Abstract
Risk for developing a learning disability (LD) or impaired intellectual functioning by age 7 was assessed in full-term children with prenatal cocaine exposure drawn from a cohort of 476 children born full term and enrolled prospectively at birth. Intellectual functioning was assessed using the Wechsler Intelligence Scale for Children–Third Edition (Wechsler,1991) shortform, and academic functioning was assessed using the Wechsler Individual Achievement Test (WIAT; Wechsler,1993) Screener by examiners blind to exposure status. LDs were categorized based on ability-achievement discrepancy scores, using the regression-based predicted achievement method described in the WIAT manual. The sample in this report included 409 children (212 cocaine-exposed, 197 non-cocaine-exposed) from the birth cohort with available data. Cumulative incidence proportions and relative risk values were estimated using STATA software (Statacorp, 2003). No differences were found in the estimate of relative risk for impaired intellectual functioning (IQ below 70) between children with and without prenatal cocaine exposure (estimated relative risk = .95;95%confidence interval [CI] = 0.65,1.39; p = .79). The cocaine-exposed children had 2.8 times greater risk of developing a LD by age 7 than non-cocaine-exposed children (95%CI = 1.05,7.67; p = .038; IQ ≥ 70 cutoff). Results remained stable with adjustment for multiple child and care-giver covariates, suggesting that children with prenatal cocaine exposure are at increased risk for developing a learning disability by age 7 when compared to their non-cocaine-exposed peers.
Academic casualty, including the continuum of learning disorders and lower intellectual functioning requiring educational assistance, has enormous impact on the individual child and family as well as the systems that must support and care for children with disabilities. Recent data from the U.S. Department of Education revealed that over 5.7 million students ages 6 through 21 years received services under the Individuals with Disabilities Education Act, Part B, during the 2000–2001 school year. This figure represents 11.5% of the estimated student enrollment in public schools for grades kindergarten through twelfth grade. Of children receiving special services, 2,887,217 (50%) were identified as having specific learning disabilities (LD) and 612,978 (10.6%) were identified as having mental retardation (U.S. Department of Education, 2001). According to the Special Education Expenditure Project, per-pupil special education expenditures for students with LD average $10,558, compared with per-pupil expenditures of $6,556 for students who do not receive special education services (Chambers, Shkolnik, & Pérez, 2003). Thus, educational costs for students with LD is 1.6 times the amount expended for students without special needs (National Center for Learning Disabilities, 2005). For students with mental retardation and LD, their diminished academic functioning and the impact on opportunities for employment and economic productivity have lifelong consequences. Thus, examination of populations who may have increased risk for these types of disabilities is important at the level of the affected individual as well as the educational and public health system levels.
Over the past decade significant concern has been raised regarding the public health impact of maternal cocaine use during pregnancy and the potential for long-term clinically devastating outcomes for infant and child development. The teratological approach examines whether there is increased risk of neurodevelopmental impairments including physical malformation, growth abnormalities, or abnormal developmental functions as a result of exposure to a toxic agent (Vorhees, 1989). Cocaine, a potential teratogen, readily crosses the placenta and is believed to impact the developing monoaminergic neurotransmitter systems, disrupting both structural and functional aspects of fetal brain development (Mayes, 1999). Monoaminergic neurotransmitters appear early in fetal brain development and are important in the development of neuronal circuitry (Volpe, 1992), with the norepinephrine and serotonin systems extending throughout the brain. Accordingly, cocaine’s influence on these systems may yield more generalized deficits, whereas the impact on monoaminergic pathways involving dopamine is likely to be more focused (Mayes, 1999). In addition, cocaine use during pregnancy is associated with maternal hypertension, decreased uterine blood flow, and fetal vasoconstriction and hypoxemia (Moore, Sorg, Miller, Key, & Resnik, 1986; Volpe, 1992) as well as nutritional deficiencies that may also cause disruptions in fetal neurodevelopment (Frank et al., 1990).
Following the increase in cocaine use among women of childbearing age in the late 1980s, early reports suggested devastating clinical outcomes for infants with prenatal cocaine exposure. Since then, over a decade of research has yielded findings from the infancy and toddler period suggesting that prenatal cocaine exposure may instead lead to subtle neurobehavioral impairments, with the potential to increase long-term risk for learning and social/behavioral difficulties in children (Carmichael Olson & Toth, 1999; Lester, LaGasse, & Seifer, 1998). Little is known, however, regarding the intellectual functioning and learning abilities of school-aged children with prenatal cocaine exposure. Few published studies to date extend beyond age 6, and published reports are often characterized by great variability in sample size, methodology, and consideration of potential confounding, mediating, and moderating influences.
Many of the early infant and preschool studies have not reported deficits in cognitive development in children with prenatal cocaine exposure. In a systematic review of prospective studies published before 2001, nine studies reported no cocaine-related effect on infant cognitive development, most often measured by the Bayley Scales of Infant Development (Frank, Augustyn, Knight, Pell, & Zuckerman, 2001). The authors noted an additional four studies that showed subtle cocaine-related cognitive impairments, but these findings dissipated with statistical adjustment for potential confounders, were primarily evident in a small subgroup within the sample, or did not include statistical control for other prenatal drug exposures. The results from the preschool-aged studies reviewed in this article are similar, with six separate reports emanating from four cohorts indicating no consistent pattern of cognitive deficits attributable specifically to prenatal cocaine exposure after consideration for other prenatal drug exposures.
More recent reports, often from larger cohort studies (summarized in Table 1), suggest mild to moderate cognitive development deficits emerging during the infancy/toddler period that are not always sustained in reports from preschool-aged cohorts. For example, several studies utilizing longitudinal methodologies have shown lower mental scores on the Bayley Scales of Infant Development across varying age points during the first 2 years of life (Behnke, Eyler, Garvan, Wobie, & Hou, 2002; Lewis, Misra, Johnson, & Rosen, 2004; Mayes, Cicchetti, Acharyya, & Zhang, 2003; Singer et al., 2002). Studies of preschool-aged children using standardized assessments of intelligence, however, show more subtle impacts on intellectual processing, typically mediated through other variables or occurring only on subtests rather than global indexes, if at all (see Table 1). Two large study cohorts reporting direct cocaine-related effects during the infancy period found only subtle sustainable effects when the children were tested at older age points during the preschool years (see Table 1; Behnke et al., 2006; Singer et al., 2004).
TABLE 1.
Infant, Toddler, and Preschool Studies Reporting on Prenatal Cocaine Exposure and Child Cognitive and Mental Development (From 2001 to the Present)
| Study | Sample Size and Exposure Groups | Measures | Assessment Age of Children | Controlled or Considered Other Drug Use in Analyses | Cocaine-Related Cognitive and Mental Development Findings |
|---|---|---|---|---|---|
| Infant/toddler studies | |||||
| Brown et al. (2004) | 83 CE children; 63 non-drug (234 birth cohort; 62% retention) | BSID | 2 years | Not applicable due to negative findings in uncontrolled models | No cocaine-related difference between CE and non-CE children; CE children had higher MDI scores when children were in nonparental care |
| Lewis, Misra, et al. (2004) | 147 CE infants; 89 non-CE (361 birth cohort; 65% retention) | BSID | 12, 18, 24, 36 months | Yes | Direct cocaine-related effects on MDI scores in longitudinal models, and at 12 and 24 months in cross-sectional models; dose-response cocaine-associated relationship observed for MDI scores |
| Mayes et al. (2003) | 265 CE; 66 non-CE; 129 non-drug (460 birth cohort; retention across visits ranged from 69%–87%) | BSID–II | 3, 6, 12, 18, 24, 36 months | Not as covariates but in the context of grouping | In longitudinal analyses, CE children had lower MDI scores compared to non-CE and non-drug exposed children across all assessment ages; the cocaine-related effect was entirely mediated through birth weight and gestational age |
| Behnke et al. (2002) | 154 CE (birth cohort); 154 non-CE (retention: 270–288 or 88%–94% for varying age points; 253 or 82% with all 3 age points) | BNBAS; BSID | Birth, 1 and 6 months | Yes | Direct cocaine-related effects at birth and 6 months in longitudinal modeling; indirect effects at birth, 1 month and 6 months mediated by prenatal alcohol and tobacco exposure and birth head circumference |
| Frank et al. (2002) | 75 CE/light; 38 CE/heavy; 90 non-CE (birth cohort 252; 81% retention) | BSID | 6, 12, 24, months | Yes | No cocaine-related main effect for level of cocaine exposure on MDI scores; heavily CE children who received early intervention had higher MDI scores than all other groups |
| Singer et al. (2002) | 218 CE (birth cohort); 197 non-CE retention: 6 months 339 (84%); 12 months 364 (90%); 2 years 379 (94%) | BSID | 6, 12 months, 2 years (corrected age) | Yes | Direct cocaine-related effect on MDI scores (6 point difference in mean MDI between the groups at 2 years); CE children were twice as likely to have a significant developmental delay. MDI scores were related to higher amounts of cocaine metabolites in infant meconium and maternal self-reported measures of amount and frequency of cocaine use during pregnancy. |
