Abstract
The “waist” area (W) of the parabrachial nucleus contains neurons that receive orosensory input and play a role in the initiation of oromotor behaviors. Immunohistochemical data indicate that neurons in W receive glutamatergic input and express glutamate receptors, but a behavioral role for glutamate neurotransmission within W has not been investigated. To determine the role of specific glutamate receptors in taste reactivity behaviors, glutamate receptor blockers were delivered into W by reverse microdialysis during intraoral infusion of 0.1 M sodium chloride, 0.1 M sucrose, 0.03 M hydrochloric acid, and 0.003 M quinine hydrochloride. Blocking α-amino-3-hydroxy-5-methyl-isoxazolepropionate (AMPA)/kainate ionotropic glutamate receptors in W with 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX) reduced ingestive taste reactivity behaviors to each tastant by 72–85% compared with baseline levels (P’s < 0.05). Blocking N-methyl-D-aspartate receptors as well as type 1 and group III metabotropic glutamate receptors had minor effects on taste reactivity responses to the tastants. These data provide strong evidence for a behavioral role of glutamatergic neurotransmission in W in conscious rats.
Keywords: AMPA receptors, gustation, orosensory, pons, reverse microdialysis
Introduction
The parabrachial nucleus (PBN) is a dorsal pontine structure composed of discrete regions that process orosensory, viscerosensory, cardiovascular, and respiratory information (Norgren and Pfaffmann 1975; Fulwiler and Saper 1984; Jhamandas et al. 1991; Baird et al. 2001; Dutschmann et al. 2004). Neurons responsive to orosensation are concentrated in the “waist” area (W) of the PBN that includes the central medial and ventral lateral subnuclei as well as neurons that bridge the brachium conjunctivum (Norgren and Pfaffmann 1975; Fulwiler and Saper 1984; Halsell and Frank 1991; Halsell and Travers 1997). In rodents, the PBN is a relay for most of the ascending orosensory fibers originating within the rostral nucleus of the solitary tract (NST; Norgren and Leonard 1973; Norgren 1978; Travers 1988; Herbert et al. 1990; Karimnamazi et al. 2002). From the PBN, ascending orosensory pathways proceed to the insular cortex via the thalamus and to ventral forebrain structures including the amygdala and hypothalamus (Norgren 1976; Saper and Loewy 1980; Halsell 1992; Krukoff et al. 1993; Karimnamazi and Travers 1998). The PBN, particularly W, also provides a descending pathway to the NST and the medullary reticular formation (Herbert et al. 1990; Krukoff et al. 1993; Karimnamazi and Travers 1998). Although the anatomy of the connections of the PBN is well understood, the functional roles of these connections are less clear. Suggesting a role for efferent projections of the PBN in the initiation of oromotor behaviors, previous studies have shown that electrical stimulation of W activates neurons in central oromotor structures as identified with fos-immunoreactivity (Morganti et al. 2007) and also elicits oromotor behaviors in conscious rats (Galvin et al. 2004). Together, these data implicate neurons in W and their efferent connections with a role in taste-related behaviors.
Because the projection from the NST to the PBN is partly glutamatergic (Jhamandas and Harris 1992; Saleh et al. 1997; Gill et al. 1999) and PBN neurons express glutamate receptor types (Chamberlin and Saper 1995; Zidichouski et al. 1996; Guthmann and Herbert 1999a, 1999b, 1999c), it is likely that glutamate is involved in the transmission of information within the ascending orosensory pathway in the pons. Specifically, neurons in W express α-amino-3-hydroxy-5-methyl-isoxazolepropionate (AMPA; Chamberlin and Saper 1995; Guthmann and Herbert 1999c) and N-methyl-D-aspartate (NMDA; Guthmann and Herbert 1999b) ionotropic as well as some (particularly group I) metabotropic glutamate receptors (Guthmann and Herbert 1999a). Of particular relevance to the suggestion that glutamate and its receptors play a behavioral role is the finding that microinjection of glutamate into W, but not other PBN subnuclei, elicits oromotor behaviors in conscious rats (Galvin et al. 2004).
Although the previous behavioral studies implicate glutamate in the PBN in the control of oromotor behaviors, the possible role of glutamatergic neurotransmission in the PBN in taste reactivity responses to orostimulation has not been addressed. Therefore, the goals of the current investigation were to demonstrate that glutamate neurotransmission in W is necessary for normal oromotor responses to taste input and to identify the glutamate receptor types within W that play a role in these behaviors. These goals were addressed using the taste reactivity test (Grill and Norgren 1978; Spector et al. 1988; Berridge 2000) to assess the effects of blocking glutamate receptors in W on the immediate behavioral responses to taste stimulation in conscious rats. Due to the prevalence of ionotropic and group I metabotropic glutamate receptors in W and the general excitatory effects of glutamate, it was hypothesized that blockade of these receptor types would reduce the number of taste reactivity behaviors performed during intraoral infusion of taste solutions in conscious rats.
Materials and methods
Animals
Data from 35 adult male Wistar rats (Hilltop Lab Animals, Scottdale, PA) are reported. All rats were housed individually in hanging stainless steel cages, exposed to a 12:12-h light:dark cycle, and given free access to water and block rodent food (L/M Animal Farms, Pleasant Plain, OH). At the time of surgery, rats weighed between 264 and 350 g (mean = 323 g). All experimental manipulations were approved by the Stetson University Animal Use and Care Committee.
Surgical techniques
Guide cannulas, microdialysis probes, and associated materials were purchased from CMA/Microdialysis (North Chelmsford, MA). To administer glutamate receptor blockers into the waist area of the PBN (W), guide cannulas (0.38–0.64 mm diameter) were implanted bilaterally just dorsal to W in all 35 rats. Prior to surgery, rats were anesthetized with sodium pentobarbital (60 mg/kg intraperitoneally [i.p.]) and their scalps shaved and cleaned with a betadine solution. After being placed in a stereotaxic device with nontraumatic ear bars (Stoelting, Wood Dale, IL) and the head held horizontally, a 1–2 cm midline incision was made in the scalp, and 2 small (2.0 mm diameter) burr holes were drilled into the skull over the PBNs. The tip of each guide cannula was placed just onto the surface of the pons using the stereotaxic coordinates of 9.3 mm caudal to bregma, 1.5 mm lateral to the midline, and 6.5 mm ventral to the skull at bregma (Paxinos and Watson 1998). The guide cannulas were secured with small screws embedded into the skull and dental acrylic.
