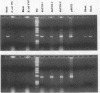
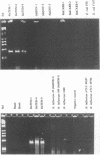
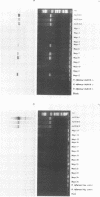
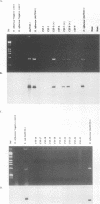
Abstract
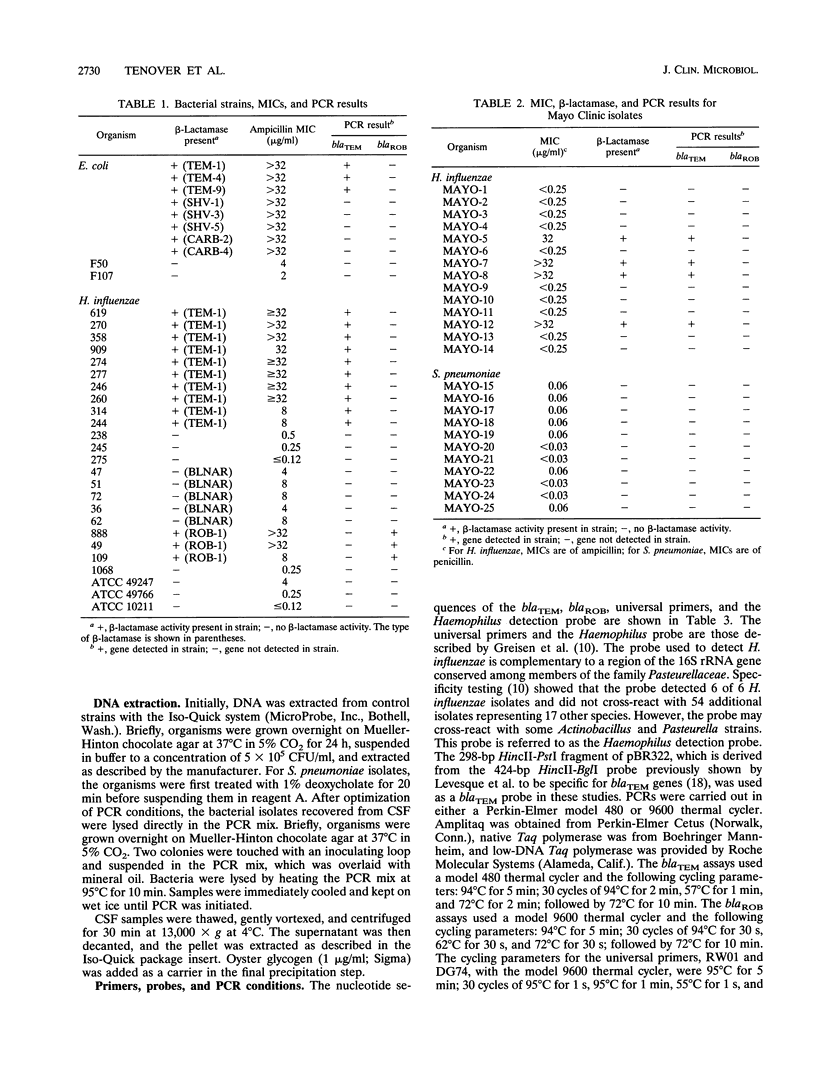
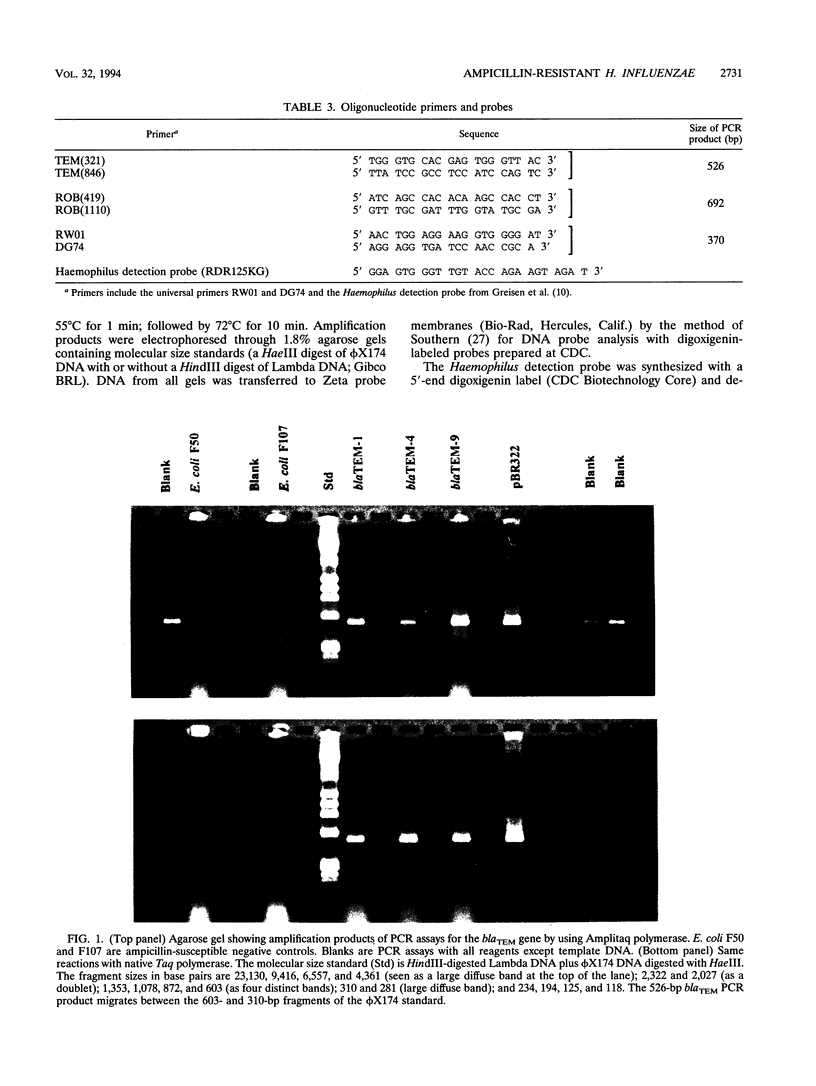
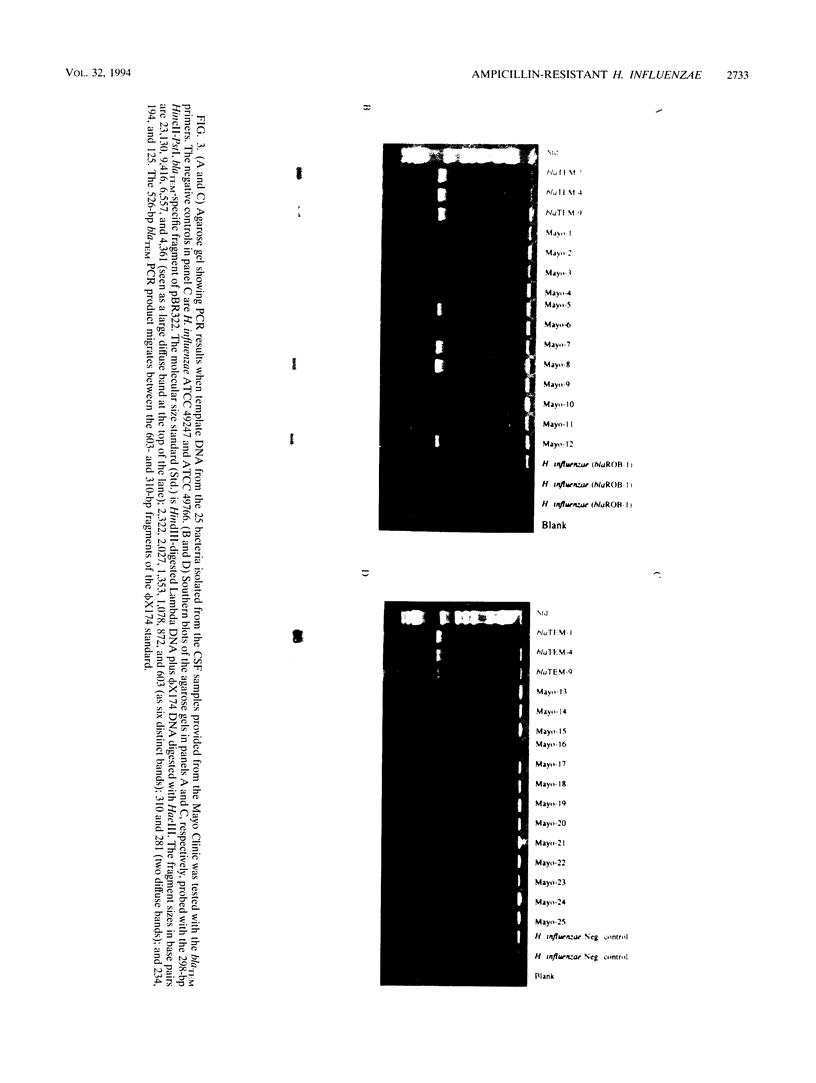
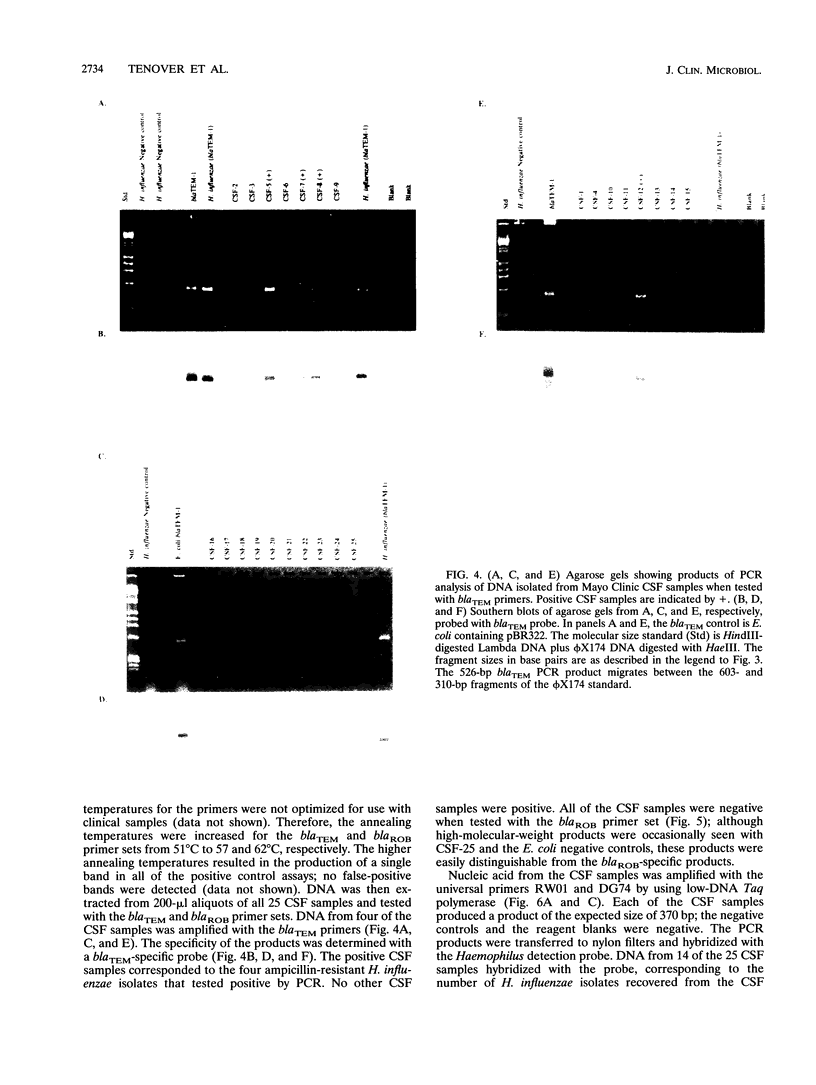
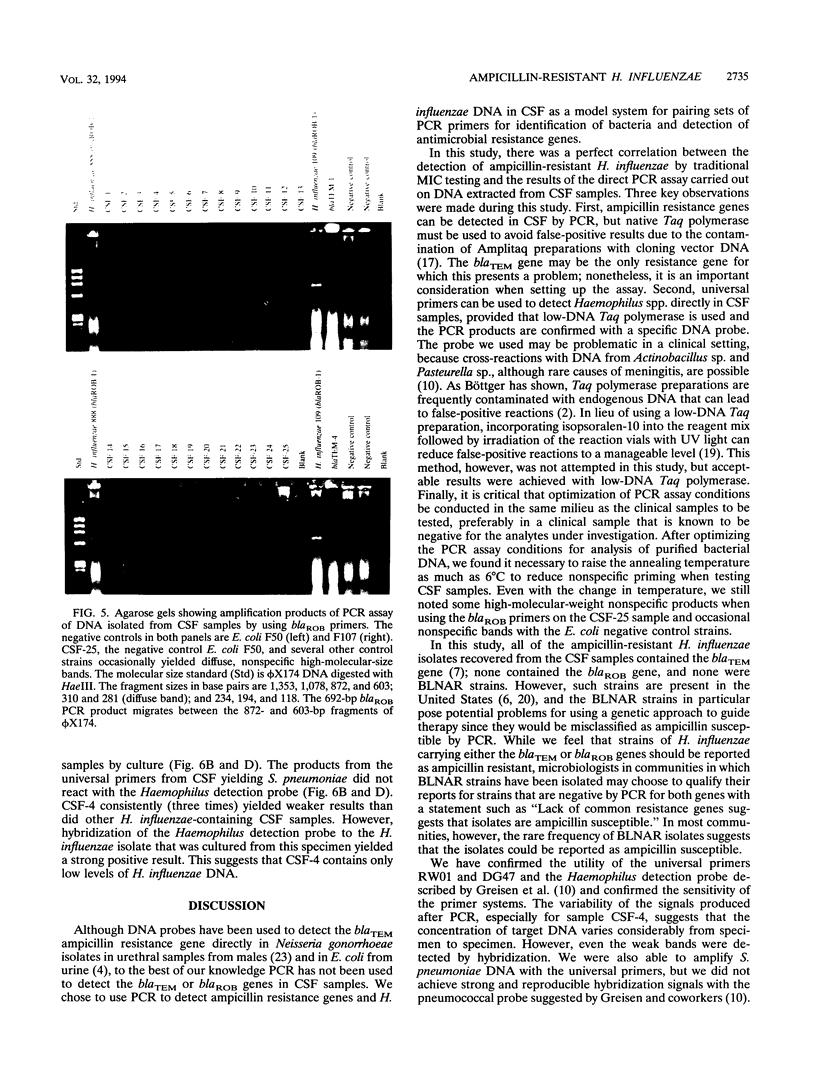
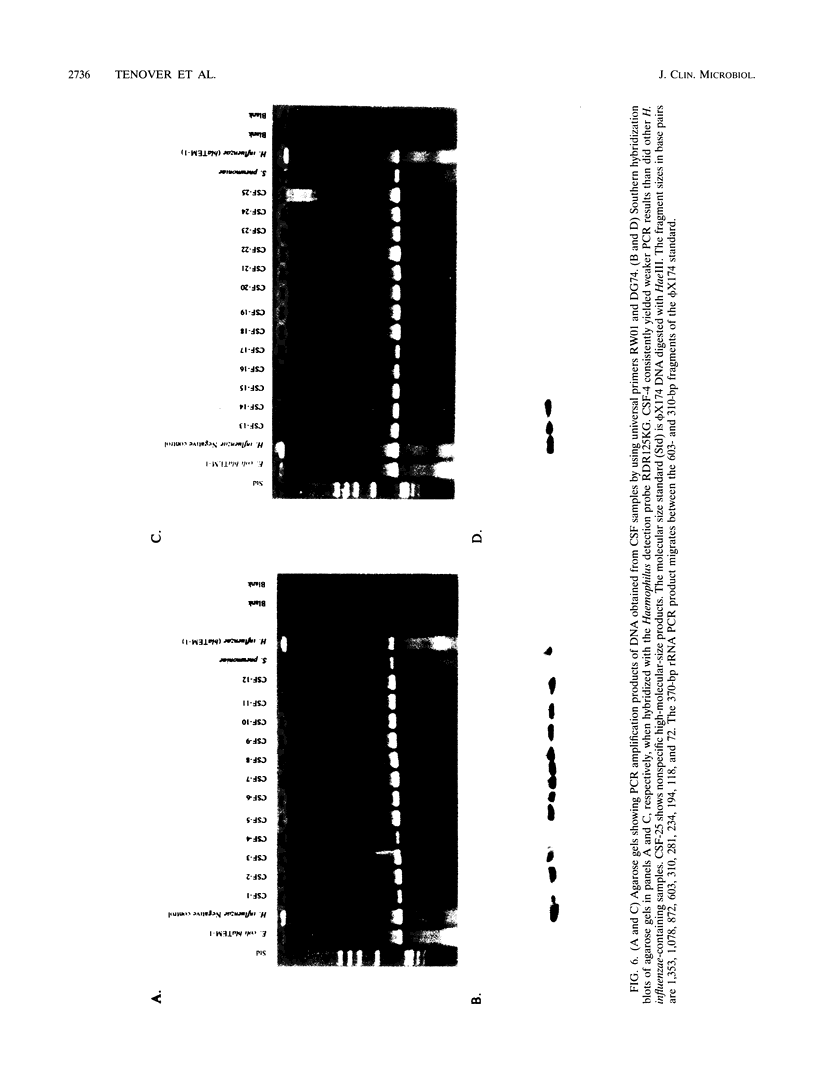
We developed PCR primers specific for the blaTEM and blaROB ampicillin resistance genes. The specificity of the primers was confirmed by testing a series of Escherichia coli isolates containing a variety of ampicillin resistance genes and a series of ampicillin-resistant and ampicillin-susceptible Haemophilus influenzae isolates. There was a perfect correlation between ampicillin MICs, the presence of beta-lactamase (as determined by the nitrocefin test), and the results with the blaTEM and blaROB primers. Isolates of H. influenzae and Streptococcus pneumoniae obtained from 25 frozen cerebrospinal fluid (CSF) specimens were also tested. Four of 14 H. influenzae isolates were positive with the blaTEM primers; none were positive with the blaROB primers. Ampicillin MICs were determined for the H. influenzae isolates, and penicillin