Abstract
Objective
To determine how phonation is affected by the presence and by alteration in the position of the supraglottic structures.
Method
Study used three excised canine larynges. A series of pressure-flow experiments were completed first on the excised larynx with false folds and epiglottis intact, then with the epiglottis removed, and finally with the false folds removed. Aerodynamic and acoustic effects were quantified with the analysis of the pressure, flow, and audio signals.
Results
The results of the study indicated that (a) elevation of the epiglottis to upright position from a horizontal position decreased subglottal pressure, increased flow (decreased laryngeal resistance), and slightly decreased fundamental frequency (b) vibration of the false vocal folds induced some irregularity into the acoustic output of the larynx, (c) the presence of the epiglottis and the false vocal folds enhanced the second partial of the acoustic signal, and (d) the absence of the epiglottis and false folds increased low frequency noise (between 0 and 300 Hz).
Conclusion
Alteration in the position of the supraglottic structures affects laryngeal aerodynamics and acoustics, possibly due to biomechanical linkage with true vocal folds. When the supraglottic structures are present they act as resonators, enhancing the second partial and when they are absent (as in persons with supraglottic laryngectomy), low frequency noise is increased perhaps due to the loss of boundary conditions or to the presence of loose tissue.
Keywords: laryngeal ventricle, false folds, epiglottis, excised canine larynges, supraglottic laryngectomy
INTRODUCTION
The effect of supraglottic laryngeal structures on phonation has been examined by an increasing number of investigators, but is still not well understood. Although it has long been known that the approximated false vocal folds can function as a sound source1, more recent observations indicate that the unapproximated false folds and the mucosa overlying the arytenoid cartilages vibrate during normal phonation2–3, presumably adding energy to the acoustic signal generated from true vocal fold vibration. Svec et al2 examined the resonance of laryngeal tissue and found that the ventricular folds, aryepiglottic folds and arytenoid cartilages, as well as the true vocal folds, responded to external excitation via a shaker on the subject’s neck. The amplitude of vibration of these supraglottic structures exceeded that of the true vocal folds during excitations below 100 Hz. Granqvist and Lindestad3 also observed covibrations in the ventricular folds and in the mucosa covering the arytenoid cartilages during high speed laryngoscopy. Alternatively, other researchers have suggested that the soft tissue lining the vocal tract may absorb energy at low frequencies due to tissue compliance4 or, that the supraglottic laryngeal spaces could function as a low-pass filter5.
The geometry and mechanical properties of the supraglottic laryngeal structures have also been examined. Agarwal et al.6 studied the false vocal folds and provided the shape and size differences between male and female human larynges from laminagraphic images obtained during phonation. Also, Haji et al.7 estimated the mechanical properties of the human false vocal folds in an excised model and reported that its elastic modulus was much less than for the true vocal folds. This measurement was improved recently through an in vitro technique by Chan et al.8, who reported an elastic modulus for the false vocal folds that was up to 10 times lower than for the true vocal folds (estimated from their Figure 7). This difference in the mechanical properties may be responsible for irregularity in the co-vibration of the true vocal folds and false vocal folds. It has been reported that when false vocal folds vibrate with large amplitude and in irregular fashion, voice roughness was observed9. Using image analysis of high-speed and kymographic recordings, Lindestad et al.9 observed that the true vocal folds vibrated in a stable fashion, but the false vocal folds vibrated in irregular fashion.
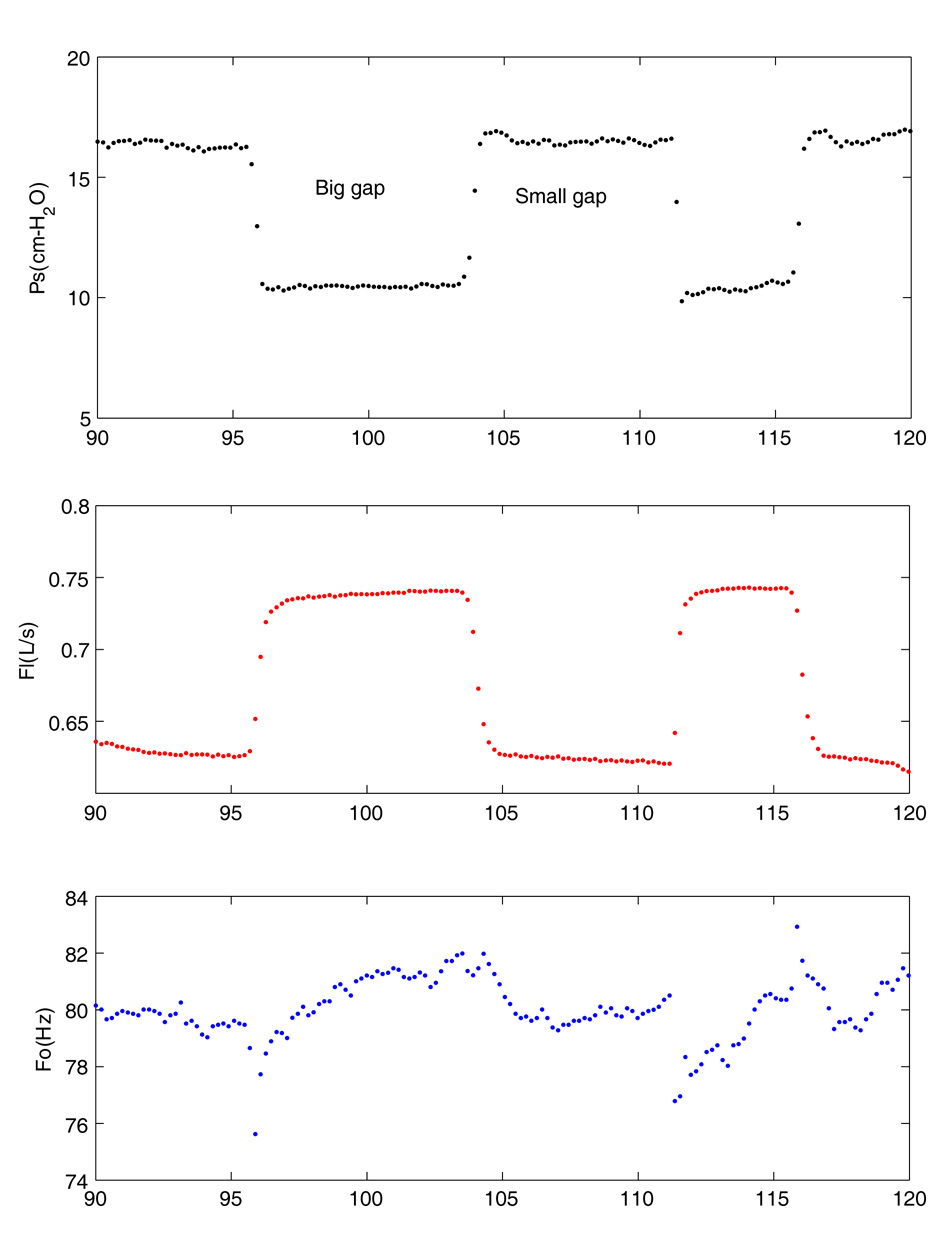
Figure 7.