During the same surgical session, intraoral cannulas were implanted bilaterally in all rats (Grill and Norgren 1978; King et al. 1999). The cannulas consisted of approximately 1.0 cm of PE-100 tubing with a Teflon washer threaded onto one end of the tubing that had been heat flanged. One side of the 5.0 mm diameter washer was cut flat so that it could rest against the rats’ gums. The other end of the tubing was connected to a 20-gauge syringe needle and inserted through the temporal muscle just anterolateral to the first maxillary molar and brought up the side of the skull, under the skin, to exit at the incision on the top of the head. On the top of the skull, the PE-100 tubing was cut and connected to about 1.5 cm of 19-gauge stainless steel tubing. The PE-100 tubing was tied to the steel tubing with suture and then affixed to the skull (just anterior to the guide cannula) with dental acrylic. Following surgery, a topical antibiotic was applied, the wounds sutured shut, and each animal placed back into its home cage after a brief recovery on a heated pad.
Reverse microdialysis of glutamate receptor blockers
Following surgery, the rats were allowed to recover for 3 days. After the recovery period, the rats were habituated to the behavioral arena by placing them in the arena for an hour on at least 2 days before behavioral studies began. The behavioral arena, an opaque plastic cylinder that had a diameter of 26 cm and was 26 cm tall, was placed on top of a glass table with a mirror mounted below it to allow videotaping of taste reactivity behaviors.
On the day of the experiment, 2 CMA/11 microdialysis probes were connected to 1.0 ml glass syringes with fluorinated ethylene propylene tubing (0.12 mm inner diameter). The syringes were filled with sterile dH2O, placed in a CMA/102 microdialysis pump, and sterile dH2O was pumped through the probes at 10 μl/min for 15 min. During this time, the probes (cuprophane membrane, 0.24 mm diameter, 6000 daltons cut off) were in 70% ethanol. After the 15-min period, the flow of dH2O was stopped, the probes placed in sterile dH2O, and the syringes filled with sterile CNS perfusion fluid that contained (in mmol/l) 147.0 NaCl, 2.7 KCl, 1.2 CaCl2, and 0.9 MgCl2. After pumping the perfusion fluid through the probes for 1 min at 10 μl/min, the flow was stopped, and the probes were inserted through the guide cannulas into the PBN. At this time, the flow of CNS perfusion fluid through the probes was restarted at 1.0 μl/min, PE-100 tubing was connected to one of the intraoral cannulas, and the rat was placed into the behavioral arena.
The subsequent experimental procedure took over 3 h. CNS perfusion fluid was pumped through the probes at 1.0 μl/min for 1 h before the infusion of tastants into the oral cavity began as well as throughout the taste reactivity procedure. Each tastant (0.1 M NaCl, 0.1 M sucrose, 0.03 M HCl, and 0.003 M QHCl) was infused through the intraoral cannula for 1 min at 0.233 ml/min using a syringe pump (Harvard Apparatus, Holliston, MA), while taste reactivity behaviors were videotaped using S-VHS equipment (Panasonic AW-E300 convertible camera and Sony SLV-R1000 video cassette recorder). Water rinses (30 s) and a 5-min rest period were given between tastants. Behavioral responses to tastants during this period were used as baseline (saline infusion control).
After the first series of intraoral infusions, a glutamate receptor blocker (Tocris Bioscience, St Louis, MO) was delivered into W bilaterally through the microdialysis probe for 1 h prior to and then during infusion of tastants and videotaping a taste reactivity responses. Each blocker was diluted in the CNS perfusion fluid at a final concentration of 10 mM. The only blocker that was not readily water soluble was kynurenic acid (KYN, an ionotropic receptor blocker, n = 4) that had to be dissolved first in a few drops of 1 M NaOH. In this case, the final pH of the microdialysis solution was adjusted to 7.0 with 1 M HCl. The other blockers used were 6-cyano-7-nitroquinoxaline-2,3-dione disodium (CNQX, AMPA/kainate receptor blocker, n = 10), DL-2-amino-5-phosphonopentanoic acid (AP5, NMDA receptor blocker, n = 7), α-methyl-4-carboxyphenylglycine (MCPG, group I and II metabotropic receptor blocker, n = 4), 6-amino-N-cyclohexyl-N,3-dimethylthiazolo[3,2-a]benzimidazole-2-carboxamide hydrochloride (YM 298198, mGluR1 blocker, n = 6), and α-methylserine-O-phosphate (MSOP, group III metabotropic receptor blocker, n = 4).
During the final phase of the experiment, CNS perfusion fluid was run through the probe for 1 h before and during videotaping the taste reactivity responses to each tastant again. Therefore, during the more than 3-h procedure, each tastant was infused into the oral cavity 3 times, for 1 min each time, before, during, and after specific glutamate receptors in the PBN were blocked with receptor antagonists. CNS perfusion fluid was pumped through the probes during the “before” and “after” periods to assess baseline and recovery levels of behavior.
Histology
At the end of the experiment, each rat was overdosed with sodium pentobarbital (80 mg/kg i.p.) and perfused intracardially with 150 ml of 0.1 M phosphate-buffered saline followed by 500 ml of 4% phosphate-buffered paraformaldehyde. The brains were removed, postfixed for 1 h, and then soaked in 30% sucrose (w/v) for 3–5 days at 4 °C. The pons was sectioned at 40 μm using a freezing microtome, and the sections mounted on gelatin and chrome alum subbed slides and allowed to dry overnight. Sections were Nissl stained with 0.1% thionin, dehydrated in alcohols, and cleared in xylenes. Coverslips were secured with Permount (Fisher Scientific, Pittsburgh, PA).
