Abstract
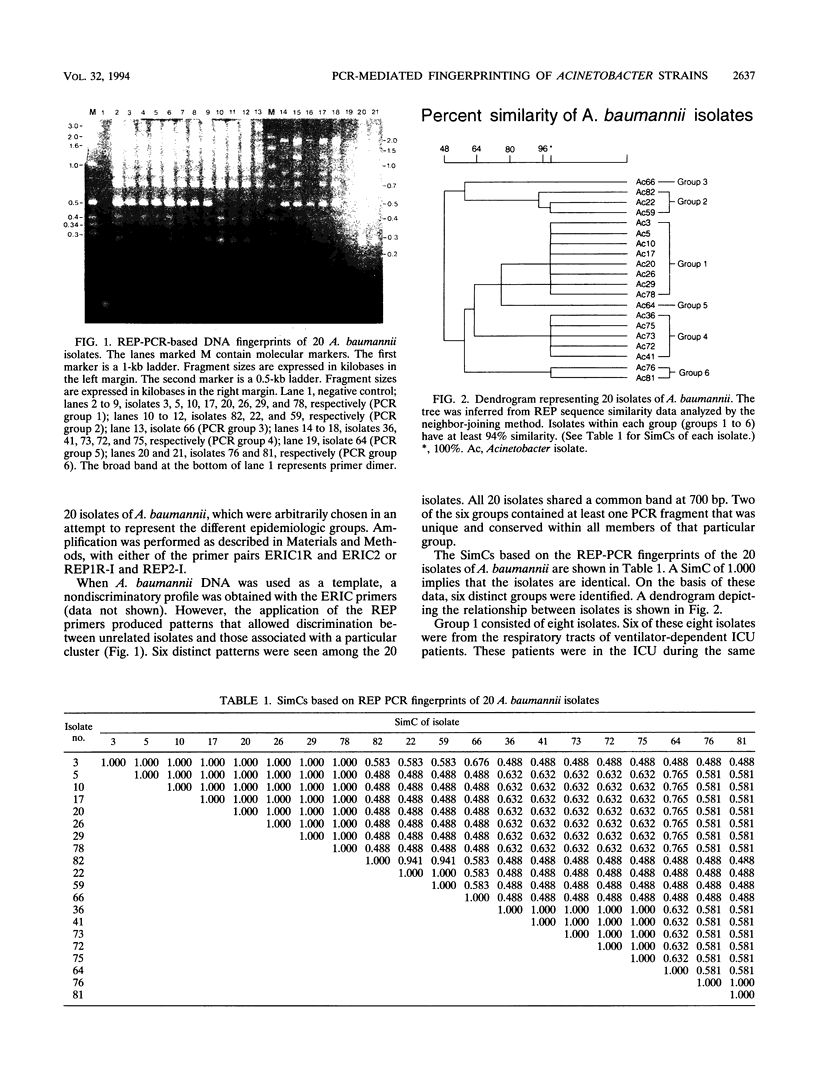
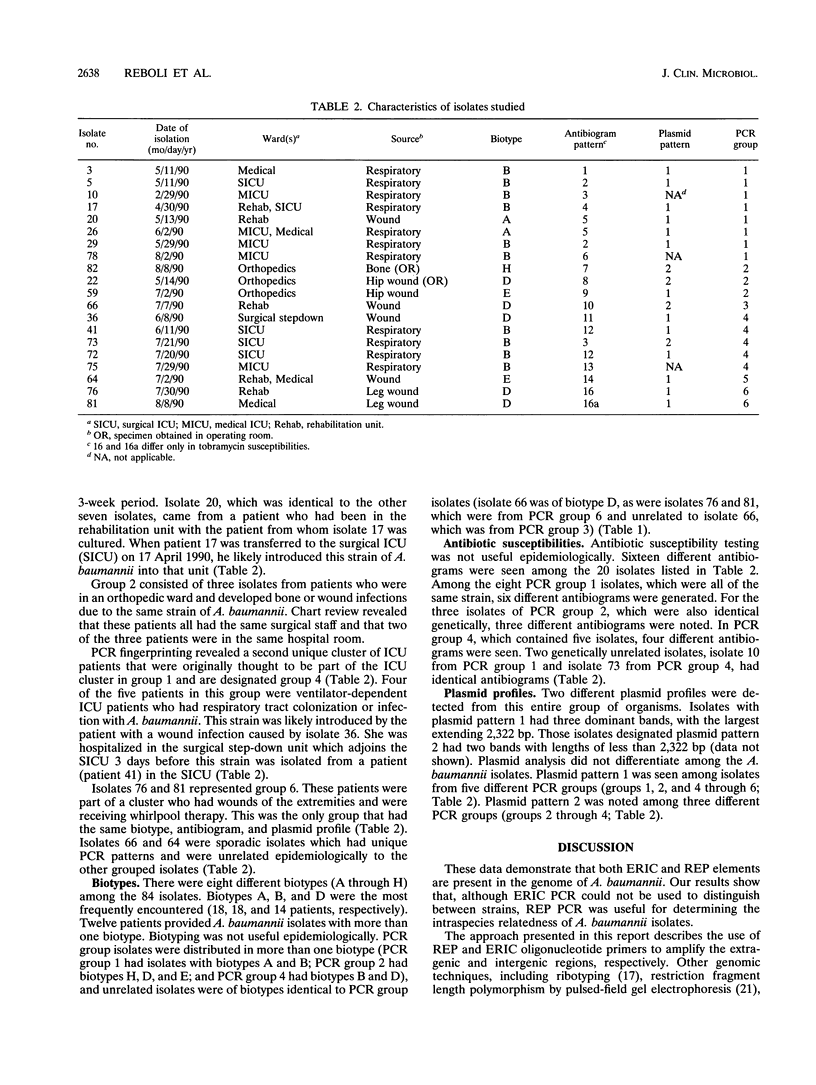
In 1990, there was a significant increase in the number of lower respiratory tract infections and surgical wound infections in the adult intensive care units of our tertiary care teaching hospital caused by Acinetobacter baumannii compared with the number in 1989. During the 5-month period from April through August 1990, 84 isolates of A. baumannii were recovered from 50 hospitalized patients. Biotyping, comparison of antibiograms, plasmid analysis, and DNA polymorphisms of 20 isolates from 20 different patients, determined by the use of repetitive element PCR with primers aimed at repetitive extragenic palindromic sequences and enterobacterial repetitive intergenic consensus sequences, were used to investigate this apparent outbreak. Biotyping, antibiograms, plasmid analysis, and enterobacterial repetitive intergenic consensus PCR were not useful epidemiologically. Repetitive element PCR-mediated DNA fingerprinting using repetitive extragenic palindromic primers discriminated between epidemic and sporadic strains of A. baumannii and demonstrated four discrete clusters which were unique epidemiologically.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M., Ismail F., Jackman P. J., Noble W. C. Fingerprinting Acinetobacter strains from clinical sources by numerical analysis of electrophoretic protein patterns. J Med Microbiol. 1984 Aug;18(1):55–64. doi: 10.1099/00222615-18-1-55. [DOI] [PubMed] [Google Scholar]
- Allardet-Servent A., Bouziges N., Carles-Nurit M. J., Bourg G., Gouby A., Ramuz M. Use of low-frequency-cleavage restriction endonucleases for DNA analysis in epidemiological investigations of nosocomial bacterial infections. J Clin Microbiol. 1989 Sep;27(9):2057–2061. doi: 10.1128/jcm.27.9.2057-2061.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann P. Isolation of Acinetobacter from soil and water. J Bacteriol. 1968 Jul;96(1):39–42. doi: 10.1128/jb.96.1.39-42.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvet P. J., Jeanjean S., Vieu J. F., Dijkshoorn L. Species, biotype, and bacteriophage type determinations compared with cell envelope protein profiles for typing Acinetobacter strains. J Clin Microbiol. 1990 Feb;28(2):170–176. doi: 10.1128/jcm.28.2.170-176.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton A. E., Anderson R. L., Werdegar D., Atlas E. Nosocomial respiratory tract infection and colonization with Acinetobacter calcoaceticus. Epidemiologic characteristics. Am J Med. 1978 Sep;65(3):507–513. doi: 10.1016/0002-9343(78)90777-5. [DOI] [PubMed] [Google Scholar]
