Abstract
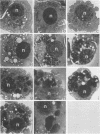
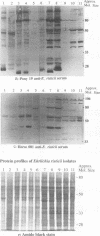
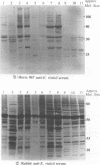
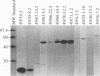
Ehrlichia risticii causes an acute infectious disease in horses called Potomac horse fever. To investigate the biological diversity of E. risticii organisms, nine E. risticii isolates derived from the peripheral blood monocytes of clinically sick horses in Ohio and Kentucky during the summers of 1991 and 1993 were compared with Illinois and Virginia isolates originally obtained from horses in Maryland in 1984. Seven of the nine isolates (081, 606, 380, 679, As, Co, and Ov) formed large morulae (tightly packed inclusions of ehrlichial organisms). The remaining isolates, including 1984 isolates, were individually dispersed or formed small morulae in the cytoplasm of P388D1 cells. In Western blot (immunoblot) analysis with four equine and one rabbit polyclonal anti-E. risticii sera, these recent E. risticii isolates showed patterns of antigenic proteins distinct from those of the 1984 isolates and could be divided into three groups: (i) 081; (ii) 606, 022, 067, 380, and 679; and (iii) As, Co, and Ov. By indirect fluorescent antibody labeling with two panels of murine anti-E. risticii (Illinois and Maryland isolates) monoclonal antibodies, isolate 081 was not labeled with any of 20 monoclonal antibodies tested. The remaining isolates were not labeled