Abstract
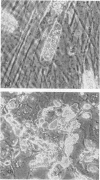
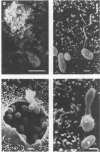
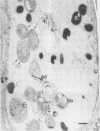
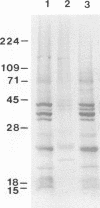
Microsporidia are primitive, spore-forming, mitochondria-lacking, eukaryotic protozoa that are obligate intracellular parasites. They are known to parasitize almost every group of animals including humans. Recently, microsporidia have increasingly been found to infect patients with AIDS. Five genera (Encephalitozoon, Enterocytozoon, Nosema, Septata, and Pleistophora) of microsporidia are known to infect humans. Enterocytozoon organisms cause gastrointestinal disease in a majority of AIDS patients with microsporidiosis. However, a smaller, but an expanding, number of patients with AIDS are being diagnosed with ocular and disseminated infection with Encephalitozoon hellem. Although microsporidial spores can be identified in clinical samples by a staining technique such as one with Weber's chromotrope stain, identification to the species level is dependent on cumbersome and time-consuming electron microscopy. We have recently isolated and established in continuous culture several strains of E. hellem from urine, bronchoalveolar lavage, and sputum samples from AIDS patients with disseminated microsporidiosis. We developed polyclonal and monoclonal antibodies and PCR primers to a strain of E. hellem that can be used successfully to identify E. hellem from other species of microsporidia either in clinical specimens or in cultures established from clinical specimens. Since patients infected with Encephalitozoon spp. are known to respond favorably to albendazole, identification of the parasite to the species level would be invaluable in the treatment of disseminated microsporidiosis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldras A. M., Orenstein J. M., Kotler D. P., Shadduck J. A., Didier E. S. Detection of microsporidia by indirect immunofluorescence antibody test using polyclonal and monoclonal antibodies. J Clin Microbiol. 1994 Mar;32(3):608–612. doi: 10.1128/jcm.32.3.608-612.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan R. T., Cali A., Owen R. L., Spencer H. C. Microsporidia: opportunistic pathogens in patients with AIDS. Prog Clin Parasitol. 1991;2:1–26. [PubMed] [Google Scholar]
- Cali A., Kotler D. P., Orenstein J. M. Septata intestinalis N. G., N. Sp., an intestinal microsporidian associated with chronic diarrhea and dissemination in AIDS patients. J Eukaryot Microbiol. 1993 Jan-Feb;40(1):101–112. doi: 10.1111/j.1550-7408.1993.tb04889.x. [DOI] [PubMed] [Google Scholar]
- Cali A., Meisler D. M., Lowder C. Y., Lembach R., Ayers L., Takvorian P. M., Rutherford I., Longworth D. L., McMahon J., Bryan R. T. Corneal microsporidioses: characterization and identification. J Protozool. 1991 Nov-Dec;38(6):215S–217S. [PubMed] [Google Scholar]
- Canning E. U., Hollister W. S. Enterocytozoon bieneusi (Microspora): prevalence and pathogenicity in AIDS patients. Trans R Soc Trop Med Hyg. 1990 Mar-Apr;84(2):181–186. doi: 10.1016/0035-9203(90)90247-c. [DOI] [PubMed] [Google Scholar]
- Chupp G. L., Alroy J., Adelman L. S., Breen J. C., Skolnik P. R. Myositis due to Pleistophora (Microsporidia) in a patient with AIDS. Clin Infect Dis. 1993 Jan;16(1):15–21. doi: 10.1093/clinids/16.1.15. [DOI] [PubMed] [Google Scholar]
- Didier E. S., Didier P. J., Friedberg D. N., Stenson S. M., Orenstein J. M., Yee R. W., Tio F. O., Davis R. M., Vossbrinck C., Millichamp N. Isolation and characterization of a new human microsporidian, Encephalitozoon hellem (n. sp.), from three AIDS patients with keratoconjunctivitis. J Infect Dis. 1991 Mar;163(3):617–621. doi: 10.1093/infdis/163.3.617. [DOI] [PubMed] [Google Scholar]
- Diesenhouse M. C., Wilson L. A., Corrent G. F., Visvesvara G. S., Grossniklaus H. E., Bryan R. T. Treatment of microsporidial keratoconjunctivitis with topical fumagillin. Am J Ophthalmol. 1993 Mar 15;115(3):293–298. doi: 10.1016/s0002-9394(14)73578-0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Orenstein J. M., Chiang J., Steinberg W., Smith P. D., Rotterdam H., Kotler D. P. Intestinal microsporidiosis as a cause of diarrhea in human immunodeficiency virus-infected patients: a report of 20 cases. Hum Pathol. 1990 May;21(5):475–481. doi: 10.1016/0046-8177(90)90003-n. [DOI] [PubMed] [Google Scholar]
