Abstract
We aimed to clarify responsiveness to angiotensin (Ang) II in the porcine basilar artery and the role of Ang II receptor subtypes by functional, radioligand binding, and cell culture studies. Ang II induced more potent contractions in the proximal part than in the distal part of isolated porcine basilar arteries. The contraction induced by Ang II was inhibited by the Ang II type 1 (AT1) receptor antagonist losartan, but the Ang II type 2 (AT2) receptor antagonist PD123319 enhanced it. After removal of the endothelium, the effect of losartan remained but the effect of PD123319 was abolished. The specific binding site of [3H]Ang II on the smooth muscle membrane was inhibited by losartan, but not by PD123319. Stimulation of angiotensin II increased nitric oxide (NO) production in cultured basilar arterial endothelial cells. This production was inhibited by PD123319 and the NO synthase inhibitor L–NG-nitroarginine. These results suggest that the contraction induced by Ang II might be mediated via the activation of AT1 receptors on the basilar arterial smooth muscle cells and be modulated via the activation of AT2 receptors on the endothelial cells, followed by NO production.
Keywords: Angiotensin II receptors, Cerebral arteries, Endothelium, Losartan, Nitric oxide, PD123319
Introduction
Angiotensin (Ang) receptor subtypes are generally classified into two main groups, Ang II type 1 (AT1) and Ang II type 2 (AT2) receptors (Widdop et al., 2003). The distributions of the two receptor subtypes are different (Timmermans et al., 1993). In general, AT2 receptors are predominant in the fetus; however, after birth the expression of AT2 receptors is restricted to some regions and AT1 receptors become predominant (Matsubara, 1998; Horiuchi et al., 1999). The AT1 receptor is concerned with vasoconstriction in various arteries, whereas the AT2 receptor is concerned with vasorelaxation in the uterine (McMullen et al., 1999; Hannan et al., 2003), mesenteric (Matrougui et al., 1999; Touyz et al., 1999; Matrougui et al., 2000), and cerebral (Tsutsumi and Saavedra, 1991) arteries. However, the role of the AT2 receptor remains unclear.
Ang II induces contraction in the basilar arteries of the monkey (Toda et al., 1990), rabbit (Zerrouk et al., 1996), and dog (Manabe et al., 1989; Yen et al., 1990). However, these reports have not demonstrated the Ang receptor subtypes responsible for contraction in these arteries. Similarly, there is no information about responsiveness of the porcine basilar artery to Ang II and its related Ang receptor subtypes. Therefore, we aimed to clarify the response of the porcine basilar artery to Ang II and determine the role of Ang II receptor subtypes in this response by functional, radioligand binding, and cell culture studies.
Material and methods
Tissue preparation
Basilar arteries were obtained from freshly slaughtered pigs (both sexes, about 6 or 7 months old, Landrace–Large White–Duroc crossbreed) at a local slaughterhouse and transferred to our laboratory in ice-cold physiological saline (119 mM NaCl, 4.7 mM KCl, 1.6 mM CaCl2, 1.2 mM MgCl2, 25 mM NaHCO3, 1.2 mM KH2PO4, and 10 mM glucose, pH 7.4) aerated with carbogen (95% (v/v) O2, 5% (v/v) CO2). Each artery was dissected free of adherent tissues.
Reagents
We used the following reagents: Ang II acetate salt, [Sar1, Thr8]angiotensin II, bradykinin, PD123319 ditrifluoroacetate salt, uridine 5′-triphosphate sodium salt (UTP), Dulbecco’s modified Eagle’s medium (DMEM), nutrient mixture F-12 HAM, penicillin, streptomycin and amphotericin B (Sigma, Saint Louis, MO, USA), heat-inactivated horse serum (Invitrogen Corp., NY, USA), fluorescent acetylated low-density lipoprotein (Harbor Bio-Product, Norwood, MA, USA), Ang II (5-L-isoleucine), [tyrosyl-3.5-3H(N)] (Perkin Elmer, Boston, MA, USA), NO2/NO3 assay kit (Wako, Osaka, Japan). Losartan potassium was a gift of Merck and Co., Inc. (Whitehouse Station, NJ, USA).
Functional study
Several (three or 4) rings approximately 4 mm long were cut from each artery. The rings were mounted vertically between two L-shaped stainless steel holders, with the upper part fixed to an isometric force transducer (TB-611T, Nihon Kohden Kogyo, Tokyo, Japan), and immersed in a 5-ml water-jacketed organ bath containing oxygenated salt solution at 37 °C (pH 7.4). Each suspended ring was left to equilibrate for at least 120 min under a resting tension of 7.5 mN. This tension was chosen because it allowed us to induce maximum contractions in the artery. KCl (60 mM) was applied every 30 min until the amplitude of the contraction reached a constant value. Changes in the KCl concentration of the physiological saline were compensated for by equimolar adjustment of the NaCl concentration. The isometric tension was displayed on an ink-writing recorder (WI-641G, Nihon Kohden Kogyo, Tokyo, Japan). The cumulative concentration–response curve of Ang II or the contraction with a single application was obtained by adding a solution of Ang II directly to the fluid in the bath. Antagonists were added to the bathing media 30 min before Ang II. There were no effects of antagonists on the resting tension.
The presence of endothelial cells was confirmed pharmacologically by testing the relaxant response to bradykinin under precontracted conditions with UTP (this response is abolished by endothelial denudation; Miyamoto et al., 1999), and morphologically by scanning and transmission electron microscopy after the experiments.
