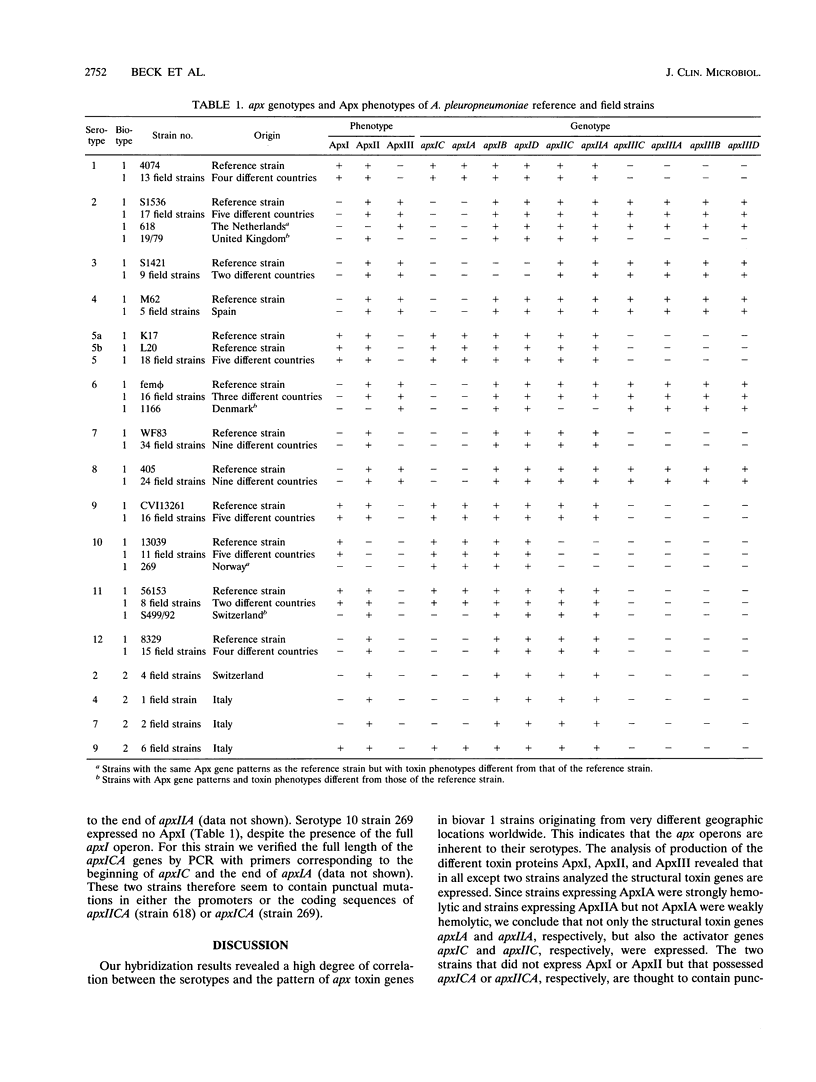
Abstract
Actinobacillus pleuropneumoniae serotype reference strains and 204 A. pleuropneumoniae field strains representing all 12 serotypes and both biovars 1 and 2, obtained from laboratories from various countries worldwide, were analyzed for the presence of the toxin genes apxIC, apxIA, apxIB, apxID, apxIIC, apxIIA, apxIIIC, apxIIIA, apxIIIB, and apxIIID by DNA-DNA hybridization with specific gene probes. Expression of the toxins ApxI, ApxII, and ApxIII was assessed by immunoblot analysis with monoclonal antibodies. The results show that the patterns of apx genes and those of the expressed Apx toxins in biovar 1 field strains are the same as those of the genes and toxins of corresponding serotype reference strain. We found only three strains which had certain apx genes missing compared with the genes in their serotype reference strains. Analysis of the expression of the three toxins showed that nearly all strains expressed their apx genes and produced the same Apx toxins as their serotype reference strain. We found only one strain that did not produce ApxI, although it contained the apxICABD genes, and one strain which did not express ApxII but which contained apxIICA. Several field strains which initially showed that their serotype did not correspond to the apx gene profile of the reference strain and which had an unexpected virulence for the given serotype revealed that their initial serotyping was erroneous. We show that the apx gene profiles are inherent to a given serotype. The method cannot differentiate between all 12 serotypes. However, it allowed us to distinguish five groups of toxin gene patterns which showed pathological, toxicological, and epidemiological significance. None of the biovar 2 strains contained apxIII genes. The apxI and apxII genes in the biovar 2 strains, however, were the same as those found in the serotype reference strains of biovar 1.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C., Potter A. A., Gerlach G. F. Isolation and molecular characterization of spontaneously occurring cytolysin-negative mutants of Actinobacillus pleuropneumoniae serotype 7. Infect Immun. 1991 Nov;59(11):4110–4116. doi: 10.1128/iai.59.11.4110-4116.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beynon L. M., Richards J. C., Perry M. B. Characterization of the Actinobacillus pleuropneumoniae serotype K11:01 capsular antigen. Eur J Biochem. 1993 May 15;214(1):209–214. doi: 10.1111/j.1432-1033.1993.tb17914.x. [DOI] [PubMed] [Google Scholar]
- Brandreth S. R., Smith I. M. Comparative virulence of some English strains of Haemophilus pleuropneumoniae serotypes 2 and 3 in the pig. Res Vet Sci. 1987 Mar;42(2):187–193. [PubMed] [Google Scholar]
- Chang Y. F., Shi J., Ma D. P., Shin S. J., Lein D. H. Molecular analysis of the Actinobacillus pleuropneumoniae RTX toxin-III gene cluster. DNA Cell Biol. 1993 May;12(4):351–362. doi: 10.1089/dna.1993.12.351. [DOI] [PubMed] [Google Scholar]
- Chang Y. F., Young R., Struck D. K. Cloning and characterization of a hemolysin gene from Actinobacillus (Haemophilus) pleuropneumoniae. DNA. 1989 Nov;8(9):635–647. doi: 10.1089/dna.1.1989.8.635. [DOI] [PubMed] [Google Scholar]
