Abstract
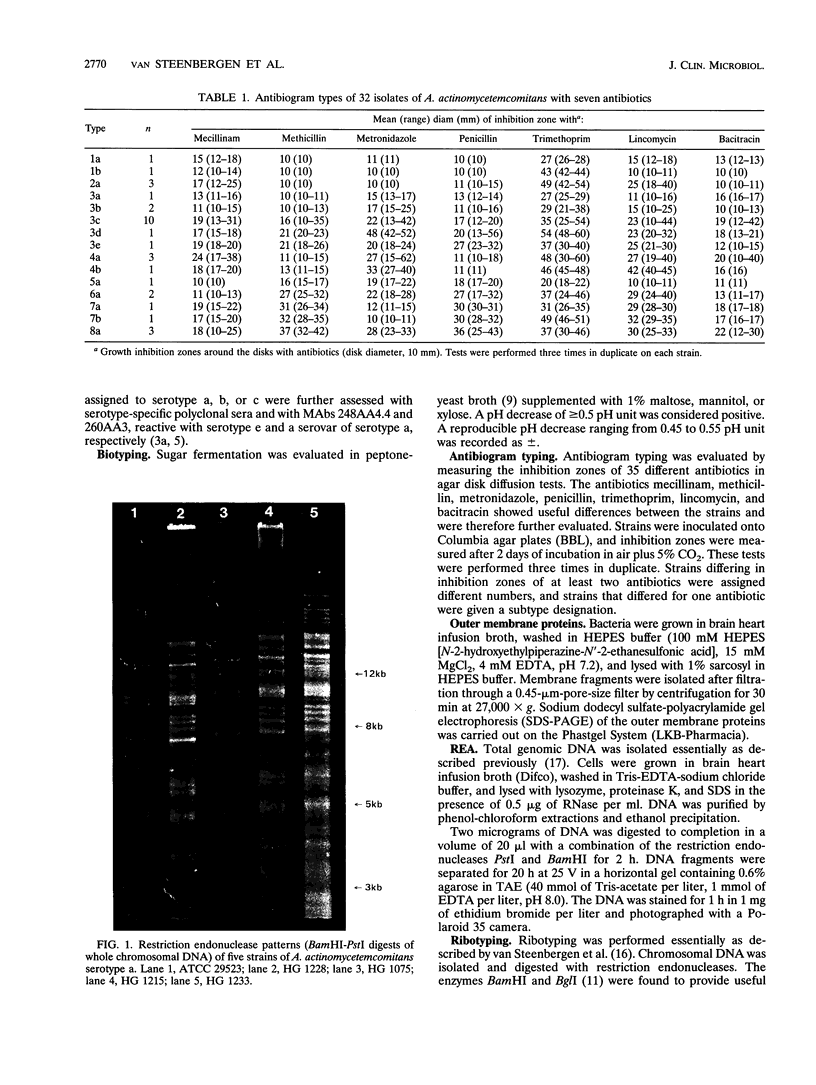
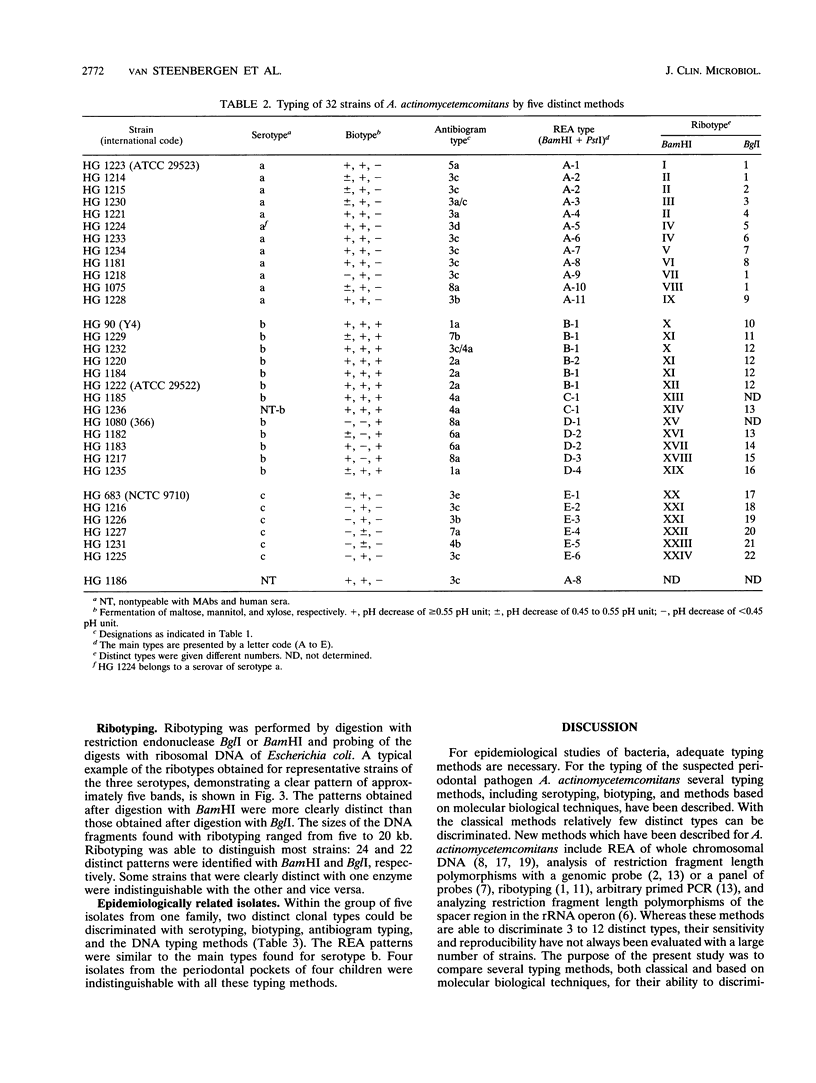
Actinobacillus actinomycetemcomitans is an important pathogen in the etiology of severe periodontitis. For epidemiological studies on the prevalence of certain pathogenic clones and transmission of this bacterium, adequate typing methods are necessary. The purpose of this study was to compare six different typing methods for A. actinomycetemcomitans. Five reference strains and 27 fresh clinical isolates from periodontitis patients were used. Serotyping showed 12 serotype a strains, 13 type b strains, 6 type c strains, and 1 nontypeable strain. Biotyping on the basis of the fermentation of mannose, mannitol, and xylose resulted in six biotypes. Antibiogram typing was evaluated by measuring the inhibition zones of seven antibiotics in agar diffusion tests. With this method eight main types which could be further differentiated into 15 subtypes were found. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis patterns of outer membrane proteins were similar among all isolates tested. Restriction endonuclease analysis (REA) of whole chromosomal DNA resulted in five main types. These five main types were further differentiated into 24 subtypes on the basis of DNA fragment differences in the high-molecular-weight region. Hybridization of DNA fragments with ribosomal DNA (ribotyping) resulted in 22 to 24 different types, depending on the restriction endonuclease used. Ribotype patterns were easy to interpret and provided an univocal distinction between different strains compared with REA results. When applied to epidemiologically related isolates, all methods were able to discriminate two clonal types among five isolates from five children from one family. We conclude that serotyping, biotyping, and outer membrane patterns were reproducible but had a low discriminatory potential. REA and ribotyping were reproducible and gave the highest number of distinct types. When the DNA typing methodis were compared, all strains tested could be distinguished. These findings confirm the heterogeneity found within the species A. actinomycetemcomitans.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alaluusua S., Saarela M., Jousimies-Somer H., Asikainen S. Ribotyping shows intrafamilial similarity in Actinobacillus actinomycetemcomitans isolates. Oral Microbiol Immunol. 1993 Aug;8(4):225–229. doi: 10.1111/j.1399-302x.1993.tb00564.x. [DOI] [PubMed] [Google Scholar]