Radioligand binding study
Isolated porcine basilar arteries for radioligand binding assay were cut longitudinally and the endothelial cells were removed by gentle rubbing with a cotton rod, followed by rinsing with physiological saline. The rinsed basilar arteries were minced with scissors and then homogenized in 8 volumes of 50 mM Tris–HCl buffer (pH 7.4) using a Polytron homogenizer at a setting of 8 for periods of 15 s with 45-s intervals in an ice-bath. The membrane homogenate was centrifuged at 500 ×g for 15 min. Then the supernatant was centrifuged at 100,000 ×g for 30 min. The pellet was resuspended in buffer solution, and the suspension was used for the binding assay as a crude membrane fraction. These procedures were all performed at a temperature of 4 °C. The protein concentration of the final suspension was measured by the method of Lowry et al. (1951), with bovine serum albumin as a standard.
Cell culture
Primary porcine basilar arterial endothelial cells were isolated by infusing 0.05% trypsin–EDTA solution into the vessel through a polyethylene tube (SP10, I.D. 0.28 mm, O.D. 0.61 mm, Natume, Tokyo, Japan) and cultured in a growth medium containing 45% DMEM, 45% nutrient mixture F-12 HAM, 10% horse serum and antibiotic mixture of 100 units/ml penicillin, 100 mg/ml streptomycin, and 2.5 mg/ml amphotericin B. The above method was referred to in a previous report of porcine brain capillary endothelial cells (Huwyler et al., 1997). When the nitrite (NO2−) and nitrate (NO3−) level was measured as an indicator of NO production, a mixed medium of DMEM and F-12 HAM without phenol red was used to avoid disturbance of the fluorometric assay (Misko et al., 1993). In this assay kit, the minimum detectable dose of NO2/NO3 is <1 µM with coefficient of variation (3.5±0.5%). The endothelial cells were characterized by their morphology using phase-contrast microscopy (Olympus, IX70, Tokyo, Japan) and by staining for fluorescent acetylated low-density lipoprotein (Voyta et al., 1984). Only endothelial cells of less than 6 passages were used. Confluent endothelial cells (3–5×106) were treated with Ang II.
Statistical analysis
Results are expressed as the mean T s.e.m. Statistical analyses were performed by Student’s t-test or the Bonferroni test after one-way analysis of variance. Significance was established when the probability level was equal to or less than 5%.
Results
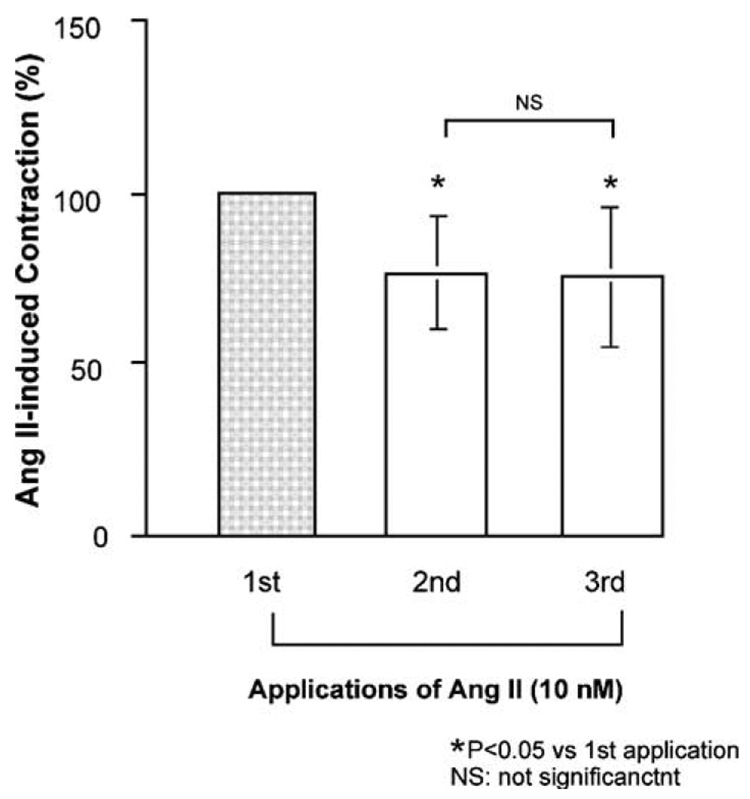
A typical isometric tension response to Ang II in isolated porcine basilar arterial rings obtained from region [a] (Fig. 1A) is illustrated in Fig. 1B. Ang II induced a concentration-dependent contraction. The maximum response induced by 10 nM Ang II was 2.2±0.2 mN; this was 19.1±2.4% of the response to 60 mM KCl. The response to all doses at region [a] was significantly higher than those in the other regions [b–d] (Fig. 1A) (Fig. 1C). Therefore, we performed the following experiments in a functional study using the section of artery isolated from region [a]. Removal of the endothelium had no significant effect on regional differences in the response to Ang II in the basilar artery (Fig. 1D). The first response to Ang II was significantly greater than the second; however, the second response was not significantly different from the third response (Fig. 2). Therefore, the second response was defined as the control, and antagonists were added to the arterial ring 30 min before the third response was elicited.
Fig. 1.
Typical response to angiotensin (Ang) II [B] in isolated porcine basilar arterial rings obtained from region a [A]. Regional differences in responsiveness to Ang II are shown in [C]. After removal of the endothelium, the regional difference in response to Ang II (10 nM) remained [D]. The regions of arteries (a–d) used in [C] and [D] were shown in [A]. Each bar represents the mean±s.e.m. from 7 [C] or 5 [D] pigs.
