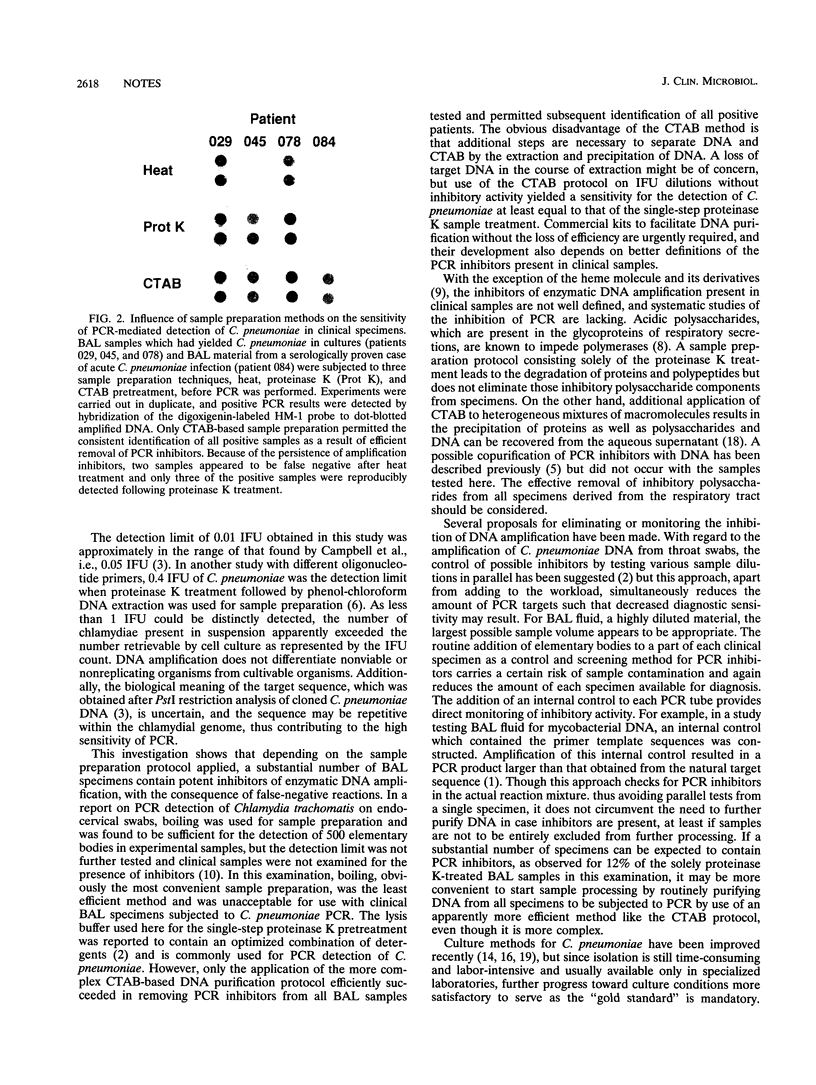
Abstract
Amplification inhibitors can lead to false-negative results for PCR. In order to evaluate the reliability of PCR for the detection of Chlamydia pneumoniae, the presence of PCR inhibitors in 75 bronchoalveolar lavage specimens was assessed after treatment by various sample preparation methods. Specimens were collected from patients with acute respiratory infections, including four cases of proven C. pneumoniae infection. Substances inhibitory to the amplification of chlamydial DNA continued to be present in 12% of the samples treated according to the commonly used single-step proteinase K digestion and in 31% of the samples processed by heat treatment. However, the complexing of DNA-contaminating proteins and polysaccharides from digested specimens to cetyltrimethylammonium bromide (CTAB) followed by DNA extraction efficiently removed inhibitors from all experimental samples and provided subsequent identification of all positive clinical samples by PCR. The CTAB method and proteinase K treatment had comparable detection limits of approximately 0.01 inclusion-forming units. CTAB-based DNA purification of respiratory specimens is recommended to increase the diagnostic sensitivity of PCR and confidence in negative results.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bretagne S., Costa J. M., Vidaud M., Tran J., Nhieu V., Fleury-Feith J. Detection of Toxoplasma gondii by competitive DNA amplification of bronchoalveolar lavage samples. J Infect Dis. 1993 Dec;168(6):1585–1588. doi: 10.1093/infdis/168.6.1585. [DOI] [PubMed] [Google Scholar]
- Campbell L. A., Perez Melgosa M., Hamilton D. J., Kuo C. C., Grayston J. T. Detection of Chlamydia pneumoniae by polymerase chain reaction. J Clin Microbiol. 1992 Feb;30(2):434–439. doi: 10.1128/jcm.30.2.434-439.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin K., Roblin P. M., Gelling M., Hammerschlag M. R., Schachter J. Infection with Chlamydia pneumoniae in Brooklyn. J Infect Dis. 1991 Apr;163(4):757–761. doi: 10.1093/infdis/163.4.757. [DOI] [PubMed] [Google Scholar]
- Gaydos C. A., Quinn T. C., Eiden J. J. Identification of Chlamydia pneumoniae by DNA amplification of the 16S rRNA gene. J Clin Microbiol. 1992 Apr;30(4):796–800. doi: 10.1128/jcm.30.4.796-800.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayston J. T. Infections caused by Chlamydia pneumoniae strain TWAR. Clin Infect Dis. 1992 Nov;15(5):757–761. doi: 10.1093/clind/15.5.757. [DOI] [PubMed] [Google Scholar]