with several monoclonal antibodies. The digestion pattern with one of the restriction enzymes, AvaII, of the PCR-amplified partial 16S rRNA gene of E. risticii from all Kentucky isolates (As, Co, and Ov) was different from that of Illinois, Virginia, and six Ohio isolates. These results indicate the presence of distinct variants of E. risticii which vary significantly in morphology, antigenic composition, and the base sequence of the 16S rRNA gene.
Full text
PDF
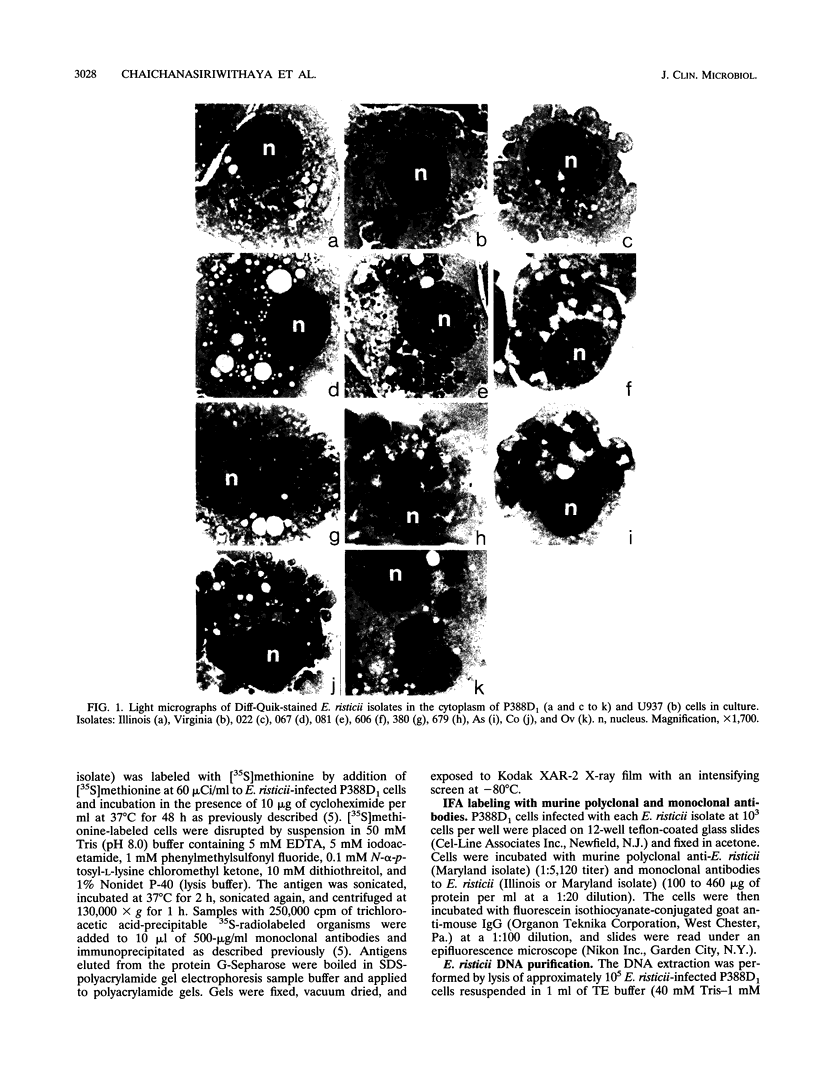
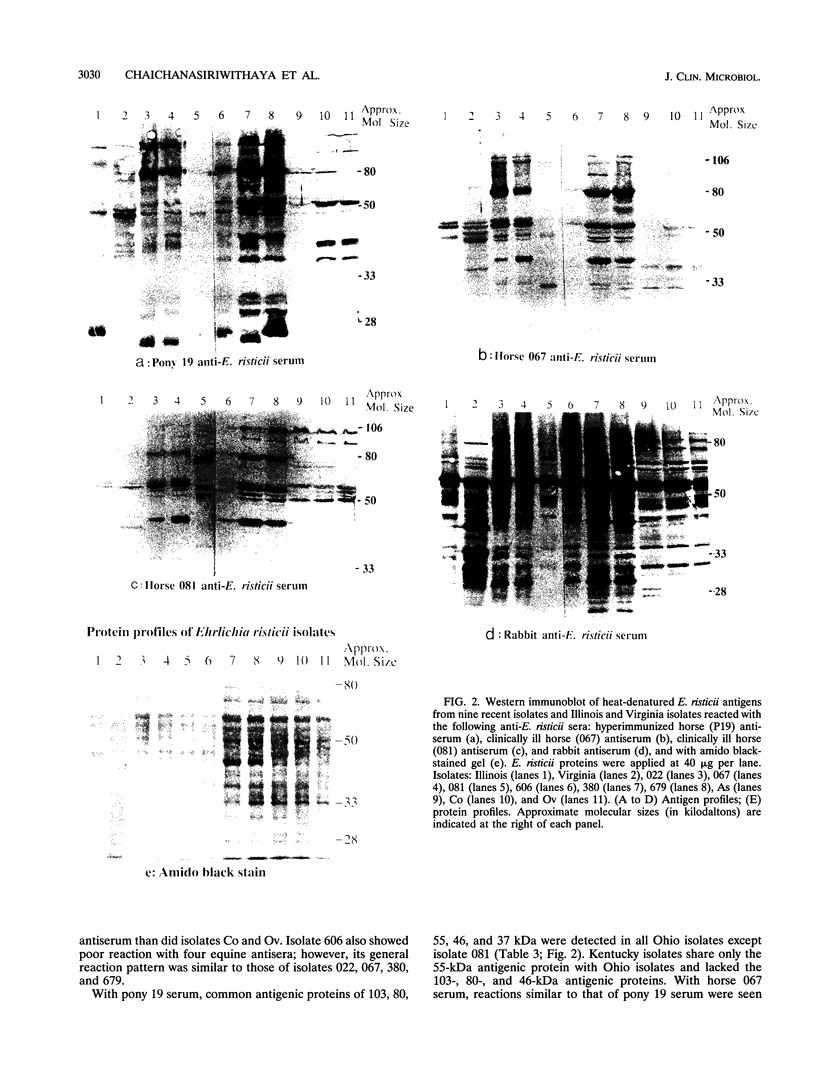
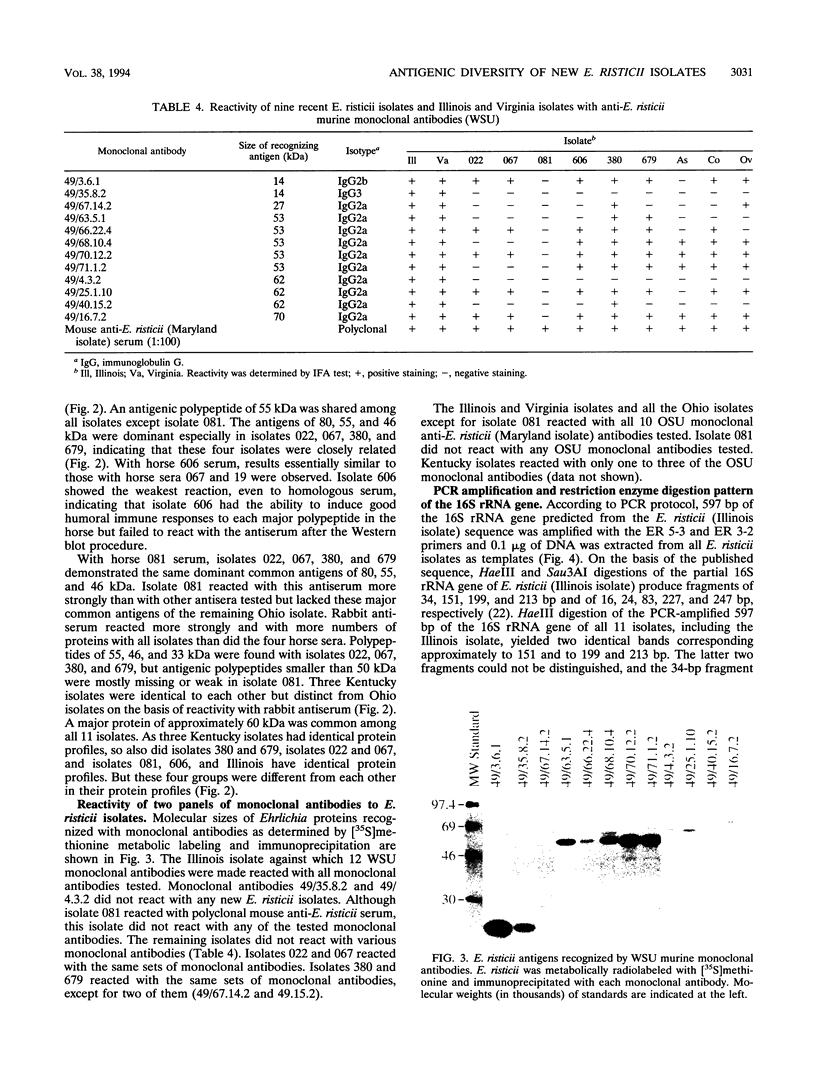
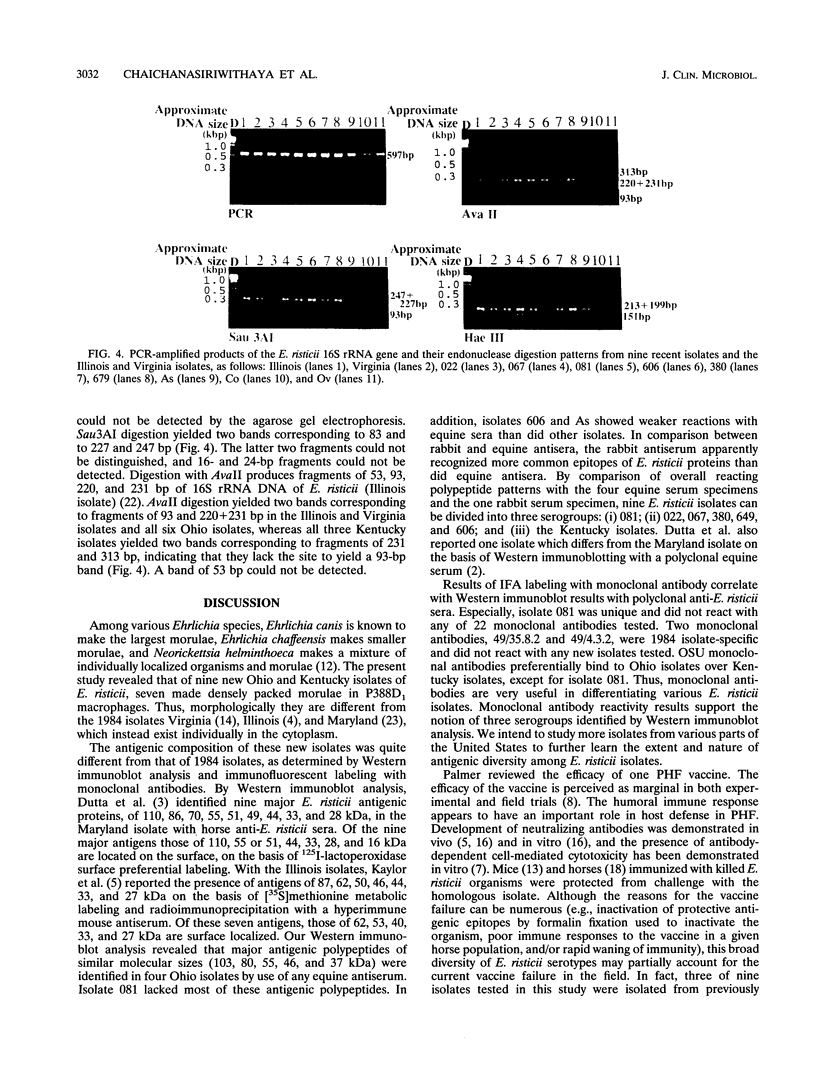

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dutta S. K., Myrup A. C., Rice R. M., Robl M. G., Hammond R. C. Experimental reproduction of Potomac horse fever in horses with a newly isolated Ehrlichia organism. J Clin Microbiol. 1985 Aug;22(2):265–269. doi: 10.1128/jcm.22.2.265-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta S. K., Shankarappa B., Thaker S. R., Mattingly-Napier B. L. DNA restriction endonuclease cleavage pattern and protein antigen profile of Ehrlichia risticii. Vet Microbiol. 1990 Oct;25(1):29–38. doi: 10.1016/0378-1135(90)90090-i. [DOI] [PubMed] [Google Scholar]
- Holland C. J., Ristic M., Cole A. I., Johnson P., Baker G., Goetz T. Isolation, experimental transmission, and characterization of causative agent of Potomac horse fever. Science. 1985 Feb 1;227(4686):522–524. doi: 10.1126/science.3880925. [DOI] [PubMed] [Google Scholar]
