Abstract
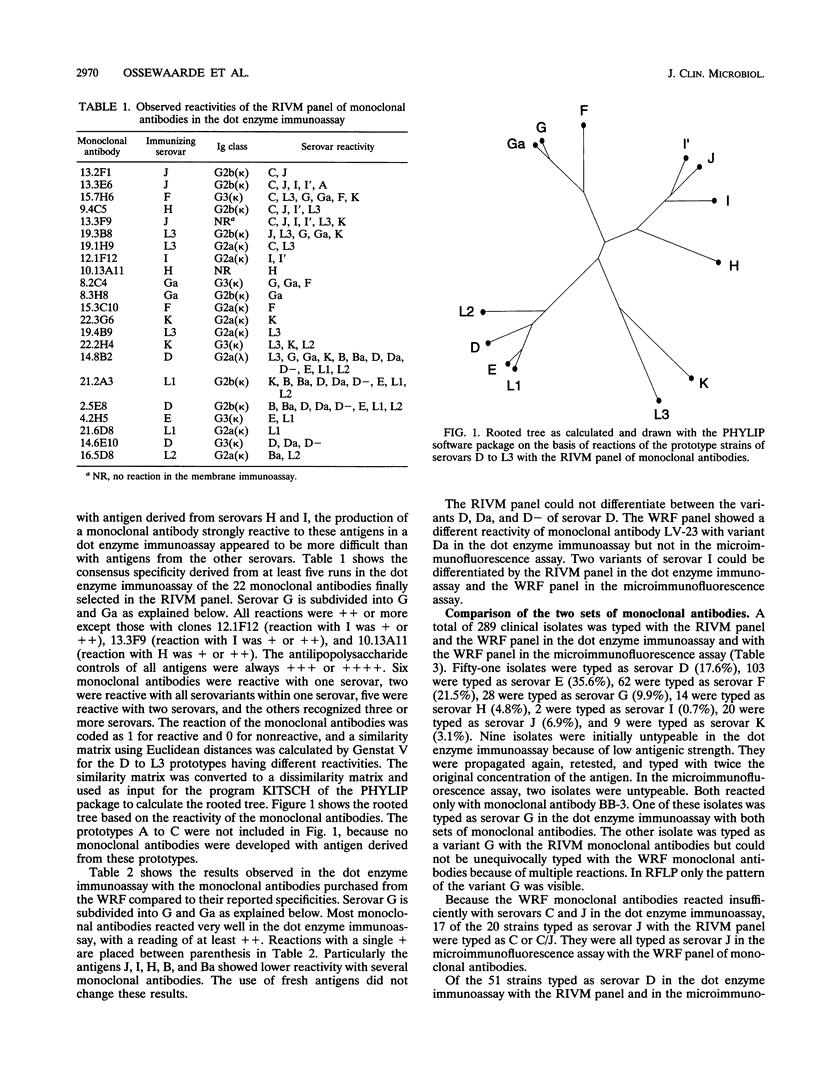
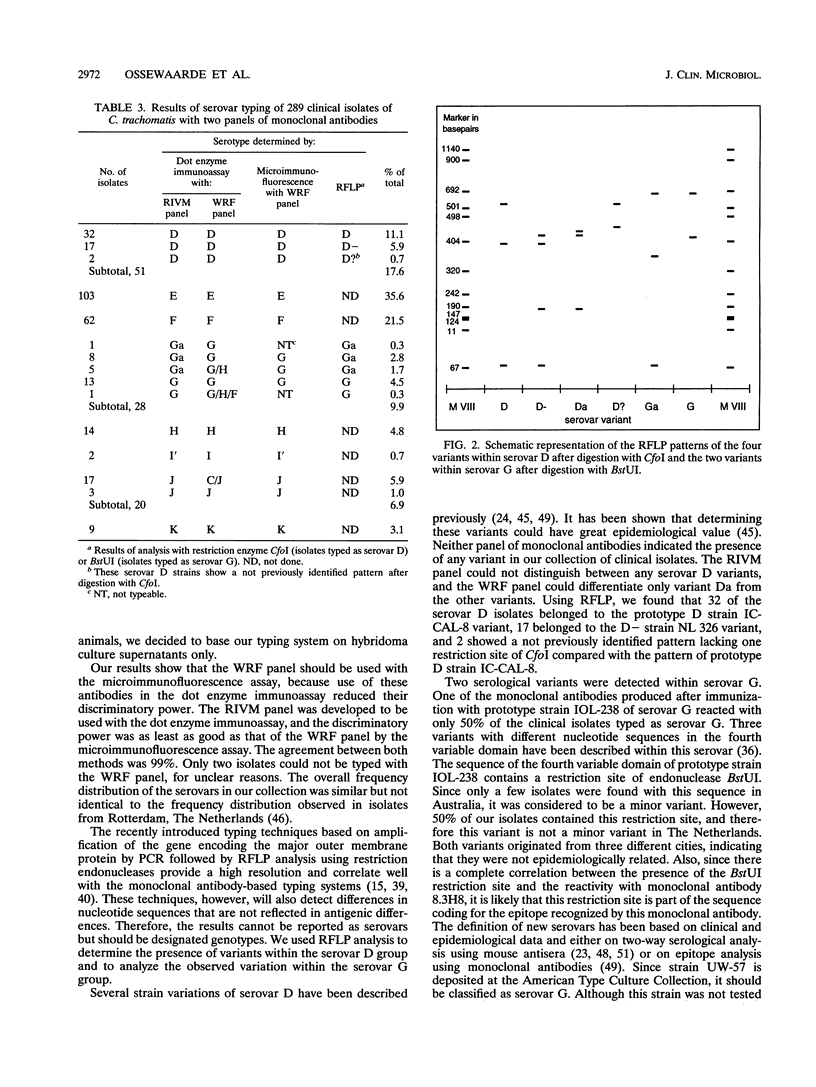
A panel of monoclonal antibodies was developed for serovar typing of clinical isolates of Chlamydia trachomatis. The panel could distinguish all 15 established serovars from one another, although the hybridomas of the panel were developed by fusions of myeloma cells and spleen cells from mice immunized with antigen derived from the urogenital serovars D to L3. The typing assay was based on a dot enzyme immunoassay, and the monoclonal antibodies that were included in the panel reacted strongly in this assay. A collection of 289 clinical isolates from The Netherlands was typed. The observed serovar frequency distribution was 51 isolates of serovar D (17.6%), 103 isolates of serovar E (35.6%), 62 isolates of serovar F (21.5%), 28 isolates of serovar G (9.9%), 14 isolates of serovar H (4.8%), 2 isolates of serovar I' (0.7%), 20 isolates of serovar J (6.9%), and 9 isolates of serovar K (3.1%). These results were confirmed by typing these isolates with a panel of monoclonal antibodies purchased from the Washington Research Foundation, Seattle. No strain variation was observed within serovar D with both panels. However, restriction fragment length polymorphism analysis of the gene encoding the major outer membrane protein showed that 32 isolates were similar to the prototype D and 17 were similar to the variant D-. The two others showed a new restriction pattern. Our panel of monoclonal antibodies contained one monoclonal antibody that divided the serovar G isolates into two groups. This differentiation was confirmed by restriction fragment length polymorphism analysis, confining this difference to a known sequence variation in variable domain IV. These data support the subdivision of serovar G into serovars G (prototype strain UW-57) and Ga (prototype strain IOL-238).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- BEDSON S. P., BARWELL C. F. The laboratory diagnosis of lymphogranuloma venereum. J Clin Pathol. 1949 Nov;2(4):241–249. doi: 10.1136/jcp.2.4.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELL S. D., Jr, SNYDER J. C., MURRAY E. S. Immunization of mice against toxic doses of homologous elementary bodies of trachoma. Science. 1959 Sep 11;130(3376):626–627. doi: 10.1126/science.130.3376.626. [DOI] [PubMed] [Google Scholar]
- BELL S. D., Jr, THEOBALD B. Differentiation of trachoma strains on the basis of immunization against toxic death of mice. Ann N Y Acad Sci. 1962 Mar 5;98:337–346. doi: 10.1111/j.1749-6632.1962.tb30556.x. [DOI] [PubMed] [Google Scholar]
- Barnes R. C., Rompalo A. M., Stamm W. E. Comparison of Chlamydia trachomatis serovars causing rectal and cervical infections. J Infect Dis. 1987 Dec;156(6):953–958. doi: 10.1093/infdis/156.6.953. [DOI] [PubMed] [Google Scholar]