Changes in aerodynamic and acoustic signals associated with varying the posterior gap between the false folds (epiglottis removed). The false folds have a small posterior gap initially. The gap is increased then decreased and then increased again.
Interest in the supraglottic structures results, in part, from observations of alteration in the position of these structures during speech and song. Speaker and singers adjust the position and shape of the false folds and epiglottis to produce different voice qualities such as sob, opera, and etc10. Alteration in the dimensions of supraglottic cavities contributes to production of the singer’s formant and resonant voice11–12. Stager et al.13 investigated activity of the false folds in persons with normal vocal folds, with vocal nodules, and with vocal hyperfunction. They reported that medial compression of the false vocal folds is used in normal laryngeal articulation (e.g. glottal stop production), but that there was an increased incidence of both medial and anterior/posterior compression in the hyperfunctional patients.
In a previous work by our group using excised canine larynges14, it was reported that the supraglottic structures generally have an increasing effects on laryngeal resistance and sound intensity. It was also reported that FVF medial compression had significant effects on laryngeal resistance, but AP compression had minor effects. Both FVF compression and AP compression contribute positively to the sound intensity.
The purpose of this study was to examine the effects of the false folds and epiglottis on the phonatory characteristics of excised canine larynges. The primary purpose of our initial series of experiments was to compare the acoustic and aerodynamic output from the excised canine larynges with different positions of supraglottic structures. It was hypothesized that these additional structures will change the frequency spectra of the source of phonation. Spectral analysis was used to examine how the distribution of energy was altered in the different conditions. Aerodynamic and EGG changes that accompany acoustic changes are reported as well.
METHODS
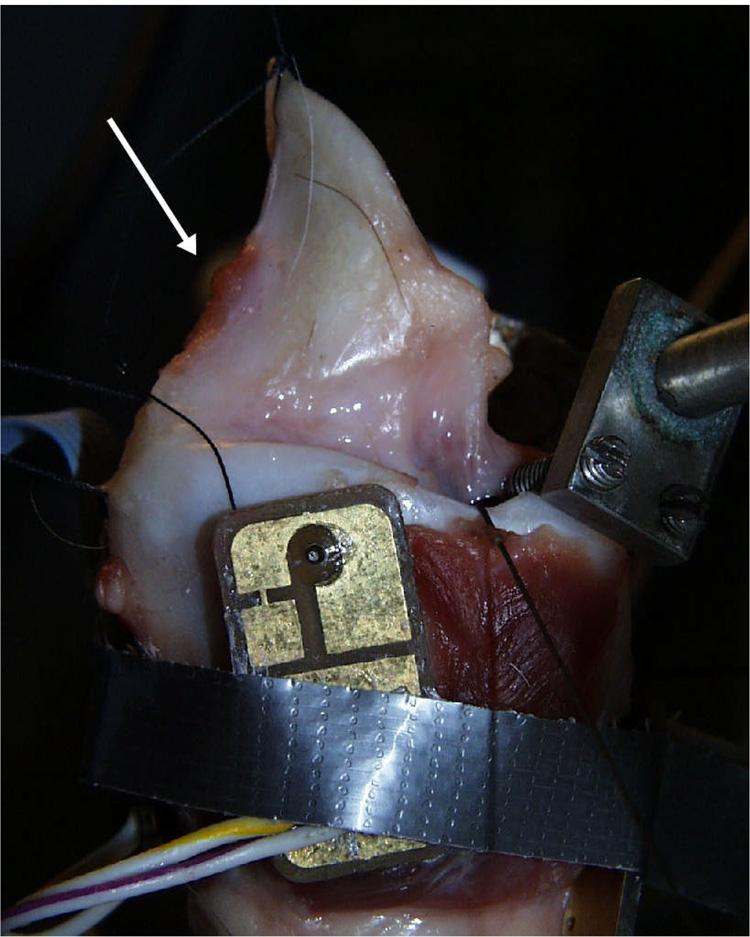
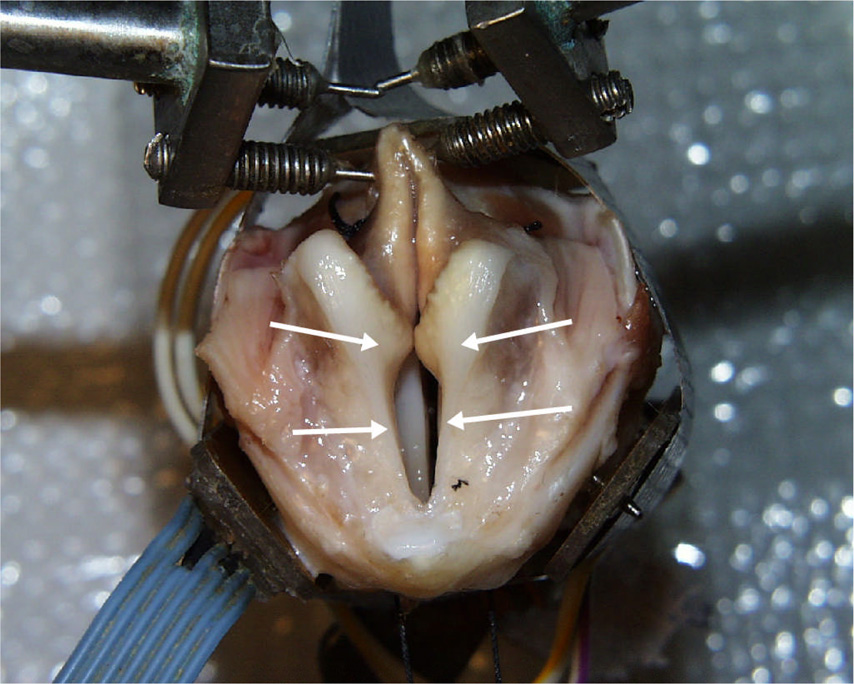
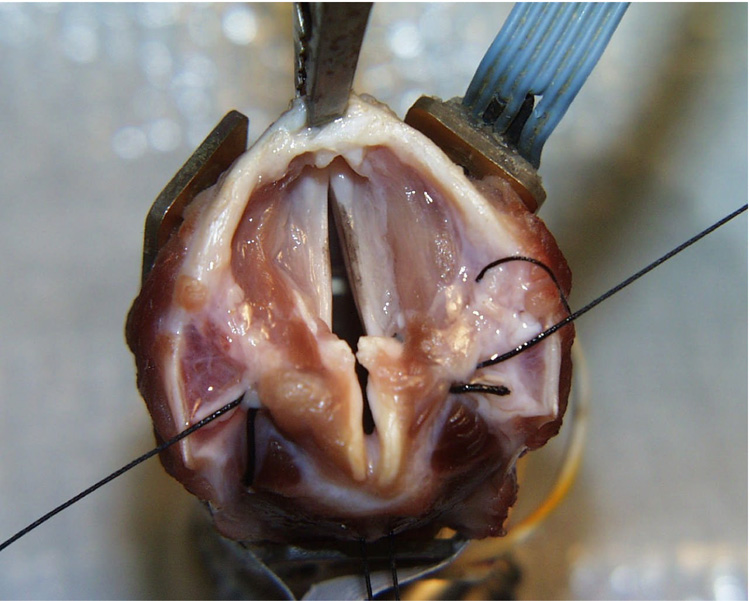
Canine specimens were obtained from large adult subjects with no evidence of trauma or head and neck disease. The experimental protocol was approved by the Institutional Animal Care and Use committee (IACUC) of the University of Iowa. All larynges were quick-frozen using liquid nitrogen, stored in a freezer (at −20°C), and slowly thawed in a refrigerator prior to use. Each larynx was prepared and mounted with the trachea over a 3/4-inch (19 mm) tube, which supplied pressurized, heated, and humidified air15–16. Airflow through the glottis was a controlled variable. Glottal adduction was accomplished either by using two-pronged probes to press the arytenoids together or by passing a suture to simulate the lateral cricoarytenoid muscle action, as in arytenoids adduction. First the excised larynx with false folds and epiglottis included was subjected to a series of pressure-flow experiments (Figure 1a). Then the epiglottis was removed and the experiment was repeated (Figure 1b). Finally, the false folds were removed and the experiment was repeated a third time (Figure 1c). Because we were interested in the effects of alteration in the position, as well as the presence, of the supraglottic structures, a suture was tied to the anterior aspect of the epiglottis at midline just inferior to the point where muscles attach (as indicated by arrow in Figure 1a). The position of the epiglottis was adjusted by changing the tension of the suture line. Sutures were also attached at the posterior and middle 1/3 positions along the medial edge of the false vocal folds to allow for manipulation of the posterior glottis and the membranous false folds (as indicated by arrow in Figure 1b). The control parameters were adduction level, subglottal pressure, lateral gap of the false folds, and the anterior-posterior positioning of the epiglottis.
