Abstract
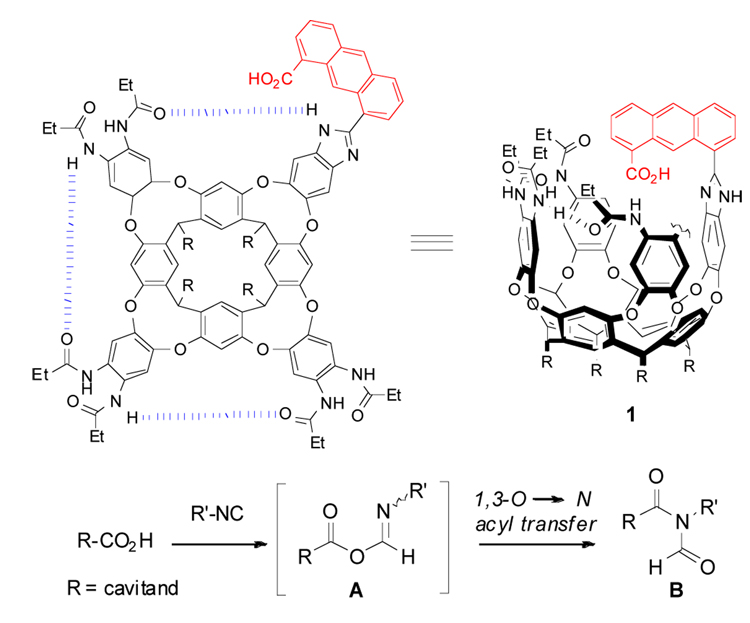
A deep cavitand with an inwardly-directed carboxylic acid function reacts with small aliphatic isonitriles to form N-acyl formamides inside the cavity. The unique isolation and stabilization of covalently bound guests within the structured environment of the cavitand allows for observation of the labile O-acyl isoimide intermediate using conventional spectroscopic methods.
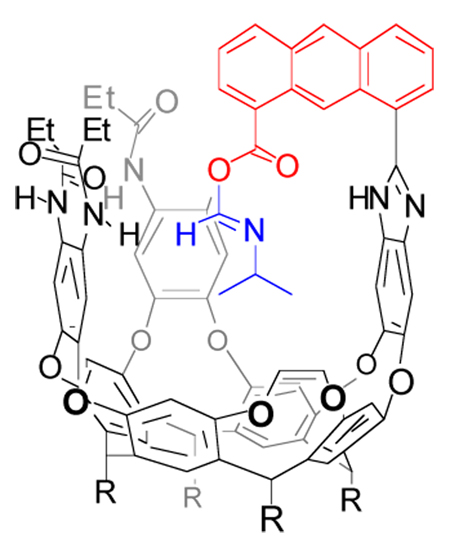
Cavitands are vase-shaped structures that have been widely used in studies of molecular recognition.1 While it is generally difficult to derivatize their (internal) concave surfaces, the upper rims are readily functionalized. Unconventional reagents such as Kemp’s triacid2 attached there can lead to receptor molecules that fold around small molecules, isolate them from bulk solution and present them with functional groups directed into the cavity.3,4 A range of chemical transformations can be promoted within the structured environment of the cavitands, including stabilization of labile carbonyl addition intermediates5 and acceleration of organic reactions.6 Here, we introduce cavitand 1 (Scheme 1) containing an “introverted” acid functionality on an anthracene skeleton. Its reaction with small, aliphatic isonitrile molecules bound inside the cavity gives elusive intermediates that are observed by conventional spectroscopic methods.
Scheme 1.
Reaction of cavitand 1 with aliphatic isonitriles.