The location of the microdialysis probes was determined by viewing brain sections with a Zeiss Axioskop microscope (Carl Zeiss, Thornwood, NY) equipped with a CCD camera (Hamamatsu Corporation, Bridgewater, NH). Images of Nissl-stained sections that included the PBN and probe sites were captured onto a Dell computer using Zeiss Image Analysis software, and W was delineated within the caudal PBN as previously described (Fulwiler and Saper 1984).
Data analysis
An investigator, who was unaware of the tape sequence being analyzed, conducted a frame-by-frame analysis of videotape during each 1 min intraoral infusion of tastant. Videotaped oromotor behaviors were analyzed as previously described (Grill and Norgren 1978; Spector et al. 1988). Ingestive oromotor behaviors scored included tongue protrusions, lip flares, mouth movements, and paw licks; the aversive behaviors included gapes, chin rubs, forelimb flails, and head shakes. The number, type, and timing of each oromotor behavior were recorded. Total ingestive or aversive scores reflect the sum of the occurrences of each individual oromotor behavior.
To be included in the analysis of the effects of glutamate receptor blockers, histological evidence had to indicate that the microdialysis probe was in W on at least one side of the brain and within at least 100 μm of W on the other side. Statistical analysis of the behavioral effects of blocking glutamate receptors was accomplished using a single-factor analysis of variance (ANOVA) on the total number of ingestive or aversive taste reactivity behaviors performed before, during, and after introduction of a specific glutamate receptor blocker. If the ANOVA revealed a significant difference (P < 0.05), post hoc Student's t-tests were used to determine differences between time periods. Because in 6 of the 10 rats in the CNQX group, the probes were more than 100 μm from W, the importance of the location of the probe was assessed by comparing the total number of ingestive and aversive behaviors performed to each tastant in these subgroups of rats using a Student's t-test. Data from the few rats in other groups that had misplaced probes were not included in the analysis.
Results
Effects of blocking ionotropic glutamate receptors
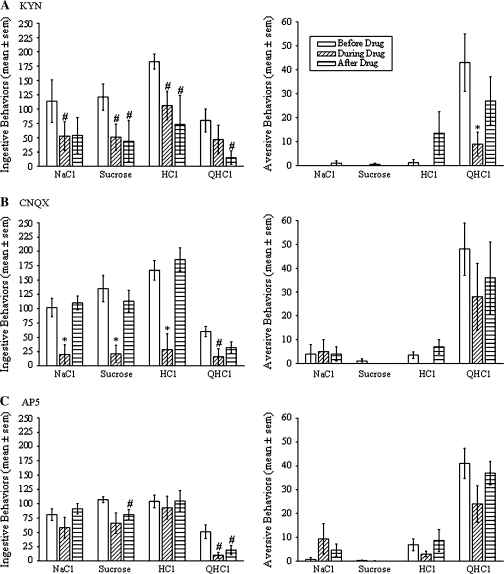
Ionotropic glutamate receptor blockers were delivered bilaterally into W by reverse microdialysis. Data on the effects of the blockers on taste reactivity behaviors are derived from rats in which the microdialysis probe was in W on one side of the brain and within at least 100 μm of W on the other side (Figure 1). Simultaneously, blocking AMPA/kainate and NMDA receptors with KYN significantly reduced taste reactivity behaviors in conscious rats (Figure 2A, n = 4). Specifically, KYN decreased the total number of ingestive taste reactivity behaviors to each tastant by 34–65% when compared with the baseline period when the probe was infused with CNS perfusion fluid (P’s < 0.05, Student's t-test). All ingestive taste reactivity behaviors (mouth movements, tongue protrusions, and lip flares) were reduced by KYN (P’s < 0.05). KYN also reduced the total number of aversive taste reactivity behaviors performed during intraoral infusion of QHCl by 80% (P < 0.01). Both gapes and chin rubs were significantly reduced by KYN (P’s < 0.05). Although the aversive responses to QHCl returned to normal levels after KYN was removed from the probe, the number of ingestive behaviors remained suppressed even after 1 h of flushing the probe with CNS perfusion fluid.
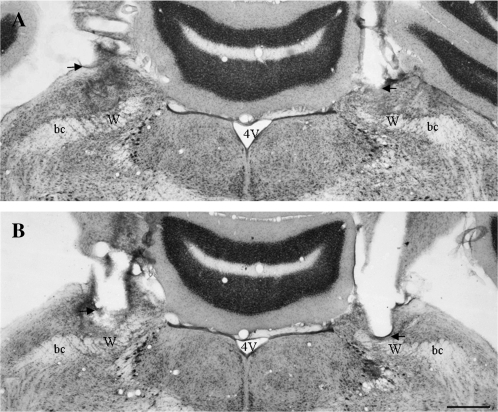
Figure 1.
Images of Nissl-stained coronal brain sections showing the location of probes for reverse microdialysis of glutamate receptor blockers bilaterally into W. The images in (A and B) are from the same rat. The image in (A) is from a section approximately 200 μm caudal to the section shown in (B). Notice that the damage due to the guide cannula ends just dorsal to W (the ventrolateral edge of the guide cannula is indicated with arrows) and that the damage due to the probe extends ventrally into W (indicated by microglial infiltration). Abbreviations are 4V, fourth ventricle; bc, brachium conjunctivum; and W, waist area. Scale bar = 0.5 mm.
Figure 2.