- Barnes R. C., Wang S. P., Kuo C. C., Stamm W. E. Rapid immunotyping of Chlamydia trachomatis with monoclonal antibodies in a solid-phase enzyme immunoassay. J Clin Microbiol. 1985 Oct;22(4):609–613. doi: 10.1128/jcm.22.4.609-613.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batteiger B. E., Lennington W., Newhall W. J., Katz B. P., Morrison H. T., Jones R. B. Correlation of infecting serovar and local inflammation in genital chlamydial infections. J Infect Dis. 1989 Aug;160(2):332–336. doi: 10.1093/infdis/160.2.332. [DOI] [PubMed] [Google Scholar]
- CHANG I., WANG S., GRAYSTON J. T. Antigenic relationshkps of trachoma virus strains in the mouse toxicity prevention test. Ann N Y Acad Sci. 1962 Mar 5;98:347–351. doi: 10.1111/j.1749-6632.1962.tb30557.x. [DOI] [PubMed] [Google Scholar]
- Carter M. W., al-Mahdawi S. A., Giles I. G., Treharne J. D., Ward M. E., Clark I. N. Nucleotide sequence and taxonomic value of the major outer membrane protein gene of Chlamydia pneumoniae IOL-207. J Gen Microbiol. 1991 Mar;137(3):465–475. doi: 10.1099/00221287-137-3-465. [DOI] [PubMed] [Google Scholar]
- Frost E. H., Deslandes S., Bourgaux-Ramoisy D. Chlamydia trachomatis serovars in 435 urogenital specimens typed by restriction endonuclease analysis of amplified DNA. J Infect Dis. 1993 Aug;168(2):497–501. doi: 10.1093/infdis/168.2.497. [DOI] [PubMed] [Google Scholar]
- Frost E. H., Deslandes S., Veilleux S., Bourgaux-Ramoisy D. Typing Chlamydia trachomatis by detection of restriction fragment length polymorphism in the gene encoding the major outer membrane protein. J Infect Dis. 1991 May;163(5):1103–1107. doi: 10.1093/infdis/163.5.1103. [DOI] [PubMed] [Google Scholar]
- Fukushi H., Hirai K. Proposal of Chlamydia pecorum sp. nov. for Chlamydia strains derived from ruminants. Int J Syst Bacteriol. 1992 Apr;42(2):306–308. doi: 10.1099/00207713-42-2-306. [DOI] [PubMed] [Google Scholar]
- Gaydos C. A., Bobo L., Welsh L., Hook E. W., 3rd, Viscidi R., Quinn T. C. Gene typing of Chlamydia trachomatis by polymerase chain reaction and restriction endonuclease digestion. Sex Transm Dis. 1992 Nov-Dec;19(6):303–308. [PubMed] [Google Scholar]
- Grayston J. T., Wang S. New knowledge of chlamydiae and the diseases they cause. J Infect Dis. 1975 Jul;132(1):87–105. doi: 10.1093/infdis/132.1.87. [DOI] [PubMed] [Google Scholar]
- Hagiwara T., Shiga S., Kojima T., Miyano A., Yamazaki S. [Distribution of serovars of Chlamydia trachomatis isolates in Japan]. Kansenshogaku Zasshi. 1991 Apr;65(4):447–450. doi: 10.11150/kansenshogakuzasshi1970.65.447. [DOI] [PubMed] [Google Scholar]