The reaction of carboxylates with nitrilium ions is well known and is the key step in the Ugi and Passerini multicomponent condensations.7 In contrast, the reaction of carboxylic acids and aliphatic isonitriles is rare.8 Recently, Danishefsky and Li9 showed that this reaction occurs under microwave heating at 150 °C for 30 minutes in CHCl3 and provides an efficient synthesis of diverse imides. The reaction proceeds via the O-acyl isoimide intermediate A followed by a 1,3-O→N acyl transfer (Mumm rearrangement)7a–d to form N-acylformamide B. In a recent departure, we investigated this process with the reactants in cylindrical capsule and showed that the reaction inside occurs at significantly lower temperatures.10 In addition, the formation of transient intermediate could be detected by 1H NMR spectroscopy but we were unable to further characterize it. The covalent attachment of the acid and its isolation inside cavitand 1 now allows the corresponding intermediate A to be characterized by NMR and IR methods.
We prepared cavitand 1 by oxidation of the previously described “introverted” aldehyde cavitand.5a Cavitand 1 folds in competitive solvents such as THF-d8 but in mesitylene-d12 that do not fit inside the cavity the peaks in the 1H NMR spectrum are broad and undefined. Upon addition of a small isonitrile molecule such as iPrNC the peaks sharpen considerably and show characteristic signals for bound guest.11 These signals appear shifted far upfield due to the magnetically shielded environment inside the cavity of 1 created by the aromatic walls (Figure 1).
Figure 1.
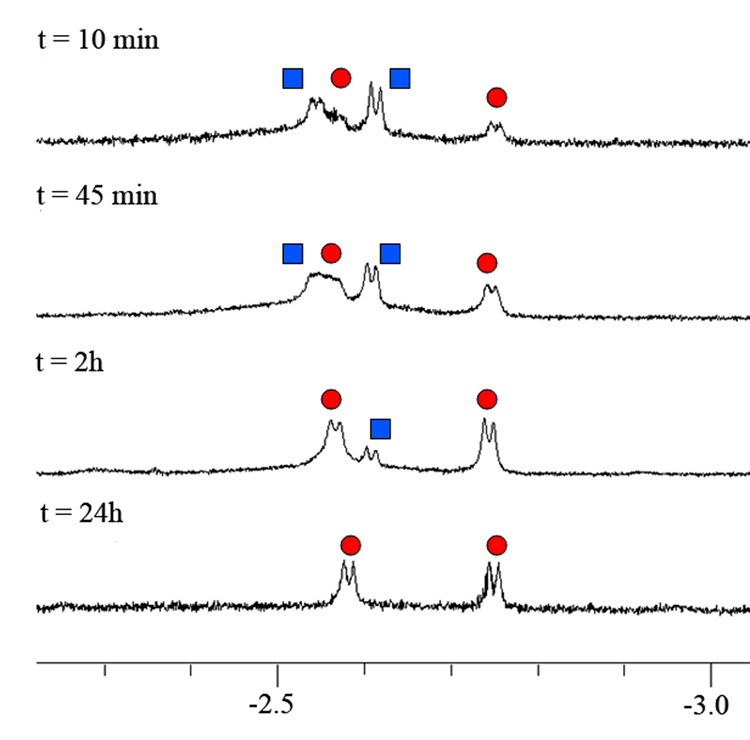
1H NMR spectra of cavitand 1 and iPrNC. Blue square shows intermediate A and red circle product B.
After addition of iPrNC to 1 in mesitylene-d12, two sets of doublets were observed in the 1H NMR spectra arising from the terminal methyl groups of A and B respectively, buried deep inside the cavity of 1. The nearby chiral environment of cavitand renders the two isopropyl methyl groups diastereotopic and they are observed as separate signals. The cyclic seam of intramolecular hydrogen-bonds that stabilize the vase-like conformation of 1 can be oriented either clockwise or counterclockwise and interconversion is slow on the NMR chemical shift timescale.12 Over the course of a few hours the doublets arising from intermediate A disappeared and were replaced by signals from the rearranged product B. The formation of B was confirmed by ESI-HRMS which gave a mass of 2157.2520 [M + H+].
The reaction was also monitored by IR spectroscopy (Figure 2). Immediately after addition of iPrNC to 1 the IR spectrum showed only one carbonyl (C=O) band (ν = 1667 cm−1) which arises from the amide carbonyls on the brim of 1.13 After 10 min the appearance of another carbonyl (C=O) band at 1771 cm−1 appeared. This value is consistent with previously reported data for structurally related α-amino isobenzimides generated from iminoaziridines (ν = 1747 cm−1).14 After 24h, the absorption at 1771 cm−1 had disappeared and was replaced by a carbonyl C=O band at 1696 cm−1, a value in good agreement with reported data for N-acyl formamides.15
Figure 2.