- Di Rienzo J. M., Spieler E. L. Identification and characterization of the major cell envelope proteins of oral strains of Actinobacillus actinomycetemcomitans. Infect Immun. 1983 Jan;39(1):253–261. doi: 10.1128/iai.39.1.253-261.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gmür R., Guggenheim B. Monoclonal antibodies for the detection of 'periodontopathic' bacteria. Arch Oral Biol. 1990;35 (Suppl):145S–151S. doi: 10.1016/0003-9969(90)90146-2. [DOI] [PubMed] [Google Scholar]
- Gmür R., McNabb H., van Steenbergen T. J., Baehni P., Mombelli A., van Winkelhoff A. J., Guggenheim B. Seroclassification of hitherto nontypeable Actinobacillus actinomycetemcomitans strains: evidence for a new serotype e. Oral Microbiol Immunol. 1993 Apr;8(2):116–120. doi: 10.1111/j.1399-302x.1993.tb00556.x. [DOI] [PubMed] [Google Scholar]
- Griffen A. L., Leys E. J., Fuerst P. A. Strain identification of Actinobacillus actinomycetemcomitans using the polymerase chain reaction. Oral Microbiol Immunol. 1992 Aug;7(4):240–243. doi: 10.1111/j.1399-302x.1992.tb00032.x. [DOI] [PubMed] [Google Scholar]
- Guthmiller J. M., Kolodrubetz D., Kraig E. A panel of probes detects DNA polymorphisms in human and non-human primate isolates of a periodontal pathogen, Actinobacillus actinomycetemcomitans. Microb Pathog. 1993 Feb;14(2):103–115. doi: 10.1006/mpat.1993.1011. [DOI] [PubMed] [Google Scholar]
- Han N., Hoover C. I., Winkler J. R., Ng C. Y., Armitage G. C. Identification of genomic clonal types of Actinobacillus actinomycetemcomitans by restriction endonuclease analysis. J Clin Microbiol. 1991 Aug;29(8):1574–1578. doi: 10.1128/jcm.29.8.1574-1578.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarela M., Asikainen S., Alaluusua S., Pyhälä L., Lai C. H., Jousimies-Somer H. Frequency and stability of mono- or poly-infection by Actinobacillus actinomycetemcomitans serotypes a, b, c, d or e. Oral Microbiol Immunol. 1992 Oct;7(5):277–279. doi: 10.1111/j.1399-302x.1992.tb00588.x. [DOI] [PubMed] [Google Scholar]