- Kaylor P. S., Crawford T. B., McElwain T. F., Palmer G. H. Passive transfer of antibody to Ehrlichia risticii protects mice from ehrlichiosis. Infect Immun. 1991 Jun;59(6):2058–2062. doi: 10.1128/iai.59.6.2058-2062.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messick J. B., Rikihisa Y. Characterization of Ehrlichia risticii binding, internalization, and proliferation in host cells by flow cytometry. Infect Immun. 1993 Sep;61(9):3803–3810. doi: 10.1128/iai.61.9.3803-3810.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J. E. Prevention of Potomac horse fever. Cornell Vet. 1989 Jul;79(3):201–205. [PubMed] [Google Scholar]
- Palmer J. E., Whitlock R. H., Benson C. E. Equine ehrlichial colitis (Potomac horse fever): recognition of the disease in Pennsylvania, New Jersey, New York, Ohio, Idaho, and Connecticut. J Am Vet Med Assoc. 1986 Jul 15;189(2):197–199. [PubMed] [Google Scholar]
- Rikihisa Y. Cross-reacting antigens between Neorickettsia helminthoeca and Ehrlichia species, shown by immunofluorescence and Western immunoblotting. J Clin Microbiol. 1991 Sep;29(9):2024–2029. doi: 10.1128/jcm.29.9.2024-2029.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Perry B. D. Causative ehrlichial organisms in Potomac horse fever. Infect Immun. 1985 Sep;49(3):513–517. doi: 10.1128/iai.49.3.513-517.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y. Protection against murine potomac horse fever by an inactivated Ehrlichia risticii vaccine. Vet Microbiol. 1991 May;27(3-4):339–350. doi: 10.1016/0378-1135(91)90159-d. [DOI] [PubMed] [Google Scholar]
