Abstract
Accurate temporal processing of sound is essential for detecting word structures in speech. Maternal smoking affects speech processing in newborns and may influence child language development; however, it is unclear how neonatal exposure to nicotine, present in cigarettes, affects the normal development of temporal processing. The present study used the gap-induced prepulse inhibition (gap-PPI) of the acoustic startle response to investigate the effects of neonatal nicotine exposure on the normal development of gap detection, a behavioral testing procedure of auditory temporal resolution. Neonatal rats were injected twice per day with saline (control), 1 mg/kg nicotine (N-1mg) or 5 mg/kg nicotine (N-5mg) from postnatal day 8 to 12 (P8–P12). During the first month after birth, rats showed poor gap-PPI in all three groups. At P45 and P60, gap-PPI in control rats improved significantly, whereas rats exposed to nicotine exhibited less improvement. At P60, the gap-detection threshold in the N-5mg group was significantly higher than in the control group, suggesting that neonatal nicotine exposure affects the normal development of gap detection acuity. Additionally, 1 hour after receiving an acute nicotine injection (1 mg/kg), gap-PPI recorded in adult rats from the N-5mg group showed a temporary significant improvement. These results suggest that neonatal nicotine exposure reduces gap-PPI implying an impairment of the normal development of auditory temporal processing by inducing changes in cholinergic systems.
Keywords: Nicotine, Startle reflex, Gap detection, Development, Auditory cortex
INTRODUCTION
The processing of complex acoustic signals, such as speech and music, requires a central auditory system able to detect rapid changes of sound intensity and spectral fluctuations (Metherate et al., 2005; Purcell et al., 2004). A reduced spectral and temporal resolution, as seen in elderly listeners, can lead to difficulties in understanding speech (Strouse et al., 1998). High spectral and temporal resolutions are critical for normal development of child language and speech comprehension (Nicholas et al., 2006). Children with language impairment score poorly on auditory processing tests, such as auditory word discrimination, temporal integration and auditory synthesis (Stollman et al., 2003). In addition, Trehub et al. found that children with poor temporal resolution are more likely to have language learning disabilities than children with better temporal resolution (Trehub et al., 1996). Recent studies have found that prelingually deaf children who received cochlear implants also require precise temporal processing capability in order to acquire language. Cochlear implant users who exhibited poor language performance had difficulties on simple temporal gap-detection tasks, such as detecting a silent gap in a continuous sound of moderate intensity. However, cochlear implant users with better language skills exhibited better gap-detection ability; some exhibited gap-detection thresholds similar to normal hearing listeners (Fu et al., 2001).
Maternal smoking, which could expose prenatal children to large doses of nicotine, has been found to be harmful to fetal brain development (Oncken et al., 2003) which may lead to auditory cognitive dysfunction (Dwyer et al., 2008). Maternal smoking has also been shown to affect the development of speech processing in infants. Key et al. investigated vowel-sound evoked event-related potentials in human two day old infants, whose mothers did or did not smoke during pregnancy (Key et al., 2007). The infants belonging to non-smoking mothers exhibited different event-related potentials to different vowel-sounds. In contrast, infants of smoking mothers exhibited no difference in event-related potentials to different vowel-sounds. Since the infants from both groups experienced no differences in postnatal environment, the event-related potential differences between them are suggested to be a result of maternal smoking during pregnancy. Given that maternal smoking and exposure of newborns to second-hand smoke are not uncommon, understanding the risk of prenatal and neonatal nicotine exposure on central auditory system development could provide important and useful information about prenatal care and child education.
In the present study, we investigated the effects of neonatal nicotine exposure on the normal development of gap detection acuity, an established behavioral testing procedure of auditory temporal resolution in rats (Friedman et al., 2004). The gap detection acuity was measured as the level and threshold of gap-induced prepulse inhibition (gap-PPI) of the acoustic startle reflex (Friedman et al., 2004; Ison et al., 2002; Walton et al., 1997). This test does not require any auditory training and allows assessment of rapid developmental changes in neonatal rats. Nicotine was given to neonatal rat pups before the onset of hearing on postnatal days 8 to 12 (P8–P12), the critical period of central auditory system development (Chang et al., 2003). Since the hearing of rats develops two weeks after the birth, the auditory development in the second postnatal week in rats is equivalent to the third trimester of human fetal development (Bayer et al., 1993).
MATERIALS and METHODS
Animals and Nicotine Exposure
Neonatal Sprague-Dawley rats (Harlan, Indianapolis, IN), both male and female, were randomly assigned into three groups: rats in the low dose nicotine group (N-1mg, n = 11) were injected with 1 mg/kg nicotine, rats in the high dose nicotine group (N-5mg, n = 10) were injected with 5 mg/kg nicotine, and control rats (n = 16) were injected with saline (10 ml/kg). Nicotine (#72290, Fluka) was diluted in saline to 0.1 mg/ml (pH 8.6). Nicotine or saline was given twice per day (one injection at 9 am and one injection at 5 pm) subcutaneously (s.c.) from P8 to P12. For acute nicotine treatment to adult rats, nicotine was diluted in saline (1 mg/ml) and injected once in a dose of 1 mg/kg (s.c.).