| Preschool studies | |||||
| Behnke et al. (2006) | 135 CE children; 141 non-CE children (birth cohort 308; 93% retention) | BSID | 3 years | Yes (in SEM context) | No group differences on MDI or PDI scores. SEM also showed no direct cocaine-related effect on child development measured by the MDI, but cocaine was related to head circumference, which in turn was related to development |
| Frank et al. (2005) | 91 CE children; 79 unexposed (birth cohort 252; 67% retention) | WPPSI–R | 4 years | Yes | No cocaine-related effect on WPPSI–R Full Scale, Performance, or Verbal IQ or any subtests; subgroup differences between heavier and lighter exposure for Object Assembly and Picture Completion subtests only, with heavier exposed children scoring higher |
| Pulsifer, Radonovich, Belcher, & Butz (2004) | 104 cocaine/opiate exposed children; 35 demographically matched controls (birth cohort 204; 68% retention) | SB–IV; Bracken Basic Concept Scale–Revised | 4 to 5 years | Yes | No SB–IV or Bracken group differences between drug-exposed and demographically matched controls. Regression models also showed no drug exposure effects on these measures |
| Singer et al. (2004) | 190 CE; 186 non-CE (415 birth cohort; retention 93%) | WPPSI–R | 4 years | Yes | No cocaine-related effect on WPPSI–R Full Scale, Performance, or Verbal IQ; prenatal cocaine exposure was related to small but significant deficits on several WPPSI–R subscales and was associated with a lower likelihood of achievement of IQ above normative means |
| Bennett, Bendersky, & Lewis (2002) | 85 CE children; 138 unexposed (birth cohort N not reported) | SB–IV | 4 years | Yes | In regression analyses, prenatal cocaine exposure negatively predicted children’s IQ, verbal reasoning, and short-term memory, but only for boys |
| Hurt, Malmud, et al. (2001) | 65 CE children; 68 controls (birth cohort 218; 61% completed both the 3- and 5-year assessments) | WPPSI–R; Batelle Developmental Inventory | 3 & 5 years | Yes (in separate regression analyses, not as a control variable) | No WPPSI–R group differences. CE children performed more poorly than controls at age 3 but not at age 5 on the Battelle; findings were attributed to postnatal environmental factors rather than prenatal cocaine exposure. |
Note. All studies included in the table were prospective, with enrollment occurring during the prenatal or immediate postpartum period. All studies used examiners blind to exposure status. Reported sample sizes and retention percentages are for cases included in data analyses for each published report. CE = cocaine-exposed with other drugs; non-CE = possible exposure to other drugs but not cocaine (usually alcohol, tobacco, and/or marijuana); non-drug = no known drug exposure; BNBAS = Brazelton Neonatal Behavioral Assessment Scale; BSID = Bayley Scales of Infant Development; MDI = Mental Development Index; PDI = Psychomotor Development Index; WPPSI–R = Wechsler Preschool and Primary Scale of Intelligence–Revised; SEM = structural equation modeling; SB–IV = Stanford–Binet Intelligence Scale(4the dition).
School-aged studies of children with prenatal cocaine exposure focusing on intellectual and academic functioning are sparse and methodologically diverse; however, the few studies reporting school-aged achievement or IQ data consistently report no between-group differences related to prenatal cocaine exposure. Wasserman et al. (1998) reported no differences in mean IQ scores between cocaine-exposed and nondrug-exposed children evaluated between 6 and 9 years of age. This sample of 206 children was retrieved from an identified birth sample of 560 children (representing 37% of the birth-identified sample), limiting conclusions due to the absence of information about the children who were not assessed beyond the birth period. Richardson, Conroy, and Day (1996) reported no differences at age 6 in IQ or achievement scores measured by the Wide Range Achievement Test–Revised. Although this cohort was enrolled prospectively with excellent retention to age 6, cocaine exposure was determined through self-report methods without toxicology verification. The cocaine-exposed group (n = 28) was small relative to the non-cocaine-exposed group (n = 523), raising concerns about statistical power and generalizability when interpreting the results.
Hurt, Giannetta, Brodsky, Malmud, and Pelham (2001) similarly reported no statistically significant differences in IQ scores, school grades and retention data, and neurological examinations at age 6 in a cohort of 115 children followed since birth, but representing only 52.5% of the original enrollees. A recent report from this cohort as the children completed fourth grade also found no cocaine-related differences in school performance measures including grade progression, grade point average, reading level, and performance on standardized tests (Hurt, Brodsky, Roth, Malmud, & Giannetta, 2005). Delaney-Black et al. (2000) also reported no cocaine-associated differences in mean IQ scores in a large school-aged follow-up sample (n = 458) of children enrolled prospectively at birth and assessed at age 6; however, a larger proportion of cocaine-exposed children from this study were categorized as having low language abilities (63% vs. 37%), with lower Verbal IQ scores reported in this group.
More important, many school-aged studies reporting IQ outcomes to date are characterized by significant cohort attrition and other methodological concerns that limit interpretation of the results. In addition, no studies have yet evaluated risk for the development of a learning disability or disorder, defined in the Diagnostic and Statistical Manual of Mental Disorders (DSM–IV–TR; American Psychiatric Association, 2000) as a discrepancy between expected achievement levels and measured intelligence, given an individual’s age and education. Evidence exists from infancy and preschool studies to suggest prenatal cocaine exposure results in subtle processing deficits that might increase risk for developing a learning disability, including deficits in visual attention, sustained attention, and inhibitory control (Bendersky, Gambini, Lastella, Bennett, & Lewis, 2003; Heffelfinger, Craft, White, & Shyken, 2002; Richardson et al., 1996), as well as early language development (Delaney-Black et al., 2000; Lewis, Singer, et al., 2004; Singer, Arendt, et al., 2001). Our own research findings through age 7 reveal cocaine-related deficits in language skills (Bandstra et al., 2002; Bandstra, Vogel, Morrow, Xue, & Anthony, 2004; Morrow et al., 2003, 2004) and sustained attention processing (Bandstra, Morrow, Anthony, Accornero, & Fried, 2001). Other aspects of neurocognitive development that may place children at increased risk for learning difficulties have begun to be identified in older children. For example, in a recent study 8- and 9-year-old children with prenatal cocaine exposure exhibited deficits in visuospatial processing (Schroder, Snyder, Sielski, & Mayes, 2004).
Due to the prevalent distribution of dopamine in the frontal cortex, impaired frontal lobe functioning in children with prenatal cocaine exposure has been hypothesized, with a suspected increased risk for learning difficulties due to underlying deficits in attention and executive functioning. This finding has been supported by animal studies linking prenatal cocaine exposure to dysfunctions in dopaminergic (D1) signal transduction and associated changes in cortical neuronal development leading to frontocingulate cortex and other brain structure abnormalities most often associated with attentional processing (Harvey, 2004).
This report investigates risk for academic casualty in children with prenatal cocaine exposure. We hypothesize that cocaine exposure will not differentially place children at risk for deficits in intellectual functioning at age 7, but will increase risk for subtle and focal learning impairments resulting in the development of a learning disability by age 7. The research questions are evaluated with consideration for fetal growth as a potential mediating influence and with evaluation of other potentially confounding influences within a large well-retained cohort of children, enrolled prospectively at birth with verification of substance exposure status using biological markers in addition to maternal self-report.
METHOD
Study Recruitment and Participant Information
The originating birth cohort of 476 infants (253 cocaine-exposed [CE] and 223 non-cocaine-exposed [NCE]) was recruited from the obstetrical service of the University of Miami Jackson Memorial Medical Center between November 1990 and July 1993 as part of a larger epidemiological study. Recruitment procedures have been extensively detailed in an earlier report (Bandstra, Morrow, Anthony, Churchill, et al., 2001). The study was approved by the Institutional Review Board and conducted under a federal Department of Health and Human Services Certificate of Confidentiality with informed consent obtained from all participants. The birth cohort was homogeneous with regard to full-term gestational age (≥37 completed weeks), low socioeconomic status, inner-city residence, and African American race/ethnicity. Exclusion criteria included maternal HIV/AIDS; prenatal exposure to opiates, methadone, amphetamines, barbiturates, benzodiazepines or phencyclidine; major congenital malformation; chromosomal aberration; or disseminated congenital infection.
The birth cohort included 253 CE infants (cocaine exposure with varying exposures to alcohol, tobacco, or marijuana) and 223 NCE infants (no cocaine exposure; 147 were drug-free and 76 were exposed to varying combinations of alcohol, tobacco, or marijuana). Prenatal cocaine exposure was determined by maternal self-report and positive assay on one or more biologic markers, including maternal urine, infant urine, and meconium. Alcohol and tobacco exposures were determined by self-report, and marijuana exposure was indicated by self-report or a positive toxicology screen. Drug-free mothers had negative self-report drug histories during and for 3 months preceding pregnancy, negative lifetime histories for cocaine use, and negative results on all available toxicology assays.
From the original birth cohort of 476 infants, 409 children (197 NCE, 212 CE) completed a valid assessment of intellectual functioning at the age 7 follow-up visit and are included in this report. Participant attrition information and analyses are presented in the results section.