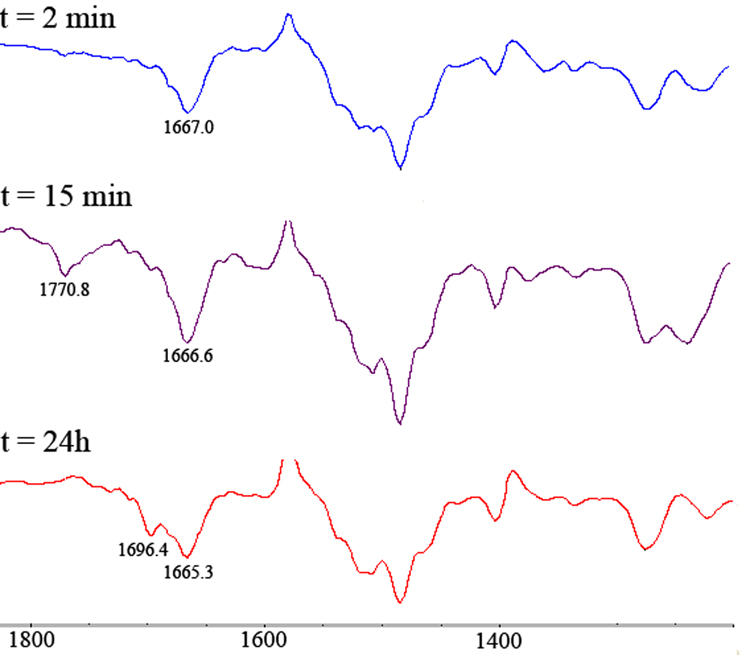
IR spectra of cavitand 1 and iPrNC.
Similar results were obtained using tBuNC as the reagent in cavitand 1.16 In this case, the 1H NMR spectra showed upfield signals for non-covalently bound guest along with O-acyl isoimide intermediate A and rearranged product B. After 24h only the signal from product B remained. IR spectroscopic analysis showed a C=O absorption at 1766 cm−1 for intermediate A and 1698 cm−1 for product B as before.
How does the cavitand facilitate the reaction between the carboxylic acid moiety and the isonitriles? First, the cavitand amplifies the concentrations of the reacting species by binding the guest: the reaction is effectively promoted from a bimolecular to a unimolecular one inside the cavity. Second, the cavitand can be thought of as a solvent cage fixed in time through synthesis. The cavitand’s aromatic walls offer an electron-rich π-surface that can interact with the bound substrate whereas the secondary amide bonds in the rim make up a polar region rich in hydrogen-bond donors. Third, the confined space can provide steric barriers that slow the rearrangement of intermediate A (Scheme 1). These features create a unique environment in the cavity not possible in bulk solution which isolates and stabilizes labile and otherwise unobservable reaction intermediates. No reaction was observed between the isonitriles and typical acids under these conditions in the absence of cavitand 1.10 Cavitands have previously shown rate accelerations and catalysis of reactions even without functional groups attached.17 In conclusion, cavitand 1 possessing an inwardly-directed carboxylic acid function binds and reacts with small isonitrile guests held inside its cavity at ambient temperature and millimolar concentrations.11 It was also possible to observe the labile O-acyl isoimide intermediate A by 1H NMR and IR spectroscopy. Elsewhere, complete encapsulation was shown to alter the reactivity of bound species and prolong the lifetimes of otherwise unstable molecules.18 Examples include isolation of iminium ions18a and siloxanes18c from aqueous media, detection of unfavored conformations19 and appearance of unknown reaction courses20 channeled by the size and shape of the host. Cavitands and capsules provide versatile, modern complements to classical kinetics and nonkinetic21 methods for the study of reaction intermediates, and offer promise as models for enzyme catalysis.
