Abstract
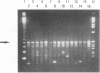
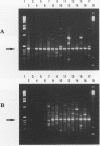
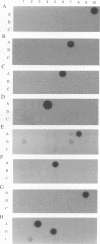
The superoxide dismutase gene has been identified as a target in screening for the presence of mycobacteria on the genus level and differentiating relevant mycobacterial species from one another by PCR. Consensus primers deduced from known superoxide dismutase gene sequences allowed the amplification of DNAs from a variety of bacteria, fungi, and protozoa. Selected amplicons from Actinomyces viscosus, Corynebacterium diphtheriae, Corynebacterium pseudodiphtheriticum, Mycobacterium avium, M. fortuitum, M. gordonae, M. intracellulare, M. kansasii, M. scrofulaceum, M. simiae, M. tuberculosis, M. xenopi, and Nocardia asteroides were subsequently cloned and sequenced. The alignment of those sequences facilitated the selection of primers targeting conserved regions present in myobacterial species but absent in nonmycobacterial species and thus allowed the genus-specific amplification of all 28 different mycobacterial species tested. A pool of genus-specific allowed the genus-specific amplification of all 28 different mycobacterial species tested. A pool of genus-specific probes recognized 23 of the 28 mycobacterial species and did not cross-react with any of the 96 nonmycobacterial species tested. In addition, probes recognizing species-specific variable regions within the superoxide dismutase genes of M. avium, M. fortuitum, M. gordonae, M. intracellulare, M. kansasii, M. scrofulaceum, M. simiae, the M. tuberculosis complex, and M. xenopi were identified. All probes recognized only the species from which they were derived and did not cross-react with any other mycobacterial species or with any of the nonmycobacterial species tested. We conclude that the superoxide dismutase gene is a suitable target for amplifying mycobacteria by PCR on the genus level, confirming correct amplification by genus-specific probes, and differentiating relevant species from one another by a set of species-specific probes.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannister J. V., Bannister W. H., Rotilio G. Aspects of the structure, function, and applications of superoxide dismutase. CRC Crit Rev Biochem. 1987;22(2):111–180. doi: 10.3109/10409238709083738. [DOI] [PubMed] [Google Scholar]
- Bowler C., Van Kaer L., Van Camp W., Van Montagu M., Inzé D., Dhaese P. Characterization of the Bacillus stearothermophilus manganese superoxide dismutase gene and its ability to complement copper/zinc superoxide dismutase deficiency in Saccharomyces cerevisiae. J Bacteriol. 1990 Mar;172(3):1539–1546. doi: 10.1128/jb.172.3.1539-1546.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson-Noël A., Gicquel B., Lecossier D., Lévy-Frébault V., Nassif X., Hance A. J. Rapid diagnosis of tuberculosis by amplification of mycobacterial DNA in clinical samples. Lancet. 1989 Nov 4;2(8671):1069–1071. doi: 10.1016/s0140-6736(89)91082-9. [DOI] [PubMed] [Google Scholar]
- Butler W. R., Kilburn J. O. High-performance liquid chromatography patterns of mycolic acids as criteria for identification of Mycobacterium chelonae, Mycobacterium fortuitum, and Mycobacterium smegmatis. J Clin Microbiol. 1990 Sep;28(9):2094–2098. doi: 10.1128/jcm.28.9.2094-2098.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böddinghaus B., Rogall T., Flohr T., Blöcker H., Böttger E. C. Detection and identification of mycobacteria by amplification of rRNA. J Clin Microbiol. 1990 Aug;28(8):1751–1759. doi: 10.1128/jcm.28.8.1751-1759.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttger E. C., Teske A., Kirschner P., Bost S., Chang H. R., Beer V., Hirschel B. Disseminated "Mycobacterium genavense" infection in patients with AIDS. Lancet. 1992 Jul 11;340(8811):76–80. doi: 10.1016/0140-6736(92)90397-l. [DOI] [PubMed] [Google Scholar]
- Danciger E., Dafni N., Bernstein Y., Laver-Rudich Z., Neer A., Groner Y. Human Cu/Zn superoxide dismutase gene family: molecular structure and characterization of four Cu/Zn superoxide dismutase-related pseudogenes. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3619–3623. doi: 10.1073/pnas.83.11.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit D., Steyn L., Shoemaker S., Sogin M. Direct detection of Mycobacterium tuberculosis in clinical specimens by DNA amplification. J Clin Microbiol. 1990 Nov;28(11):2437–2441. doi: 10.1128/jcm.28.11.2437-2441.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenach K. D., Cave M. D., Bates J. H., Crawford J. T. Polymerase chain reaction amplification of a repetitive DNA sequence specific for Mycobacterium tuberculosis. J Infect Dis. 1990 May;161(5):977–981. doi: 10.1093/infdis/161.5.977. [DOI] [PubMed] [Google Scholar]
- Fries J. W., Patel R. J., Piessens W. F., Wirth D. F. Genus- and species-specific DNA probes to identify mycobacteria using the polymerase chain reaction. Mol Cell Probes. 1990 Apr;4(2):87–105. doi: 10.1016/0890-8508(90)90011-n. [DOI] [PubMed] [Google Scholar]