Measures
Infant Birth Assessments
During the immediate postpartum period, research staff performed a standardized research interview and collected the biological specimens. Trained research personnel, blinded to drug exposure status, performed the Ballard gestational age assessment (Ballard, Novak, & Driver, 1979) within 36 hours of delivery and obtained occipital-frontal head circumference and recumbent crown-heel birth length. Pertinent medical and demographic data were collected from the hospital record at birth.
Drug Exposure Measures at Birth
Maternal interview
Mothers were interviewed at birth regarding their drug use during pregnancy. Standardized drug use questions were asked by trimester and included number of weeks used, most days per week, fewest days per week, usual days per week, and usual dose per day. Dosage was measured in number of cigarettes smoked; number of marijuana joints smoked; number of drinks of beer, wine, or hard liquor; and number of cocaine lines/rocks; recorded in increments of usual daily dose; usual days per week; and number of weeks used. Standard definitions were used for determining 1-drink units for each type of alcohol (beer 12 oz., wine 5 oz., and liquor 1.5oz.). Pregnancy exposure composites were calculated for each drug by multiplying the number of weeks used by the usual days per week and the usual dose per day.
Biological markers (urine and meconium)
Screening of maternal and infant urine and infant meconium for cocaine metabolite (benzoylecgonine) was performed by EMIT® (Syva D.A.U.), at a cut-off of 150 ng/ml urine and 150 ng/gm meconium, respectively. Cocaine-positive specimens were confirmed by gas chromatography/mass spectrometry (GC/MS) (Mulé & Casella, 1988). Urine specimens were assayed by EMIT® for marijuana, opiates, amphetamines, barbiturates, benzodiazepines, and phencyclidine. Meconium specimens were assayed by EMIT® for marijuana and opiates. In the original cohort, 100% had at least one biological marker, 96% had at least two biologic markers, and 68% had all three biological markers available for analysis.
Child Measures Conducted at 7-Year Follow Up
All child measures were administered by trained research associates blinded to the drug exposure status of the child.
Wechsler Intelligence Scale for Children–Third Edition (WISC–III) short form
A short form of the WISC–III (Wechsler, 1991) was selected for use at the 7-year research visit to minimize participant burden due to the overall duration of the test day. The WISC–III short form included the following subtests: Information, Similarities, Vocabulary, Picture Arrangement, and Block Design. An estimated Full Scale IQ Score (M = 100, SD = 15) was calculated from these five subtests based on the method developed by Sattler (1992), who reported an internal reliability coefficient of .94 and a validity coefficient of .90, representing the relation between the short form and the full form of the WISC–III.
Wechsler Individual Achievement Test (WIAT) Screener
The Screener version of the WIAT (Wechsler, 1993) was selected for use at the 7-year research visit to assess academic achievement in basic educational areas that are fundamental for later learning. The WIAT Screener included the following subtests: Basic Reading, Spelling, and Mathematics Reasoning. In addition, the Numerical Operations subtest was also administered. The Basic Reading subtest assessed the child’s ability to identify lower case letters, identify and produce rhyming words, associate phonetic sounds with letters and letter groups, and decode simple words. The Spelling subtest assessed the child’s ability to write his or her name, write letters that represent certain phonetic sounds in words, and spell simple words. The Mathematics Reasoning task assessed the child’s knowledge of mathematics concepts such as “more/less” or“taller/shorter,” ability to count pictured objects, perform simple addition and subtraction operations with picture cues after listening to a problem read by the examiner, and answer questions involving money and time concepts. The Numerical Operations subtest asked the child to discriminate numbers from letters, identify a specific number from a larger array, write numbers from dictation, use number–object correspondence in counting, and perform simple addition and subtraction operations by using paper and pencil. Standards cores (M = 100, SD = 15)were generated for each individual subtest, as well as the Mathematics composite and WIAT Screener composite.
Additional Covariate Measures
Child hearing
Hearing was assessed using play audiometry techniques at age 3. For children unable to complete the play audiometry task, visual reinforcement audiometry in the sound field was used. All testing and interpretations were performed at the University of Miami Mailman Center for Child Development by a licensed, certified pediatric audiologist. For analyses in this study, behavioral audiometry results were coded as normal in at least one ear or bilaterally abnormal. Minimum response levels of 30 dB were considered abnormal.
Blood lead level
At the age 3- and 5-year follow-up visits, screening lead levels were performed by capillary sample and processed at the State of Florida Department of Health Laboratory. Abnormal capillary lead levels (i.e., ≥ 10 mg/dL), were confirmed by repeat specimen obtained by venipuncture. The higher blood level at either the 3- or 5-year visit was categorized dichotomously as <10 and ≥10 mg/dL and used as a single composite covariate in the analyses.
Caregiver psychosocial interview
A structured psychosocial interview covering extensive family, demographic, and drug use information was conducted with the mother/primary caregiver of each child at the 7-year visit. The primary caregiver was defined as any family member or custodial guardian responsible for the physical, emotional, and financial well-being of the child. Biological mothers residing with and parenting the child were always prioritized for interview purposes as the primary caregiver. The caregiver demographic and drug use covariates included in the analyses were drawn from this structured interview.
Data Reduction
Categorization of Intellectual Functioning Levels Based on IQ Scores
The WISC–III short form, Full Scale IQ, was used to categorize children as average (80 and above), borderline (70–79), and mentally deficient (below 70). The terminology mental retardation was not utilized because IQ scores were not interpreted within the context of an adaptive behavior measure, which is necessary to make the diagnosis of mental retardation according to DSM–IV–TR (American Psychiatric Association, 2000). The categorization of IQ scores represents a continuum of intellectual functioning from which to evaluate the impact of prenatal cocaine exposure in relation to clinically meaningful categories.
Categorization of LD Subtypes Using IQ and Achievement Scores
A discrepancy-based model was used to categorize children as having a LD, based on conventional practices used in the majority of U.S. public schools at the time the data were collected. Although the validity of discrepancy-based approaches to identify LD has been questioned on statistical and theoretical grounds, (Aaron, 1997; Shaywitz, Fletcher, Holahan, & Shaywitz, 1992; Stanovich & Stanovich, 1997; Swanson, 2000), alternative approaches have also been met with criticism (Dean & Burns, 2002; Naglieri & Reardon, 1993; Swanson, 2000). Given the current controversy in the field regarding the definition and diagnosis of LD, the conventional approach most similar to that used to determine eligibility for special education services in the public schools was used in this study. Ability-achievement discrepancy scores were calculated using regression-based predicted achievement methods and discrepancy score cutoffs (p < .05) as described in the WIAT manual. Due to the lower than normative mean values of the WISC–III and WIAT test scores for the study population, two different IQ cutoff scores were used to specify LD cases (inclusion based on the standard of IQ > 80 and also based on an IQ > 70).
Statistical Analyses
Initial data review procedures included visual inspection of frequency distributions and longitudinal plots for individual subjects. Relative risk estimates for cognitive deficits and cumulative incidence proportions estimating the risk of being categorized as LD by age 7 were estimated using STATA software (Statacorp, 2003) with a generalized linear model for binary responses that permits statistical adjustment for multiple covariates. Estimates for risk differences and for relative risk (i.e., via the odds ratio), 95% confidence intervals, and exact p values are presented for interpretation of results. Regression models were first restricted to a set of covariates specified in advance (child sex, age at exam, and prenatal exposures to alcohol, tobacco, and marijuana). Additional covariates were then added sequentially to assess the stability of the observed cocaine-LD risk estimates with varying model specifications. This approach allowed for identification of individual covariates that potentially attenuate the effect estimate, within the bounds of a sample size that limited the simultaneous inclusion of all potential covariates.
RESULTS
Sample Characteristics
Attrition Information
A total of 415 children representing 87% of the original cohort (217 CE and 198 NCE) returned for the 7-year assessment. Of these, 6 children did not complete valid IQ assessments and were not included in the primary analyses (5 due to extreme cognitive deficits and 1 due to logistical issues related to completing the tests). The study groups were not statistically different in the proportion of children with incomplete exams, although the cell sizes were small (CE: 2.3% , n = 5, vs. NCE: 0.5%, n = 1; p = .218).
A total of 409 children (212 CE and 197 NCE) from the original cohort of 476 children completed valid WISC–III intellectual assessments at the 7-year follow-up visit and were included in the primary cognitive analyses. Attrition analyses showed no differences with respect to study group status and maternal and infant characteristics at birth (variables listed in Table 2) between the 409 children included in the cognitive analyses and the remaining 67 who either did not have complete data (n = 6) or did not attend the 7-year assessment visit (n = 61). Of the 409 children with WISC–III scores, 6 did not have complete WIAT data due primarily to logistical and rescheduling issues, resulting in 403 children being included in the LD analyses. Attrition analyses also indicated no appreciable differences when evaluating the subgroup of 403 children with both WISC–III and WIAT assessment data who were included in the LD analyses.
TABLE 2.