Figure 1.
Canine larynges with (a) lateral view of larynx with epiglottis and false folds intact (EGG electrode on lateral aspect of larynx and vocal folds adducted using prongs), with upright epiglottis (b) epiglottis removed and false folds intact, and (c) epiglottis and false folds removed (vocal folds adducted with sutures). The arrow in Figure 1A indicates the point of attachment for the suture used to manipulate the position of the epiglottis. The arrows in Figure 1B indicate the point of attachment for the sutures used to manipulate the position of the false folds.
The mean subglottal pressure was monitored with a wall mounted water manometer (Dwyer No. 1230-8) at a location 10 cm below the glottis. The time-varying subglottal pressure was recorded using a pressure transducer (Microswitch 136PC01G1) at the same location as the manometer tap. Flow rate was measured with a pneumatic transducer. EGG, pressure, flow rate and microphone signals were monitored on a 4-channel Tektronix digital oscilloscope and digitized at 10k samples/sec per channel, using a 14-bit A/D board and Windaq software (Dataq Instruments) on a Pentium computer. The mean subglottal pressure, mean flow rate, and sound pressure level were recorded manually for each oscillating condition of the larynx during the experiment. A superior-view video image was recorded with a stroboscopic light to reveal oscillatory motion. The sound of the larynx was recorded with a microphone positioned 6 inches from the larynx and recorded on a Sony portable DAT recorder and on a PC computer with Windaq software and later digitized with the Sound Forge software (Sonic Foundry). This was the signal used to obtain the Fast Fourier (FFT) spectra of the signal. The microphone signal was also calibrated against a sound level meter (Extech Instruments, model 407762) for intensity measurement during one of the excised larynx experiments.
For each structure a series of pressure-flow sweeps and sustained oscillations were recorded on the computer for 10–20 seconds. For the cases with varying false-fold gap or anterior-posterior positioning of the epiglottis, long data sets of 2–3 minutes were recorded for later analysis. The EGG signal, subglottal pressure, and mean flow rate were converted to physical quantities in MATLAB. The phonatory section was divided into 100 to1000 segments (depending on the length of data) such that each segment included at least 10 cycles. The mean values of pressure, flow rate, fundamental frequency, pressure amplitude (AC pressure), and sound intensity for each segment were calculated. The EGG signal was also used to calculate the closed quotient and speed quotient by using the peak derivative time markers.
RESULTS
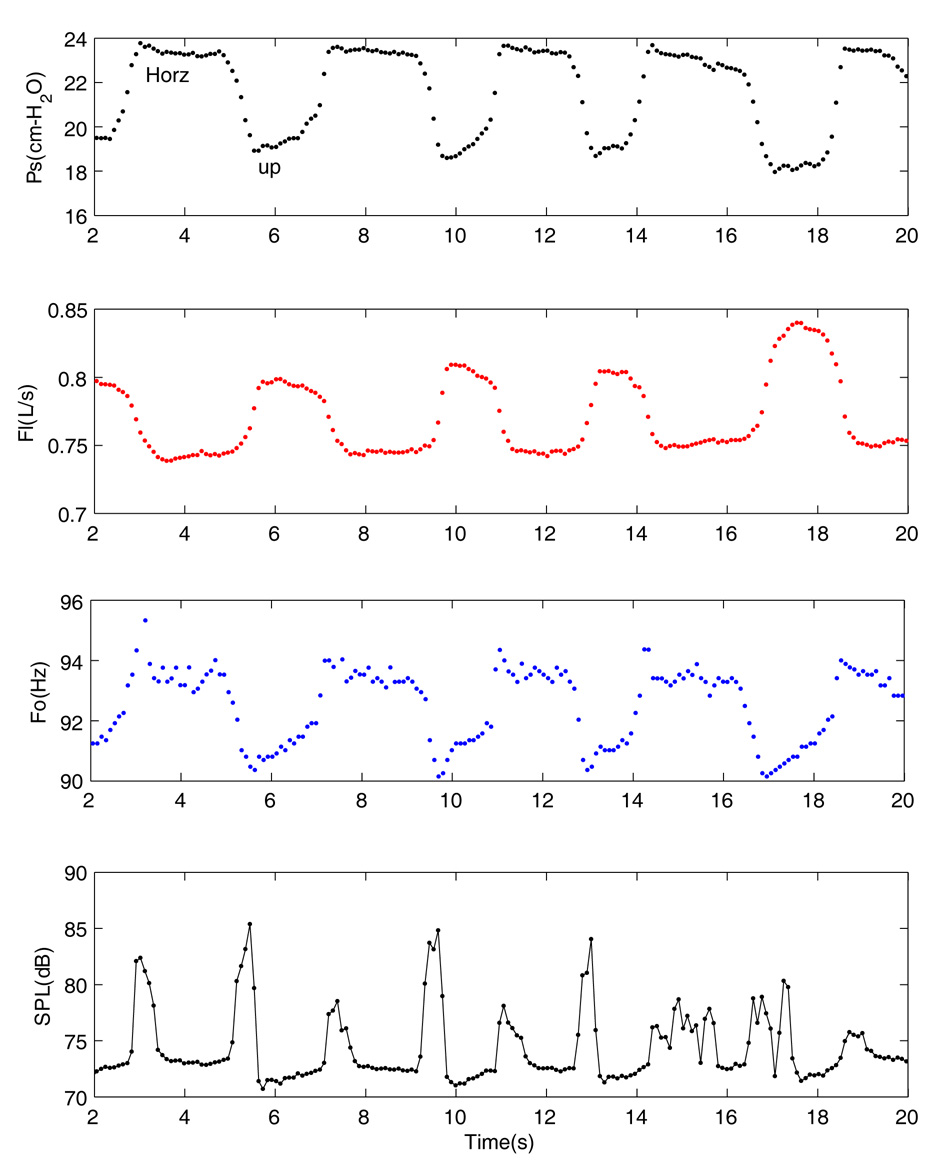
Figure 2 shows the effects of the epiglottis position on the performance of the larynx. This was a recording of a relatively slow manipulation of the epiglottis, in which alternating positions were held for 2 to 4 seconds. In this figure, each data point represents the mean value calculated for a segment of 10–15 cycles. Subglottal pressure and flow rate are shown in the first and second graphs from the top. The mean oscillation frequency, calculated with a zero crossing technique in MATLAB from the EGG signal, is shown in the third graph and finally, the sound pressure level calculated from the microphone signal is plotted in the fourth panel. At the start of the record (left edge of Figure 2, Time = 5 s), tension was applied to the suture and the epiglottis was in an upright position. When the tension on the suture was released, the epiglottis resumed a horizontal position. As the epiglottis was moved from a horizontal to an upright position, subglottal pressure decreased, flow rate increased (indicating decreased glottal resistance), and frequency decreased slightly. When the suture was released, they returned to their original values. This effect was repeatable within each experiment. A sudden increase of sound intensity was associated with movement of the epiglottis that showed itself with repeated spikes on the SPL curves.