Fig. 2.
Effects of repeated application of angiotensin (Ang) II (10 nM) on isolated porcine basilar artery. Each bar represents the mean±s.e.m. for 8 pigs.
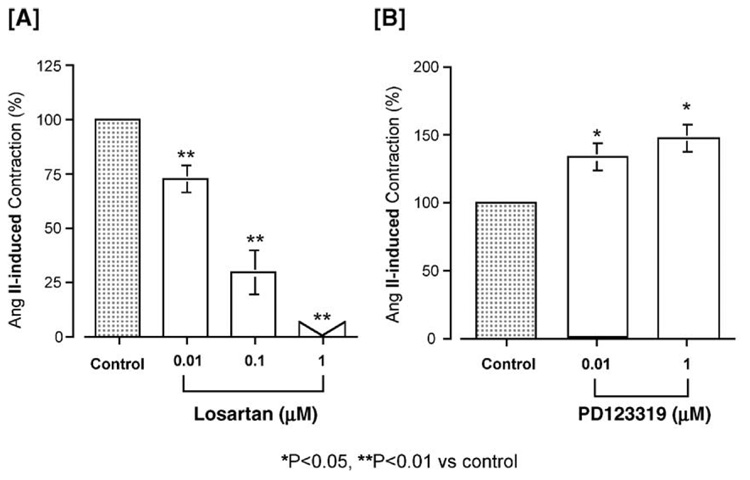
The basilar arteries were treated with a non-selective Ang II receptor antagonist, [Sar1, Thr8]Ang II. This antagonist inhibited the Ang II (10 nM)-induced contraction in a concentration-dependent manner. The pIC50 of [Sar1, Thr8]Ang II was 8.81±0.21. We then examined the effects of the selective AT1 receptor antagonist losartan (0.01 to 1 µM) and the selective AT2 receptor antagonist PD123319 (0.01 and 1 µM) on the Ang II (10 nM)-induced contraction. Losartan significantly inhibited the Ang II-induced contraction, with a pIC50 of 7.33±0.14 (Fig. 3A) and abolished it at a concentration of 1 µM, whereas PD123319 significantly enhanced the contraction in a concentration-dependent manner (Fig. 3B).
Fig. 3.
Effects of the selective AT1 antagonist losartan [A] and the selective AT2 antagonist PD123319 [B] on angiotensin (Ang) II (10 nM)-induced contraction (control) in isolated porcine basilar artery. Each bar represents the mean±s.e.m. for 8 pigs.
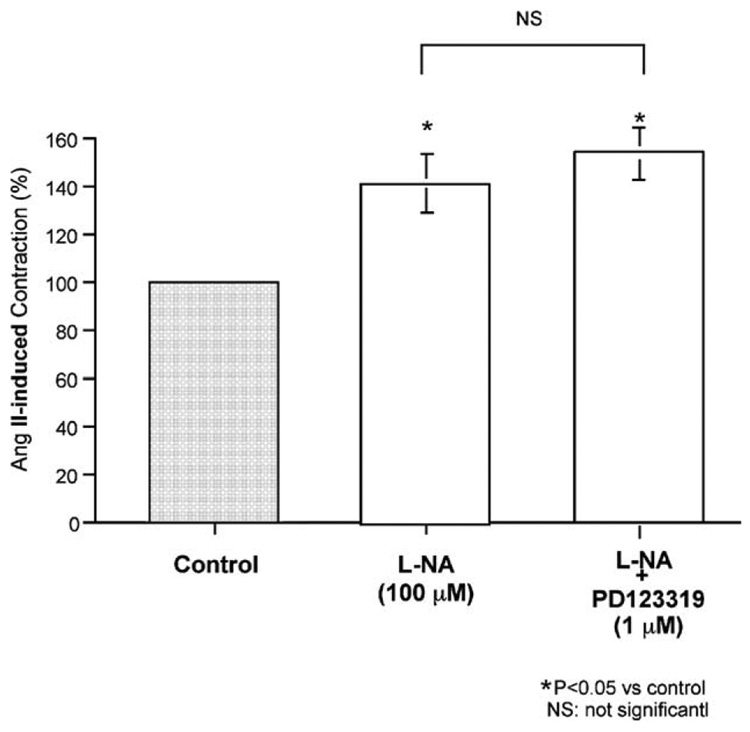
Fig. 4 shows that the NO synthase inhibitor L–NG-nitroarginine (L-NA) (100 µM) significantly enhanced Ang II (10 nM)-induced contraction. However, addition of PD123319 (1 µM) to the L-NA had no effect on enhancement of the Ang II (10 nM)-induced contraction.
Fig. 4.
Effects of L–NG-nitroarginine (L-NA) (an NO synthase inhibitor) and L-NA plus PD123319 (a selective AT2 antagonist) on the angiotensin (Ang) II (10 nM)-induced contraction (control) in isolated porcine basilar artery. Each bar represents the mean±s.e.m. for 8 pigs.