- Chang Y. F., Young R., Struck D. K. The Actinobacillus pleuropneumoniae hemolysin determinant: unlinked appCA and appBD loci flanked by pseudogenes. J Bacteriol. 1991 Aug;173(16):5151–5158. doi: 10.1128/jb.173.16.5151-5158.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R., Mittal K. R., Malo R. Porcine pleuropneumonia associated with Haemophilus pleuropneumoniae serotype 3 in Quebec. Vet Rec. 1984 Dec 15;115(24):628–629. doi: 10.1136/vr.115.24.628. [DOI] [PubMed] [Google Scholar]
- Dom P., Haesebrouck F. Comparative virulence of NAD-dependent and NAD-independent Actinobacillus pleuropneumoniae strains. Zentralbl Veterinarmed B. 1992 Jun;39(4):303–306. doi: 10.1111/j.1439-0450.1992.tb01173.x. [DOI] [PubMed] [Google Scholar]
- Fales W. H., Morehouse L. G., Mittal K. R., Bean-Knudsen C., Nelson S. L., Kintner L. D., Turk J. R., Turk M. A., Brown T. P., Shaw D. P. Antimicrobial susceptibility and serotypes of Actinobacillus (Haemophilus) pleuropneumoniae recovered from Missouri swine. J Vet Diagn Invest. 1989 Jan;1(1):16–19. doi: 10.1177/104063878900100106. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frey J., Beck M., Stucki U., Nicolet J. Analysis of hemolysin operons in Actinobacillus pleuropneumoniae. Gene. 1993 Jan 15;123(1):51–58. doi: 10.1016/0378-1119(93)90538-e. [DOI] [PubMed] [Google Scholar]
- Frey J., Bosse J. T., Chang Y. F., Cullen J. M., Fenwick B., Gerlach G. F., Gygi D., Haesebrouck F., Inzana T. J., Jansen R. Actinobacillus pleuropneumoniae RTX-toxins: uniform designation of haemolysins, cytolysins, pleurotoxin and their genes. J Gen Microbiol. 1993 Aug;139(8):1723–1728. doi: 10.1099/00221287-139-8-1723. [DOI] [PubMed] [Google Scholar]
- Frey J., Nicolet J. Hemolysin patterns of Actinobacillus pleuropneumoniae. J Clin Microbiol. 1990 Feb;28(2):232–236. doi: 10.1128/jcm.28.2.232-236.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez C. B., Tascon R. I., Vazquez J. A., Rodriguez Ferri E. F. Cross-reactivity between Actinobacillus pleuropneumoniae serotypes comparing different antigens and serological tests. Res Vet Sci. 1991 May;50(3):308–310. doi: 10.1016/0034-5288(91)90129-c. [DOI] [PubMed] [Google Scholar]
- Gygi D., Nicolet J., Hughes C., Frey J. Functional analysis of the Ca(2+)-regulated hemolysin I operon of Actinobacillus pleuropneumoniae serotype 1. Infect Immun. 1992 Aug;60(8):3059–3064. doi: 10.1128/iai.60.8.3059-3064.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzana T. J., Todd J., Ma J. N., Veit H. Characterization of a non-hemolytic mutant of Actinobacillus pleuropneumoniae serotype 5: role of the 110 kilodalton hemolysin in virulence and immunoprotection. Microb Pathog. 1991 Apr;10(4):281–296. doi: 10.1016/0882-4010(91)90012-y. [DOI] [PubMed] [Google Scholar]
- Jansen R., Briaire J., Kamp E. M., Gielkens A. L., Smits M. A. Cloning and characterization of the Actinobacillus pleuropneumoniae-RTX-toxin III (ApxIII) gene. Infect Immun. 1993 Mar;61(3):947–954. doi: 10.1128/iai.61.3.947-954.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R., Briaire J., Kamp E. M., Gielkens A. L., Smits M. A. Structural analysis of the Actinobacillus pleuropneumoniae-RTX-toxin I (ApxI) operon. Infect Immun. 1993 Sep;61(9):3688–3695. doi: 10.1128/iai.61.9.3688-3695.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R., Briaire J., Kamp E. M., Smits M. A. Comparison of the cytolysin II genetic determinants of Actinobacillus pleuropneumoniae serotypes. Infect Immun. 1992 Feb;60(2):630–636. doi: 10.1128/iai.60.2.630-636.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komal J. P., Mittal K. R. Grouping of Actinobacillus pleuropneumoniae strains of serotypes 1 through 12 on the basis of their virulence in mice. Vet Microbiol. 1990 Nov;25(2-3):229–240. doi: 10.1016/0378-1135(90)90080-f. [DOI] [PubMed] [Google Scholar]