Figure 2.
Changes in aerodynamic and acoustic signals associated with altering the position of the epiglottis, upright (Up) and horizontal (Horz). Waveforms from the top to bottom are: subglottal pressure, flow rate, fundamental frequency, and sound pressure calculated from the microphone signal. Each data points on these waveforms correspond to the mean values of the corresponding signal calculated for 10–20 cycles.
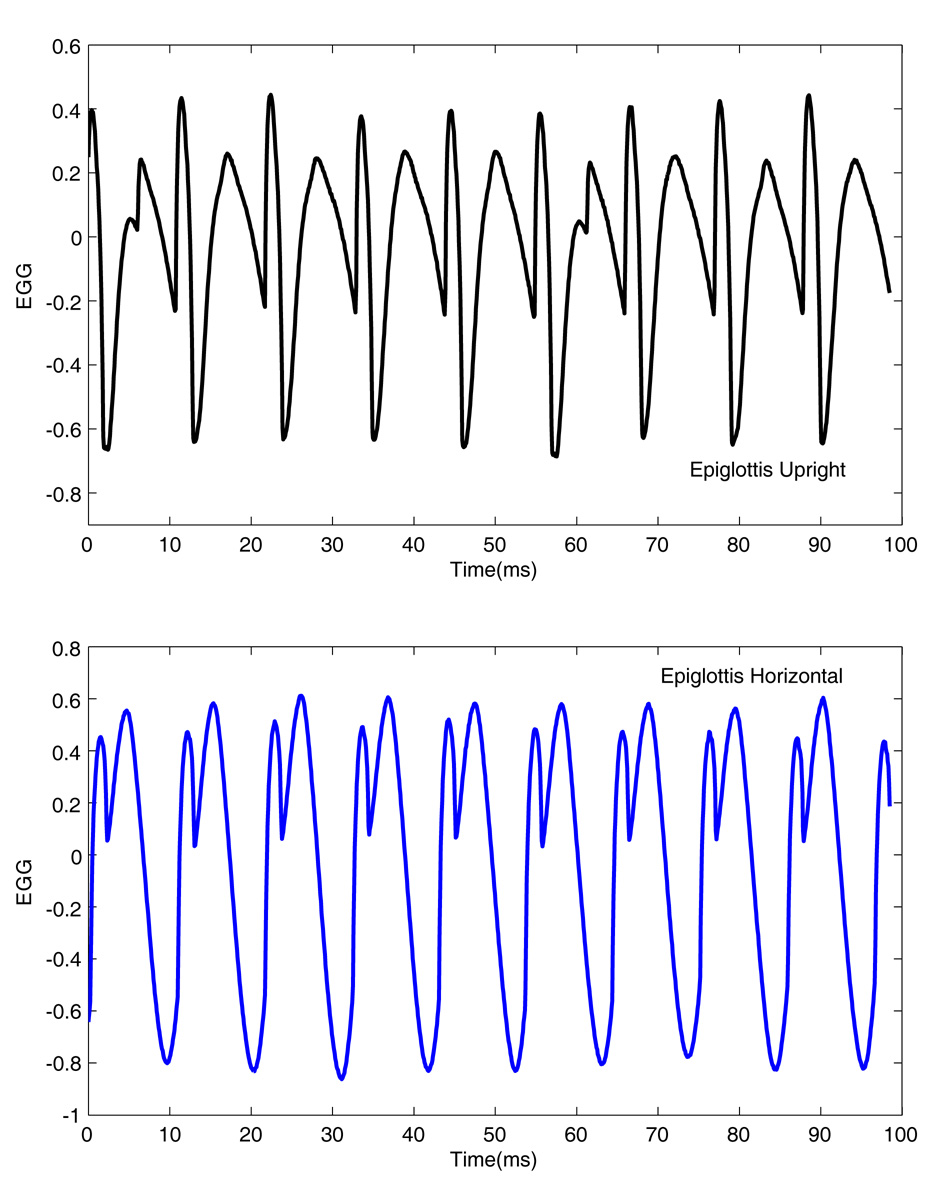
The oscillation of the true and false vocal folds may be different at different positions of the epiglottis. This can be observed through the EGG waveforms in Figure 3. There are major differences between these two oscillating conditions that are observed in these waveforms. With upright epiglottis (top panel), the EGG appears to have double closure or double contact. This special shape of the EGG waveforms makes it difficult to calculate the closed quotient or speed quotient for the oscillation. In the horizontal position (bottom panel), besides the double contact, the subglottal pressure waveform shows some strong harmonics. With the upright epiglottis, the fundamental frequency was decreased from 93.7 to 90.7 Hz, the mean subglottal pressure was decreased from 23.6 to 19.2 cm-H2O, and the mean flow rate was increased from 744 to 798 ml/s.
Figure 3.
EGG waveforms obtained with an upright epiglottis and a horizontal epiglottis.