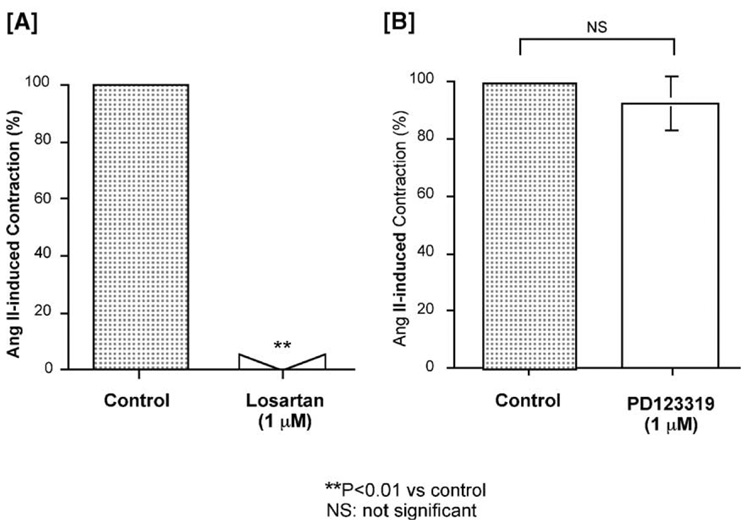
After removal of the endothelium, losartan abolished the Ang II-induced contraction at the same concentration (1 µM) at which it abolished it in the presence of endothelium. However, PD123319 no longer enhanced the Ang II-induced contraction (Fig. 5).
Fig. 5.
Effects of the selective AT1 antagonist losartan [A] and the selective AT2 antagonist PD123319 [B] on the angiotensin (Ang) II (10 nM)-induced contraction (control) in isolated porcine basilar artery, after removal of the endothelium. Each bar represents the mean±s.e.m. for 7 pigs.
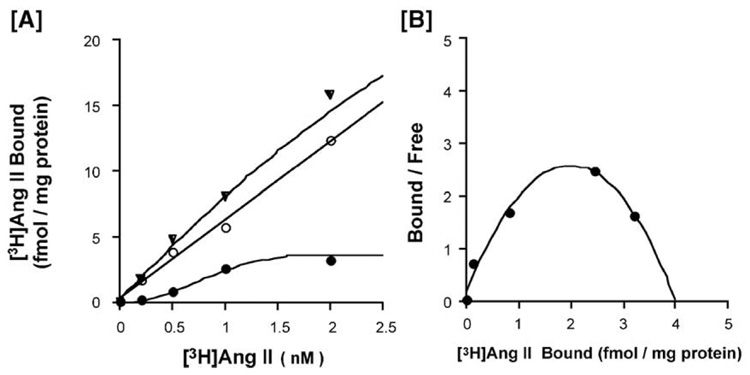
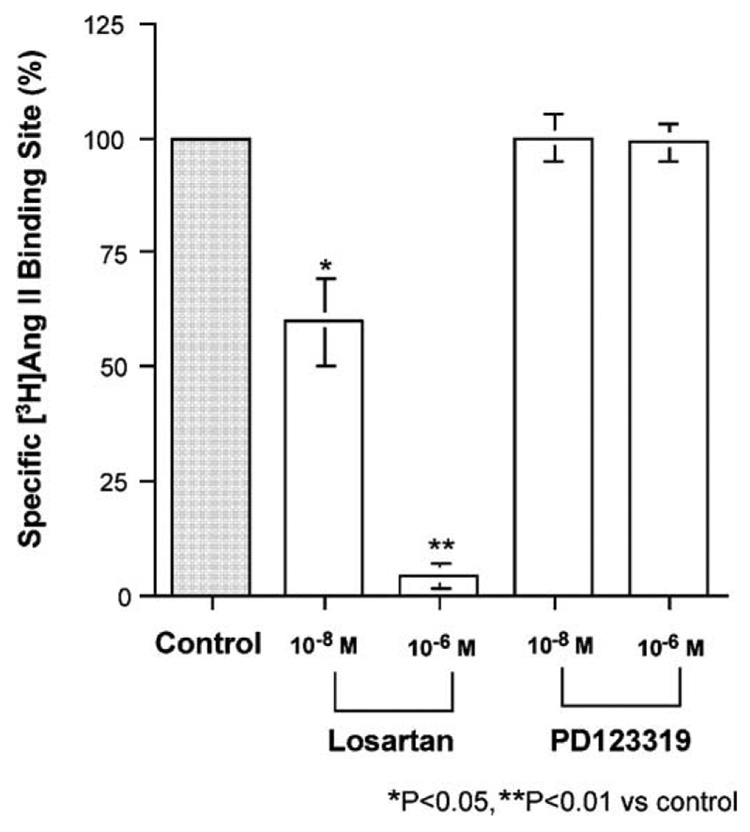
Fig. 6 shows a typical pattern obtained in the binding assay of [3H]Ang II to the membrane fraction from porcine basilar arteries without endothelium in the absence (total binding) and presence (nonspecific binding) of 100 µM Ang II. Similar results were obtained from two other experiments. The specific binding, which was calculated as the difference between the total and non-specific bindings, appeared to be saturable. The Bmax value was 4.15 ±1.17 fmol/mg protein (n =3). A Scatchard plot of the specific binding showed a positive cooperativity. The Hill coefficient was 1.77 ±0.18 (n =3). Losartan inhibited the specific [3H]Ang II (1 nM) binding site with a pIC50 of 7.76±0.25 (n =3) in a concentration-dependent manner; however, the selective AT2 receptor antagonist PD123319 did not (Fig. 7).
Fig. 6.
Typical [3H]angiotensin (Ang) II binding to the membrane fraction from porcine basilar artery smooth muscle (A), and a Scatchard plot (B). ▼: total binding, ○: non-specific binding, ●: specific binding.
Fig. 7.