All procedures used in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of State University of New York at Buffalo.
Acoustic Startle Reflex
The prepulse inhibition of acoustic startle reflex (PPI) test was utilized to monitor temporal processing development from P20 to P60. The testing procedure has been described previously (Yang et al., 2007). Each rat was positioned in a wire mesh cage mounted on an acrylic glass base that rested on a sensitive piezoelectric transducer (Radio Shack Corp.). Three different sizes of wire mesh cages were used for rats at different age (P20 to P60) to restrict the rat’s movement within the calibrated sound field (824 audiometer, Larson Davis). The output of the piezoelectric transducer was sent to a low-pass filter set at 1000 Hz (LPF-300, World Precision Instruments) and then connected to an analog-digital converter (RP2.1, Tucker-Davis Technologies (TDT), FL). The root mean square (RMS) amplitude of the first 100 ms response after the onset of the sound stimulus was measured using custom software (Matlab, The MathWorks Inc.). Sound signals were generated by a second digital signal processor (RP2.1, TDT) controlled by custom software, amplified (SA1; TDT), and presented by a high frequency dome tweeter (FT28D, Fostex, NJ) located 25 cm above the rat. The startle eliciting stimulus consisted of a broadband noise burst presented at 100 dB SPL (1 to 30 kHz bandwidth, 20 ms duration, 0.1 ms rise/fall time, 100 kHz sampling rate).
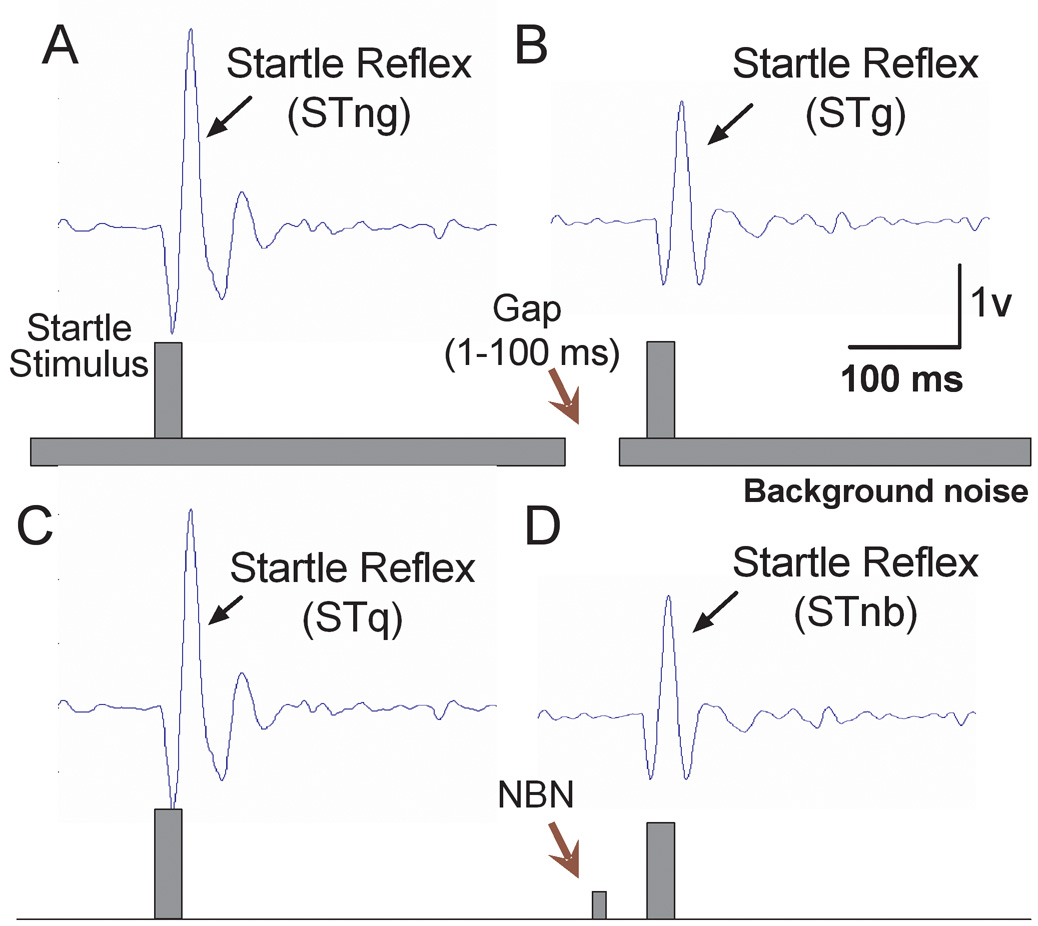
The amplitude of acoustic startle responses were measured for all three groups of rats at P20, P28, P35, P45 and P60 using two different testing procedures: gap-PPI and narrow band prepulse inhibition (NB-PPI). For the gap-PPI test, the startle stimulus was embedded in a white noise background (60 dB SPL) with or without a silent gap immediately prior to the startle stimulus. The duration of the silent gap varied from 1 to 100 ms and the offset of the gap preceded the onset of the startle stimulus by 60 ms. The RMS amplitude of the acoustic startle was measured in the presence of continuous noise with no gap (STng) (Figure 1A), or continuous noise containing a gap (STg) (Figure 1B). When the gap precedes the startle stimulus, it reduces the amplitude of the startle response in normal hearing rats (Figure 1A vs. 1B), i.e. inhibits the startle response (Ison et al., 2002; Yang et al., 2007). Extent of gap-PPI in % was defined as: (STng-STg)/STng × 100%. Ten trials for each condition contained either no gap or gaps with durations of 1, 2, 4, 6, 8, 10, 15, 25, 50 and 100 ms (semilogarithmic scale), presented in random order. The inter-trial interval varied from 17 to 23 seconds. Startle responses in the gap condition were compared to the response in the no-gap condition using the Student’s t-test and significance level was set to P<0.05. The gap detection threshold was defined as minimum duration of gap needed to induce significant inhibition of the startle response.