- Rikihisa Y., Reed S. M., Sams R. A., Gordon J. C., Pretzman C. I. Serosurvey of horses with evidence of equine monocytic ehrlichiosis. J Am Vet Med Assoc. 1990 Nov 15;197(10):1327–1332. [PubMed] [Google Scholar]
- Rikihisa Y. The tribe Ehrlichieae and ehrlichial diseases. Clin Microbiol Rev. 1991 Jul;4(3):286–308. doi: 10.1128/cmr.4.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y., Wada R., Reed S. M., Yamamoto S. Development of neutralizing antibody in horses infected with Ehrlichia risticii. Vet Microbiol. 1993 Jul;36(1-2):139–147. doi: 10.1016/0378-1135(93)90135-t. [DOI] [PubMed] [Google Scholar]
- Weisburg W. G., Dobson M. E., Samuel J. E., Dasch G. A., Mallavia L. P., Baca O., Mandelco L., Sechrest J. E., Weiss E., Woese C. R. Phylogenetic diversity of the Rickettsiae. J Bacteriol. 1989 Aug;171(8):4202–4206. doi: 10.1128/jb.171.8.4202-4206.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Dasch G. A., Kang Y. H., Westfall H. N. Substrate utilization by Ehrlichia sennetsu and Ehrlichia risticii separated from host constituents by renografin gradient centrifugation. J Bacteriol. 1988 Nov;170(11):5012–5017. doi: 10.1128/jb.170.11.5012-5017.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]