- Saarela M., Asikainen S., Jousimies-Somer H., Asikainen T., von Troil-Lindén B., Alaluusua S. Hybridization patterns of Actinobacillus actinomycetemcomitans serotypes a-e detected with an rRNA gene probe. Oral Microbiol Immunol. 1993 Apr;8(2):111–115. doi: 10.1111/j.1399-302x.1993.tb00555.x. [DOI] [PubMed] [Google Scholar]
- Slots J., Listgarten M. A. Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in human periodontal diseases. J Clin Periodontol. 1988 Feb;15(2):85–93. doi: 10.1111/j.1600-051x.1988.tb00999.x. [DOI] [PubMed] [Google Scholar]
- Slots J., Liu Y. B., DiRienzo J. M., Chen C. Evaluating two methods for fingerprinting genomes of Actinobacillus actinomycetemcomitans. Oral Microbiol Immunol. 1993 Dec;8(6):337–343. doi: 10.1111/j.1399-302x.1993.tb00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slots J., Reynolds H. S., Genco R. J. Actinobacillus actinomycetemcomitans in human periodontal disease: a cross-sectional microbiological investigation. Infect Immun. 1980 Sep;29(3):1013–1020. doi: 10.1128/iai.29.3.1013-1020.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Steenbergen T. J., Menard C., Tijhof C. J., Mouton C., De Graaff J. Comparison of three molecular typing methods in studies of transmission of Porphyromonas gingivalis. J Med Microbiol. 1993 Dec;39(6):416–421. doi: 10.1099/00222615-39-6-416. [DOI] [PubMed] [Google Scholar]
- Zambon J. J., Slots J., Genco R. J. Serology of oral Actinobacillus actinomycetemcomitans and serotype distribution in human periodontal disease. Infect Immun. 1983 Jul;41(1):19–27. doi: 10.1128/iai.41.1.19-27.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambon J. J., Sunday G. J., Smutko J. S. Molecular genetic analysis of Actinobacillus actinomycetemcomitans epidemiology. J Periodontol. 1990 Feb;61(2):75–80. doi: 10.1902/jop.1990.61.2.75. [DOI] [PubMed] [Google Scholar]
- van Steenbergen T. J., Van der Velden U., Abbas F., de Graaff J. Microflora and bacterial DNA restriction enzyme analysis in young adults with periodontitis. J Periodontol. 1991 Apr;62(4):235–241. doi: 10.1902/jop.1991.62.4.235. [DOI] [PubMed] [Google Scholar]