Figure 1.
Prepulse inhibition paradigm used to record gap detection and noise-burst induced prepulse inhibition. (A) The acoustic startle response was recorded from rats elicited by a white noise burst presented at 100 dB SPL for 20 ms. (B) A gap (ranging from 1 to 100 ms) embedded in a continuous white noise (60 dB SPL) 60 ms prior to the startle acoustic signal inhibits the startle response by reducing the amplitude. This inhibition is defined as gap-PPI. (C–D) A narrow-band noise (NBN) burst (60 dB SPL, 20 ms) 60 ms prior to the startle eliciting sound induced inhibition on the startle reflex amplitude defined as NB-PPI. STng: startle reflex elicited by an acoustic stimulus in continuous noise without a preceding gap; STg: startle reflex elicited by an acoustic stimulus in continuous noise with a preceding gap; STq: startle reflex elicited by an acoustic stimulus without a preceding stimulus; STnb: startle reflex elicited by an acoustic stimulus with a preceding narrow band noise.
In the NB-PPI test, the startle stimulus was either presented alone (Figure 1C) or preceded by a 60 dB SPL narrowband (1000 Hz bandwidth) noise-burst (50 ms, 5 ms rise/fall time) centered at 6, 12, 16, 20 and 24 kHz (Figure 1D). The onset of noise-burst prepulse preceded the onset of the startle stimulus by 60 ms. The RMS of the acoustic startle was measured in quiet (STq) or with the noise-burst preceding the startle stimulus (STnb). When the noise-burst prepulse precedes the startle stimulus, it reduces the amplitude of the startle response (Figure 1C vs. 1D), i.e. the startle is inhibited. STnb and STq were presented in pairs in randomized order. Extent of NB-PPI in % was defined as: (STq-STnb)/STq × 100%. Ten stimulus pairs (20 trials) were presented at each frequency with random inter-trial interval (17–23 seconds).
Auditory Brainstem Response (ABR) Recordings
Hearing thresholds were evaluated by ABR tests. During the tests, rats were anesthetized under isoflurane (1.5%). The vertex was used as the non-inverted recording site, the ipsilateral pinna was used as the inverted recording site and contralateral pinna as the ground. The lead of the active electrode was connected to a headstage (RA4LI, TDT) using a flexible, low noise cable. The headstage was connected to a preamplifier (RA16PA), the output of which was routed to a digital signal processing module (RX5-2, Pentusa Base Station, TDT) before being further processed with software (BioSigRP version 4.4, TDT; digital band pass filter:100–3000 Hz) for the ABR recording. The sound stimulus was a tone-burst (duration was 5 ms duration, 1 ms rise/fall time) at 4, 8, 12, 16 and 20 kHz generated with a real-time digital-analog converter (RP2.1, TDT). The repetition rate was 21 times/second. Sound stimuli were presented through a high frequency dome tweeter (FT28D, Fostex). Sound levels were calibrated using free-field microphones (1/2” 2540 and ¼” 2520, Larson Davis) sound level meter (824, Larson Davis).
Data Analysis
Graphs and statistic analyses were performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA). The Student’s t-test and one-way ANOVA test were used to calculate the significant changes. P<0.05 was used as confidence level. Results are presented as Mean ± Standard Error of Mean.