Effects of reverse microdialysis of kynurenic acid (A, KYN, n = 4), 6-cyano-7-nitroquinoxaline-2,3-dione disodium (B, CNQX, n = 4), and DL-2-amino-5-phosphonopentanoic acid (C, AP5, n = 7) bilaterally into W on ingestive (left) and aversive (right) taste reactivity behaviors. Each bar indicates the mean (±standard error of the mean) number of behaviors performed during 1 min of intraoral infusion of 0.1 M NaCl, 0.1 M sucrose, 0.03 M HCl, and 0.003 M QHCl. Each triplet of bars shows the number of behaviors performed before, during, and after reverse microdialysis of the ionotropic receptor blocker into W. Asterisks indicate a significant difference compared with the behaviors performed both before and after delivery of the blocker, and the pound sign indicates a significant difference compared with the behaviors performed before introduction of the blocker (P < 0.05, Student's t-test).
Blocking AMPA/kainate receptors with CNQX also reduced taste reactivity behaviors. Specifically, CNQX significantly reduced the total ingestive taste reactivity behaviors to each tastant by 72–85% compared with the number of behaviors performed prior to drug delivery (Figure 2B, P’s < 0.05, n = 4). Again, this reduction was due to a decrease in the number of each of the types of ingestive behavior (P’s < 0.05). Unlike KYN, CNQX did not significantly alter aversive taste reactivity behaviors to QHCl. On the other hand, blocking NMDA receptors with AP5 had minimal effects on taste reactivity behaviors. The only significant effect of AP5 was a 79% reduction in the total number of ingestive behaviors during intraoral infusion of QHCl (Figure 2C, P < 0.05, n = 7). The 23% decrease in the number of aversive behaviors to QHCl caused by delivery of AP5 into W was not statistically significant.
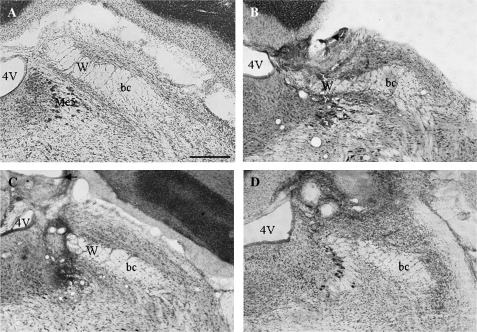
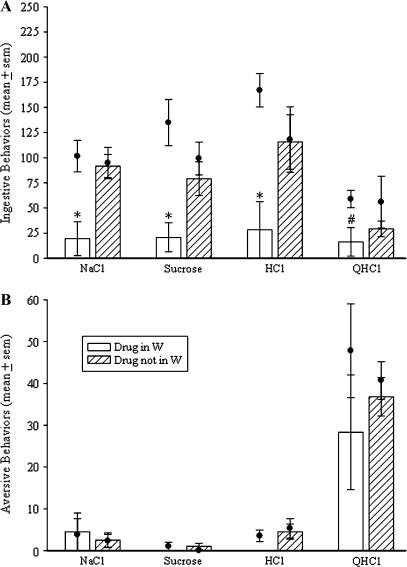
Delivery of CNQX into the PBN but outside of W did not alter taste reactivity responses (Figures 3 and 4) demonstrating that the behavioral effects of this drug were site specific. Specifically, rats in which CNQX was delivered into W (n = 4) performed significantly fewer ingestive behaviors to NaCl, sucrose, and HCl than rats in which CNQX was delivered medial, dorsal, lateral, or rostral to W (Figure 4, P < 0.05, n = 6). The ingestive and aversive responses to QHCl were not significantly different between these 2 groups of rats (Figure 4).
Figure 3.
Images of Nissl-stained coronal sections of the right PBN showing the location of the microdialysis probe used to deliver CNQX in 3 rats. (A) Image showing normal PBN histology including the waist region (W). (B) Image showing the location of a probe that delivered CNQX to W (the ventrolateral edge of the guide cannula is indicated with arrows, and the probe location is indicated by the site of microglial infiltration). (C) Image showing the location of a probe that delivered CNQX medial to W. (D) Image showing the location of a probe that delivered drug dorsal and rostral to W. Abbreviations are 4V, fourth ventricle; bc, brachium conjunctivum; and W, waist area. Scale bar = 0.5 mm.
Figure 4.
Effects of reverse microdialysis of CNQX into W (n = 4) compared with the effects of the delivery of CNQX within the PBN but outside of W (n = 6) on ingestive (A) and aversive (B) taste reactivity behaviors. Each bar indicates the mean (±standard error of the mean [SEM]) number of behaviors performed during 1 min of intraoral infusion of 0.1 M NaCl, 0.1 M sucrose, 0.03 M HCl, and 0.003 M QHCl. Each dot indicates the mean (±SEM) number of behaviors performed during 1 min intraoral infusions of the same tastants before drug delivery (baseline). Asterisks indicate a significant difference from baseline as well as from the behaviors performed by the rats in which CNQX was delivered outside of W. The pound sign indicates a significant difference from baseline but not the other group of rats (P < 0.05, Student's t-test).
Effects of blocking metabotropic glutamate receptors
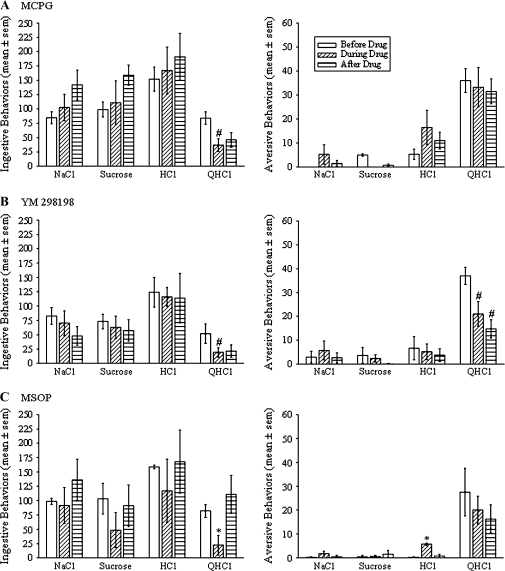
To discern a possible role of metabotropic glutamate receptors in W in taste reactivity behaviors, 3 different metabotropic glutamate receptor antagonists were delivered into W by reverse microdialysis during intraoral infusion of tastants. MCPG, which blocks groups I and II (types 1, 5, 2, and 3) metabotropic glutamate receptors, did not alter the taste reactivity responses to NaCl, sucrose, or HCl. However, MCPG did significantly reduce the total number of ingestive behaviors during intraoral infusion of QHCl by 56% over the number of behaviors performed during the flow of CNS perfusion fluid through the probe prior to drug delivery (Figure 5A, P < 0.05, n = 4). Similarly, only blocking type 1 metabotropic glutamate receptors (mGluR1) with YM 298198 reduced ingestive oromotor behaviors to QHCl (Figure 5B, P < 0.05, n = 6). In addition, YM 298198 reduced the number of aversive behaviors performed during intraoral infusion of QHCl by 44% (P < 0.05, Student’s t-test). Finally, blocking group III (types 4, 6, 7, and 8) metabotropic glutamate receptors with MSOP reduced ingestive behaviors during QHCl presentation and increased aversive behaviors to HCl (Figure 5C, P’s < 0.05, n = 4), with no other statistically significant effects.