- Ito J. I., Jr, Lyons J. M., Airo-Brown L. P. Variation in virulence among oculogenital serovars of Chlamydia trachomatis in experimental genital tract infection. Infect Immun. 1990 Jun;58(6):2021–2023. doi: 10.1128/iai.58.6.2021-2023.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltenboeck B., Kousoulas K. G., Storz J. Two-step polymerase chain reactions and restriction endonuclease analyses detect and differentiate ompA DNA of Chlamydia spp. J Clin Microbiol. 1992 May;30(5):1098–1104. doi: 10.1128/jcm.30.5.1098-1104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. C., Wang S. P., Grayston J. T., Alexander E. R. TRIC type K, a new immunologic type of Chlamydia trachomatis. J Immunol. 1974 Aug;113(2):591–596. [PubMed] [Google Scholar]
- Lampe M. F., Suchland R. J., Stamm W. E. Nucleotide sequence of the variable domains within the major outer membrane protein gene from serovariants of Chlamydia trachomatis. Infect Immun. 1993 Jan;61(1):213–219. doi: 10.1128/iai.61.1.213-219.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J., Ossewaarde J. M., Walboomers J. M., Meijer C. J., van den Brule A. J. Improved PCR sensitivity for direct genotyping of Chlamydia trachomatis serovars by using a nested PCR. J Clin Microbiol. 1994 Feb;32(2):528–530. doi: 10.1128/jcm.32.2.528-530.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncan T., Eb F., Orfila J. Monoclonal antibodies in serovar determination of 53 Chlamydia trachomatis isolates from Amiens, France. Res Microbiol. 1990 Jul-Aug;141(6):695–701. doi: 10.1016/0923-2508(90)90063-v. [DOI] [PubMed] [Google Scholar]
- NICHOLS R. L., MCCOMB D. E. SEROLOGIC STRAIN DIFFERENTIATION IN TRACHOMA. J Exp Med. 1964 Oct 1;120:639–654. doi: 10.1084/jem.120.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhall W. J., 5th, Terho P., Wilde C. E., 3rd, Batteiger B. E., Jones R. B. Serovar determination of Chlamydia trachomatis isolates by using type-specific monoclonal antibodies. J Clin Microbiol. 1986 Feb;23(2):333–338. doi: 10.1128/jcm.23.2.333-338.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näher H., Petzoldt D. Chlamydia-trachomatis-Serovars und das klinische Bild der urogenitalen Infektionen. Hautarzt. 1991 May;42(5):298–301. [PubMed] [Google Scholar]
- Ossewaarde J. M., Manten J. W., Hooft H. J., Hekker A. C. An enzyme immunoassay to detect specific antibodies to protein and lipopolysaccharide antigens of Chlamydia trachomatis. J Immunol Methods. 1989 Oct 24;123(2):293–298. doi: 10.1016/0022-1759(89)90233-0. [DOI] [PubMed] [Google Scholar]
- Ossewaarde J. M., Plantema F. H., Rieffe M., Nawrocki R. P., de Vries A., van Loon A. M. Efficacy of single-dose azithromycin versus doxycycline in the treatment of cervical infections caused by Chlamydia trachomatis. Eur J Clin Microbiol Infect Dis. 1992 Aug;11(8):693–697. doi: 10.1007/BF01989972. [DOI] [PubMed] [Google Scholar]