Maternal and Infant Characteristics at Birth Enrollment
| Non-CEa |
CEb |
|||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | M | SD | % | n | M | SD | % | n |
| Maternal | ||||||||
| Age (in years)* | 23.8 | 5.4 | 28.9 | 4.9 | ||||
| Education (in years) | 11.3 | 1.39 | 11.1 | 1.42 | ||||
| Unemployed* | 83.3 | 164 | 94.8 | 201 | ||||
| Never married | 87.8 | 173 | 91.0 | 193 | ||||
| Prenatal care ≥4 visits* | 84.3 | 166 | 69.3 | 147 | ||||
| Infant | ||||||||
| Birth weight (in grams)* | 3,299 | 510 | 2,962 | 467 | ||||
| Birth length (in centimeters)* | 50.8 | 2.3 | 48.9 | 2.5 | ||||
| Birth head circumference (in centimeters)* | 33.8 | 1.5 | 33.0 | 1.5 | ||||
| Gestational age (in weeks)* | 39.7 | 1.4 | 39.3 | 1.4 | ||||
| Male | 50.3 | 99 | 50.0 | 106 | ||||
Note. N = 409. CE = cocaine-exposed.
n = 197.
n = 212.
p < .01.
Sample Descriptive Information
Table 2 presents a description of selected maternal and infant characteristics at birth for the 409 children included in this report, and Table 3 summarizes maternal drug use patterns during pregnancy. Table 4 depicts selected social and demographic characteristics of the primary caregiver measured at the 7-year assessment and child characteristics measured during the preschool assessment visits that were included as covariates in the analyses. For descriptive purposes, Table 5 summarizes mean WISC–III and WIAT subtest and composite scores by group.
TABLE 3.
Maternal Self-Reported Drug Use during Pregnancy
| Non-CEa |
CEb |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dosage |
Reporting Use |
Dosage |
Reporting Use |
|||||||
| Variable | Mdn | Min | Max | % | n | Mdn | Min | Max | % | n |
| Alcohol (no. of drinks)c | 57 | 2 | 1,680 | 29.4 | 58 | 105 | 1 | 5,226 | 66.5 | 141 |
| Tobacco (no. of cigarettes)c,d | 1,043 | 21 | 5,880 | 15.2 | 30 | 2,184 | 1 | 8,820 | 76.9 | 163 |
| Marijuana (no. of joints)c | 32 | 1 | 807 | 11.7 | 23 | 28 | 1 | 1,229 | 44.8 | 95 |
| Cocaine/crack(no. of lines/rocks) | 144 | 1 | 19,320 | 68.4 | 145 | |||||
Note. N = 409. Maternal substance use is expressed as median, minimum, and maximum due to skewed distributions. Median values based only on mothers who reported or acknowledged drug use at birth within the subsample of 409 children; drug use composites were calculated as follows: (number of weeks used) × (usual number of days per week) × (usual dose per day). CE = cocaine-exposed.
n = 197.
n = 212.
p < .01, between-group comparison of percentage reporting drug use (Columns 2 and 4; p < .01 for these comparisons).
p < .05, between-group comparison of median maternal drug use (Columns 1 and 3).
TABLE 4.
Child and Caregiver Characteristics Assessed at Follow Up
| Non-CEa |
CEb |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Result |
Result |
|||||||||
| Variable | nc | M | SD | % | n | nc | M | SD | % | n |
| Assessed at 7-year research visit | ||||||||||
| Child’s examination age (in months) | 197 | 88.7 | 4.2 | 212 | 88.6 | 3.9 | ||||
| Biological mother as caregiver** | 197 | 95.9 | 189 | 208 | 59.1 | 123 | ||||
| Caregiver education (in years)* | 197 | 11.7 | 1.5 | 207 | 11.2 | 2.0 | ||||
| Caregiver unemployment** | 197 | 28.4 | 56 | 206 | 56.8 | 117 | ||||
| Caregiver past year cocaine use** | 197 | 3.1 | 6 | 207 | 19.8 | 41 | ||||
| Caregiver past year marijuana use | 197 | 15.7 | 31 | 207 | 17.9 | 37 | ||||
| Caregiver past year alcohol use | 197 | 57.9 | 114 | 207 | 55.6 | 115 | ||||
| Caregiver past year tobacco use** | 197 | 23.9 | 47 | 207 | 54.1 | 112 | ||||
| Assessed at 3- or 5-year research visit | ||||||||||
| Child bilateral hearing deficit (assessed at 3 years) | 188 | 3.2 | 6 | 200 | 3.0 | 6 | ||||
| Child blood lead ≥10mg/dL (assessed at 3/5 years) | 193 | 8.3 | 16 | 210 | 13.8 | 29 | ||||
| Head Start/Prekindergarten* (assessed at 5 years) | 187 | 70.1 | 131 | 196 | 60.2 | 118 | ||||
Note. N = 409. CE = cocaine-exposed.
n = 197.
n = 212.
Group ns for covariates are drawn from those children who attended the 7-year follow-up visit with available Wechsler Intelligence Scale for Children–Third Edition scores and had the associated covariate data available.
p < .05.
p < .01, for between-group comparisons.
TABLE 5.
WISC–III and WIAT Standard Scores Assessed at 7-Year Research Visit
| Non-CE |
CE |
|||
|---|---|---|---|---|
| Test | M | SD | M | SD |
| WISC–III Short Form Full Scale IQa | 83.1 | 13.7 | 81.9 | 13.2 |
| WIAT Screenerb | 91.5 | 12.7 | 89.1 | 11.8 |
| Basic reading | 92.6 | 11.8 | 90.8 | 10.8 |
| Spelling | 93.1 | 12.2 | 91.1 | 11.8 |
| Math reasoning | 93.1 | 12.3 | 90.9 | 10.7 |
| Numerical operations | 95.9 | 13.2 | 93.4 | 13.3 |
| Math composite | 92.8 | 14.3 | 90.2 | 13.0 |
Note. WISC–III = Wechsler Intelligence Scale for Children–Third Edition; WIAT = Wechsler Individual Achievement Test; CE = cocaine-exposed.
n = 409.
n = 403.
Analyses
Risk for Intellectual Functioning Deficits
WISC–III short form Full Scale IQ scores were categorized as previously described: average IQ (80 and above), borderline IQ (70–79), and mentally deficient IQ (below 70). Table 6 depicts the percentage and number of children in each group falling into each IQ categorization based on available IQ scores. A baseline regression model, unadjusted for covariates, yielded results consistent with a null odds ratio of relative risk for intellectual impairment between children with and without prenatal cocaine exposure (estimated relative risk = .95; 95% confidence interval [CI] = 0.65, 1.39; p = .79). Similar null results were obtained when sex of the child and test age was statistically controlled and when children who met criteria for any learning disability were excluded from the analyses. An additional 5 children attended the 7-year research visit but could not be assessed due to extreme intellectual deficiencies and were not included in the overall analyses or descriptive tables. An ancillary analysis, however, was conducted that included these cases in the IQ < 70 group with no change in the overall results (CE = 21.3% versus NCE = 19.7%; p = .678).
TABLE 6.
WISC–III Categorization by Full Scale IQ Scorea
| Non-CEb |
CEc |
|||
|---|---|---|---|---|
| IQ | % | n | % | n |
| ≥80: Average | 59.9 | 118 | 61.8 | 131 |
| 70–79: Borderline | 20.8 | 41 | 18.4 | 39 |
| < 70: Mentally deficient | 19.3 | 38 | 19.8 | 42 |
Note. N = 409. WISC–III = Wechsler Intelligence Scale for Children–Third Edition; CE = cocaine-exposed.
Derived from the WISC–III Short Form as described in the Methods section.
n = 197.
n = 212.
Given the large percentage of CE children residing in nonmaternal caregiving environments (see Table 4), additional analyses were conducted to determine whether caregiving status may have obscured group differences in intellectual functioning due to the potential ameliorating influence of an alternative caregiving environment. The caregiving environment of the CE group was further subdivided into three groups: biological mother caregiving (59.1%), biological relative caregiving (30.8%), and nonrelative caregiving (10.1%). IQ scores were not different when compared within the CE group across the three subgroups or when the NCE children (96% of whom reside with their biological mother) were included as an additional comparison group. Mean IQ scores and standard deviations for these subgroups are NCE: 83.1, ± 13.7; CE biological mom: 81.9, ± 13.1; CE relative caregiver: 82.8, ± 13.0; and CE non-relative caregiver: 80.0, ± 13.1. Due to the absence of statistically significant findings relating prenatal cocaine exposure to deficits in intellectual functioning, analysis of mediation and subgroup differences and additional covariate adjustments were not conducted.
Risk for Being Categorized as LD
A total of 20 children were classified as having LD in at least one of the academic areas measured using the IQ ≥ 70 cutoff (15 CE children and 5 NCE children), and 19 were classified using the IQ ≥ 80 cutoff (14 CE children and 5 NCE children). Specifically, these children met or exceeded the IQ-achievement discrepancy level needed for statistical significance using the WIAT predicted achievement model for diagnosing LD. Estimated risk was 7.3% for CE children and 2.6% for NCE children using the IQ ≥ 70 cutoff condition (estimated risk difference for LD = 4.7%; 95% CI = 0.01, 0.09; p = .038).