Figure 5.
Effects of reverse microdialysis of α-methyl-4-carboxyphenylglycine (A, MCPG, n = 4), 6-amino-N-cyclohexyl-N,3-dimethylthiazolo[3,2-a]benzimidazole-2-carboxamide hydrochloride (B, YM 298198, n = 6), and α-methylserine-O-phosphate (C, MSOP, n = 4) bilaterally into W on ingestive (left) and aversive (right) taste reactivity behaviors. Each bar indicates the mean (±standard error of the mean) number of behaviors performed during 1 min of intraoral infusion of 0.1 M NaCl, 0.1 M sucrose, 0.03 M HCl, and 0.003 M QHCl. Each triplet of bars shows the number of behaviors performed before, during, and after reverse microdialysis of the metabotropic receptor blocker into W. Asterisks indicate a significant difference compared with the behaviors performed both before and after delivery of the blocker, and the pound sign indicates a significant difference compared with the behaviors performed before introduction of the blocker (P < 0.05, Student's t-test).
Discussion
Although neurons in W receive taste input (Norgren and Pfaffmann 1975; Halsell and Frank 1991; Halsell and Travers 1997) and are involved in the initiation of oromotor behaviors (Galvin et al. 2004), the neural processes in W that lead to behavioral responses to taste inputs are not well understood. In particular, the neurotransmitters responsible for activation of neurons in W during taste stimulation remain unidentified. To determine if glutamatergic neurotransmission in W plays a role in the performance of taste reactivity behaviors, glutamate receptor antagonists were delivered into W by reverse microdialysis during intraoral infusion of tastants in conscious rats. The current data provide support for the hypothesis that glutamate is a neurotransmitter in the taste pathway in the pons. AMPA/kainate ionotropic glutamate receptors in W may be particularly important for normal behavioral responses to taste input, whereas NMDA, and type 1, and group III metabotropic glutamate receptors likely play a minor role.
Technical considerations
Before postulating the implications of the data from the current study, some technical considerations should be addressed. Tissue damage caused by the probe, the area of diffusion of the drugs, the dose of the drugs reached within tissue surrounding the probe, and the time course of drug application and removal are topics of concern. Due to the nature of the microdialysis technique, it is impossible to prevent all damage to neural tissue. Our histological results showed that most of the damage was dorsal to W due to the placement of the guide cannula. However, because the microdialysis probe itself did extend into the PBN, we cannot dismiss the possibility that tissue damage caused behavioral changes. Nevertheless, it is unlikely that damage to W influenced behavior because the probe was in place and infused with CNS perfusion fluid for 1 h before baseline behavioral measurements were taken, and the effects of blocking glutamate receptors were assessed by comparison to these baseline periods. Obviously, the distance of drug diffusion from the probe site depends upon the molecular structure of the drug and local tissue properties. Again, to insure that glutamate receptors in W were sufficiently blocked, to be included in the analysis the probes delivering antagonists had to be in W in one PBN and within 100 μm of W on the other side. The site specificity of the effects of CNQX (Figures 3 and 4) suggests that the effective concentration of the drug (at least for CNQX) was reached only relatively close to the probe. Rats in which the probe was misplaced by more than 100 μm from W responded normally to tastants during CNQX delivery. Although each antagonist was used at 10 mM, the concentration reached at neurons near the probe is unknown. Based on preliminary data using KYN 10 mM, it was selected (King et al. 2004) and clearly was effective for several antagonists. However, despite the pharmacological level of this dose, it is possible that other effects of blockers would be apparent at higher doses. The timing of drug application and removal depends upon the delivery technique. During preliminary studies (King et al. 2004; King and Dorné 2007), glutamate receptor blockers were either injected acutely (200–400 nl/10 s) immediately prior to the intraoral infusion of tastants or delivered chronically via osmotic pumps. Using the former approach, it was difficult to demonstrate that the injection successfully delivered the drug to the targeted area and that the drug still was present during the intraoral infusion of tastants that followed. Not surprisingly, the effects of acute injections of glutamate receptor blockers were very variable. Although the chronic delivery of KYN into W very effectively reduced taste reactivity behaviors, this chronic treatment with KYN also caused undesirable side effects (lethargy and motor impairment) that altered the behavior of the rats. The reverse microdialysis technique employed in the current study minimized the technical issues associated with the other delivery techniques by reliably exposing the neurons in W to drug over an hour that allowed for the presentation of the 4 main tastants without behavioral side effects of drug delivery.
A final issue to consider when interpreting the current data relates to the inherent variability in behavioral data. Because each behavioral event (mouth movement, tongue protrusion, gape, etc) was counted as an individual behavior, the total number of behaviors performed in 1 min could be relatively high (over 100). Although the number of behaviors performed during the baseline period, while CNS perfusion fluid without drug was delivered into the PBN, was relatively consistent there were some intra- and intergroup differences. For example, the number of ingestive behaviors performed during the baseline period to intraoral infusion of HCl was significantly lower in the AP5 group than in the CNQX and KYN groups (P’s < 0.05). The lower baseline could mask potential effects of receptor blockade, but because each group of rats served as its own control (receiving each tastant before, during, and after drug delivery) possible influences of the variability in baseline were likely minimal.