Supplementary Material
Synthesis of cavitand 1 and spectral data characterization of 1 and the reaction intermediates. This material is available free of charge at http://pubs.acs.org.
Acknowledgements
We are grateful to the Skaggs Institute and the National Institute of Health (GM 50174) for support. P.R. is a Swedish Knut and Alice Wallenberg post-doctoral fellow.
REFERENCES
- 1.(a) Cram DJ. Science. 1983;219:1177–1183. doi: 10.1126/science.219.4589.1177. [DOI] [PubMed] [Google Scholar]; (b) Rudkevich DM, Rebek J., Jr Eur. J. Org. Chem. 1999:1991–2005. [Google Scholar]; (c) Purse BW, Rebek J., Jr Proc. Natl. Acad. Sci. U.S.A. 2005;102:10777–10782. doi: 10.1073/pnas.0501731102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kemp DS, Petrakis KS. J. Org. Chem. 1981;46:5140–5143. [Google Scholar]
- 3.Wash PL, Renslo AR, Rebek J. Angew. Chem. Int. Ed. Engl. 2000;40:1221–1222. [PubMed] [Google Scholar]; b) Iwasawa T, Wash PL, Gibson C, Rebek J. Tetrahedron. 2007;63:6506–6511. doi: 10.1016/j.tet.2007.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Renslo AR, Rebek J. Angew. Chem. Int. Ed. 2000;40:3281–3283. doi: 10.1002/1521-3773(20000915)39:18<3281::aid-anie3281>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]; d) Purse BW, Rebek J. Proc. Natl. Acad. Sci., U.S.A. 2006;103:2530–2534. doi: 10.1073/pnas.0511149103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butterfield SM, Rebek J. J. Am. Chem. Soc. 2006;128:15366–15367. doi: 10.1021/ja0663374. [DOI] [PubMed] [Google Scholar]
- 5.(a) Iwasawa T, Hooley RJ, Rebek J., Jr Science. 2007;317:493–496. doi: 10.1126/science.1143272. [DOI] [PubMed] [Google Scholar]; (b) Hooley RJ, Restorp P, Iwasawa T, Rebek J., Jr J. Am. Chem. Soc. 2007;129:15639–15643. doi: 10.1021/ja0756366. [DOI] [PubMed] [Google Scholar]
- 6.(a) Gissot A, Rebek J. J. Am. Chem. Soc. 2004;126:7424–7425. doi: 10.1021/ja049074r. [DOI] [PubMed] [Google Scholar]; (b) Purse BW, Ballester P, Rebek J. J. Am. Chem. Soc. 2003;125:14682–14683. doi: 10.1021/ja036595q. [DOI] [PubMed] [Google Scholar]; (c) Shenoy SR, Pinacho Crisostomo FR, Iwasawa T, Rebek J., Jr J. Am. Chem. Soc. 2008;130:5658–5659. doi: 10.1021/ja801107r. [DOI] [PubMed] [Google Scholar]
- 7.(a) Schwarz JSP. J. Org. Chem. 1972;37:2906–2908. [Google Scholar]; (b) Brady K, Hegarty AF. J. Chem. Soc. Perkin Trans. II. 1980:121–126. [Google Scholar]; (c) Hegarty AF. Acc. Chem. Res. 1980;13:448–454. [Google Scholar]; (d) Passerini M. Gazz. Chim. Ital. 1921;51:181. [Google Scholar]; (e) Ugi I, Meyr R. Angew. Chem. 1958;70:702–703. [Google Scholar]; (f) Dömling A, Ugi I. Angew. Chem. Int. Ed. 2000;39:3168–3210. doi: 10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 8.(a) Gautier A. Liebig’s Annalen. 1986;151:240–243. [Google Scholar]; (b) Shaabani A, Soleimani E, Rezayan AH. Tetrahedron Lett. 2007;48:6137–6141. [Google Scholar]
- 9.Li X, Danishefsky S. J. Am. Chem. Soc. 2008;130:5446–5447. doi: 10.1021/ja800612r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hou J, Ajami D, Rebek J., Jr J. Am. Chem. Soc. In press. IR spectroscopic analysis of the reactions inside the cylindrical capsule described in this reference were inconclusive due to overlapping C=O bands with those of the capsule.