Effects of the selective AT1 receptor antagonist losartan and the selective AT2 receptor antagonist PD123319 on [3H]angiotensin (Ang) II (1 nM) binding sites (control) in the membrane of porcine basilar artery smooth muscle. Each bar represents the mean±s.e.m. for 3 independent experiments performed in duplicate.
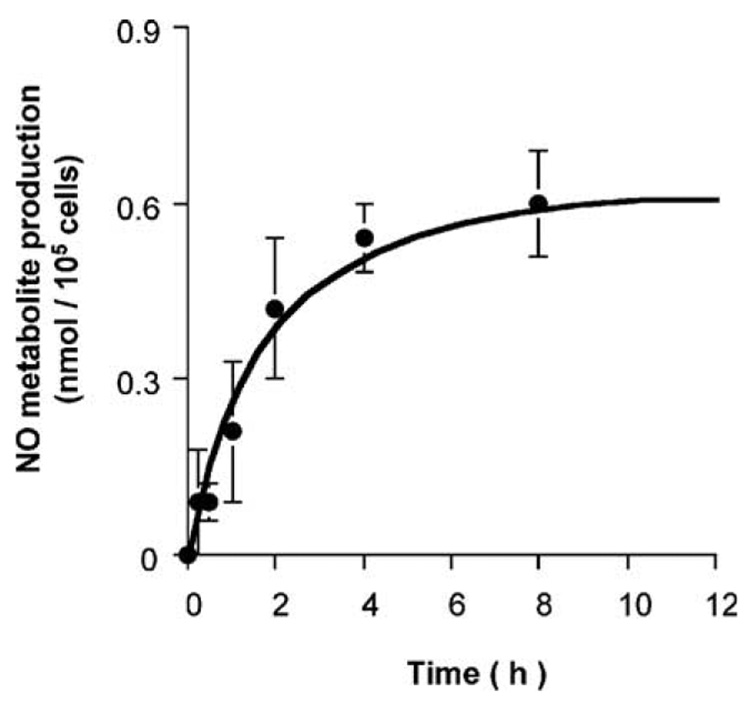
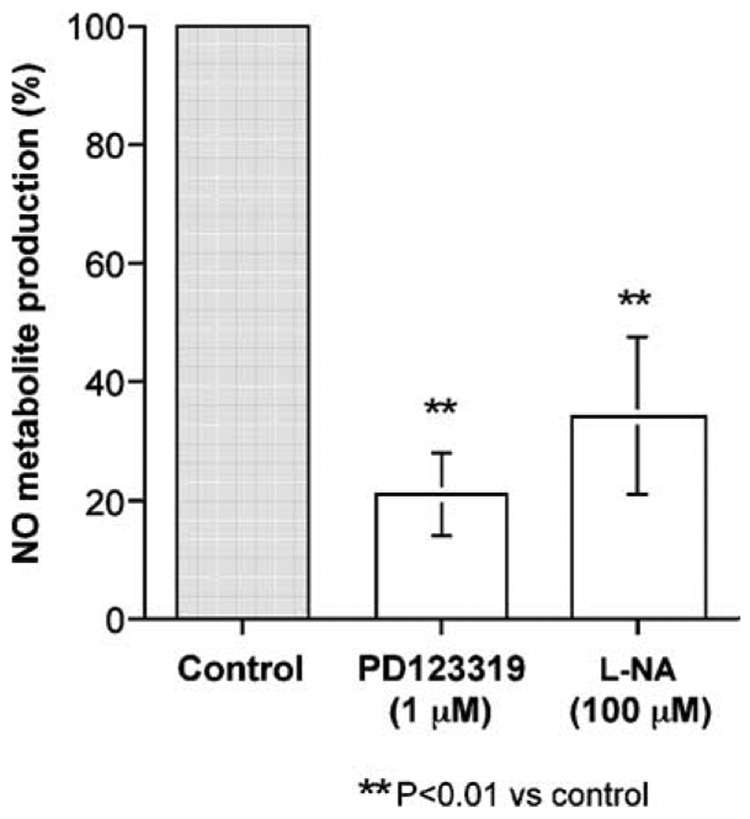
To confirm NO production from porcine basilar endothelial cells stimulated by Ang II, the total amounts of NO2− and NO3− (NO final metabolites) were measured using an NO2/NO3 assay kit. This assay kit was suitable for the measurement of 1–10 µM NO2/NO3. Stimulation of the cultured porcine basilar arterial endothelial cells with 0.1 µM Ang II released NO metabolites, and their production reached a plateau after 8 h (Fig. 8). NO production 2 h after stimulation by Ang II (0.1 µM) was significantly inhibited by PD123319 (1 µM) or L-NA (100 µM) (Fig. 9).
Fig. 8.
Time cores of NO metabolite (NO2−/NO3 −) production after angiotensin (Ang) II (0.1 µM) treatment of cultured porcine basilar artery endothelial cells. Each bar represents the mean±s.e.m. for 3 independent experiments in duplicate.
Fig. 9.
Effects of PD123319 (1 µM) or L–NG-nitroarginine (L-NA) (100 µM) on NO metabolite production 2 h after angiotensin II (0.1 µM) treatment of cultured porcine basilar arterial endothelial cells. Each bar represents the mean±s.e.m. for 3 independent experiments in duplicate.
Discussion
Our results showed that Ang II induced contractions in the porcine basilar artery through activation of AT1 receptors located on the smooth muscle cells, whereas AT2 receptors were located on the endothelial cells and modified the Ang II-induced contraction by production of NO.