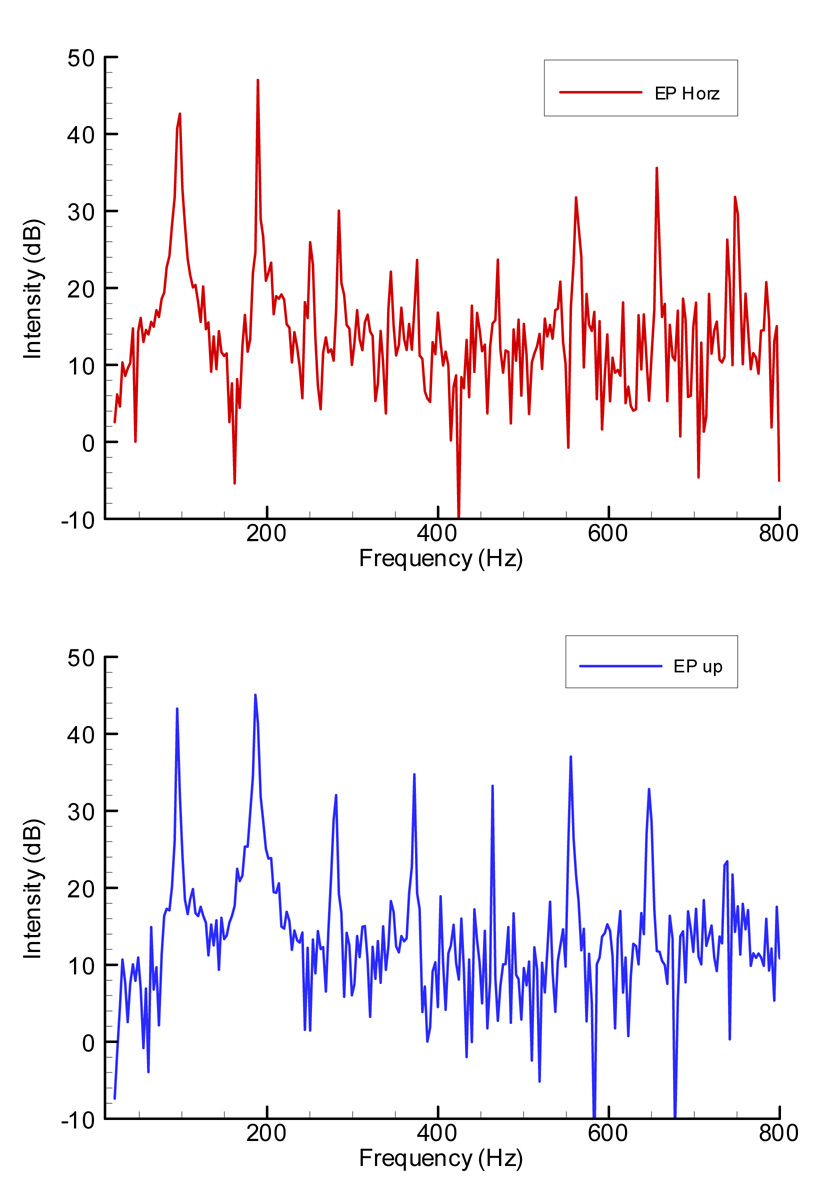
The manipulation of the epiglottis was also performed at a much slower rate, such that each condition lasted for at least 10 seconds. This allowed examination of the spectrogram of the microphone signal and extraction of its harmonic contents. The changes in the measured variables were consistent with those previous shown in Figure 2. Using a Fast Fourier Transform (FFT) in MATLAB, spectrums were calculated and plotted in Figure 4. The top panel shows the FFT spectrum of the microphone signal from the larynx with the epiglottis in horizontal position, with mean flow rate of 730 ml/s, mean subglottal pressure of 23.7 cmH2O, and mean SPL of 73.2 dB. The first partial (fundamental) had an amplitude of 43.4 dB and the second partial had a stronger amplitude of 46.9 dB. The bottom panel shows the FFT spectrum from the larynx with the epiglottis in upright position. The mean flow rate was 780 ml/s, mean pressure 20.1 cm-H2O, and mean SPL of 73.5 dB. Similarly, the second partial at 46.3 dB was stronger than the fundamental at 43.4 dB.
Figure 4.
Acoustic spectra of the microphone signal during slow alterations in the position of the epiglottis. The top figure was calculated from 5-second microphone signal with epiglottis in a horizontal position. The bottom graph was calculated from the 5-seceond segment with the epiglottis in an upright position.
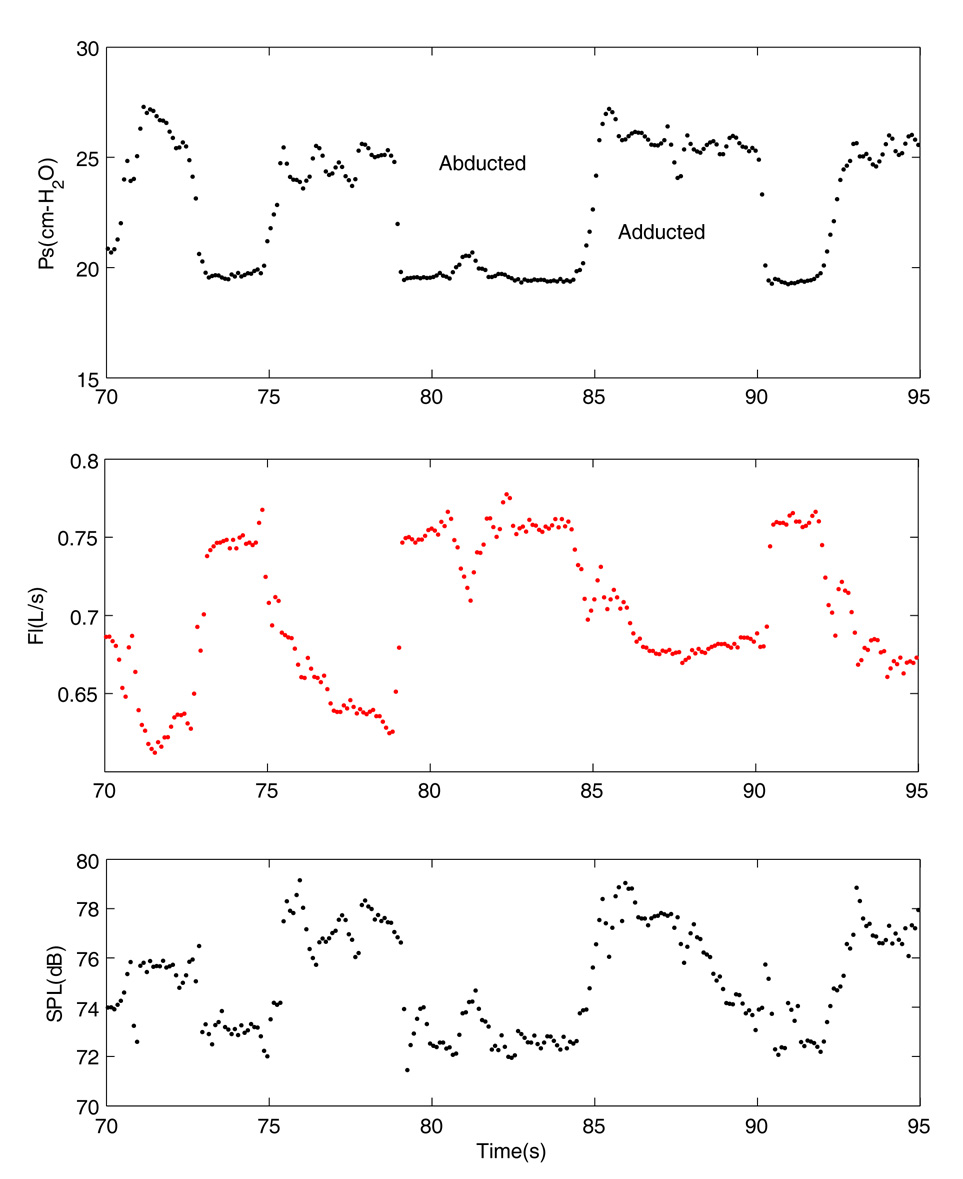
Figure 5 shows the mean glottal parameters during the manipulation of the false vocal folds. In this case, the false vocal folds vibrated along with the true vocal folds (in an excised larynx without epiglottis) and the false vocal folds were adducted at the mid-membranous location and released repeatedly. This adduction not only made changes in the pressure, flow, and sound intensity, it created some irregularity in the vibrations of the false vocal folds. As the false folds were medialized, the mean subglottal pressure increased from 19.5 to 26 cm-H2O and mean flow rate decreased from 755 to 680 ml/s. Because of the false folds vibrations, the EGG waveforms had double peaks. The fundamental frequency of the adducted condition was 107.8 Hz compared to 99.1 Hz for the abducted condition. Due to the shape of the EGG waveforms, the pitch extraction algorithm may erroneously provide the frequency of the second partial instead of the fundamental.
