [PubMed] [Google Scholar]
- 35.Lem J, Krasnoperova NV, Calvert PD, et al. Morphological, physiological, and biochemical changes in rhodopsin knockout mice. Proc Nat Acad Sci USA. 1999;19:736–741. doi: 10.1073/pnas.96.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deretic D. A role for rhodopsin in a signal transduction cascade that regulates membrane trafficking and photoreceptor polarity. Vis Res. 2006;46:4427–4433. doi: 10.1016/j.visres.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 37.Noorwez SM, Malhotra R, McDowell JH, Smith KA, Krebs MP, Kaushal S. Retinoids assist the cellular folding of the autosomal dominant retinitis pigmentosa opsin mutant P23H. J Biol Chem. 2004;279:16278–16284. doi: 10.1074/jbc.M312101200. [DOI] [PubMed] [Google Scholar]
- 38.Tam BM, Moritz OL, Hurd LB, Papermaster DS. Identification of an outer segment targeting signal in the COOH terminus of rhodopsin using transgenic Xenopus laevis. J Cell Biol. 2000;151:1369–1380. doi: 10.1083/jcb.151.7.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deretic D, Williams AH, Ransom N, Morel V, Hargrave PA, Arendt A. Rhodopsin C terminus, the site of mutations causing retinal disease, regulates trafficking by binding to ADP-ribosylation factor 4 (ARF4) Proc Natl Acad Sci USA. 2005;102:3301–3306. doi: 10.1073/pnas.0500095102. [DOI] [PMC free article] [PubMed] [Google Scholar]