The contraction induced by Ang II was most potent (2.2±0.2 mN) at the proximal part of the porcine basilar artery. The magnitude of the contraction was weaker than those induced by histamine (7.7±0.9 mN) (Miyamoto and Nishio, 1993) and serotonin (9.8±1.5 mN) (Miyamoto et al., 1994) in the same artery. The Bmax of [3H]Ang II (4.15 fmol/mg protein) was lower than those of [3H]mepyramine (a selective H1 antagonist; 95.6 fmol/mg protein), [3H]cimetidine (a selective H2 antagonist; 459.8 fmol/mg protein) (Miyamoto and Nishio, 1993), and [3H]serotonin (high affinity site: 29.5 fmol/mg protein, low affinity site: 1950 fmol/mg protein) (Miyamoto et al., 1994). The lowest Bmax for specific binding sites might induce the lowest magnitude of contraction to vasoconstrictors. Regional differences were observed in the Ang II-induced contraction; however, this phenomenon was not observed in histamine- and serotonin-induced contractions or in norepinephrine-induced relaxation (Miyamoto et al., 1993) in porcine basilar arteries. As shown in Fig. 2, the 2nd responses to Ang II were weaker than 1st responses, but the 3rd responses were not significantly different from 2nd responses. Precise reasons about the loss of response to Ang II between the 1st and 2nd application were unclear. But the 2nd responses were abolished by losartan, so the contraction on the 2nd application was Ang II specific. The similar phenomena were observed in bovine basilar arteries applied with histamine (Miyamoto and Nishio, 1994). The regional difference in Ang II-induced contractions remained after removal of the endothelial cells (Fig. 1D). These results suggest that the different levels of responsiveness to Ang II within the basilar artery are caused by differences in the distribution of AT1 receptors located on the smooth muscle cells.
The selective AT1 receptor antagonist losartan inhibited the Ang II-induced contraction with a pIC50 of 7.33 (Fig. 3A), and the same effect remained after removal of the basilar arterial endothelial cells (Fig. 5A). Moreover, the radioligand binding study using the membrane fraction obtained from the smooth musculature of the basilar arteries showed that losartan, not PD123319, inhibited the specific binding sites of [3H]Ang II with a pIC50 of 7.76 (Fig. 7). These two pIC50 values (obtained from the functional and binding studies) were not significantly different. These results suggest that AT1 receptors are located on the smooth muscle cells and are responsible for vasoconstriction.
The selective AT2 receptor antagonist PD123319 (Widdop et al., 1993), enhanced Ang II-induced contraction. This effect was abolished by removal of the basilar arterial endothelial cells. These results suggest that AT2 receptors are located on the endothelial cells and are responsible for vasorelaxation. This suggestion is supported by the results of our three other experiments: (1) PD123319 had no effect on Ang II-induced contraction after inhibition of NO production by the NO synthase inhibitor L-NA (Fig. 4); (2) the specific binding site of [3H]Ang II on the membrane fraction obtained from smooth muscles was not inhibited by PD123319 (Fig. 5B); and (3) Ang II increased NO production from porcine basilar arterial endothelial cells in tissue culture medium (Fig. 8). These results suggest that the relaxing factor induced by Ang II might be NO. NO production by AT2 receptor activation on endothelial cells has also been reported in canine coronary arteries (Seyedi et al., 1995), rat aorta (Gohlke et al., 1998; Pueyo et al., 1998), rat skeletal muscle microcirculation (Nora et al., 1998), and porcine and rat pulmonary arteries (Hill-Kapturczak et al., 1999; Olson et al., 2004).
The evaluation of Ang II binding using intact basilar arteries might support the functional data directly. However, small pieces of arteries would preclude picking up the receptors, especially on the endothelial cells. So, in this study we did not evaluate Ang II binding in intact arteries. It seems important to evaluate the AT2 receptors on porcine basilar arterial endothelial cells, so near future radioligand binding study would be done using cultured endothelial cells.
The Scatchard plots and Hill coefficient (1.77) of the specific binding of [3H]Ang II showed a positive cooperativity in porcine basilar arterial smooth muscle membrane fractions. These results indicate that the resting state of the Ang receptor in smooth muscle is a low affinity state, and that interaction with Ang II induces a portion of the receptors into a high affinity “excited” state (Moore and Kwok, 1987). Positive cooperativity (Hill coefficient for Ang II, 1.41) has been reported in rat aorta smooth muscle cells (Moore and Scanlon, 1989). However, the Scatchard plots of the specific binding of [3H]Ang II form a straight line in the rat liver (Widdowson et al., 1993). It remains unclear why our Scatchard plot was not a straight line and why the Hill coefficient was more than unity. Further studies might be needed to clarify these points.
On the physiological condition, when Ang II acts on the basilar artery for contraction via AT1 receptor stimulation, the endothelium would act to prevent too much contraction via AT2 receptor stimulation and to keep the basilar arterial blood flow via releasing NO. In the case of diabetes (Trauernicht et al., 2003) or hypertension, for example, spontaneous hypertensive rat (SHR) (Bueno et al., 2005) or a renin-dependent model of hypertensive rat (Callera et al., 2000), the endothelium would be injured and dysfunctional because NO production was low and/or acetylcholine-induced relaxation was impaired. Therefore AT2 receptors might not act on the release of NO. It might be possible to speculate that diabetes or hypertension bring the blood flow reduction in the basilar artery. This speculation might be supported by a similar set of studies using SHR or diabetic rat.