Table 7 presents risk estimates from a succession of regression models, starting with a baseline model unadjusted for any covariates, for both IQ cutoff conditions. In the IQ ≥ 70 cutoff condition, the CE children had an estimated 2.8 times greater risk of developing a learning disability by age 7 than NCE children (95% CI = 1.05, 7.67; p = .039). When analyses were rerun using the clinical IQ ≥ 80 cutoff condition, the estimates were not appreciably different (estimated risk = 2.7; 95% CI = 0.97, 7.22; p = .057; data presented in Table 7).
TABLE 7.
Magnitude of the Estimated Effect of Prenatal Cocaine Exposure on Risk of Developing a Learning Disability (LD) by Age 7
| Risk Ratio for Developing an LDa |
Risk Ratio for Developing an LDb |
|||||
|---|---|---|---|---|---|---|
| Variable | Estimated Risk Ratio | 95% CI | p | Estimated Risk Ratio | 95% CI | p |
| Model 1: Unadjusted for any covariates | 2.8 | 1.05, 7.67 | .039 | 2.7 | 0.97, 7.22 | .057 |
| Model 2: Covariate adjustment for child sex and exam age | 2.8 | 1.05, 7.64 | .039 | 2.6 | 0.97, 7.19 | .057 |
| Model 3: Subsumes Model 2 and adds covariate adjustment for prenatal exposure to alcohol, marijuana, and tobacco | 3.2 | 1.09, 9.12 | .034 | 3.4 | 1.17, 9.82 | .025 |
| Model 4: Subsumes Model 3 and adds covariate adjustment for fetal growthc as a potential mediating variable | 2.5 | 0.85, 7.64 | .096 | 2.6 | 0.87, 7.92 | .087 |
| Each additional model subsumes Model 3 and statistically adjusts for each of the following covariates individually: | ||||||
| Maternal covariates at birth | ||||||
| Maternal age | 3.1 | 1.02, 9.45 | .045 | 3.4 | 1.10, 10.37 | .034 |
| Maternal education | 3.2 | 1.09, 9.12 | .034 | 3.4 | 1.17, 9.81 | .025 |
| Maternal employment | 2.9 | 1.00, 8.29 | .050 | 3.1 | 1.06, 8.85 | .038 |
| Maternal marital status | 3.1 | 1.08, 9.14 | .036 | 3.2 | 1.11, 9.43 | .031 |
| Prenatal care | 3.0 | 1.02, 8.72 | .045 | 3.1 | 1.08, 9.19 | .036 |
| Caregiver characteristics at Age 7 | ||||||
| Biological mother caregiving | 3.3 | 1.13, 9.86 | .029 | 3.5 | 1.17, 10.41 | .025 |
| Caregiver education | 3.2 | 1.11, 9.38 | .031 | 3.5 | 1.20, 10.20 | .022 |
| Caregiver employment | 4.4 | 1.47, 12.94 | .008 | 4.8 | 1.60, 14.07 | .005 |
| Caregiver past year cocaine use | 3.4 | 1.16, 9.74 | .026 | 3.6 | 1.24, 10.43 | .019 |
| Caregiver past year marijuana use | 3.2 | 1.11, 9.27 | .031 | 3.5 | 1.19, 10.00 | .022 |
| Caregiver past year alcohol use | 3.3 | 1.15, 9.71 | .027 | 3.6 | 1.23, 10.50 | .019 |
| Caregiver past year tobacco use | 3.0 | 1.02, 8.91 | .047 | 3.1 | 1.03, 9.08 | .045 |
| Child characteristics | ||||||
| Child bilateral hearing deficit (%) (measured at 3 years) | 2.9 | 1.01, 8.60 | .048 | 3.2 | 1.09, 9.26 | .034 |
| Child blood lead ≥10mg/dL (%) (assessed at 3/5 years) | 3.6 | 1.23, 10.27 | .019 | 3.7 | 1.29, 10.85 | .015 |
| Child attended Head Start/prekindergarten (measured at 5 years) | 3.1 | 1.08, 9.07 | .036 | 3.5 | 1.19, 10.09 | .023 |
| Caldwell HOMEd Learning Environment (measured at 4½ years) | 3.2 | 1.11, 9.15 | .032 | 3.4 | 1.18, 9.82 | .024 |
Note. Using Model 3 as the base model, each additional variable is added individually. CI = confidence interval.
IQ cutoff≥70.
IQ cutoff≥80.
Fetal growth measured by a latent construct comprised of birth weight, length, and head circumference.
Risk estimates for LD in both IQ cutoff conditions did not change appreciably with statistical adjustment for child sex and exam age (Model 2), with risk estimates ranging from 2.6 to 2.8, or with the addition of covariate terms for prenatal exposure to alcohol, marijuana, and tobacco (Model 3; risk estimates ranging from 3.2–3.4). Although not the focus of this report, prenatal marijuana use was also independently related to risk for LD in the IQ ≥ 70 condition (estimated risk = 1.2; 95% CI = 1.04, 1.48; p = .017; data are not presented in table). This finding was not significant in the IQ ≥ 80 condition (p = .09). Reported alcohol and tobacco use did not independently increase risk for LD in either IQ cutoff condition.
Building from Model 3, additional child and caregiver covariates measured at birth through age 7 were added successively, introduced one by one (as shown in Table 7), with little change in the risk estimates for LD related to prenatal cocaine exposure. In each of these models, a general pattern of statistically robust (i.e., p < .05) risk estimates were observed in both IQ cutoff conditions. Marijuana also remained a stable, independent predictor of risk for LD in each covariate adjusted model for the IQ ≥ 70 condition only.
Due to the reported influence of prenatal cocaine exposure on fetal growth previously shown in this cohort (Bandstra, Morrow, Anthony, Churchill, et al., 2001) as well as other studies, fetal growth and gestational age were probed as potential mediators of the relation between prenatal cocaine exposure and the risk of developing LD by age7. The approach involved introducing the fetal growth term to the terms included in Model 3 (Table 7), and checking for attenuation of risk estimates that would support an inference of mediation. As indicated in Model 4 (Table7), the addition of the fetal growth term indicated no appreciable attenuation of the relation between prenatal cocaine exposure and risk of developing LD. Similar results occurred when gestational age was added separately to the model (data not shown in table). Product terms were also added to probe for male–female differences in the size of the risk estimates. These terms did not improve the fit of the regression models, indicating no male–female variation in the risk estimates for developing LD.
Analysis of LD Subtypes
Results are presented descriptively for each LD subtype in Figure 1. The cell counts for each LD subtype were small and should be interpreted with caution; however, analyses indicate that the Math Achievement Composite score reached statistical significance in both IQ cutoff conditions (estimated risk for IQ ≥ 70 cutoff = 9.5; 95% CI = 1.22, 73.30; p = .031; estimated risk for IQ ≥ 80 cutoff = 8.5; 95% CI = 1.09, 66.60; p = .041) and remained significant after adjusting for child sex; age; prenatal exposures to alcohol, tobacco, and marijuana; and the additional maternal, child, and caregiver covariates presented in Table 6.
FIGURE 1.
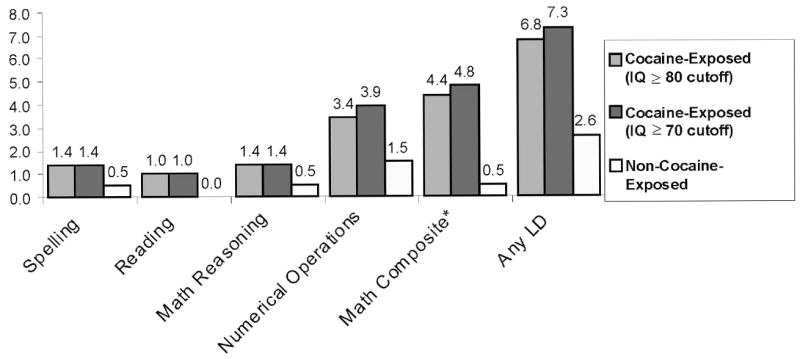
Percentage of children in each specific LD category.
Analysis of Low Achievement Scores
Exploratory analyses were also conducted to evaluate low achievement scores only, as an alternative nondiscrepancy based approach to identifying learning problems. Using standardized scores, cutoffs were set for achievement scores lower than 80 and lower than 70. Comparisons of WIAT achievement scores (using a categorical variable of <80 or ≥80 on any WIAT subtest) indicated no between group differences in an uncontrolled model (<80 CE = 30.8%, <80 NCE = 24%; p = .13) that reached significance when IQ scores were statistically controlled (p = .05). The same analysis using a categorical cutoff of <70 or ≥70 indicated no significant group differences in the uncontrolled and IQ controlled model, perhaps due to the lower number of children scoring below 70 on any of the WIAT subtests (<70 CE = 5.3%, <70 NCE = 3.6%; p = .41). Analyses suggest that using low achievement scores in a nondiscrepancy IQ model yielded results that were less robust but trended in a similar direction as the discrepancy-based approach.