- MacDonald J., Rycroft A. N. Molecular cloning and expression of ptxA, the gene encoding the 120-kilodalton cytotoxin of Actinobacillus pleuropneumoniae serotype 2. Infect Immun. 1992 Jul;60(7):2726–2732. doi: 10.1128/iai.60.7.2726-2732.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal K. R., Bourdon S. Cross-reactivity and antigenic heterogeneity among Actinobacillus pleuropneumoniae strains of serotypes 4 and 7. J Clin Microbiol. 1991 Jul;29(7):1344–1347. doi: 10.1128/jcm.29.7.1344-1347.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal K. R., Higgins R., Lariviere S. Evaluation of slide agglutination and ring precipitation tests for capsular serotyping of Haemophilus pleuropneumoniae. J Clin Microbiol. 1982 Jun;15(6):1019–1023. doi: 10.1128/jcm.15.6.1019-1023.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal K. R., Higgins R., Lariviere S. Quantitation of serotype-specific and cross-reacting group-specific antigens by coagglutination and immunodiffusion tests for differentiating Actinobacillus (Haemophilus) pleuropneumoniae strains belonging to cross-reacting serotypes 3, 6, and 8. J Clin Microbiol. 1988 May;26(5):985–989. doi: 10.1128/jcm.26.5.985-989.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal K. R., Higgins R., Larivière S., Leblanc D. A 2-mercaptoethanol tube agglutination test for diagnosis of Haemophilus pleuropneumoniae infection in pigs. Am J Vet Res. 1984 Apr;45(4):715–719. [PubMed] [Google Scholar]
- Nielsen R. Haemophilus pleuropneumoniae (Actinobacillus pleuropneumoniae). Serotypes 8, 3 and 6. Serological response and cross immunity in pigs. Nord Vet Med. 1985 Jul-Aug;37(4):217–227. [PubMed] [Google Scholar]
- Nielsen R. New diagnostic techniques: a review of the HAP group of bacteria. Can J Vet Res. 1990 Apr;54 (Suppl):S68–S72. [PubMed] [Google Scholar]
- Nielsen R., O'Connor P. J. Serological characterization of 8 Haemophilus pleuropneumoniae strains and proposal of a new serotype: serotype 8. Acta Vet Scand. 1984;25(1):96–106. doi: 10.1186/BF03547283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp V. J., Ross R. F., Erickson B. Z. Serotyping of Haemophilus pleuropneumoniae by rapid slide agglutination and indirect fluorescent antibody tests in swine. Am J Vet Res. 1985 Jan;46(1):185–192. [PubMed] [Google Scholar]
- Rycroft A. N., Williams D., McCandlish I. A., Taylor D. J. Experimental reproduction of acute lesions of porcine pleuropneumonia with a haemolysin-deficient mutant of Actinobacillus pleuropneumoniae. Vet Rec. 1991 Nov 16;129(20):441–443. doi: 10.1136/vr.129.20.441. [DOI] [PubMed] [Google Scholar]
- Turk J. R., Fales W. H., Maddox C. W., Ramos J. A., Fischer J. R., Johnson G. C., Kreeger J. M., Miller M. A., Pace L. W., Turnquist S. E. Pleuropneumonia in Missouri swine. J Vet Diagn Invest. 1993 Jan;5(1):101–103. doi: 10.1177/104063879300500123. [DOI] [PubMed] [Google Scholar]
- Welch R. A. Pore-forming cytolysins of gram-negative bacteria. Mol Microbiol. 1991 Mar;5(3):521–528. doi: 10.1111/j.1365-2958.1991.tb00723.x. [DOI] [PubMed] [Google Scholar]