- Holland S. M., Gaydos C. A., Quinn T. C. Detection and differentiation of Chlamydia trachomatis, Chlamydia psittaci, and Chlamydia pneumoniae by DNA amplification. J Infect Dis. 1990 Oct;162(4):984–987. doi: 10.1093/infdis/162.4.984. [DOI] [PubMed] [Google Scholar]
- Kuo C. C., Chen H. H., Wang S. P., Grayston J. T. Identification of a new group of Chlamydia psittaci strains called TWAR. J Clin Microbiol. 1986 Dec;24(6):1034–1037. doi: 10.1128/jcm.24.6.1034-1037.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Grayston J. T. Factors affecting viability and growth in HeLa 229 cells of Chlamydia sp. strain TWAR. J Clin Microbiol. 1988 May;26(5):812–815. doi: 10.1128/jcm.26.5.812-815.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Shor A., Campbell L. A., Fukushi H., Patton D. L., Grayston J. T. Demonstration of Chlamydia pneumoniae in atherosclerotic lesions of coronary arteries. J Infect Dis. 1993 Apr;167(4):841–849. doi: 10.1093/infdis/167.4.841. [DOI] [PubMed] [Google Scholar]
- Maass M., Essig A., Marre R., Henkel W. Growth in serum-free medium improves isolation of Chlamydia pneumoniae. J Clin Microbiol. 1993 Nov;31(11):3050–3052. doi: 10.1128/jcm.31.11.3050-3052.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne G., Lefebvre J. Specificity of the microimmunofluorescence assay for the serodiagnosis of Chlamydia pneumoniae infections. Can J Microbiol. 1992 Nov;38(11):1185–1189. doi: 10.1139/m92-194. [DOI] [PubMed] [Google Scholar]
- Roblin P. M., Dumornay W., Hammerschlag M. R. Use of HEp-2 cells for improved isolation and passage of Chlamydia pneumoniae. J Clin Microbiol. 1992 Aug;30(8):1968–1971. doi: 10.1128/jcm.30.8.1968-1971.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shor A., Kuo C. C., Patton D. L. Detection of Chlamydia pneumoniae in coronary arterial fatty streaks and atheromatous plaques. S Afr Med J. 1992 Sep;82(3):158–161. [PubMed] [Google Scholar]
- Wong K. H., Skelton S. K., Chan Y. K. Efficient culture of Chlamydia pneumoniae with cell lines derived from the human respiratory tract. J Clin Microbiol. 1992 Jul;30(7):1625–1630. doi: 10.1128/jcm.30.7.1625-1630.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Franchis R., Cross N. C., Foulkes N. S., Cox T. M. A potent inhibitor of Taq polymerase copurifies with human genomic DNA. Nucleic Acids Res. 1988 Nov 11;16(21):10355–10355. doi: 10.1093/nar/16.21.10355. [DOI] [PMC free article] [PubMed] [Google Scholar]