- Cunha B. A., Klimek J. J., Gracewski J., McLaughlin J. C., Quintiliani R. A common source outbreak of Acinetobacter pulmonary infections traced to Wright respirometers. Postgrad Med J. 1980 Mar;56(653):169–172. doi: 10.1136/pgmj.56.653.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkshoorn L., Aucken H. M., Gerner-Smidt P., Kaufmann M. E., Ursing J., Pitt T. L. Correlation of typing methods for Acinetobacter isolates from hospital outbreaks. J Clin Microbiol. 1993 Mar;31(3):702–705. doi: 10.1128/jcm.31.3.702-705.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkshoorn L., Michel M. F., Degener J. E. Cell envelope protein profiles of Acinetobacter calcoaceticus strains isolated in hospitals. J Med Microbiol. 1987 Jun;23(4):313–319. doi: 10.1099/00222615-23-4-313. [DOI] [PubMed] [Google Scholar]
- Dunkle L. M., Sippel C. J. Rapid microprocedure for extraction of plasmid DNA from Staphylococcus aureus. J Infect Dis. 1984 Jun;149(6):921–923. doi: 10.1093/infdis/149.6.921. [DOI] [PubMed] [Google Scholar]
- Ellsworth D. L., Rittenhouse K. D., Honeycutt R. L. Artifactual variation in randomly amplified polymorphic DNA banding patterns. Biotechniques. 1993 Feb;14(2):214–217. [PubMed] [Google Scholar]
- French G. L., Casewell M. W., Roncoroni A. J., Knight S., Phillips I. A hospital outbreak of antibiotic-resistant Acinetobacter anitratus: epidemiology and control. J Hosp Infect. 1980 Jun;1(2):125–131. doi: 10.1016/0195-6701(80)90044-4. [DOI] [PubMed] [Google Scholar]
- Garner J. S., Jarvis W. R., Emori T. G., Horan T. C., Hughes J. M. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988 Jun;16(3):128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- Gerner-Smidt P. Ribotyping of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex. J Clin Microbiol. 1992 Oct;30(10):2680–2685. doi: 10.1128/jcm.30.10.2680-2685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson E., Clément J. M., Brutlag D., Hofnung M. A family of dispersed repetitive extragenic palindromic DNA sequences in E. coli. EMBO J. 1984 Jun;3(6):1417–1421. doi: 10.1002/j.1460-2075.1984.tb01986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lus P., Fields B. S., Benson R. F., Martin W. T., O'Connor S. P., Black C. M. Comparison of arbitrarily primed polymerase chain reaction, ribotyping, and monoclonal antibody analysis for subtyping Legionella pneumophila serogroup 1. J Clin Microbiol. 1993 Jul;31(7):1940–1942. doi: 10.1128/jcm.31.7.1940-1942.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouby A., Carles-Nurit M. J., Bouziges N., Bourg G., Mesnard R., Bouvet P. J. Use of pulsed-field gel electrophoresis for investigation of hospital outbreaks of Acinetobacter baumannii. J Clin Microbiol. 1992 Jun;30(6):1588–1591. doi: 10.1128/jcm.30.6.1588-1591.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräser Y., Klare I., Halle E., Gantenberg R., Buchholz P., Jacobi H. D., Presber W., Schönian G. Epidemiological study of an Acinetobacter baumannii outbreak by using polymerase chain reaction fingerprinting. J Clin Microbiol. 1993 Sep;31(9):2417–2420. doi: 10.1128/jcm.31.9.2417-2420.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartstein A. I., Morthland V. H., Rourke J. W., Jr, Freeman J., Garber S., Sykes R., Rashad A. L. Plasmid DNA fingerprinting of Acinetobacter calcoaceticus subspecies anitratus from intubated and mechanically ventilated patients. Infect Control Hosp Epidemiol. 1990 Oct;11(10):531–538. doi: 10.1086/646087. [DOI] [PubMed] [Google Scholar]