MICs were determined for the S. pneumoniae isolates. Only the four PCR-positive H. influenzae isolates had elevated MICs of ampicillin and were beta-lactamase positive. None of the H. influenzae isolates contained the blaROB gene, and none of the S. pneumoniae isolates produced positive reactions with either primer set. We then used universal primers directed to conserved regions of rRNA and a Haemophilus detection probe to identify which of the 25 frozen samples of CSF contained H. influenzae. Fourteen of the 25 CSF specimens were positive for H. influenzae, which correlated with the number of organisms obtained by culture of the CSF samples. Four of the CSF samples were positive with the blaTEM primer set, and these correlated with the four H. influenzae isolates that were positive when tested directly by PCR. The blaTEM assay required the use of native Taq polymerase because Amplitaq preparations were contaminated with vector DNA that contained the blaTEM-1 gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams W. G., Deaver K. A., Cochi S. L., Plikaytis B. D., Zell E. R., Broome C. V., Wenger J. D. Decline of childhood Haemophilus influenzae type b (Hib) disease in the Hib vaccine era. JAMA. 1993 Jan 13;269(2):221–226. [PubMed] [Google Scholar]
- Brakstad O. G., Aasbakk K., Maeland J. A. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J Clin Microbiol. 1992 Jul;30(7):1654–1660. doi: 10.1128/jcm.30.7.1654-1660.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttger E. C. Frequent contamination of Taq polymerase with DNA. Clin Chem. 1990 Jun;36(6):1258–1259. [PubMed] [Google Scholar]
- Carter G. I., Towner K. J., Pearson N. J., Slack R. C. Use of a non-radioactive hybridisation assay for direct detection of gram-negative bacteria carrying TEM beta-lactamase genes in infected urine. J Med Microbiol. 1989 Feb;28(2):113–117. doi: 10.1099/00222615-28-2-113. [DOI] [PubMed] [Google Scholar]
- Coffey T. J., Dowson C. G., Daniels M., Zhou J., Martin C., Spratt B. G., Musser J. M. Horizontal transfer of multiple penicillin-binding protein genes, and capsular biosynthetic genes, in natural populations of Streptococcus pneumoniae. Mol Microbiol. 1991 Sep;5(9):2255–2260. doi: 10.1111/j.1365-2958.1991.tb02155.x. [DOI] [PubMed] [Google Scholar]
- Daum R. S., Murphey-Corb M., Shapira E., Dipp S. Epidemiology of rob beta-lactamase among ampicillin-resistant Haemophilus influenzae isolates in the United States. J Infect Dis. 1988 Mar;157(3):450–455. doi: 10.1093/infdis/157.3.450. [DOI] [PubMed] [Google Scholar]