- Schwartz D. A., Bryan R. T., Hewan-Lowe K. O., Visvesvara G. S., Weber R., Cali A., Angritt P. Disseminated microsporidiosis (Encephalitozoon hellem) and acquired immunodeficiency syndrome. Autopsy evidence for respiratory acquisition. Arch Pathol Lab Med. 1992 Jun;116(6):660–668. [PubMed] [Google Scholar]
- Schwartz D. A., Visvesvara G. S., Diesenhouse M. C., Weber R., Font R. L., Wilson L. A., Corrent G., Serdarevic O. N., Rosberger D. F., Keenen P. C. Pathologic features and immunofluorescent antibody demonstration of ocular microsporidiosis (Encephalitozoon hellem) in seven patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1993 Mar 15;115(3):285–292. doi: 10.1016/s0002-9394(14)73577-9. [DOI] [PubMed] [Google Scholar]
- Schwartz D. A., Visvesvara G. S., Leitch G. J., Tashjian L., Pollack M., Holden J., Bryan R. T. Pathology of symptomatic microsporidial (Encephalitozoon hellem) bronchiolitis in the acquired immunodeficiency syndrome: a new respiratory pathogen diagnosed from lung biopsy, bronchoalveolar lavage, sputum, and tissue culture. Hum Pathol. 1993 Sep;24(9):937–943. doi: 10.1016/0046-8177(93)90106-q. [DOI] [PubMed] [Google Scholar]
- Shadduck J. A. Human microsporidiosis and AIDS. Rev Infect Dis. 1989 Mar-Apr;11(2):203–207. doi: 10.1093/clinids/11.2.203. [DOI] [PubMed] [Google Scholar]
- Shadduck J. A., Meccoli R. A., Davis R., Font R. L. Isolation of a microsporidian from a human patient. J Infect Dis. 1990 Sep;162(3):773–776. doi: 10.1093/infdis/162.3.773. [DOI] [PubMed] [Google Scholar]
- Visvesvara G. S., Peralta M. J., Brandt F. H., Wilson M., Aloisio C., Franko E. Production of monoclonal antibodies to Naegleria fowleri, agent of primary amebic meningoencephalitis. J Clin Microbiol. 1987 Sep;25(9):1629–1634. doi: 10.1128/jcm.25.9.1629-1634.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossbrinck C. R., Baker M. D., Didier E. S., Debrunner-Vossbrinck B. A., Shadduck J. A. Ribosomal DNA sequences of Encephalitozoon hellem and Encephalitozoon cuniculi: species identification and phylogenetic construction. J Eukaryot Microbiol. 1993 May-Jun;40(3):354–362. doi: 10.1111/j.1550-7408.1993.tb04928.x. [DOI] [PubMed] [Google Scholar]
- Vossbrinck C. R., Maddox J. V., Friedman S., Debrunner-Vossbrinck B. A., Woese C. R. Ribosomal RNA sequence suggests microsporidia are extremely ancient eukaryotes. 1987 Mar 26-Apr 1Nature. 326(6111):411–414. doi: 10.1038/326411a0. [DOI] [PubMed] [Google Scholar]
- Weber R., Bryan R. T., Owen R. L., Wilcox C. M., Gorelkin L., Visvesvara G. S. Improved light-microscopical detection of microsporidia spores in stool and duodenal aspirates. The Enteric Opportunistic Infections Working Group. N Engl J Med. 1992 Jan 16;326(3):161–166. doi: 10.1056/NEJM199201163260304. [DOI] [PubMed] [Google Scholar]
- Weber R., Kuster H., Visvesvara G. S., Bryan R. T., Schwartz D. A., Lüthy R. Disseminated microsporidiosis due to Encephalitozoon hellem: pulmonary colonization, microhematuria, and mild conjunctivitis in a patient with AIDS. Clin Infect Dis. 1993 Sep;17(3):415–419. doi: 10.1093/clinids/17.3.415. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Cali A., Levee E., LaPlace D., Tanowitz H., Simon D., Wittner M. Diagnosis of Encephalitozoon cuniculi infection by western blot and the use of cross-reactive antigens for the possible detection of microsporidiosis in humans. Am J Trop Med Hyg. 1992 Oct;47(4):456–462. doi: 10.4269/ajtmh.1992.47.456. [DOI] [PubMed] [Google Scholar]
- Zierdt C. H., Gill V. J., Zierdt W. S. Detection of microsporidian spores in clinical samples by indirect fluorescent-antibody assay using whole-cell antisera to Encephalitozoon cuniculi and Encephalitozoon hellem. J Clin Microbiol. 1993 Nov;31(11):3071–3074. doi: 10.1128/jcm.31.11.3071-3074.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gool T., Snijders F., Reiss P., Eeftinck Schattenkerk J. K., van den Bergh Weerman M. A., Bartelsman J. F., Bruins J. J., Canning E. U., Dankert J. Diagnosis of intestinal and disseminated microsporidial infections in patients with HIV by a new rapid fluorescence technique. J Clin Pathol. 1993 Aug;46(8):694–699. doi: 10.1136/jcp.46.8.694. [DOI] [PMC free article] [PubMed] [Google Scholar]