RESULTS
Neonatal Nicotine Exposure on Gap Detection Development
To determine the effects of neonatal nicotine exposure on temporal processing acuity development, gap-PPIs were recorded in control rats (saline) and nicotine treated rats (N-1mg and N-5mg) from P20 to P60 (Figure 2A–E). At P20 to P35, there was no significant difference between the control group and either of the nicotine groups (One-way ANOVA, Figure 2A–C). At P20, no group showed significant inhibition at gap durations shorter than 25 ms. When the gap increased to 50 ms and 100 ms, gap-PPI was present, ranging from 20 to 50%. At P28, the amplitude of gap-PPI showed a significant increase from about −30% PPI at 4 ms (an enhanced startle reflex known as facilitation (Schmajuk et al., 2005)) to about 30–50% at 100 ms gap duration. At P35, no negative gap-PPI was observed at 1−10 ms and the gap-PPI amplitude reached a plateau at 25–100 ms in all three groups. At P45 and P60, the gap-PPI in the N-5mg group was significantly smaller than in the N-1mg group and the saline group (Figure 2D, one-way ANOVA, F(2, 29) = 5.14, P = 0.01; Figure 2E, one-way ANOVA, F(2, 29) = 3.4, P = 0.04). These results showed that the gap-PPI recorded in high dose nicotine treated rats (N-5mg) showed less improvement during development from P20 to P60 compared to those treated with saline or low dose nicotine (N-1mg).
Figure 2.
Effects of neonatal nicotine exposure on the developmental change of gap detection. At P20 and P28, gap-PPI showed no difference between the nicotine exposed groups (N-1mg and N-5mg) and the saline group (A, B). At P35, gap-PPI recorded in the N-5mg group was slightly but not significantly smaller than in the saline and the N-1mg groups (C). At P45 and P60, the gap-induced PPI was significantly lower than in the N-1mg group and the saline group (D, E). Rats in the N-5mg group showed a significantly elevated gap detection threshold compared to the N-1mg and the saline group (F) (* P<0.05, ** P<0.01).
Figure 2F shows the average gap-detection threshold in the N-1mg, N-5mg and saline group. Consistent with gap-PPI amplitude changes, at P20, P28 and P35 there was no significant difference in threshold between neonatal nicotine exposed rats and the saline treated rats. At P45, the average gap-detection thresholds in the N-5mg group was 38 ± 11 ms (Mean ± SEM; n = 10) which was significantly higher than in the N-1mg group (8.3 ± 2.1 ms, n = 11) and the saline group (6.9 ± 0.9 ms, n = 14) (One-way ANOVA, F(2, 35) = 8.8, P = 0.001). At P60, the gap-detection threshold of the N-5mg group was 32 ± 13 ms, significantly higher than the N-1mg group (4.2 ± 0.5 ms, n = 10) and the saline group (4.7 ± 0.6 ms, n = 11) (One-way ANOVA, F(2, 35) = 6.1, P < 0.005). These results suggest that the gap-PPI and gap detection threshold was significantly increased by a higher dose of neonatal nicotine exposure.
Noise-Burst Induced PPI (NB-PPI)
To determine whether the background sound used in the gap detection test was audible (Willott et al., 1994), we measured the NB-PPI. The prepulse was a narrow band noise centered at 6, 12, 16, 20, or 24 kHz at 60 dB SPL, the same sound level as the continuous noise floor used in the gap detection test during period from P20 to P60. The average NB-PPI gradually increased from approximately 30% at P20 to 50% at P60 (Figure 3A). There was no significant difference between the nicotine exposed groups and the saline group (One-way ANOVA). These findings indicate that neonatal nicotine exposure had no significant effects on NB-PPI.
Figure 3.
(A) Neonatal nicotine exposure did not show any significant effects on the narrowband noise induced pre-pulse inhibition of startle response (NB-PPI). (B) ABR thresholds recorded from neonatal nicotine exposed rats were slightly lower than saline treated rats (Student’s t-test, * P<0.05).
ABR Test
To evaluate any peripheral hearing loss that could be induced by neonatal nicotine exposure, ABR tests were conducted to determine hearing thresholds of the rats in the saline group and the N-5mg group at P60. The average ABR thresholds in the saline group (n = 6) were 51 ± 3 dB, 25 ± 5 dB, 36 ± dB, 41 ± 4 dB and 46 ± 4 dB at 4, 8, 12, 16 and 20 kHz respectively; whereas in the N-5mg group the thresholds were 37 ± 3 dB, 20 ± 4 dB, 30 ± 3 dB, 39 ± 3 dB and 39 ± 4 dB at 4, 8, 12, 16 and 20 kHz respectively. The ABR threshold recorded in the N-5mg group was slightly lower than in the saline group (Figure 3B), the difference was significant at 4 kHz (Student’s t-test, P = 0.02), but not at other frequencies. Therefore, the ABR test eliminated the possibility that neonatal nicotine exposure can induce a hearing loss which might have affected the gap-PPI of the startle reflex.