- Ossewaarde J. M., Rieffe M., Rozenberg-Arska M., Ossenkoppele P. M., Nawrocki R. P., van Loon A. M. Development and clinical evaluation of a polymerase chain reaction test for detection of Chlamydia trachomatis. J Clin Microbiol. 1992 Aug;30(8):2122–2128. doi: 10.1128/jcm.30.8.2122-2128.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossewaarde J. M., Rieffe M. Storage conditions of Chlamydia trachomatis antigens. Eur J Clin Microbiol Infect Dis. 1989 Jul;8(7):658–660. doi: 10.1007/BF01968154. [DOI] [PubMed] [Google Scholar]
- Ossewaarde J. M., de Vries A., van den Hoek J. A., van Loon A. M. Enzyme immunoassay with enhanced specificity for detection of antibodies to Chlamydia trachomatis. J Clin Microbiol. 1994 Jun;32(6):1419–1426. doi: 10.1128/jcm.32.6.1419-1426.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson K., Osser S. Lack of evidence of a relationship between genital symptoms, cervicitis and salpingitis and different serovars of Chlamydia trachomatis. Eur J Clin Microbiol Infect Dis. 1993 Mar;12(3):195–199. doi: 10.1007/BF01967111. [DOI] [PubMed] [Google Scholar]
- Poole E., Lamont I. Chlamydia trachomatis serovar differentiation by direct sequence analysis of the variable segment 4 region of the major outer membrane protein gene. Infect Immun. 1992 Mar;60(3):1089–1094. doi: 10.1128/iai.60.3.1089-1094.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez P., Vekris A., de Barbeyrac B., Dutilh B., Bonnet J., Bebear C. Typing of Chlamydia trachomatis by restriction endonuclease analysis of the amplified major outer membrane protein gene. J Clin Microbiol. 1991 Jun;29(6):1132–1136. doi: 10.1128/jcm.29.6.1132-1136.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez P., de Barbeyrac B., Persson K., Dutilh B., Bebear C. Evaluation of molecular typing for epidemiological study of Chlamydia trachomatis genital infections. J Clin Microbiol. 1993 Aug;31(8):2238–2240. doi: 10.1128/jcm.31.8.2238-2240.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayada C., Denamur E., Orfila J., Catalan F., Elion J. Rapid genotyping of the Chlamydia trachomatis major outer membrane protein by the polymerase chain reaction. FEMS Microbiol Lett. 1991 Sep 15;67(1):73–78. doi: 10.1016/0378-1097(91)90447-i. [DOI] [PubMed] [Google Scholar]
- Suchland R. J., Stamm W. E. Simplified microtiter cell culture method for rapid immunotyping of Chlamydia trachomatis. J Clin Microbiol. 1991 Jul;29(7):1333–1338. doi: 10.1128/jcm.29.7.1333-1338.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vretou E., Mentis A., Psarrou E., Tsoumaris L., Conidou G., Spiliopoulou D. Unusual prevalence of the rare serovar Da of Chlamydia trachomatis in Greece detected by monoclonal antibodies. Sex Transm Dis. 1992 Mar-Apr;19(2):78–83. [PubMed] [Google Scholar]
- WANG S. P., GRAYSTON J. T. CLASSIFICATION OF TRACHOMA VIRUS STRAINS BY PROTECTION OF MICE FROM TOXIC DEATH. J Immunol. 1963 Jun;90:849–856. [PubMed] [Google Scholar]