- Elwell L. P., De Graaff J., Seibert D., Falkow S. Plasmid-linked ampicillin resistance in haempohilus influenza type b. Infect Immun. 1975 Aug;12(2):404–410. doi: 10.1128/iai.12.2.404-410.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootz T. D., Tenover F. C., Young S. A., Gordon K. P., Plorde J. J. Comparison of three DNA hybridization methods for detection of the aminoglycoside 2"-O-adenylyltransferase gene in clinical bacterial isolates. Antimicrob Agents Chemother. 1985 Jul;28(1):69–73. doi: 10.1128/aac.28.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greisen K., Loeffelholz M., Purohit A., Leong D. PCR primers and probes for the 16S rRNA gene of most species of pathogenic bacteria, including bacteria found in cerebrospinal fluid. J Clin Microbiol. 1994 Feb;32(2):335–351. doi: 10.1128/jcm.32.2.335-351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J. M., Roberts G. D., Stockman L., Felmlee T. A., Persing D. H. Detection of a genetic locus encoding resistance to rifampin in mycobacterial cultures and in clinical specimens. Diagn Microbiol Infect Dis. 1994 Apr;18(4):219–227. doi: 10.1016/0732-8893(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Jacoby G. A., Medeiros A. A. More extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 1991 Sep;35(9):1697–1704. doi: 10.1128/aac.35.9.