Role of glutamate receptors in the PBN in taste reactivity behaviors
PBN neurons, including neurons in W, express several glutamate receptor types (Chamberlin and Saper 1995; Zidichouski et al. 1996; Guthmann and Herbert 1999a, 1999b, 1999c), and therefore, it is assumed that glutamatergic mechanisms play a functional role within this nucleus. In fact, glutamate neurotransmission in the lateral PBN has been shown to be involved in cardiovascular function (Chamberlin and Saper 1992; Saleh et al. 1997; Hayward 2007) and conditioned taste aversion (Vales et al. 2006). However, possible roles of glutamatergic neurotransmission in W in taste-related behaviors were largely unexplored. Data from the current study indicate a role for glutamate and its receptors in W in taste reactivity responses. Delivery of 3 types of ionotropic glutamate receptor antagonists into W by reverse microdialysis in conscious rats suggests that the activation of AMPA/kainate receptors in W is necessary for normal ingestive behavioral responses to taste input. Specifically, blocking AMPA/kainate receptors significantly reduced the ingestive taste reactivity responses to salty, sweet, sour, and bitter tastes. Consistent with a major role for AMPA/kainate receptors, blocking NMDA receptors had fewer effects; however, it is clear that NMDA receptors in W also play a role in taste-related behaviors. NMDA receptors in W seem to be particularly important for both ingestive and aversive behavioral responses to quinine. In fact, the only significant effect of NMDA receptor blockade was on ingestive responses to this bitter tastant (Figure 2C). Although blocking NMDA receptors in W with AP5 did not significantly alter aversive responses to quinine, neither did blockade of AMPA/kainate receptors with CNQX. So, it seems that aversive behaviors to quinine may require the activation of both AMPA/kainate and NMDA receptors within W; an inference supported by the effectiveness of KYN in blocking aversive taste reactivity responses to quinine (Figure 2A).
Several types of metabotropic glutamate receptors have been found within W (Guthmann and Herbert 1999a). Specifically, dendrites in W are strongly immunoreactive for mGluR1, whereas a moderate amount of group II metabotropic receptor labeling is seen in neuropil in W. This distribution of these receptors as well as the fact that group I metabotropic receptors are the main postsynaptic metabotropic glutamate receptor type (Cartmell and Schoepp 2000) suggests that group I receptors may affect neuronal function within W. The current behavioral study supports this view and also suggests a possible role for group II metabotropic receptors. It is interesting that blocking group I and group II metabotropic glutamate receptors simultaneously with MCPG did not reduce aversive responses to Q while specifically blocking mGluR1 with YM 298198 did. Perhaps, the modulatory effects of activating mGluR5 (the other group I receptor type) and group II receptors (mGluR2 and mGluR3) oppose the behavioral effects of mGluR1. The current data also suggest a role for group III metabotropic receptors, which tend to be presynaptic (Cartmell and Schoepp 2000). Overall, the behavioral evidence suggests that type 1 (mGluR1) and group III metabotropic glutamate receptors play a particularly important role in taste reactivity responses to quinine.
Role of neurons in the PBN in taste reactivity behaviors
In rodents, the PBN is a relay for most of the ascending taste pathway (Norgren and Leonard 1973; Norgren 1978; Travers 1988; Herbert et al. 1990; Karimnamazi et al. 2002). However, although taste reactivity behaviors are altered, they are not abolished by bilateral ablation of the gustatory PBN (Spector 1995). Therefore, brainstem circuitry caudal to the PBN (the NST and its local projections) may be sufficient for relatively normal taste reactivity responses to intraoral infusion of tastants. Nevertheless, the PBN is an important site of gustatory and viscerosensory integration (Baird et al. 2001; Karimnamazi et al. 2002), descending projections from the PBN terminate within motor and premotor regions of the medullary reticular formation (Herbert et al. 1990; Krukoff et al. 1993; Karimnamazi and Travers 1998), ascending projections from the PBN reach areas involved in evaluating sensory input (Norgren 1976; Saper and Loewy 1980; Halsell 1992; Krukoff et al. 1993; Karimnamazi and Travers 1998), and activation of neurons in W elicits ingestive oromotor behaviors (Galvin et al. 2004). Therefore, although not necessary for all taste reactivity behaviors, the PBN certainly has an influence on the brainstem circuits that generate these behaviors as well as forebrain structures that influence taste-related activities. The current data support a role for neurons in W in taste reactivity responses by demonstrating the reversible alteration of these behaviors due to the temporary application of glutamate receptor blockers in intact rats.
The taste reactivity test assesses behavioral responses to small quantities of taste stimuli infused into the oral cavity (Grill and Norgren 1978). The results of taste reactivity tests can be interpreted as an indication of potential solution intake, as a measure of reflexive responses to taste input, and as an overall indication of the palatability of the infused substances (Grill and Norgren 1978; Spector et al. 1988; Berridge 2000). The behavioral effects of the glutamate receptor antagonists therefore could be due to a change in the activity of neurons in W that project to the medulla to influence motor output or could be due to a change in the activity of neurons that project to the ventral forebrain where the hedonic value of tastants is assessed. Clearly, both mechanisms could account for the behavioral effects described in the current study.