- Hartstein A. I., Rashad A. L., Liebler J. M., Actis L. A., Freeman J., Rourke J. W., Jr, Stibolt T. B., Tolmasky M. E., Ellis G. R., Crosa J. H. Multiple intensive care unit outbreak of Acinetobacter calcoaceticus subspecies anitratus respiratory infection and colonization associated with contaminated, reusable ventilator circuits and resuscitation bags. Am J Med. 1988 Nov;85(5):624–631. doi: 10.1016/s0002-9343(88)80233-x. [DOI] [PubMed] [Google Scholar]
- Hoffmann S., Mabeck C. E., Vejlsgaard R. Bacteriuria caused by Acinetobacter calcoaceticus biovars in a normal population and in general practice. J Clin Microbiol. 1982 Sep;16(3):443–451. doi: 10.1128/jcm.16.3.443-451.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton J. A report of a further hospital outbreak caused by a multi-resistant Acinetobacter anitratus. J Hosp Infect. 1982 Sep;3(3):305–309. doi: 10.1016/0195-6701(82)90051-2. [DOI] [PubMed] [Google Scholar]
- Hulton C. S., Higgins C. F., Sharp P. M. ERIC sequences: a novel family of repetitive elements in the genomes of Escherichia coli, Salmonella typhimurium and other enterobacteria. Mol Microbiol. 1991 Apr;5(4):825–834. doi: 10.1111/j.1365-2958.1991.tb00755.x. [DOI] [PubMed] [Google Scholar]
- Johnson D. R., Love-Dixon M. A., Brown W. J., Levine D. P., Downes F. P., Hall W. N. Delayed detection of an increase in resistant Acinetobacter at a Detroit hospital. Infect Control Hosp Epidemiol. 1992 Jul;13(7):394–398. doi: 10.1086/646556. [DOI] [PubMed] [Google Scholar]
- Kogan S. C., Doherty M., Gitschier J. An improved method for prenatal diagnosis of genetic diseases by analysis of amplified DNA sequences. Application to hemophilia A. N Engl J Med. 1987 Oct 15;317(16):985–990. doi: 10.1056/NEJM198710153171603. [DOI] [PubMed] [Google Scholar]
- Lupski J. R., Weinstock G. M. Short, interspersed repetitive DNA sequences in prokaryotic genomes. J Bacteriol. 1992 Jul;174(14):4525–4529. doi: 10.1128/jb.174.14.4525-4529.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslow J. N., Mulligan M. E., Arbeit R. D. Molecular epidemiology: application of contemporary techniques to the typing of microorganisms. Clin Infect Dis. 1993 Aug;17(2):153–164. doi: 10.1093/clinids/17.2.153. [DOI] [PubMed] [Google Scholar]
- Patterson J. E., Vecchio J., Pantelick E. L., Farrel P., Mazon D., Zervos M. J., Hierholzer W. J., Jr Association of contaminated gloves with transmission of Acinetobacter calcoaceticus var. anitratus in an intensive care unit. Am J Med. 1991 Nov;91(5):479–483. doi: 10.1016/0002-9343(91)90183-x. [DOI] [PubMed] [Google Scholar]
- Ramphal R., Kluge R. M. Acinetobacter calcoaceticus variety anitratus: an increasing nosocomial problem. Am J Med Sci. 1979 Jan-Feb;277(1):57–66. doi: 10.1097/00000441-197901000-00007. [DOI] [PubMed] [Google Scholar]