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko K., Onodera O., Miyatake T., Tsuji S. Rapid diagnosis of tuberculous meningitis by polymerase chain reaction (PCR). Neurology. 1990 Oct;40(10):1617–1618. doi: 10.1212/wnl.40.10.1617. [DOI] [PubMed] [Google Scholar]
- Kristiansen B. E., Ask E., Jenkins A., Fermer C., Rådstrøm P., Skøld O. Rapid diagnosis of meningococcal meningitis by polymerase chain reaction. Lancet. 1991 Jun 29;337(8757):1568–1569. doi: 10.1016/0140-6736(91)93262-8. [DOI] [PubMed] [Google Scholar]
- Larder B. A., Kemp S. D. Multiple mutations in HIV-1 reverse transcriptase confer high-level resistance to zidovudine (AZT). Science. 1989 Dec 1;246(4934):1155–1158. doi: 10.1126/science.2479983. [DOI] [PubMed] [Google Scholar]
- Lawyer F. C., Stoffel S., Saiki R. K., Myambo K., Drummond R., Gelfand D. H. Isolation, characterization, and expression in Escherichia coli of the DNA polymerase gene from Thermus aquaticus. J Biol Chem. 1989 Apr 15;264(11):6427–6437. [PubMed] [Google Scholar]
- Levesque R. C., Medeiros A. A., Jacoby G. A. Molecular cloning and DNA homology of plasmid-mediated beta-lactamase genes. Mol Gen Genet. 1987 Feb;206(2):252–258. doi: 10.1007/BF00333581. [DOI] [PubMed] [Google Scholar]