In conclusion, Ang II induced more potent contractions in the proximal parts of isolated porcine basilar arteries than in the distal parts. The response appears to be composed of a main contraction (induced by the activation of AT1 receptors on smooth muscle cells) and relaxation (induced by the activation of AT2 receptors on endothelial cells, followed by NO production).
Acknowledgment
This study was supported by Grants-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (no. 13660302 and no. 16580242).
References
- Bueno R, de Sotomayor MA, Perez-Guerrero C, Gomez-Amores L, Vazquez CM, Herrera MD. L-carnitine and propionyl-L-carnitine improve endothelial dysfunction in spontaneously hypertensive rats: Different participation of NO and COX-products. Life Sciences. 2005;77:2082–2097. doi: 10.1016/j.lfs.2005.01.035. [DOI] [PubMed] [Google Scholar]
- Callera GE, Varanda WA, Bendhack LM. Impaired relaxation to acetylcholine in 2K-1C hypertensive rat aortas involves changes in membrane hyperpolarization instead of an abnormal contribution of endothelial factors. General Pharmacology. 2000;34:379–389. doi: 10.1016/s0306-3623(01)00075-1. [DOI] [PubMed] [Google Scholar]
- Gohlke P, Pees C, Unger T. AT2 receptor stimulation increases aortic cyclic GMP in SHRSP by a kinin-dependent mechanism. Hypertension. 1998;31:349–355. doi: 10.1161/01.hyp.31.1.349. [DOI] [PubMed] [Google Scholar]
- Hannan RE, Davis EA, Widdop RE. Functional role of angiotensin II AT2 receptor in modulation of AT1 receptor-mediated contraction in rat uterine artery: involvement of bradykinin and nitric oxide. British Journal of Pharmacology. 2003;140:987–995. doi: 10.1038/sj.bjp.0705484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Kapturczak N, Kapturczak MH, Block ER, Patel JM, Malinski T, Madsen KM, Tisher CC. Angiotensin II-stimulated nitric oxide release from porcine pulmonary endothelium is mediated by angiotensin IV. Journal of the American Society of Nephrology. 1999;10:481–491. doi: 10.1681/ASN.V103481. [DOI] [PubMed] [Google Scholar]
- Horiuchi M, Akishita M, Dzau VJ. Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension. 1999;33:613–621. doi: 10.1161/01.hyp.33.2.613. [DOI] [PubMed] [Google Scholar]
- Huwyler J, Fricker G, Török M, Schneider M, Drewe J. Transport of clonidine across cultured brain microvessel endothelial cells. Journal of Pharmacology and Experimental Therapeutics. 1997;282:81–85. [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Manabe K, Shirahase H, Usui H, Kurahashi K, Fujiwara M. Endothelium-dependent contractions induced by angiotensin I and angiotensin II in canine cerebral artery. Journal of Pharmacology and Experimental Therapeutics. 1989;251:317–320. [PubMed] [Google Scholar]
- Matrougui K, Loufrani L, Heymes C, Lévy BI, Henrion D. Activation of AT2 receptors by endogenous angiotensin II is involved in flow-induced dilation in rat resistance arteries. Hypertension. 1999;34:659–665. doi: 10.1161/01.hyp.34.4.659. [DOI] [PubMed] [Google Scholar]
- Matrougui K, Lévy BI, Henrion D. Tissue angiotensin II and endothelin-1 modulate differently the response to flow in mesenteric resistance arteries of normotensive and spontaneously hypertensive rats. British Journal of Pharmacology. 2000;130:521–526. doi: 10.1038/sj.bjp.0703371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara H. Pathophysiological role of angiotensin II type 2 receptor in cardiovascular and renal diseases. Circulation Research. 1998;83:1182–1191. doi: 10.1161/01.res.83.12.1182. [DOI] [PubMed] [Google Scholar]
- McMullen JR, Gibson KJ, Lumbers ER, Burrell JH, Wu J. Interactions between AT1 and AT2 receptors in uterine arteries from pregnant ewes. European Journal of Pharmacology. 1999;378:195–202. doi: 10.1016/s0014-2999(99)00454-9. [DOI] [PubMed] [Google Scholar]
- Misko TP, Schilling RJ, Salvemini D, Moore WM, Currie MG. A fluorometric assay for the measurement of nitrite in biological samples. Analytical Biochemistry. 1993;214:11–16. doi: 10.1006/abio.1993.1449. [DOI] [PubMed] [Google Scholar]
- Miyamoto A, Nishio A. Characterization of histamine receptors in isolated pig basilar artery by functional and radioligand binding studies. Life Sciences. 1993;53:1259–1266. doi: 10.1016/0024-3205(93)90570-s. [DOI] [PubMed] [Google Scholar]
- Miyamoto A, Nishio A. Vasomotor effects of histamine on bovine and equine basilar arteries in vitro. Veterinary Research Communications. 1994;18:447–456. doi: 10.1007/BF01839422. [DOI] [PubMed] [Google Scholar]
- Miyamoto A, Ito K, Nishio A. Characterization of β-adrenoceptors in pig basilar artery from functional and radioligand binding studies. Japanese Journal of Pharmacology. 1993;61:93–99. doi: 10.1254/jjp.61.93. [DOI] [PubMed] [Google Scholar]