Acute Nicotine Treatment
In order to test whether acute nicotine treatment affects temporal processing in adult rats, nicotine injections were given to the rats in both the saline group and N-5mg group to see how it affects gap-PPI. Figure 4A shows that acute nicotine injection (1 mg/kg, s.c.) significantly improved the gap-PPI in the N-5mg group. These changes were significant at gap durations of 4–100 ms (paired t-test, P value equals to 0.004, 0.002, 0.02, 0.04, 0.04, 0.03 and 0.04 for gap durations of 4, 6, 10, 15, 25, 50 and 100 ms respectively). The gap detection threshold in the N-5mg group also underwent a significant decrease (gap detection acuity improvement) after nicotine injection, from 13.6 ± 5.4 ms (n = 11) 1h before nicotine injection to 5.5 ms ± 2.0 ms (n = 11) 1h after nicotine injection (Student’s t-test, P = 0.002, Figure 4B). In order to evaluate long term effects of the acute nicotine treatment, rats were retested four days after the injection. The gap detection threshold was returned to 13.3 ± 7.4 ms (n = 11) and gap-PPI had dropped to the level measured before acute nicotine treatment (Figure 4A, dashed line). These results suggest that the nicotine induced gap detection impairment (threshold increase) is reversible, but only temporarily. In the saline group, acute nicotine injections induced no significant improvement in gap-PPI (Figure 4C) and gap-detection threshold (Figure 4D). The gap-detection threshold was 4.6 ± 1.2 ms (n = 6) before nicotine injection and 3.7 ± 0.3 ms (n = 6) 1 h after nicotine injection (Figure 4D).
Figure 4.
Effects of acute nicotine treatment (1 mg/kg) on the gap-induced prepulse inhibition of startle reflex (gap-PPI) in adult rats. (A) Nicotine improved gap-PPI recorded from N-5mg group. (B) One hour after nicotine treatment, gap-detection thresholds was significantly decreased. After 4 days, the acute changes had disappeared, ruling out permanent changes caused by acute nicotine treatment. The nicotine treatment on the saline group showed a slight enhancement on gap-PPI (C), but did not show a significant change on gap detection threshold (D) (* P<0.05).
The NB-PPI in the N-5mg group also showed significant increase after nicotine injection (1 mg/kg, s.c., Figure 5A). The average NB-PPI before the nicotine injection was 51% ± 23%, 54% ± 18 %, 52% ± 18%, 53% ± 20% and 52% ± 20% at 6, 12, 16, 20 and 24 kHz respectively (n = 10); and increased to 62% ± 10%, 65% ± 13%, 67% ± 8%, 68% ± 8% and 66% ± 7% at 6, 12, 16, 20 and 24 kHz (n = 11) respectively 1 h after nicotine injection. The difference was significant at 16, 20 and 24 kHz (Student’s t-test, P value was 0.03, 0.03 and 0.04 at 16, 20 and 24 kHz respectively), but not significant at 6 and 12 kHz. Four days after nicotine injection, the nicotine induced NB-PPI increase had disappeared (Figure 5A, dashed line). In the saline group, there was no significant change in NB-PPI after nicotine injection (Figure 5B). The average NB-PPI before nicotine injection was 60% ± 9%, 49% ± 16%, 57% ± 14%, 51% ± 4% and 62% ± 12% at 6, 12, 16, 20 and 24 kHz respectively; and 1h after nicotine injection the NB-PPI was 54% ± 28%, 51% ± 17%, 51% ± 18%, 62% ± 16% and 54% ± 17% at 6, 12, 16, 20 and 24 kHz respectively.
Figure 5.
Effects of acute nicotine treatment (1 mg/kg) on the noise-burst induced prepulse inhibition of the startle reflex (NB-PPI) at P60. (A) NB-PPI was increased after the nicotine treatment recorded from N-5mg group. This enhancement had disappeared after 4 days. (B) The nicotine treatment on the saline group did not show any significant changes on NB-PPI (* P<0.05).
DISCUSSION
In the present study, gap-PPI was used to assess the effects of neonatal nicotine exposure on the normal development of temporal acuity in rats. We found that neonatal nicotine exposure significantly stunted the normal development of gap detection. A high dose of nicotine exposure (5 mg/kg), at P8 to P12, resulted in a reduced gap-PPI as well as a significantly higher gap-detection threshold, indicating poor auditory temporal processing in juvenile and adult rats. These results suggest that nicotine exposure to prenatal or neonatal infants, as a result of maternal or secondhand smoking, may affect development of gap detection acuity, implying temporal processing difficulty. Since phonologic processing skills are crucial for language development (Faden et al., 2000), temporal processing deficits could dramatically influence a child’s normal language development.
The lack of a significant increase in ABR thresholds in nicotine exposed rats, compared to control rats, suggests that the neonatal nicotine-induced temporal processing deficits are not related to a peripheral hearing deficit. These ABR results are consistent with a previous study which showed that infants exposed to nicotine and infants from a nicotine-free environment had similar brainstem auditory evoked response (Trammer et al., 1992). In addition, absence of significant changes in NB-PPI in nicotine exposed rats indicates that neonatal nicotine exposure did not affect hearing itself or the physiological mechanisms responsible for startle inhibition.