- Haas W. H., Butler W. R., Woodley C. L., Crawford J. T. Mixed-linker polymerase chain reaction: a new method for rapid fingerprinting of isolates of the Mycobacterium tuberculosis complex. J Clin Microbiol. 1993 May;31(5):1293–1298. doi: 10.1128/jcm.31.5.1293-1298.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hance A. J., Grandchamp B., Lévy-Frébault V., Lecossier D., Rauzier J., Bocart D., Gicquel B. Detection and identification of mycobacteria by amplification of mycobacterial DNA. Mol Microbiol. 1989 Jul;3(7):843–849. doi: 10.1111/j.1365-2958.1989.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Hermans P. W., Schuitema A. R., Van Soolingen D., Verstynen C. P., Bik E. M., Thole J. E., Kolk A. H., van Embden J. D. Specific detection of Mycobacterium tuberculosis complex strains by polymerase chain reaction. J Clin Microbiol. 1990 Jun;28(6):1204–1213. doi: 10.1128/jcm.28.6.1204-1213.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs K. A., Rudersdorf R., Neill S. D., Dougherty J. P., Brown E. L., Fritsch E. F. The thermal stability of oligonucleotide duplexes is sequence independent in tetraalkylammonium salt solutions: application to identifying recombinant DNA clones. Nucleic Acids Res. 1988 May 25;16(10):4637–4650. doi: 10.1093/nar/16.10.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao C. C., Yen T. S., You J. B., Maa J. S., Fiss E. H., Chang C. H. Detection and identification of Mycobacterium tuberculosis by DNA amplification. J Clin Microbiol. 1990 Sep;28(9):1877–1880. doi: 10.1128/jcm.28.9.1877-1880.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R. J., Fries J. W., Piessens W. F., Wirth D. F. Sequence analysis and amplification by polymerase chain reaction of a cloned DNA fragment for identification of Mycobacterium tuberculosis. J Clin Microbiol. 1990 Mar;28(3):513–518. doi: 10.1128/jcm.28.3.513-518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B. C., Dwyer B. Rapid, simple method for typing isolates of Mycobacterium tuberculosis by using the polymerase chain reaction. J Clin Microbiol. 1993 Feb;31(2):329–334. doi: 10.1128/jcm.31.2.329-334.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Salin M. L., Duke M. V., Oesterhelt D., Ma D. P. Cloning and determination of the nucleotide sequence of the Mn-containing superoxide dismutase gene from Halobacterium halobium. Gene. 1988 Oct 15;70(1):153–159. doi: 10.1016/0378-1119(88)90113-8. [DOI] [PubMed] [Google Scholar]
- Shawar R. M., el-Zaatari F. A., Nataraj A., Clarridge J. E. Detection of Mycobacterium tuberculosis in clinical samples by two-step polymerase chain reaction and nonisotopic hybridization methods. J Clin Microbiol. 1993 Jan;31(1):61–65. doi: 10.1128/jcm.31.1.61-65.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöbring U., Mecklenburg M., Andersen A. B., Miörner H. Polymerase chain reaction for detection of Mycobacterium tuberculosis. J Clin Microbiol. 1990 Oct;28(10):2200–2204. doi: 10.1128/jcm.28.10.2200-2204.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl D. A., Urbance J. W. The division between fast- and slow-growing species corresponds to natural relationships among the mycobacteria. J Bacteriol. 1990 Jan;172(1):116–124. doi: 10.1128/jb.172.1.116-124.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Avila H. Structure and gene expression of the E. coli Mn-superoxide dismutase gene. Nucleic Acids Res. 1986 Jun 11;14(11):4577–4589. doi: 10.1093/nar/14.11.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takewaki S., Okuzumi K., Ishiko H., Nakahara K., Ohkubo A., Nagai R. Genus-specific polymerase chain reaction for the mycobacterial dnaJ gene and species-specific oligonucleotide probes. J Clin Microbiol. 1993 Feb;31(2):446–450. doi: 10.1128/jcm.31.2.446-450.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangaraj H. S., Lamb F. I., Davis E. O., Colston M. J. Nucleotide and deduced amino acid sequence of Mycobacterium leprae manganese superoxide dismutase. Nucleic Acids Res. 1989 Oct 25;17(20):8378–8378. doi: 10.1093/nar/17.20.8378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry D., Cave M. D., Eisenach K. D., Crawford J. T., Bates J. H., Gicquel B., Guesdon J. L. IS6110, an IS-like element of Mycobacterium tuberculosis complex. Nucleic Acids Res. 1990 Jan 11;18(1):188–188. doi: 10.1093/nar/18.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whipple D. L., Le Febvre R. B., Andrews R. E., Jr, Thiermann A. B. Isolation and analysis of restriction endonuclease digestive patterns of chromosomal DNA from Mycobacterium paratuberculosis and other Mycobacterium species. J Clin Microbiol. 1987 Aug;25(8):1511–1515. doi: 10.1128/jcm.25.8.1511-1515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. J., Madej R., Persing D. H. The polymerase chain reaction: clinical applications. Adv Clin Chem. 1992;29:161–196. doi: 10.1016/s0065-2423(08)60224-3. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeager H., Jr, Lacy J., Smith L. R., LeMaistre C. A. Quantitative studies of mycobacterial populations in sputum and saliva. Am Rev Respir Dis. 1967 Jun;95(6):998–1004. doi: 10.1164/arrd.1967.95.6.998. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Lathigra R., Garbe T., Catty D., Young D. Genetic analysis of superoxide dismutase, the 23 kilodalton antigen of Mycobacterium tuberculosis. Mol Microbiol. 1991 Feb;5(2):381–391. doi: 10.1111/j.1365-2958.1991.tb02120.x. [DOI] [PubMed] [Google Scholar]
- Zolg J. W., Chen G. X., Plitt J. R. Detection of pyrimethamine resistance in Plasmodium falciparum by mutation-specific polymerase chain reaction. Mol Biochem Parasitol. 1990 Mar;39(2):257–265. doi: 10.1016/0166-6851(90)90064-s. [DOI] [PubMed] [Google Scholar]