Level of Prenatal Cocaine Exposure and Risk for LD
Additional analyses were conducted to determine whether increasing levels of prenatal cocaine exposure further increased risk for developing LD. A latent variable for maternal cocaine/crack usage during pregnancy was measured by separate categorical summaries of first, second, and third trimester self-report (0 = no self-reported cocaine/crack use, 1 = 1–24 uses, 2 = 25–180 uses, 3 = 180+ uses; a priori cutoffs represent approximate thirds of the distribution from the originating birth sample [n = 476], with the condition of a minimum of 30 infants per cell). A second latent variable to quantify cocaine exposure level by bioassay was also constructed, measured by cocaine-positive (yes–no) infant urine, infant meconium, and maternal urine, and log-transformed levels of cocaine metabolite benzoylecgonine determined by GC/MS assays. These self-report and bioassay results were then combined into a single summary latent variable, allowing all available self-report and bioassay information to be taken into account in estimating level of prenatal cocaine exposure. Models to estimate risk were run in the same sequence as Table 7, Models 1 through 4 (unadjusted, adjusted for sex and age, adjusted for other prenatal drug exposures, adjusted for fetal growth), and in all cases level of prenatal cocaine exposure was not related to an increased risk for developing LD.
DISCUSSION
Following the dramatic rise in maternal cocaine use during pregnancy in the 1980s and early 1990s and early reports of severe perinatal and postnatal birth outcomes for infants with prenatal cocaine exposure, the supposition of most longitudinal research studies was that prenatal cocaine exposure would be associated with severe developmental deficits similar to those associated with prenatal alcohol exposure. Subsequent research from large, prospectively enrolled cohorts has revealed a more balanced perspective, with cocaine-exposed children showing modest to moderate decrements in global developmental functioning during infancy, with an attenuation of these findings during the preschool years (see studies referenced in Table 1). These findings have not been extended to the early school-aged years, with the few available published studies reporting no cocaine-associated decrements in intellectual functioning (Delaney-Black et al., 2000; Hurt, Giannetta, Brodsky, Malmud, & Pelham,2001; Richardsonetal.,1996; Wassermanetal.,1998). This report from the Miami Prenatal Cocaine Study (PCS) addresses the continuum of academic casualty, investigating risk for intellectual functioning deficits and LD during the early school years in a large well-retained cohort of children born full-term and enrolled prospectively at birth with cocaine exposure status reported through maternal self-report and maternal and infant urine and infant meconium toxicology assays.
Consistent with previously published studies, results indicate no differences in global intellectual functioning between children with and without prenatal cocaine exposure. Several studies have reported that children with prenatal cocaine exposure who reside in nonrelative foster care or adoptive care attain higher IQ scores than cocaine-exposed children living with parents or other biological relatives, suggesting that the caregiving environment may mitigate the impact of prenatal cocaine exposure on outcomes such as intelligence (Brown, Bakeman, Coles, Platzman, & Lynch, 2004; Singer et al., 2004). Children with prenatal cocaine exposure are more often likely to be placed with alternate care providers, as is evidenced in this cohort with 10% residing with a nonrelative caregiver, 31% residing with a biological relative, and only 59% residing with the biological mother at age 7. Results from this study did not indicate differences in IQ when compared across these subgroups or when compared with children without prenatal cocaine exposure (96% resided with their biological mother). Although the results of these analyses were not significant, it is of interest that the lowest mean IQ scores occurred in the group of cocaine-exposed children living in nonrelative care. It is possible that the most seriously affected children from this cohort were more likely to be placed in a nonrelative foster care setting. This caregiving placement, however, did not replicate Singer etal.’s (2004) results, in which cocaine-exposed children in nonrelative care had IQ scores that were more similar to non-cocaine-exposed children and higher than cocaine-exposed children living with a biological parent or relative.
Although this current study does not identify significant decrements in intellectual functioning in relation to prenatal cocaine exposure, between 19% and 20% of the children in each group had IQ scores that fell in the mentally deficient range (<70). The children represented in this study reside primarily in economically disadvantaged inner-city neighborhoods characterized by high levels of unemployment, drug trafficking, teen pregnancy, family instability and domestic violence. Given the overall distribution of IQ scores in this cohort and the social and economic disadvantage that typified the home environments of both the cocaine-exposed and non-cocaine-exposed children, even subtle differences in mean intellectual functioning specific to prenatal cocaine exposure would have likely required a much larger cohort size to detect. In light of these interpretive issues, one might ascertain that the potential teratological impact of prenatal cocaine exposure on global child intellectual functioning could not be separated from the multifaceted environmental risk factors that play a formative role in development during early childhood.
By age 7, however, children with prenatal cocaine exposure were approximately three times more likely to meet criteria for a LD than children without prenatal cocaine exposure. This finding represents one of the first reports of school-related learning difficulties in a well-controlled study of the effects of prenatal cocaine exposure. Results were very robust with adjustment for prenatal exposure to other drugs including alcohol, marijuana, and tobacco and after consideration for numerous potential exogenous and endogenous child and care-giver influences. The evidence does not support an inference of mediation by fetal growth or gestational age, despite the reported influence of prenatal cocaine exposure on intrauterine growth parameters in this cohort. Also, no male–female differences were found in the magnitude of cocaine-associated risk. Although exposure to cocaine prenatally was robustly related to increased risk for developing LD, gradient increases in the level of prenatal exposure to cocaine did not provide additional information related to LD risk. Although not the focus of this study, maternal-reported prenatal marijuana use presented a modest independent contribution to the risk for developing LD that was not evident for reported tobacco or alcohol exposure.
Analysis of LD subtypes indicated that children with prenatal cocaine exposure were at greater risk for developing a math-related LD. The small number of cases relative to the cohort size makes it difficult to interpret the absence of statistical findings for reading or spelling; however, math processing appeared to be more affected. This finding raises questions about the underlying neuropsychological processes that might be affected by prenatal cocaine exposure. Studies suggest that cortical regions associated with mathematical competence are widely dispersed in the brain and may involve such areas as the right hemisphere, the language-dominant hemisphere, frontal lobe, parietal lobe, occipital lobe, temporal lobe, and higher association areas of the dominant hemisphere in mediating this complex set of functions (Cohen, Dehaene, Chochon, Lehericy, & Naccache, 2000; Fulbright et al., 2000; Kazui, Kitagaki, & Mori, 2000; Whalen, McCloskey, & Lesser, 1997). Preliminary results from recent MRI studies have identified elevated creatine levels in the white matter of the frontal lobes in cocaine-exposed children at age 8, suggesting a long-term impact on the metabolic function of the frontal regions of the brain potentially influencing impulse control, sustained attention, and goal-directed behavior (Smith et al., 2001). Thus, further examination of visual-spatial, executive function, language, and other cognitive processing abilities in this cohort may provide important information about the neuropsychological functions most affected by prenatal cocaine exposure.
In this report, average IQ scores fell in the lower part of the low average range for both the cocaine-exposed and non-cocaine-exposed groups. This low IQ range made it difficult to reach the significant ability-achievement discrepancies required when using the conventional discrepancy approach to LD categorization; thus, the actual rates and increased risk for LD in children with prenatal cocaine exposure may have been underestimated. Although the percentage of children meeting criteria for LD was relatively low (7.3% for the cocaine-exposed children and 2.6% for the non-cocaine-exposed children), the overall risk difference between the groups of 4.7% has potentially significant clinical and economic ramifications for service providers as the associated distributional shift results in an increased number of cocaine-exposed children qualifying for special education services than would be expected in the normative population (Lester et al., 1998).
Several study characteristics should also be noted when interpreting results. The cohort included only full-term, healthy African American infants born to mothers residing in socially disadvantaged inner-city neighborhoods. Results from this study suggest that African American children with prenatal cocaine exposure are at increased risk for developing a learning disability by age 7 when compared with a racially and demographically similar comparison group. Generalization to other samples or other populations is, however, uncertain and replication studies are needed.
It is also plausible that the mechanisms by which cocaine influences child development may differ in samples of children with more varied risk levels, different racial/ethnic backgrounds, or who were born prematurelyor with other health conditions. This suggestion is evident in a study reported by Singer, Hawkins, Huang, Davillier,and Baley (2001), in which a dramatic 13-point mean difference in expressive language functioning was observed between cocaine-exposed and non-cocaine-exposed very low birth weight children. Finally, the veracity of self-report methodologies for collecting substance use information is difficult to ascertain. Although our study is strengthened by its use of a combination of self-report and bioassay methodologies to determine prenatal exposure to cocaine and other drugs, classification errors may still have occurred, with potential biases that might have drawn the study estimates to the null.
Most research has approached the study of prenatal cocaine exposure using a teratological model developed from animal studies. Teratogenic effects are believed to be expressed on a continuum based on the severity and timing of the teratological exposure during prenatal development, with potential outcomes including abnormal developmental functions, growth abnormalities, physical malformation, and death (Vorhees, 1989). In human models, research of this nature is further complicated by the prevalence of polydrug exposure in addition to exposure to the drug of interest (i.e., cocaine). Although statistical models control for the potential effects of other drugs while evaluating the statistically independent contribution of prenatal cocaine exposure, it is difficult to ascertain the potential supra-additive or synergistic effects of polydrug use in human studies in which precision in measuring the timing and dosing of pregnancy drug use is at best a very rough estimate and largely dependent on the veracity of self-report methods. Accordingly, a limitation of this study includes difficulty specifying with precision the timing, duration, and dosing of cocaine and other drug exposures during gestation and, thus, difficulty identifying the specific mechanism of suspected effects (e.g., neurogenesis, neuronal migration, synaptogenesis, etc.). It is also difficult to identify critical periods of gestation or other windows of vulnerability for the affected process in fetal neural ontogeny.