- Miyamoto A, Sakota T, Nishio A. Characterization of 5-hydroxytryptamine receptors on the isolated pig basilar artery by functional and radioligand binding studies. Japanese Journal of Pharmacology. 1994;65:265–273. doi: 10.1254/jjp.65.265. [DOI] [PubMed] [Google Scholar]
- Miyamoto A, Ishiguro S, Nishio A. Stimulation of bradykinin B2-receptors on endothelial cells induces relaxation and contraction in porcine basilar artery in vitro. British Journal of Pharmacology. 1999;128:241–247. doi: 10.1038/sj.bjp.0702783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GJ, Kwok YC. Angiotensin receptors in resting smooth muscle are the low affinity sites observed in binding studies. Life Sciences. 1987;41:505–511. doi: 10.1016/0024-3205(87)90228-1. [DOI] [PubMed] [Google Scholar]
- Moore GJ, Scanlon MN. Methods for analyzing and interpreting cooperativity in dose– response curves — I. Antagonist effects on angiotensin receptors in smooth muscle. General Pharmacology. 1989;20:193–198. doi: 10.1016/0306-3623(89)90014-1. [DOI] [PubMed] [Google Scholar]
- Nora EH, Munzenmaier DH, Hansen-Smith FM, Lombard JH, Greene AS. Localization of the ANG II type 2 receptor in the microcirculation of skeletal muscle. American Journal of Physiology. 1998;275:H1395–H1403. doi: 10.1152/ajpheart.1998.275.4.h1395. [DOI] [PubMed] [Google Scholar]
- Olson S, Oeckler R, Li X, Du L, Traganos F, Zhao X, Burke-Wolin T. Angiotensin II stimulates nitric oxide production in pulmonary artery endothelium via the type 2 receptor. American Journal of Physiology. 2004;287:L559–L568. doi: 10.1152/ajplung.00312.2003. [DOI] [PubMed] [Google Scholar]
- Pueyo ME, Arnal J-F, Rami J, Michel J-B. Angiotensin II stimulates the production of NO and peroxynitrite in endothelial cells. American Journal of Physiology. 1998;274:C214–C220. doi: 10.1152/ajpcell.1998.274.1.C214. [DOI] [PubMed] [Google Scholar]
- Seyedi N, Xu X, Nasjletti A, Hintze TH. Coronary kinin generation mediates nitric oxide release after angiotensin receptor stimulation. Hypertension. 1995;26:164–170. doi: 10.1161/01.hyp.26.1.164. [DOI] [PubMed] [Google Scholar]
- Timmermans PB, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JA, Smith RD. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacological Reviews. 1993;45:205–251. [PubMed] [Google Scholar]
- Toda N, Ayaziki K, Okamura T. Modifications by endogenous prostaglandins of angiotensin II-induced contractions in dog and monkey cerebral and mesenteric arteries. Journal of Pharmacology and Experimental Therapeutics. 1990;252:374–379. [PubMed] [Google Scholar]
- Touyz RM, Endemann D, He G, Li J-S, Schiffrin EL. Role of AT2 receptors in angiotensin II-stimulated contraction of small mesenteric arteries in young SHR. Hypertension. 1999;33:366–372. doi: 10.1161/01.hyp.33.1.366. [DOI] [PubMed] [Google Scholar]
- Trauernicht AK, Sun H, Patel KP, Mayhan WG. Enalapril prevents impaired nitric oxide synthase-dependent dilatation of cerebral arterioles in diabetic rats. Stroke. 2003;34:2698–2703. doi: 10.1161/01.STR.0000092121.62649.DC. [DOI] [PubMed] [Google Scholar]
- Tsutsumi K, Saavedra JM. Characterization of AT2 angiotensin II receptors in rat anterior cerebral arteries. American Journal of Physiology. 1991;261:H667–H670. doi: 10.1152/ajpheart.1991.261.3.H667. [DOI] [PubMed] [Google Scholar]
- Voyta JC, Netland PA, Via DP, Zetter BR. Specific labeling of endothelial cells using fluorescent acetylated low-density lipoprotein. Journal of Cell Biology. 1984;99:81A. doi: 10.1083/jcb.99.6.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdop RE, Jones ES, Hannan RE, Gaspari TA. Angiotensin AT2 receptors: cardiovascular hope or hype? British Journal of Pharmacology. 2003;140:809–824. doi: 10.1038/sj.bjp.0705448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdop RE, Gardiner SM, Kemp PA, Bennett T. Central administration of PD 123319 or EXP-3174 inhibits effects of angiotensin II. American Journal of Physiology. 1993;264:H117–H125. doi: 10.1152/ajpheart.1993.264.1.H117. [DOI] [PubMed] [Google Scholar]
- Widdowson PS, Renouard A, Vilaine J-P. Binding of [3H]angiotensin II and [3H]DuP 753 (losartan) to rat liver homogenates reveals multiple sites. Relationship to AT1a- and AT1b-type angiotensin receptors and novel nonangiotensin binding sites. Peptides. 1993;14:829–837. doi: 10.1016/0196-9781(93)90121-v. [DOI] [PubMed] [Google Scholar]
- Yen M-H, Sheu Y-Z, Chiou W-F, Wu C-C. Differential modulation by basilar and mesenteric endothelium of angiotensin-induced contraction in canine arteries. European Journal of Pharmacology. 1990;180:209–216. doi: 10.1016/0014-2999(90)90304-o. [DOI] [PubMed] [Google Scholar]
- Zerrouk A, Auguet M, Delaflotte S, Chabrier PE. Effects of angiotensin I and angiotensin II in blood vessels: greater influence of converting enzyme activity in the rabbit basilar artery. Naunyn-Schmiede-berg’s Archives Pharmacology. 1996;354:466–473. doi: 10.1007/BF00168438. [DOI] [PubMed] [Google Scholar]