- Raz R., Alroy G., Sobel J. D. Nosocomial bacteremia due to Acinetobacter calcoaceticus. Infection. 1982;10(3):168–171. doi: 10.1007/BF01640769. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sherertz R. J., Sullivan M. L. An outbreak of infections with Acinetobacter calcoaceticus in burn patients: contamination of patients' mattresses. J Infect Dis. 1985 Feb;151(2):252–258. doi: 10.1093/infdis/151.2.252. [DOI] [PubMed] [Google Scholar]
- Stern M. J., Ames G. F., Smith N. H., Robinson E. C., Higgins C. F. Repetitive extragenic palindromic sequences: a major component of the bacterial genome. Cell. 1984 Jul;37(3):1015–1026. doi: 10.1016/0092-8674(84)90436-7. [DOI] [PubMed] [Google Scholar]
- TAPLIN D., ZAIAS N. THE HUMAN SKIN AS A SOURCE OF MIMA-HERELLEA INFECTIONS. JAMA. 1963 Dec 7;186:952–955. doi: 10.1001/jama.1963.63710100030023a. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Nagano Y. Rapid procedure for isolation of plasmid DNA and application to epidemiological analysis. J Clin Microbiol. 1984 Oct;20(4):608–613. doi: 10.1128/jcm.20.4.608-613.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub W. H. Acinetobacter baumannii serotyping for delineation of outbreaks of nosocomial cross-infection. J Clin Microbiol. 1989 Dec;27(12):2713–2716. doi: 10.1128/jcm.27.12.2713-2716.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versalovic J., Koeuth T., Lupski J. R. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991 Dec 25;19(24):6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versalovic J., Woods C. R., Jr, Georghiou P. R., Hamill R. J., Lupski J. R. DNA-based identification and epidemiologic typing of bacterial pathogens. Arch Pathol Lab Med. 1993 Nov;117(11):1088–1098. [PubMed] [Google Scholar]
- Vila J., Almela M., Jimenez de Anta M. T. Laboratory investigation of hospital outbreak caused by two different multiresistant Acinetobacter calcoaceticus subsp. anitratus strains. J Clin Microbiol. 1989 May;27(5):1086–1089. doi: 10.1128/jcm.27.5.1086-1089.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods C. R., Jr, Versalovic J., Koeuth T., Lupski J. R. Analysis of relationships among isolates of Citrobacter diversus by using DNA fingerprints generated by repetitive sequence-based primers in the polymerase chain reaction. J Clin Microbiol. 1992 Nov;30(11):2921–2929. doi: 10.1128/jcm.30.11.2921-2929.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn F. J. Use of repetitive (repetitive extragenic palindromic and enterobacterial repetitive intergeneric consensus) sequences and the polymerase chain reaction to fingerprint the genomes of Rhizobium meliloti isolates and other soil bacteria. Appl Environ Microbiol. 1992 Jul;58(7):2180–2187. doi: 10.1128/aem.58.7.2180-2187.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Soolingen D., Hermans P. W., de Haas P. E., Soll D. R., van Embden J. D. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J Clin Microbiol. 1991 Nov;29(11):2578–2586. doi: 10.1128/jcm.29.11.2578-2586.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]