Many contemporary teratological models include possibilities for independent and interdependent effects of a toxin expressed as a function of genetics and the rearing environment. Clearly, children who are raised by a drug-using parent are likely to be at increased risk for poor developmental and social outcomes due to chaotic caregiving environments, which may include parental neglect and abuse, ongoing caregiver substance use and severe mental health issues, family instability and homelessness, and exposure to violence, as well as the many environmental disparities associated with poverty (Mayes, 2002; Mayes & Ward, 2003). As research on the impact of prenatal cocaine exposure progresses, studies will need to include increased precision in identifying a priori hypotheses regarding not only the teratogenic effects of prenatal cocaine exposure but also more explicitly the social and environmental contexts in which they are expressed. These specifications include potential mediators, moderators and other important contextual determinants and inclusion of more complex modeling approaches. In addition, future follow-up assessments of the Miami PCS will make it possible to assess the stability of the currently reported LD findings as the cohort moves into the middle-school-age range. As the children in this cohort mature, it should be possible to assess the influence of higher cortical processes on learning and to delineate more precisely the predictors of the various subtypes of LD in relation to prenatal cocaine exposure.
Acknowledgments
This research was supported by the National Institutes of Health (NIH) National Institute on Drug Abuse (RO1 DA 06556); the NIH Center for Research Resources, University of Miami General Clinical Research Center (MO1–RR 05280); and a National Institute on Drug Abuse Research Training Award (T32 DA 07292 P.I. to James C. Anthony). Support was also provided by the Florida Healthy Start Program, the Health Foundation of South Florida, and the Kenneth A. Lattman Foundation.
Contributor Information
Connie E. Morrow, Department of Pediatrics, University of Miami School of Medicine
Jan L. Culbertson, Department of Pediatrics, University of Oklahoma Health Sciences Center
Veronica H. Accornero, Department of Pediatrics, University of Miami School of Medicine
Lihua Xue, Department of Pediatrics, University of Miami School of Medicine.
James C. Anthony, Department of Epidemiology, Michigan State University College of Human Medicine
Emmalee S. Bandstra, Department of Pediatrics, University of Miami School of Medicine
References
- Aaron PG. The impending demise of the discrepancy formula. Review of Education Research. 1997;67:461–502. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 2000. text rev. [Google Scholar]
- Ballard JL, Novak KK, Driver M. A simplified score for assessment of fetal maturation of newly born infants. Journal of Pediatrics. 1979;95:769–774. doi: 10.1016/s0022-3476(79)80734-9. [DOI] [PubMed] [Google Scholar]
- Bandstra ES, Morrow CE, Anthony JC, Accornero VH, Fried PA. Longitudinal investigation of task persistence and sustained attention in children with prenatal cocaine exposure. Neurotoxicology and Teratology. 2001;23:545–559. doi: 10.1016/s0892-0362(01)00181-7. [DOI] [PubMed] [Google Scholar]
- Bandstra ES, Morrow CE, Anthony JC, Churchill SS, Chitwood DD, Steele BM, et al. Intrauterine growth of full-term infants: Impact of prenatal cocaine exposure. Pediatrics. 2001;108:1309–1319. doi: 10.1542/peds.108.6.1309. [DOI] [PubMed] [Google Scholar]
- Bandstra ES, Morrow CE, Vogel AL, Fifer RC, Ofir AY, Dausa AT, et al. Longitudinal influence of prenatal cocaine exposure on child language functioning. Neurotoxicology and Teratology. 2002;24:297–308. doi: 10.1016/s0892-0362(02)00192-7. [DOI] [PubMed] [Google Scholar]
- Bandstra ES, Vogel A, Morrow CE, Xue L, Anthony JC. Severity of prenatal cocaine exposure and child language functioning through age seven years: A longitudinal latent growth curve analysis. Substance Use Misuse. 2004;39:25–59. doi: 10.1081/JA-120027765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnke M, Eyler FD, Garvan CW, Wobie K, Hou W. Cocaine exposure and developmental outcome from birth to 6 months. Neurotoxicology and Teratology. 2002;24:283–295. doi: 10.1016/s0892-0362(02)00191-5. [DOI] [PubMed] [Google Scholar]
- Behnke M, Eyler FD, Warner TD, Garvan CW, Hou W, Wobie K. Outcome from a prospective, longitudinal study of prenatal cocaine use: Preschool development at 3 years of age [Electronic version] Journal of Pediatric Psychology. 2006;31:41–49. doi: 10.1093/jpepsy/jsj027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendersky M, Gambini G, Lastella A, Bennett DS, Lewis M. Inhibitory motor control at five years as a function of prenatal cocaine exposure. Journal of Developmental and Behavioral Pediatrics. 2003;24:345–351. doi: 10.1097/00004703-200310000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DS, Bendersky M, Lewis M. Children’s intellectual and emotional-behavioral adjustment at 4 years as a function of cocaine exposure, maternal characteristics, and environmental risk. Developmental Psychology. 2002;38:648–658. doi: 10.1037//0012-1649.38.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JV, Bakeman R, Coles CD, Platzman KA, Lynch ME. Prenatal cocaine exposure: A comparison of 2-year-old children in parental and nonparental care. Child Development. 2004;75:1282–1295. doi: 10.1111/j.1467-8624.2004.00739.x. [DOI] [PubMed] [Google Scholar]
- Caldwell BM, Bradley RH, editors. Manual for the Home Observation for Measurement of the Environment (H.O.M.E.) Little Rock: University of Arkasas; 1984. Rev. ed. [Google Scholar]
- Carmichael Olson H, Toth SK. Samples in research on prenatal cocaine exposure: Vexing problems and practical solutions. Journal of Drug Issues. 1999;29:237–252. [Google Scholar]
- Chambers J, Shkolnik J, Pérez M. Total expenditures for students with disabilities, 1999–2000: Spending variation by disability. Palo Alto, CA: Center for Special Education Finance; 2003. Retrieved October 12, 2005 from http://www.csef-air.org/publications/seep/national/Final_SEEP_Report_5.PDF. [Google Scholar]
- Cohen L, Dehaene S, Chochon F, Lehericy S, Naccache L. Language and calculation within the parietal lobe: A combined cognitive, anatomical and fMRI study. Neuropsychologia. 2000;38:1426–1440. doi: 10.1016/s0028-3932(00)00038-5. [DOI] [PubMed] [Google Scholar]
- Dean VJ, Burns MK. Inclusion of intrinsic processing difficulties in LD diagnostic models: A critical review. Learning Disability Quarterly. 2002;25:170–177. [Google Scholar]
- Delaney-Black V, Covington C, Templin T, Kershaw T, Nordstrom-Klee B, Ager J, et al. Expressive language development of children exposed to cocaine prenatally: Literature review and report of a prospective cohort study. Journal of Communication Disorders. 2000;33:463–480. doi: 10.1016/s0021-9924(00)00033-2. [DOI] [PubMed] [Google Scholar]
- Frank DA, Augustyn M, Knight WG, Pell T, Zuckerman B. Growth, development, and behavior in early childhood following prenatal cocaine exposure: A systematic review. Journal of the American Medical Association. 2001;285:1613–1625. doi: 10.1001/jama.285.12.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DA, Bauchner H, Parker S, Huber AM, Kyei-Aboagye K, Cabral H, et al. Neonatal body proportionality and body composition after in utero exposure to cocaine and marijuana. Journal of Pediatrics. 1990;117:622–626. doi: 10.1016/s0022-3476(05)80702-4. [DOI] [PubMed] [Google Scholar]
- Frank DA, Jacobs RR, Beeghly M, Augustyn M, Bellinger D, Cabral H, et al. Level of prenatal cocaine exposure and scores on the Bayley Scales of Infant Development: Modifying effects of caregiver, early intervention, and birth weight. Pediatrics. 2002;110:1143–1152. doi: 10.1542/peds.110.6.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DA, Rose-Jacobs R, Beeghly M, Wilbur M, Bellinger D, Cabral H. Level of prenatal cocaine exposure and 48-month IQ: Importance of preschool enrichment. Neurotoxicology and Teratology. 2005;27:15–28. doi: 10.1016/j.ntt.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Fulbright RK, Molfese DL, Stevens AA, Skudlarski P, Lacadie CM, Gore JC. Cerebral activation during multiplication: A functional MR imaging study of number processing. American Journal of Neuroradiology. 2000;21:1048–1054. [PMC free article] [PubMed] [Google Scholar]