The anatomical correlate to the nicotine-induced gap detection impairment is not known. One strong candidate is the auditory cortex (AC). Our results on gap-PPI and NB-PPI are similar to those from previous studies on rats with bilateral AC lesions, which showed a higher threshold in gap-PPI, but not in NB-PPI (Bowen et al., 2003; Cooke et al., 2007). This suggests that the reduction of gap detection performance induced by neonatal nicotine exposure during P8 to P12, the most sensitive period for AC function development (de Villers-Sidani et al., 2007), may be due to AC impairment. Moreover, the function of the AC goes through a significant developmental change from infancy to childhood. Tuning properties of AC neurons and temporal processing acuity showed strong correlation in animal studies (Chang et al., 2005). Neonatal rats have an undefined AC response to acoustic signals and poor temporal acuity (Chang et al., 2003). The tuning curves of AC neurons became sharper and temporal resolution improved significantly as they reach maturity. The gap detection threshold also improved from over 20 ms at P15 to 5–10 ms at P60, a finding which correlates with the critical period of AC function development (Friedman et al., 2004). Finally, the auditory cortex plays an important role in processing speech. Lesions in the AC caused by brain injury or stroke have been seen to impair temporal processing and also affect language skills in human subjects (Karbe et al., 1990; Wittmann et al., 2004). Patients who have temporal lobe lesions often suffer severe agnosia for verbal and nonverbal sounds (Rosati et al., 1982; Saygin et al., 2003).
Other central auditory regions, i.e., in the brainstem and the thalamus, are also involved in auditory temporal processing. For example, a recent study used electrophysiological recordings from the dorsal nucleus of the lateral lemniscus in the brainstem showed that fast temporal processing of monaural and binaural modulations of acoustic signals occurs at early regions in the auditory system (Siveke et al., 2008). Temporal resolution is crucial for the integration of interaural time difference cues for horizontal localization of low frequency sound, which occurs at the level of the superior olivary complex (Moore et al., 1983). The speech evoked brainstem response recorded in children with language learning difficulties exhibit poor temporal resolution suggesting the difficulty may originate as a neural encoding deficit in structures as early as the auditory brainstem (Johnson et al., 2007). Recent studies have shown that the medial geniculate body (MGB) can modulate the input signals from the inferior colliculus, showing phase locking and synchronizing of the neural response (Bartlett et al., 2007; Wallace et al., 2007). This suggests that the MGB may also be involved in the temporal processing of complex acoustical signals (Bartlett et al., 2007; Johnson et al., 2007). Thus, nicotine-induced changes in gap detection or temporal processing may also be related to changes in regions earlier than AC. Physiological investigations are required to localize anatomical correlates to nicotine induced gap-detection impairment.
We found that acute nicotine exposure to adult rats, which were exposed to nicotine at P8 to P12, could temporarily improve their gap detection acuity. This suggests that nicotinic receptors are involved in gap detection processing and that the impaired gap detection acuity induced by neonatal nicotine exposure can be compensated by nicotine treatment during adulthood. Interestingly, the acute nicotine treatment-induced gap-PPI enhancement was only found in neonatal nicotine exposed rats, not in saline treated rats. This evidence suggests that the nicotine-induced gap detection deficits and subsequent improvement after acute nicotine treatment may be due to a dysfunction of cholinergic system components, such as ACh synthesis or nicotinic receptors. Liang et al. found neonatal nicotine exposure can affect nicotinic regulation of AC function as well as impair auditory learning of avoidance conditioning in adult rats (Liang et al., 2006). In addition, Abreu-Villaca et al. found that prenatal nicotine exposure produced a cholinergic transmission hypoactivity in the cerebral cortex and midbrain as well as a robust nAChR upregulation resulting from adolescent nicotine exposure (Abreu-Villaca et al., 2004). These findings may explain why acute nicotine could induce an enhancement on gap-PPI and NB-PPI in neonatal nicotine exposed rats, not the control rats in our study. Gap detection deficits may be related to cholinergic hypoactivity, and improvement after acute nicotine exposure in the adult rats may be either due to compensation of the hypoactivity or to an increased sensitivity towards nicotine due to receptor upregulation. Our results also suggest that the nicotine induced gap detection impairment (threshold increase) is reversible. This finding may open up for possibilities for clinical treatment of nicotine-induced impairment of gapdetection, or of auditory temporal processing in general.
In summary, we found that neonatal nicotine exposure has deleterious effects on the normal development of gap detection acuity suggesting temporal processing deficits. Since temporal processing is crucial for the acquisition of language and speech, our results suggest neonatal nicotine exposure-induced temporal processing deficits may be one source of speech production and language deficits in children of smoking mothers. Further research is underway to understand mechanisms and localize the nicotine induced functional changes in the cholinergic system.