- Meier A., Persing D. H., Finken M., Böttger E. C. Elimination of contaminating DNA within polymerase chain reaction reagents: implications for a general approach to detection of uncultured pathogens. J Clin Microbiol. 1993 Mar;31(3):646–652. doi: 10.1128/jcm.31.3.646-652.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelman P. M., Chaffin D. O., Stull T. L., Rubens C. E., Mack K. D., Smith A. L. Characterization of non-beta-lactamase-mediated ampicillin resistance in Haemophilus influenzae. Antimicrob Agents Chemother. 1984 Aug;26(2):235–244. doi: 10.1128/aac.26.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni H., Knight A. I., Cartwright K., Palmer W. H., McFadden J. Polymerase chain reaction for diagnosis of meningococcal meningitis. Lancet. 1992 Dec 12;340(8833):1432–1434. doi: 10.1016/0140-6736(92)92622-m. [DOI] [PubMed] [Google Scholar]
- Perine P. L., Totten P. A., Holmes K. K., Sng E. H., Ratnam A. V., Widy-Wersky R., Nsanze H., Habte-Gabr E., Westbrook W. G. Evaluation of a DNA-hybridization method for detection of African and Asian strains of Neisseria gonorrhoeae in men with urethritis. J Infect Dis. 1985 Jul;152(1):59–63. doi: 10.1093/infdis/152.1.59. [DOI] [PubMed] [Google Scholar]
- Persing D. H., Mathiesen D., Marshall W. F., Telford S. R., Spielman A., Thomford J. W., Conrad P. A. Detection of Babesia microti by polymerase chain reaction. J Clin Microbiol. 1992 Aug;30(8):2097–2103. doi: 10.1128/jcm.30.8.2097-2103.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relman D. A., Loutit J. S., Schmidt T. M., Falkow S., Tompkins L. S. The agent of bacillary angiomatosis. An approach to the identification of uncultured pathogens. N Engl J Med. 1990 Dec 6;323(23):1573–1580. doi: 10.1056/NEJM199012063232301. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Telenti A., Imboden P., Marchesi F., Schmidheini T., Bodmer T. Direct, automated detection of rifampin-resistant Mycobacterium tuberculosis by polymerase chain reaction and single-strand conformation polymorphism analysis. Antimicrob Agents Chemother. 1993 Oct;37(10):2054–2058. doi: 10.1128/aac.37.10.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ketel R. J., de Wever B., van Alphen L. Detection of Haemophilus influenzae in cerebrospinal fluids by polymerase chain reaction DNA amplification. J Med Microbiol. 1990 Dec;33(4):271–276. doi: 10.1099/00222615-33-4-271. [DOI] [PubMed] [Google Scholar]