Figure 5.
Variations of glottal mean parameters during medial adduction of the false vocal folds. Epiglottis was removed. The description of the waveforms is similar to those of figure 2.
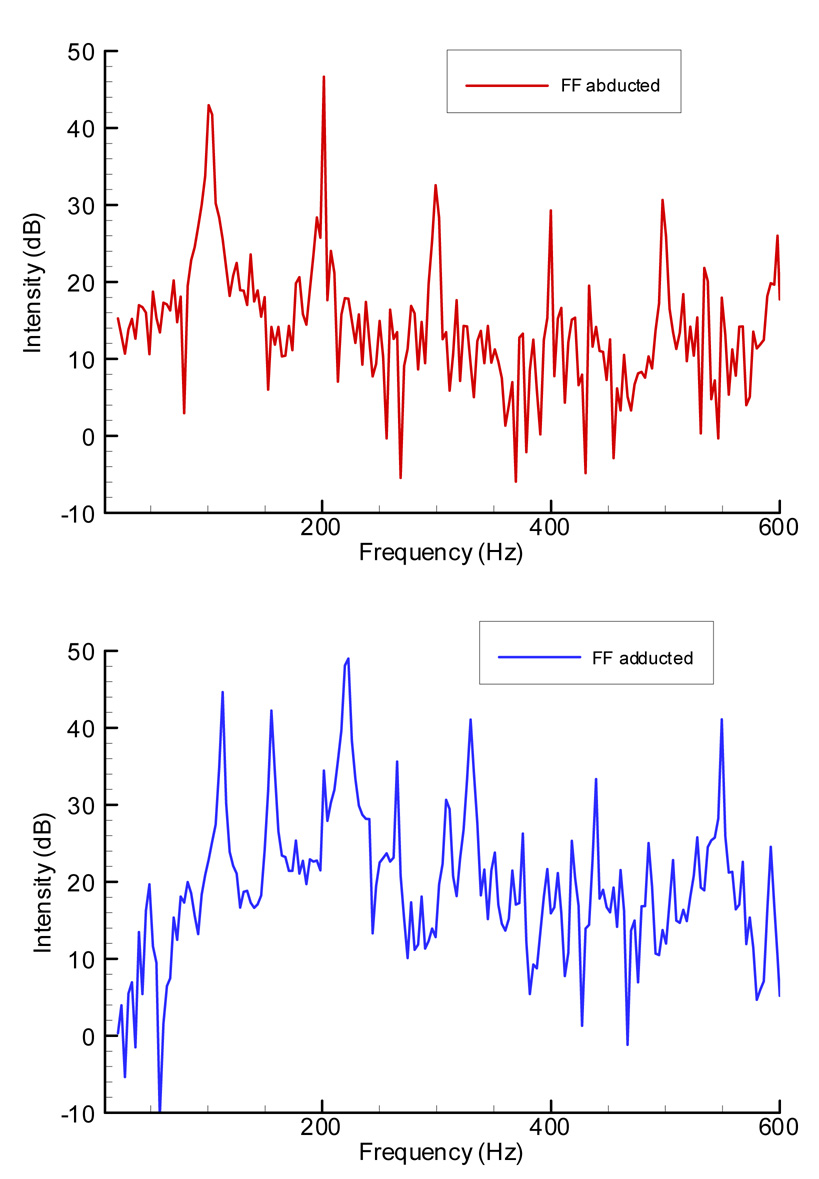
The irregularity of the false folds oscillation can be detected better through the FFT spectrum of the microphone signal from these two conditions. Figure 6 demonstrates these instabilities clearly. In the top panel, the audio spectrum of the larynx with false folds before adduction indicates amplification of the second partial from 43.8 to 47 dB. However, in the bottom panel with adducted FVF, in addition to a second partial (223Hz) that is stronger than the fundamental (113 Hz) by 4.3 dB, a subharmonic of 156 Hz appears with an intensity of 42.3 dB. This suggests the irregularity of the vibration is due to the participation of false vocal folds. The false vocal folds oscillated in both conditions and the frequency resolution in the graph was about 3 Hz.
Figure 6.
Acoustic spectra of the microphone signal recorded during adduction of the mid-membranous false vocal folds. The top figure was calculated from a one-second microphone signal with FVF in abducted position. The bottom graph was calculated from the one-second segment for the adducted FVF position.
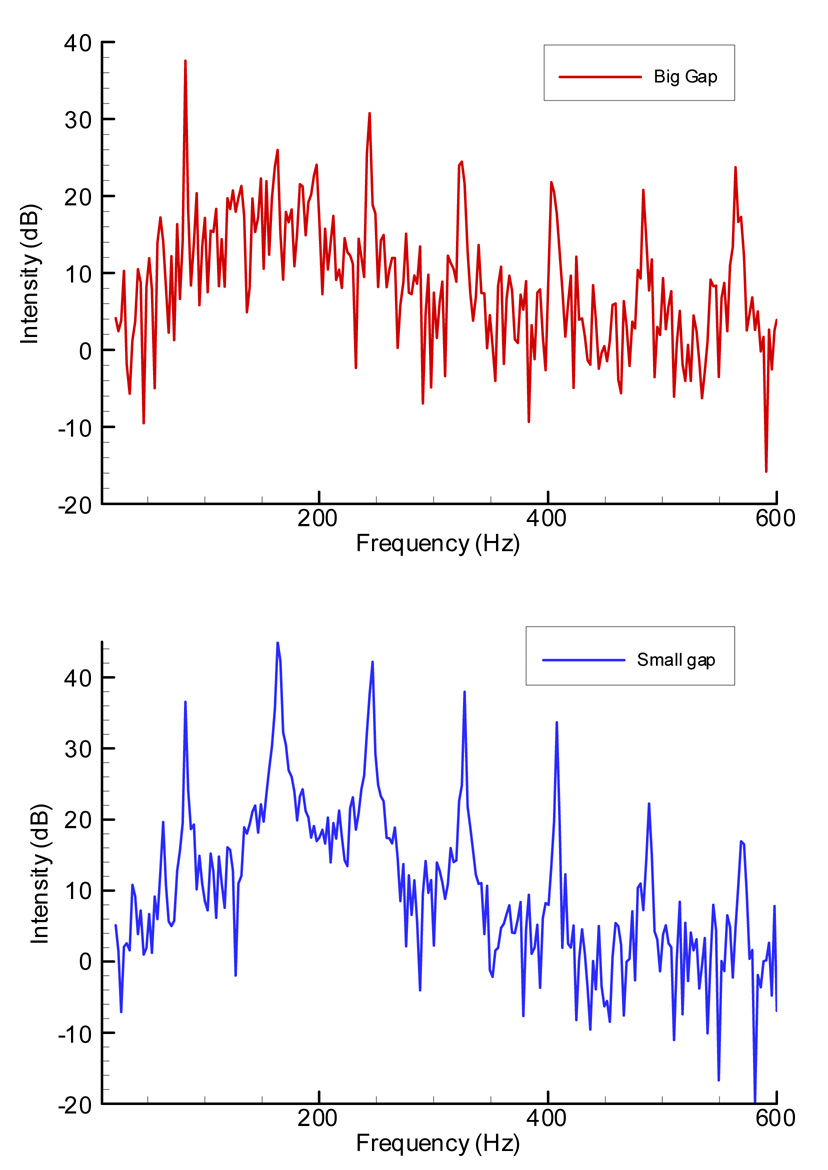
Figure 7 shows the effects of variation in the posterior gap of the false folds on the larynx with the epiglottis removed. In this experiment, the posterior gap was increased by applying tension to sutures attached to the posterior false folds (above the vocal processes). When the gap was increased, mean subglottal pressure decreased about 30% and mean flowrate increased about 15% (decreasing glottal resistance), as seen in the top two graphs. There was no significant change in the mean fundamental frequency. However, the sound of the larynx changed with this process. To explain this change, the FFT spectrum of the microphone signal was compared for two conditions. As shown in Figure 8, during the small posterior gap condition, the second partial become stronger than the fundamental and there was more energy in the higher partials as well.