Anatomical and physiological data suggest that neurons in different PBN subnuclei are involved in behavioral responses to different tastes (Ogawa et al. 1984; Yamamoto et al. 1994; Halsell and Travers 1997; King et al. 2003). The different effects of glutamate receptor blockers on behavioral responses to quinine compared with the other tastants used in the current study also suggest that a distinctive population of PBN neurons influences responses to bitter tastes. Specifically, it appears that both AMPA/kainate and NMDA receptors are necessary for normal taste reactivity responses to quinine, whereas primarily AMPA/kainate receptors are involved in the responses to the other tastants. Furthermore, all but one of the significant effects of metabotropic receptor blockers was on responses to quinine, not the other tastants. In addition to potential differences in the glutamate receptors they express, PBN neurons responsive to quinine may be located more diffusely within the PBN than neurons responsive to sweet and salty stimuli, as suggested by fos-immunohistochemical studies (Yamamoto et al. 1994: Travers et al. 1999). Therefore, the delivery of glutamate receptor blockers into W only may have influenced a small percentage of quinine-responsive neurons. Perhaps, blocker infusions into the external PBN subnuclei would have additional effects on the taste reactivity behaviors to this bitter tastant.
Conclusions
The behavioral effects of intraoral infusion of tastants are at least partly due to the activation of glutamate receptors within W. The activation of AMPA/kainate ionotropic glutamate receptors in W is particularly important for normal taste reactivity responses, whereas NMDA receptors as well as type 1 and group III metabotropic glutamate receptors may play a minor role. Therefore, it is likely that glutamate is one of the neurotransmitters involved in the processing of orosensory information within the PBN.
Funding
National Institutes of Health RO1 grant (DC007854 to M.S.K.); a National Institutes of Health R25 grant (GM071956 to Dr Ram Nayar at Daytona State College to support L.A.W.).
Acknowledgments
We are grateful to the other undergraduate students who contributed to this research: Kristina Campbell, Tricia Dorné, Kate Egan, Gerald Keller, Melanie King, Stephanie May, and Andre Uflacker and to Dr Camille T. King for reviewing previous versions of the manuscript.
References
- Baird JP, Travers JB, Travers SP. Parametric analysis of gastric distension responses in the parabrachial nucleus. Am J Physiol. 2001;281:R1568–R1580. doi: 10.1152/ajpregu.2001.281.5.R1568. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24:173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Saper CB. Topographic organization of cardiovascular responses to electrical and glutamate microstimulation of the parabrachial nucleus in the rat. J Comp Neurol. 1992;326:245–262. doi: 10.1002/cne.903260207. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Saper CB. Differential distribution of AMPA-selective glutamate receptors subunits in the parabrachial nucleus of the rat. Neuroscience. 1995;68:435–443. doi: 10.1016/0306-4522(95)00129-7. [DOI] [PubMed] [Google Scholar]
- Dutschmann M, Mörschel M, Kron M, Herbert H. Development of adaptive behaviour of the respiratory network: implications for the pontine Kolliker-Fuse nucleus. Resp Physiol Neurobiol. 2004;143:155–165. doi: 10.1016/j.resp.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res Rev. 1984;7:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- Galvin KE, King CT, King MS. Stimulation of specific regions of the parabrachial nucleus elicits ingestive oromotor behaviors in conscious rats. Behav Neurosci. 2004;18:163–172. doi: 10.1037/0735-7044.118.1.163. [DOI] [PubMed] [Google Scholar]
- Gill CF, Madden JM, Roberts BP, Evans LD, King MS. A subpopulation of neurons in the rat rostral nucleus of the solitary tract that project to the parabrachial nucleus express glutamate-like immunoreactivity. Brain Res. 1999;821:251–262. doi: 10.1016/s0006-8993(98)01270-0. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Guthmann A, Herbert H. Distribution of metabotropic glutamate receptors in the parabrachial nucleus in the parabrachial and kolliker-fuse nuclei of the rat. Neuroscience. 1999a;89:873–881. doi: 10.1016/s0306-4522(98)00387-x. [DOI] [PubMed] [Google Scholar]
- Guthmann A, Herbert H. Expression of N-methyl-D-aspartate receptor subunits in the rat parabrachial and kolliker-fuse nuclei and in selected pontomedullary brainstem nuclei. J Comp Neurol. 1999b;415:501–517. [PubMed] [Google Scholar]
- Guthmann A, Herbert H. In situ hybridization analysis of flip/flop splice variants of AMPA-type glutamate receptor subunits in the rat parabrachial and kolliker-fuse nuclei. Mol Brain Res. 1999c;74:145–157. doi: 10.1016/s0169-328x(99)00281-8. [DOI] [PubMed] [Google Scholar]
- Halsell CB. Organization of parabrachial nucleus efferents to the thalamus and amygdala in the golden hamster. J Comp Neurol. 1992;317:57–78. doi: 10.1002/cne.903170105. [DOI] [PubMed] [Google Scholar]
- Halsell CB, Frank ME. Mapping study of the parabrachial taste-responsive area for the anterior tongue in the golden hamster. J Comp Neurol. 1991;306:708–722. doi: 10.1002/cne.903060412. [DOI] [PubMed] [Google Scholar]
- Halsell CB, Travers SP. Anterior and posterior oral cavity responsive neurons are differentially distributed among parabrachial subnuclei in rat. J Neurophysiol. 1997;78:920–938. doi: 10.1152/jn.1997.78.2.920. [DOI] [PubMed] [Google Scholar]