- Wagenvoort J. H., Suchland R. J., Stamm W. E. Serovar distribution of urogenital Chlamydia trachomatis strains in The Netherlands. Genitourin Med. 1988 Jun;64(3):159–161. doi: 10.1136/sti.64.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T., Gale J. L. Three new immunologic types of trachoma-inclusion conjunctivitis organisms. J Immunol. 1973 Mar;110(3):873–879. [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T. Serotyping of Chlamydia trachomatis by indirect fluorescent-antibody staining of inclusions in cell culture with monoclonal antibodies. J Clin Microbiol. 1991 Jul;29(7):1295–1298. doi: 10.1128/jcm.29.7.1295-1298.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. P., Grayston J. T. Three new serovars of Chlamydia trachomatis: Da, Ia, and L2a. J Infect Dis. 1991 Feb;163(2):403–405. doi: 10.1093/infdis/163.2.403. [DOI] [PubMed] [Google Scholar]
- Wang S. P., Kuo C. C., Barnes R. C., Stephens R. S., Grayston J. T. Immunotyping of Chlamydia trachomatis with monoclonal antibodies. J Infect Dis. 1985 Oct;152(4):791–800. doi: 10.1093/infdis/152.4.791. [DOI] [PubMed] [Google Scholar]
- Wang S. P., Kuo C. C., Grayston J. T. A simplified method for immunological typing of trachoma-inclusion conjunctivitis-lymphogranuloma venereum organisms. Infect Immun. 1973 Mar;7(3):356–360. doi: 10.1128/iai.7.3.356-360.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Grayston J. T. Chlamydia trachomatis immunotype J. J Immunol. 1975 Dec;115(6):1711–1716. [PubMed] [Google Scholar]
- Westerwoudt R. J., Naipal A. M., Harrisson C. M. Improved fusion technique. II. Stability and purity of hybrid clones. J Immunol Methods. 1984 Mar 30;68(1-2):89–101. doi: 10.1016/0022-1759(84)90139-x. [DOI] [PubMed] [Google Scholar]
- Workowski K. A., Suchland R. J., Pettinger M. B., Stamm W. E. Association of genital infection with specific Chlamydia trachomatis serovars and race. J Infect Dis. 1992 Dec;166(6):1445–1449. doi: 10.1093/infdis/166.6.1445. [DOI] [PubMed] [Google Scholar]
- de StGroth S. F., Scheidegger D. Production of monoclonal antibodies: strategy and tactics. J Immunol Methods. 1980;35(1-2):1–21. doi: 10.1016/0022-1759(80)90146-5. [DOI] [PubMed] [Google Scholar]
- van den Hoek J. A., van Haastrecht H. J., Fennema J. S., Kint J. A., van Doornum G. J., Coutinho R. A. Vórkomen en risicofactoren van infectie met Chlamydia trachomatis bij bezoekers van een geslachtsziektenpolikliniek in Amsterdam. Ned Tijdschr Geneeskd. 1989 Dec 2;133(48):2392–2396. [PubMed] [Google Scholar]
- van der Pol B. J., Jones R. B. Comparison of immunotyping of Chlamydia trachomatis by indirect fluorescent-antibody staining and radioimmunoassay. J Clin Microbiol. 1992 Apr;30(4):1014–1015. doi: 10.1128/jcm.30.4.1014-1015.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]