Figure 8.
Acoustic spectra of the microphone signal during false vocal folds posterior gap changes. The top figure was calculated from a one-second segment of the microphone signal recorded with a large posterior gap. The bottom figure was calculated from one-second segment recorded with a small posterior gap.
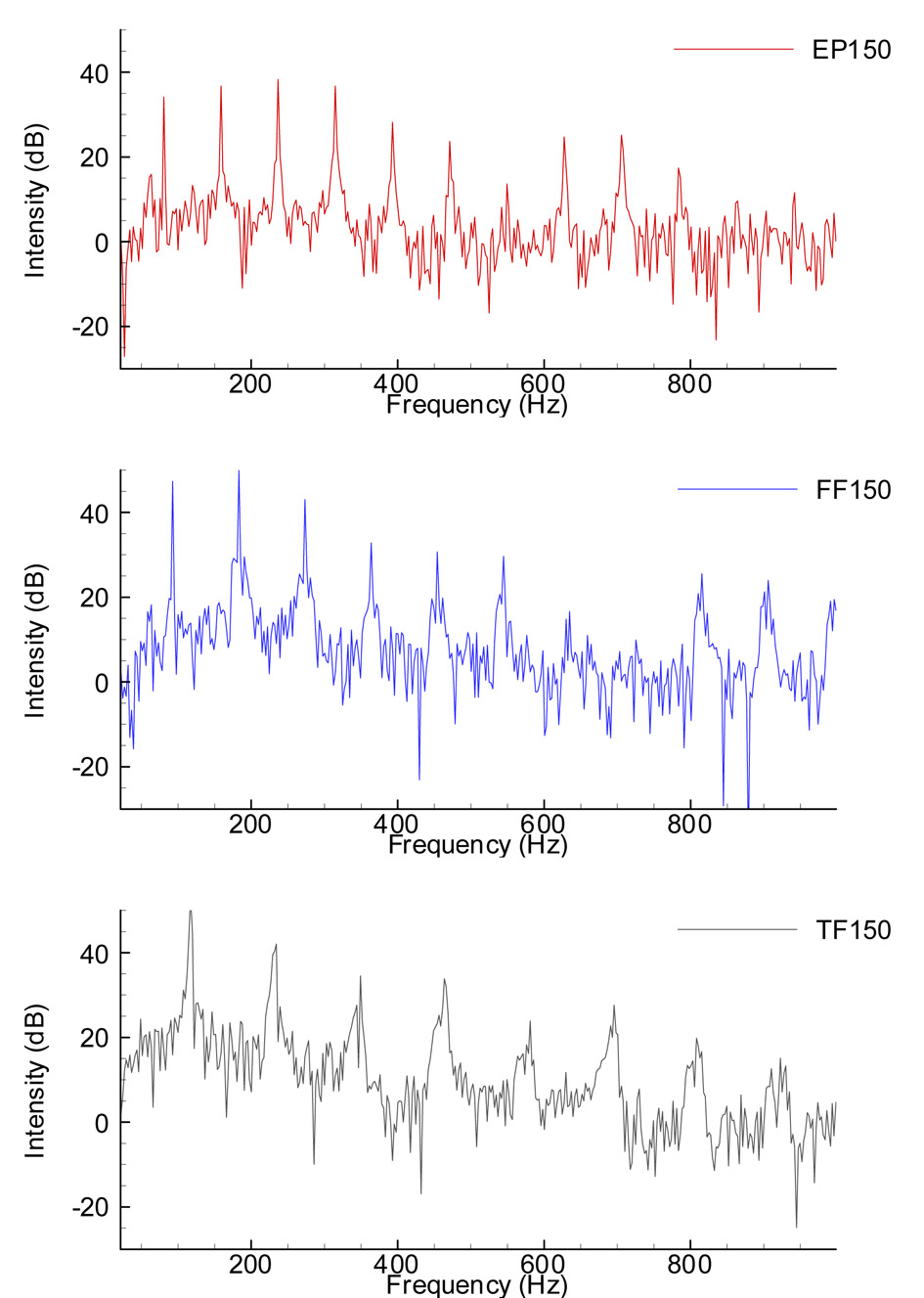
Figure 9 shows the effects of removal of the supraglottic structures on the acoustic spectrum. The top graph shows the FFT spectrum for the intact larynx including epiglottis. The middle graph shows the FFT spectrum for the larynx with false folds (but no structures above the false folds) and the lower graph shows the spectrum for the true folds. These results were obtained from one of the canine larynges used in this experiment for a constant adduction level of 150-gram weight and a subglottal level of 20 cm H2O. Progressive removal of the supraglottic structures resulted in a noticeable change in the spectrum, primarily by increasing the low frequency noise (between 0 and 300 Hz). The spectrum for the true vocal folds alone (without the supraglottic structures) typically had a substantially greater amount of noise between 0 and 300 Hz, with a peak around 150 Hz, than the spectrum for the larynx with the supraglottic structures intact. Alteration in the position of the epiglottis or false folds or in the length or adduction of the vocal folds altered the amplitude and frequency of the harmonics without altering the basic finding regarding low frequency spectral noise. Increasing true vocal fold adduction resulted in a spectrum with a greater number of harmonics and increasing vocal fold length resulted in a higher fundamental frequency, without altering amplitude of the low frequency noise. These findings were repeatable within and across experiments.
Figure 9.
Change in the acoustic spectra of the acoustic signal with progressive removal of the supraglottic structures. Acoustic signal recorded during phonation at constant subglottal pressure (20 cm H2O) and adduction (150 grams). The top graph is for true vocal folds, false folds, and epiglottis intact, the middle graph for true and false folds but no epiglottis, and the bottom graph for true vocal folds without epiglottis or false folds.