- Hayward LF. Midbrain modulation of the cardiac baroreflex involves excitation of lateral parabrachial neurons in the rat. Brain Res. 2007;1145:117–127. doi: 10.1016/j.brainres.2007.01.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert H, Moga MM, Saper CB. Connections of the parabrachial nucleus with the nucleus of the solitary tract and the medullary reticular formation in the rat. J Comp Neurol. 1990;293:540–580. doi: 10.1002/cne.902930404. [DOI] [PubMed] [Google Scholar]
- Jhamandas JH, Aippersbach SE, Harris KH. Cardiovascular influences on rat parabrachial nucleus: an electrophysiological study. Am J Physiol. 1991;260:R225–R231. doi: 10.1152/ajpregu.1991.260.1.R225. [DOI] [PubMed] [Google Scholar]
- Jhamandas JH, Harris KH. Excitatory amino acids may mediate nucleus tractus solitarius input to rat parabrachial neurons. Am J Physiol. 1992;263:R324–R330. doi: 10.1152/ajpregu.1992.263.2.R324. [DOI] [PubMed] [Google Scholar]
- Karimnamazi H, Travers JB. Differential projections from the gustatory responsive parabrachial regions to the medulla and forebrain. Brain Res. 1998;813:283–302. doi: 10.1016/s0006-8993(98)00951-2. [DOI] [PubMed] [Google Scholar]
- Karimnamazi H, Travers SP, Travers JB. Oral and gastric input to the parabrachial nucleus in the rat. Brain Res. 2002;957:193–206. doi: 10.1016/s0006-8993(02)03438-8. [DOI] [PubMed] [Google Scholar]
- King CT, Deyrup LD, Dodson SE, Galvin KE, Garcea M, Spector AC. Effects of gustatory nerve transection and regeneration on quinine-stimulated Fos-like immunoreactivity in the parabrachial nucleus of the rat. J Comp Neurol. 2003;465:296–308. doi: 10.1002/cne.10851. [DOI] [PubMed] [Google Scholar]
- King CT, Travers SP, Rowland NE, Garcea M, Spector AC. Glossopharyngeal nerve transection eliminates quinine-stimulated fos-like immunoreactivity in the nucleus of the solitary tract: implications for a functional topography of gustatory nerve input in rats. J Neurosci. 1999;19:3107–3121. doi: 10.1523/JNEUROSCI.19-08-03107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MS, Dorné TB. Reverse microdialysis of ionotropic glutamate receptor blockers into the parabrachial nucleus reduced taste reactivity behaviors in conscious rats [abstract #266] Sarasota (FL): Association for Chemoreception Sciences; 2007. [Google Scholar]
- King MS, Keller GS, Uflacker AB. Blocking glutamate receptors in the parabrachial nucleus reduces aversive oromotor responses to quinine in conscious rats [abstract #207] Sarasota (FL): Association for Chemoreception Sciences; 2004. [Google Scholar]
- Krukoff TL, Harris KH, Jhamandas JH. Efferent projections from the parabrachial nucleus demonstrated with the anterograde tracer Phaseolus vulgaris leucoagglutinin. Brain Res Bull. 1993;30:163–172. doi: 10.1016/0361-9230(93)90054-f. [DOI] [PubMed] [Google Scholar]
- Morganti JM, Odegard AK, King MS. The number and location of Fos-like immunoreactive neurons in the central gustatory system following electrical stimulation of the parabrachial nucleus in conscious rats. Chem Senses. 2007;32:543–555. doi: 10.1093/chemse/bjm023. [DOI] [PubMed] [Google Scholar]
- Norgren R. Taste pathways to hypothalamus and amygdala. J Comp Neurol. 1976;166:17–30. doi: 10.1002/cne.901660103. [DOI] [PubMed] [Google Scholar]
- Norgren R. Projections from the nucleus of the solitary tract in the rat. Neuroscience. 1978;3:207–218. doi: 10.1016/0306-4522(78)90102-1. [DOI] [PubMed] [Google Scholar]
- Norgren R, Leonard CM. Ascending central gustatory pathways. J Comp Neurol. 1973;150:217–238. doi: 10.1002/cne.901500208. [DOI] [PubMed] [Google Scholar]
- Norgren R, Pfaffmann C. The pontine taste area in the rat. Brain Res. 1975;91:99–117. doi: 10.1016/0006-8993(75)90469-2. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Hayama T, Ito S. Location and taste responses of parabrachio-thalamic relay neurons in rats. Exp Neurol. 1984;83:507–517. doi: 10.1016/0014-4886(84)90119-5. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. New York: Academic Press; 1998. [Google Scholar]
- Saleh TM, Bauce LG, Pittman QJ. Glutamate release in parabrachial nucleus and baroreflex alterations after vagal afferent activation. Am J Physiol. 1997;272:R1631–R1640. doi: 10.1152/ajpregu.1997.272.5.R1631. [DOI] [PubMed] [Google Scholar]
- Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- Spector AC. Gustatory function in the parabrachial nuclei: implications from lesion studies in rats. Rev Neurosci. 1995;6:143–175. doi: 10.1515/revneuro.1995.6.2.143. [DOI] [PubMed] [Google Scholar]
- Spector AC, Breslin P, Grill HJ. Taste reactivity as a dependent measure of the rapid formation of conditioned taste aversion: a tool for the neural analysis of taste-visceral associations. Behav Neurosci. 1988;102:942–952. doi: 10.1037//0735-7044.102.6.942. [DOI] [PubMed] [Google Scholar]
- Travers JB. Efferent projections from the anterior nucleus of the solitary tract of the hamster. Brain Res. 1988;457:1–11. doi: 10.1016/0006-8993(88)90051-0. [DOI] [PubMed] [Google Scholar]
- Travers JB, Urbanek K, Grill HJ. Fos-like immunoreactivity in the brain stem following oral quinine stimulation in decerebrate rats. Am J Physiol. 1999;277:R384–R394. doi: 10.1152/ajpregu.1999.277.2.R384. [DOI] [PubMed] [Google Scholar]
- Vales K, Zach P, Beilavska E. Metabotropic glutamate receptor antagonists but not NMDA antagonists affect conditioned taste aversion acquisition in the parabrachial nucleus of rats. Exp Brain Res. 2006;169:50–57. doi: 10.1007/s00221-005-0127-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Shimura T, Sakai N, Ozaki N. Representation of hedonics and quality of taste stimuli in the parabrachial nucleus of the rat. Physiol Behav. 1994;56:1197–1202. doi: 10.1016/0031-9384(94)90366-2. [DOI] [PubMed] [Google Scholar]
- Zidichouski JA, Easaw JC, Jhamandas JH. Glutamate receptor subtypes mediate excitatory synaptic responses of rat lateral parabrachial neurons. Am J Physiol. 1996;270:H1557–H1567. doi: 10.1152/ajpheart.1996.270.5.H1557. [DOI] [PubMed] [Google Scholar]