Acknowledgements
We thank Dr. Ison and Dr. Allen at the University at Rochester for generously sharing the custom software for startle reflex testing. This project is supported by grants from National Institute of Health (DC008685-01) and National Organization for Hearing Research.
List of the abbreviations
- ABR
auditory brainstem response
- AC
auditory cortex
- gap-PPI
gap-induced prepulse inhibition
- MGB
medial geniculate body
- nAChR
nicotinic acetylcholine receptors
- NB-PPI
narrow band noise induced prepulse inhibition
- P8
postnatal day 8
- RMS
root mean square
- s.c.
subcutaneously
- SPL
sound pressure level
- STg
startle response measured in a gap trial
- STnb
startle response measured in a noise-burst trial
- STng
startle response measured in a no-gap trial
- STq
startle response measured in a no-prepulse stimulus trial
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Wei Sun, Email: weisun@buffalo.edu.
Anna Hansen, Email: aghansen@buffalo.edu.
Liyan Zhang, Email: liyanzhang007@hotmail.com.
Jianzhong Lu, Email: jlu6@buffalo.edu.
Daniel Stolzberg, Email: djs32@buffalo.edu.
Kari Suzanne Kraus, Email: skkraus@buffalo.edu.
REFERENCES
- Abreu-Villaca Y, Seidler FJ, Tate CA, Cousins MM, Slotkin TA. Prenatal nicotine exposure alters the response to nicotine administration in adolescence: effects on cholinergic systems during exposure and withdrawal. Neuropsychopharmacology. 2004;29:879–890. doi: 10.1038/sj.npp.1300401. [DOI] [PubMed] [Google Scholar]
- Bartlett EL, Wang X. Neural representations of temporally modulated signals in the auditory thalamus of awake primates. J Neurophysiol. 2007;97:1005–1017. doi: 10.1152/jn.00593.2006. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- Bowen GP, Lin D, Taylor MK, Ison JR. Auditory cortex lesions in the rat impair both temporal acuity and noise increment thresholds, revealing a common neural substrate. Cereb Cortex. 2003;13:815–822. doi: 10.1093/cercor/13.8.815. [DOI] [PubMed] [Google Scholar]
- Chang EF, Merzenich MM. Environmental noise retards auditory cortical development. Science. 2003;300:498–502. doi: 10.1126/science.1082163. [DOI] [PubMed] [Google Scholar]
- Chang EF, Bao S, Imaizumi K, Schreiner CE, Merzenich MM. Development of spectral and temporal response selectivity in the auditory cortex. Proc Natl Acad Sci U S A. 2005;102:16460–16465. doi: 10.1073/pnas.0508239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke JE, Zhang H, Kelly JB. Detection of sinusoidal amplitude modulated sounds: deficits after bilateral lesions of auditory cortex in the rat. Hear Res. 2007;231:90–99. doi: 10.1016/j.heares.2007.06.002. [DOI] [PubMed] [Google Scholar]
- de Villers-Sidani E, Chang EF, Bao S, Merzenich MM. Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat. J Neurosci. 2007;27:180–189. doi: 10.1523/JNEUROSCI.3227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Res C Embryo Today. 2008;84:30–44. doi: 10.1002/bdrc.20118. [DOI] [PubMed] [Google Scholar]
- Faden VB, Graubard BI. Maternal substance use during pregnancy and developmental outcome at age three. J Subst Abuse. 2000;12:329–340. doi: 10.1016/s0899-3289(01)00052-9. [DOI] [PubMed] [Google Scholar]
- Friedman JT, Peiffer AM, Clark MG, Benasich AA, Fitch RH. Age and experience-related improvements in gap detection in the rat. Brain Res Dev Brain Res. 2004;152:83–91. doi: 10.1016/j.devbrainres.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Fu QJ, Galvin JJ, 3rd, Wang X. Recognition of time-distorted sentences by normal-hearing and cochlear-implant listeners. J Acoust Soc Am. 2001;109:379–384. doi: 10.1121/1.1327578. [DOI] [PubMed] [Google Scholar]
- Ison JR, Castro J, Allen P, Virag TM, Walton JP. The relative detectability for mice of gaps having different ramp durations at their onset and offset boundaries. J Acoust Soc Am. 2002;112:740–747. doi: 10.1121/1.1490352. [DOI] [PubMed] [Google Scholar]
- Johnson KL, Nicol TG, Zecker SG, Kraus N. Auditory brainstem correlates of perceptual timing deficits. J Cogn Neurosci. 2007;19:376–385. doi: 10.1162/jocn.2007.19.3.376. [DOI] [PubMed] [Google Scholar]
- Karbe H, Szelies B, Herholz K, Heiss WD. Impairment of language is related to left parieto-temporal glucose metabolism in aphasic stroke patients. J Neurol. 1990;237:19–23. doi: 10.1007/BF00319662. [DOI] [PubMed] [Google Scholar]