- Harvey JA. Cocaine effects on the developing brain: Current status. Neuroscience and Biobehavioral Reviews. 2004;27:751–764. doi: 10.1016/j.neubiorev.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Heffelfinger AK, Craft S, White DA, Shyken J. Visual attention in preschool children prenatally exposed to cocaine: Implications for behavioral regulation. Journal of the International Neuropsychological Society. 2002;8:12–21. [PubMed] [Google Scholar]
- Hurt H, Brodsky NL, Roth H, Malmud E, Giannetta JM. School performance of children with gestational cocaine exposure. Neurotoxicology and Teratology. 2005;27:203–211. doi: 10.1016/j.ntt.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Hurt H, Giannetta J, Brodsky NL, Malmud E, Pelham T. Are there neurologic correlates of in utero cocaine exposure at age 6 years? Journal of Pediatrics. 2001;138:911–913. doi: 10.1067/mpd.2001.113709. [DOI] [PubMed] [Google Scholar]
- Hurt H, Malmud E, Betancourt LM, Brodsky NL, Giannetta JM. A prospective comparison of developmental outcome of children with in utero cocaine exposure and controls using the Battelle Developmental Inventory. Journal of Developmental and Behavioral Pediatrics. 2001;22:27–34. doi: 10.1097/00004703-200102000-00005. [DOI] [PubMed] [Google Scholar]
- Kazui H, Kitagaki H, Mori E. Cortical activation during retrieval of arithmetical facts and actual calculation: A functional magnetic resonance imaging study. Psychiatry & Clinical Neurosciences. 2000;54:479–485. doi: 10.1046/j.1440-1819.2000.00739.x. [DOI] [PubMed] [Google Scholar]
- Lester BM, LaGasse LL, Seifer R. Cocaine exposure and children: The meaning of subtle effects. Science. 1998;282:633–634. doi: 10.1126/science.282.5389.633. [DOI] [PubMed] [Google Scholar]
- Lewis BA, Singer LT, Short EJ, Minnes S, Arendt R, Weishampel P, et al. Four-year language outcomes of children exposed to cocaine in utero. Neurotoxicology and Teratology. 2004;26:617–627. doi: 10.1016/j.ntt.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Lewis MW, Misra S, Johnson HL, Rosen TS. Neurological and developmental outcomes of prenatally cocaine-exposed offspring from 12 to 36 months. American Journal of Drug and Alcohol Abuse. 2004;30:299–320. doi: 10.1081/ada-120037380. [DOI] [PubMed] [Google Scholar]
- Mayes LC. Developing brain and in utero cocaine exposure: Effects on neural ontogeny. Development and Psychopathology. 1999;11:685–714. doi: 10.1017/s0954579499002278. [DOI] [PubMed] [Google Scholar]
- Mayes LC. A behavioral teratogenic model of the impact of prenatal cocaine exposure on arousal regulatory systems. Neurotoxicology and Teratology. 2002;24:385–395. doi: 10.1016/s0892-0362(02)00200-3. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Cicchetti D, Acharyya S, Zhang H. Developmental trajectories of cocaine-and-other-drug-exposed and non-cocaine-exposed children. Journal of Developmental and Behavioral Pediatrics. 2003;24:323–335. doi: 10.1097/00004703-200310000-00003. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Ward A. Principles of neurobehavioral teratology. In: Cicchetti D, Walker E, editors. Neurodevelopmental mechanisms in psychopathology. Cambridge: Cambridge University Press; 2003. pp. 3–33. [Google Scholar]
- Moore TR, Sorg J, Miller L, Key T, Resnik R. Hemodynamic effects of intravenous cocaine on the pregnant ewe and fetus. American Journal of Obstetrics and Gynecology. 1986;155:883–888. doi: 10.1016/s0002-9378(86)80044-8. [DOI] [PubMed] [Google Scholar]
- Morrow CE, Bandstra ES, Anthony JC, Ofir AY, Xue L, Reyes M. The influence of prenatal cocaine exposure on early language development: Longitudinal findings from 4 months through three years of age. Journal of Developmental and Behavioral Pediatrics. 2003;24:39–50. doi: 10.1097/00004703-200302000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow CE, Vogel AL, Anthony JC, Ofir AY, Dausa AT, Bandstra ES. Expressive and receptive language functioning in preschool children with prenatal cocaine exposure. Journal of Pediatric Psychology. 2004;29:543–554. doi: 10.1093/jpepsy/jsh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulé S, Casella GA. Confirmation and quantitation of cocaine, benzoylecgonine, ecgonine methyl ester in human urine by GC/MS. Journal of Analytical Toxicology. 1988;12:153–155. doi: 10.1093/jat/12.3.153. [DOI] [PubMed] [Google Scholar]
- Naglieri JA, Reardon SM. Traditional IQ is irrelevant to learning disabilities—intelligence is not. Journal of Learning Disabilities. 1993;26:127–133. doi: 10.1177/002221949302600205. [DOI] [PubMed] [Google Scholar]
- National Center for Learning Disabilities. Learning disabilities FAQ. Retrieved June 17, 2005 from http://www.ld.org/press/NCLDFAQ.cfm.
- Pulsifer MB, Radonovich K, Belcher HM, Butz AM. Intelligence and school readiness in preschool children with prenatal drug exposure. Child Neuropsychology. 2004;10:89–101. doi: 10.1080/09297040490911104. [DOI] [PubMed] [Google Scholar]
- Richardson GA, Conroy ML, Day NL. Prenatal cocaine exposure: Effects on the development of school-age children. Neurotoxicology and Teratology. 1996;18:627–634. doi: 10.1016/s0892-0362(96)00121-3. [DOI] [PubMed] [Google Scholar]
- Sattler JM, editor. Assessment of children: Revised and updated. 3. San Diego, CA: Author; 1992. [Google Scholar]
- Schroder MD, Snyder PJ, Sielski I, Mayes L. Impaired performance of children exposed in utero to cocaine on a novel test of visuospatial working memory. Brain and Cognition. 2004;55:409–412. doi: 10.1016/j.bandc.2004.02.062. [DOI] [PubMed] [Google Scholar]
- Shaywitz BA, Fletcher JM, Holahan JM, Shaywitz SE. Discrepancy compared to low achievement definitions of reading disability: Results from the Connecticut Longitudinal Study. Journal of Learning Disabilities. 1992;25:639–648. doi: 10.1177/002221949202501003. [DOI] [PubMed] [Google Scholar]
- Singer LT, Arendt R, Minnes S, Farkas K, Salvator A, Kirchner HL, et al. Cognitive and motor outcomes of cocaine-exposed infants. Journal of the American Medical Association. 2002;287:1952–1960. doi: 10.1001/jama.287.15.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Arendt R, Minnes S, Salvator A, Siegel AC, Lewis BA. Developing language skills of cocaine-exposed infants. Pediatrics. 2001;107:1057–1064. doi: 10.1542/peds.107.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer LT, Hawkins S, Huang J, Davillier M, Baley J. Developmental outcomes and environmental correlates of very low birthweight, cocaine-exposed infants. Early Human Development. 2001;64:91–103. doi: 10.1016/s0378-3782(01)00182-7. [DOI] [PubMed] [Google Scholar]
- Singer LT, Minnes S, Short E, Arendt R, Farkas K, Lewis B, et al. Cognitive outcomes of preschool children with prenatal cocaine exposure. Journal of the American Medical Association. 2004;291:2448–2456. doi: 10.1001/jama.291.20.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LM, Chang L, Yonekura ML, Gilbride K, Kuo J, Poland RE, et al. Brain proton magnetic resonance spectroscopy and imaging in children exposed to cocaine in utero. Pediatrics. 2001;107:227–231. doi: 10.1542/peds.107.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanovich KE, Stanovich P. Further thoughts on aptitude/achievement discrepancy. Educational Psychology in Practice. 1997;13:3–8. [Google Scholar]
- StataCorp. Stata Statistical Software. College Station, TX: Author; 2003. [Google Scholar]
- Swanson HL. Issues facing the field of learning disabilities. Learning Disability Quarterly. 2000;23:37–50. [Google Scholar]
- U.S. Department of Education. Twenty-fourth annual report to Congress on the implementation of the Individuals with Disabilities Education Act. Washington, DC: Author; 2001. [Google Scholar]
- Volpe JJ. Effect of cocaine use on the fetus. New England Journal of Medicine. 1992;327:399–407. doi: 10.1056/NEJM199208063270607. [DOI] [PubMed] [Google Scholar]
- Vorhees CV. Concepts in teratology and developmental toxicology derived from animal research. Annals of the New York Academy of Sciences. 1989;562:31–41. doi: 10.1111/j.1749-6632.1989.tb21005.x. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Kline JK, Bateman DA, Chiriboga C, Lumey LH, Friedlander H, et al. Prenatal cocaine exposure and school-age intelligence. Drug and Alcohol Dependence. 1998;50:203–210. doi: 10.1016/s0376-8716(98)00037-4. [DOI] [PubMed] [Google Scholar]
- Whalen J, McCloskey M, Lesser R. Localizing arithmetic processes in the brain: Evidence from a transient deficit during cortcal stimulation. Journal of Cognitive Neuroscience. 1997;9:409–417. doi: 10.1162/jocn.1997.9.3.409. [DOI] [PubMed] [Google Scholar]