DISCUSSION AND CONCLUSIONS
Altering the position of the supraglottic structures affected the laryngeal aerodynamics and acoustics. As might be expected, we observed that, with elevation of the epiglottis to upright position, the epilarynx tube assumed the shape of a more elongated open tube. In addition, we observed that the tissue lining the inside of the tube became tauter and the false vocal fold gap increased. These observations were consistent with the findings that, with the upright epiglottis, subglottal pressure decreased and flow rate increased (indicating decreased laryngeal resistance). The finding of a slight decrease in fundamental frequency may have been due to the decreased subglottal pressure. The position of the structures may affect the adduction of the vocal folds due to biomechanical linkage between the epiglottis, the false folds, and possibly the true folds as well.
A second finding was that vibration of the false vocal folds induced some irregularity into the acoustic output of the larynx at high pressures. Increasing the mid membranous gap between the false vocal folds decreased subglottal pressure, increased flowrate and changed the frequency and mode of vibration, probably indicating a reduction in false vocal folds vibration.
A third finding was that, in addition to the aerodynamic changes seen with manipulation of the position of the supraglottic structures, there were changes in the harmonic structure of the acoustic output due to the presence of the supraglottic structures. The epiglottis (in both the upright and horizontal position) and the false vocal folds (without a posterior gap) seemed to enhance the second partial of the acoustic signal. In other words, the supraglottic structures act as a resonator.
A fourth finding, which is related to an aspect of the acoustic spectra that typically receives little attention, was that low frequency noise was increased in the absence of supraglottic structures. The increased low frequency noise in the absence of the false folds and epiglottis may reflect the impact of loss of natural boundary conditions. Patients who have undergone supraglottic laryngectomy experience this situation. Reports of persons with supraglottic laryngectomy generally describe the voice as functional but weak5, despite the fact that the true vocal folds have been preserved. The voice may be weak because there is noise being generated by loose tissue, or there may be vocal fold asymmetry due to loss of stabilizing supraglottic structures. Alternatively, it could be that the vibration of the supraglottic structures helps to reinforce the periodicity of true vocal fold vibration. We observed vibration of the supraglottic tissue, particularly in high pressure/high flow conditions. These observations were consistent with the findings of Svec et al.2, and Granqvist & Lindestad3. In addition to vibration of the (unapproximated) false folds and the mucosa overlying the arytenoid cartilages noted by these investigators, we also observed vibration of the epiglottis and of the laryngeal tissue that forms the anterior wall of the pyriform sinuses. The supraglottic laryngeal structures may contribute to modulation of the airflow. Vibration of the supraglottic tissue may increase the modulated (harmonic) energy.
The number of partials observed in the acoustic spectra was largely determined by true vocal fold adduction and secondarily by the presence and position of the supraglottic structures. In future studies, vocal fold adduction needs to be adjusted to maximize the number of partials in the signal to improve our ability to observe the effects of supraglottic structures on formant frequencies.
ACKNOWLEDGMENT
National Institute on Deafness and other Communication Disorders, Grant No DC03566 supported this work. The authors would like to thank Sanyukta Jaiswal and Jaclyn Curiel for their assistance in data collection.
REFERENCES
- 1.Saunders WH. Dysphonia plica ventricularis; an overlooked condition causing chronic hoarseness. Annals of Otology, Rhinology & Laryngology. 1956;65(3):665–673. doi: 10.1177/000348945606500308. [DOI] [PubMed] [Google Scholar]
- 2.Svec JG, Horacek J, Sram F, Vesely J. Resonance properties of the vocal folds: In vivo laryngoscopic investigation of the externally excited laryngeal vibrations. J Acoust Soc Am. 2000;108:1397–1407. doi: 10.1121/1.1289205. [DOI] [PubMed] [Google Scholar]
- 3.Granqvist S, Lindestad P-Å. A method of applying Fourier analysis to high-speed laryngoscopy. J Acoustic Soc Amer. 2001;110/6:3193–3197. doi: 10.1121/1.1397321. [DOI] [PubMed] [Google Scholar]
- 4.Riede T, Fitch WT. Vocal tract length and acoustics of vocalization in the domestic dog Canis familiaris. J. Exp. Biol. 1999;202:2859–2869. doi: 10.1242/jeb.202.20.2859. [DOI] [PubMed] [Google Scholar]
- 5.van den Berg J. On the role of the laryngeal ventricle in voice production. Folia Phoniatr (Basel) 1955;7(2):57–69. doi: 10.1159/000262703. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal M, Scherer RC, Hollien H. The false vocal folds: shape and size in frontal view during phonation based on laminagraphic tracings. J Voice. 2003;17(2):97–113. doi: 10.1016/s0892-1997(03)00012-2. [DOI] [PubMed] [Google Scholar]
- 7.Haji T, Mori K, Omori K, Isshiki N. Viscoelasticity of the Vocal Fold. Folia Phoniatrica. 1992;44(1–2):26–27. [Google Scholar]
- 8.Chan RW, Fu M, Tirunagari N. Elasticity of the human false vocal fold. Ann Otol Rhinol Laryngol. 2006;115(5):370–381. doi: 10.1177/000348940611500510. [DOI] [PubMed] [Google Scholar]
- 9.Lindestad PA, Blixt V, Pahlberg-Olsson J, Hammarberg B. Ventricular fold vibration in voice production: a high-speed imaging study with kymographic, acoustic and perceptual analyses of a voice patient and a vocally healthy subject. Logoped Phoniatr Vocol. 2004;29(4):162–170. doi: 10.1080/14015430410020339. [DOI] [PubMed] [Google Scholar]
- 10.Yanagisawa E, Estill J, Kmucha ST, Leder SB. The contribution of aryepiglottic constriction to “ringing” voice quality – A videolaryngoscopic study with acoustic analysis. J Voice. 1989;3:342–350. [Google Scholar]
- 11.Sundberg J. Articulatory interpretation of the “singing formant”. J Acoust Soc Am. 1974;55:838–844. doi: 10.1121/1.1914609. [DOI] [PubMed] [Google Scholar]
- 12.Titze IR. Acoustic interpretation of resonant voice. J Voice. 2001;15:519–528. doi: 10.1016/S0892-1997(01)00052-2. [DOI] [PubMed] [Google Scholar]
- 13.Stager SV, Bielamowicz SA, Regnell JR, Gupta A, Barkmeier JM. Supraglottic activity: Evidence of vocal hyperfunction or laryngeal articulation? J Speech Hear Res. 2000;43(1):229–238. doi: 10.1044/jslhr.4301.229. [DOI] [PubMed] [Google Scholar]
- 14.Alipour F, Jaiswal S, Finnegan E. Aerodynamic and acoustic effects of false folds and epiglottis in excised larynx models. Ann Otol Rhinol Laryngol. 2006 doi: 10.1177/000348940711600210. (in press) [DOI] [PubMed] [Google Scholar]
- 15.Slavit DH, Lipton RJ, McCaffrey TV. Phonatory vocal fold function in the excised canine larynx. Otolaryngology - Head & Neck Surgery. 1990;103(6):947–956. doi: 10.1177/019459989010300611. [DOI] [PubMed] [Google Scholar]
- 16.Alipour F, Scherer RC, Finnegan EM. Pressure-flow relationship during phonation as a function of adduction. J. Voice. 1997;11(2):187–194. doi: 10.1016/s0892-1997(97)80077-x. [DOI] [PubMed] [Google Scholar]