- Key AP, Ferguson M, Molfese DL, Peach K, Lehman C, Molfese VJ. Smoking during pregnancy affects speech-processing ability in newborn infants. Environ Health Perspect. 2007;115:623–629. doi: 10.1289/ehp.9521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K, Poytress BS, Chen Y, Leslie FM, Weinberger NM, Metherate R. Neonatal nicotine exposure impairs nicotinic enhancement of central auditory processing and auditory learning in adult rats. Eur J Neurosci. 2006;24:857–866. doi: 10.1111/j.1460-9568.2006.04945.x. [DOI] [PubMed] [Google Scholar]
- Metherate R, Kaur S, Kawai H, Lazar R, Liang K, Rose HJ. Spectral integration in auditory cortex: mechanisms and modulation. Hear Res. 2005;206:146–158. doi: 10.1016/j.heares.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Moore MJ, Caspary DM. Strychnine blocks binaural inhibition in lateral superior olivary neurons. J Neurosci. 1983;3:237–242. doi: 10.1523/JNEUROSCI.03-01-00237.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas JG, Geers AE. Effects of early auditory experience on the spoken language of deaf children at 3 years of age. Ear Hear. 2006;27:286–298. doi: 10.1097/01.aud.0000215973.76912.c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oncken CA, Kranzler HR. Pharmacotherapies to enhance smoking cessation during pregnancy. Drug Alcohol Rev. 2003;22:191–202. doi: 10.1080/09595230100100633. [DOI] [PubMed] [Google Scholar]
- Purcell DW, John SM, Schneider BA, Picton TW. Human temporal auditory acuity as assessed by envelope following responses. J Acoust Soc Am. 2004;116:3581–3593. doi: 10.1121/1.1798354. [DOI] [PubMed] [Google Scholar]
- Rosati G, De Bastiani P, Paolino E, Prosser S, Arslan E, Artioli M. Clinical and audiological findings in a case of auditory agnosia. J Neurol. 1982;227:21–27. doi: 10.1007/BF00313543. [DOI] [PubMed] [Google Scholar]
- Saygin AP, Dick F, Wilson SM, Dronkers NF, Bates E. Neural resources for processing language and environmental sounds: evidence from aphasia. Brain. 2003;126:928–945. doi: 10.1093/brain/awg082. [DOI] [PubMed] [Google Scholar]
- Schmajuk NA, Larrauri JA. Neural network model of prepulse inhibition. Behav Neurosci. 2005;119:1546–1562. doi: 10.1037/0735-7044.119.6.1546. [DOI] [PubMed] [Google Scholar]
- Siveke I, Ewert SD, Grothe B, Wiegrebe L. Psychophysical and physiological evidence for fast binaural processing. J Neurosci. 2008;28:2043–2052. doi: 10.1523/JNEUROSCI.4488-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strouse A, Ashmead DH, Ohde RN, Grantham DW. Temporal processing in the aging auditory system. J Acoust Soc Am. 1998;104:2385–2399. doi: 10.1121/1.423748. [DOI] [PubMed] [Google Scholar]
- Trammer RM, Aust G, Koster K, Obladen M. Narcotic and nicotine effects on the neonatal auditory system. Acta Paediatr. 1992;81:962–965. doi: 10.1111/j.1651-2227.1992.tb12154.x. [DOI] [PubMed] [Google Scholar]
- Trehub SE, Henderson JL. Temporal resolution in infancy and subsequent language development. J Speech Hear Res. 1996;39:1315–1320. doi: 10.1044/jshr.3906.1315. [DOI] [PubMed] [Google Scholar]
- Wallace MN, Anderson LA, Palmer AR. Phase-locked responses to pure tones in the auditory thalamus. J Neurophysiol. 2007;98:1941–1952. doi: 10.1152/jn.00697.2007. [DOI] [PubMed] [Google Scholar]
- Walton JP, Frisina RD, Ison JR, O'Neill WE. Neural correlates of behavioral gap detection in the inferior colliculus of the young CBA mouse. J Comp Physiol [A] 1997;181:161–176. doi: 10.1007/s003590050103. [DOI] [PubMed] [Google Scholar]
- Willott JF, Carlson S, Chen H. Prepulse inhibition of the startle response in mice: relationship to hearing loss and auditory system plasticity. Behav Neurosci. 1994;108:703–713. doi: 10.1037//0735-7044.108.4.703. [DOI] [PubMed] [Google Scholar]
- Wittmann M, Burtscher A, Fries W, von Steinbuchel N. Effects of brain-lesion size and location on temporal-order judgment. Neuroreport. 2004;15:2401–2405. doi: 10.1097/00001756-200410250-00020. [DOI] [PubMed] [Google Scholar]
- Yang G, Lobarinas E, Zhang L, Turner J, Stolzberg D, Salvi R, Sun W. Salicylate induced tinnitus: Behavioral measures and neural activity in auditory cortex of awake rats. Hear Res. 2007;226:244–253. doi: 10.1016/j.heares.2006.06.013. [DOI] [PubMed] [Google Scholar]