- 11.For experimental details, see Supporting Information.
- 12.(a) Shivanyuk A, Rissanen K, Korner SK, Rudkevich DM, Rebek J., Jr Helv. Chim. Acta. 2000;83:1778–1790. [Google Scholar]; (b) Hooley RJ, Iwasawa T, Rebek J., Jr Am. Chem. Soc. 2007;129:15330–15339. doi: 10.1021/ja0759343. [DOI] [PubMed] [Google Scholar]
- 13.Renslo AR, Tucci FC, Rudkevich DM, Rebek J., Jr J. Am. Chem. Soc. 2000;122:4573–4582. [Google Scholar]
- 14.Quast H, Aldenkortt S. Chem. Eur. J. 1996;2:462–469. [Google Scholar]
- 15.Johnson RA, Murray HC, Reineke LM. J. Org. Chem. 1969;34:3834–3837. doi: 10.1021/jo01264a021. [DOI] [PubMed] [Google Scholar]
- 16.See Supporting Information.
- 17.(a) Hooley RJ, Rebek J., Jr Org. Biomol. Chem. 2007;5:3631–3636. doi: 10.1039/b713104f. [DOI] [PubMed] [Google Scholar]; (b) Butterfield S, Rebek J., Jr Chem. Comm. 2007:1605–1607. doi: 10.1039/b700319f. [DOI] [PubMed] [Google Scholar]
- 18.a) Dong VM, Fiedler D, Carl B, Bergman RG, Raymond KN. J. Am. Chem. Soc. 2006;128:14464–14465. doi: 10.1021/ja0657915. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Ziegler M, Brumaghim JL, Raymond KN. Angew. Chem. Int. Ed. 2000;39:4119–4121. doi: 10.1002/1521-3773(20001117)39:22<4119::aid-anie4119>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]; c) Yoshizawa M, Kusukawa T, Fujita M, Yamaguchi K. J. Am. Chem. Soc. 2000;129:6311–6312. doi: 10.1021/ja010875t. [DOI] [PubMed] [Google Scholar]; d) Iwasawa T, Mann E, Rebek J. J. Am. Chem. Soc. 2006;128:9308–9309. doi: 10.1021/ja062768a. [DOI] [PubMed] [Google Scholar]; e) Kawano M, Kobayashi Y, Ozeki T, Fujita M. J. Am. Chem. Soc. 2006;128:6558–6559. doi: 10.1021/ja0609250. [DOI] [PubMed] [Google Scholar]; f) Fiedler D, Bergman RG, Raymond KN. Angew. Chem. Int. Ed. 2004;43:6748–6751. doi: 10.1002/anie.200461776. [DOI] [PubMed] [Google Scholar]; g) Sato S, Iida J, Suzuki K, Ozeki T, Fujita M. Science. 2007;313:1273–1276. doi: 10.1126/science.1129830. [DOI] [PubMed] [Google Scholar]; h) Murase T, Sato S, Fujita M. Angew. Chem. Int. Ed. 2007;46:1083–1085. doi: 10.1002/anie.200603561. [DOI] [PubMed] [Google Scholar]; i) Chen J, Rebek J., Jr Org. Lett. 2002;4:327–329. doi: 10.1021/ol0168115. [DOI] [PubMed] [Google Scholar]
- 19.Scarso A, Trembleau L, Rebek J. Angew. Chem. Int. Ed. 2003;42:5499–5502. doi: 10.1002/anie.200352235. [DOI] [PubMed] [Google Scholar]
- 20.Yoshizawa M, Tamura M, Fujita M. Science. 2006;312:251–254. doi: 10.1126/science.1124985. [DOI] [PubMed] [Google Scholar]
- 21.Rebek J., Jr Tetrahedron. 1979;35:723–731. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Synthesis of cavitand 1 and spectral data characterization of 1 and the reaction intermediates. This material is available free of charge at http://pubs.acs.org.