Abstract
Transfer of exogenous genetic material into the mammalian inner ear using viral vectors has been characterized over the last decade. A number of different viral vectors have been shown to transfect the varying cell types of the nonprimate mammalian inner ear. Several routes of delivery have been identified for introduction of vectors into the inner ear while minimizing injury to existing structures and at the same time ensuring widespread distribution of the agent throughout the cochlea and the rest of the inner ear.
These studies raise the possibility that gene transfer may be developed as a potential strategy for treating inner ear dysfunction in humans. Furthermore, a recent report showing successful transfection of excised human vestibular epithelia offers proof of principle that viral gene transfer is a viable strategy for introduction and expression of exogenous genetic material to restore function to the inner ear. Human vestibular epithelia were harvested from patients undergoing labyrinthectomy, either for intractable Ménière’s disease or vestibular schwannoma resection, and cultured for as long as 5 days. In those experiments, recombinant, multiply-deleted, replication-deficient adenoviral vectors were used to transfect and express a reporter gene as well as the functionally relevant gene, wild-type KCNQ4, a potassium channel gene that when mutated causes the autosomal dominant HL DFNA2.
Here, we review the current state of viral-mediated gene transfer in the inner ear and discuss different viral vectors, routes of delivery, and potential applications of gene therapy. Emphasis is placed on experiments demonstrating viral transfection of human inner ear tissue and implications of these findings and for the future of gene therapy in the human inner ear.
Keywords: Gene transfer, transfection, adenovirus, human, vestibular epithelia
INTRODUCTION
Hearing loss (HL) is the most common sensory impairment in humans; an estimated 28 million Americans suffer from HL, and 1 to 3 per 1,000 children are born with some form of HL.1 Significant progress has been made in the last several years identifying and characterizing the individual genes and proteins involved in hereditary hearing impairment (for detailed list, see Hereditary Hearing Loss Homepage, http://webhost.ua.ac.be/hhh/), many of which may be potential targets for gene therapy. Nonetheless, experimentation, including gene transfer studies in the human inner ear, is currently limited because of ethical and practical considerations.
Despite the limitations, viral-mediated gene transfer is emerging as a promising gene therapy technique for treating both genetic and acquired forms of HL. Several viral vectors, including adenovirus,2-4 adeno-associated virus (AAV),5,6 herpes simplex virus (HSV),7,8 vaccinia virus,8 lentivirus,9 and nonviral cationic liposomes10,11 have been shown to transfect the various cell types of the mammalian inner ear. Potential applications of gene transfer in genetic hearing and balance dysfunction include expression of exogenous proteins, gene rescue or targeted gene replacement, and targeted inhibition of mutant gene expression with antisense or siRNA sequences.
More specifically, for acquired inner ear dysfunction, gene therapy could be used to express functional proteins as protective factors against oxidative stress for both hair cells12,13and neurons14-17 to ameliorate or prevent aminoglycoside or noise-induced ototoxicity. Hair cell regeneration is another promising application of gene therapy. Gene transfer of the basic helix-loop-helix transcription factor mouse atonal homologue 1 (Math1) into the scala media of deafened guinea pig cochleae induced new hair cell formation18 and improved auditory brainstem response (ABR) thresholds compared with controls after an aminoglycoside deafening regimen.19
Here, we review the evolution of gene transfer in the inner ear with emphasis on a new model system to study gene therapy in human inner ear tissue. We begin with basic principles of gene transfer in the inner ear and review different viral vectors and possible routes of delivery into the inner ear. Potential applications of gene therapy are discussed, and the final section focuses on a series of experiments demonstrating the feasibility of gene transfer in human vestibular epithelium.
BASIC PRINCIPLES
As the mechanisms underlying cochlear physiology and the genetic basis of HL unravel, a greater percentage of HL, both age-related and congenital, will likely be attributed to specific gene mutations. Approximately 45 different genes have been identified that cause nonsyndromic hereditary HL when mutated, and another 30 genes associated with syndromic HL have also been identified.
Despite the current limited applicability to human systems, viral-mediated gene therapy may be developed as an important tool for treating genetic forms of HL and balance dysfunction. Gene therapy is the addition, or transfer, of a segment of genetic material into a cell that results in expression of a transgenic protein and ultimately a change in the function or behavior of the cell. The change in phenotype of the cell can result in the transient or chronic production of a new protein, replacement of a defective protein (gene rescue), or suppression of a dominant-negative protein.
Studies of viral-mediated gene transfer in the inner ear have advanced for several reasons. The bony housing of the sensory epithelium prevents, or at least limits, unwanted systemic viral diffusion. The isolation of this organ system makes it ideal for the study and potential application of gene transfer. The low-volume, fluid-filled chambers of the inner ear are in continuity, allowing spread of virus throughout all regions of the inner ear. Although simple diffusion of vectors throughout the fluid-filled cochlea may appear to be an advantage of this system, numerous studies show a gradient of transfection when virus is introduced into the scala tympani via the round window: greatest at the injection site (basal end of cochlea) and least at the apex for all vectors studied, including adenovirus,11 cationic liposomes,10,11 HSV-1, and vaccinia virus vectors.8 An important consideration for future studies will be the uniform distribution of virus throughout the entire cochlea (or vestibular labyrinth).
The varied insults, both acquired and genetic, that cause HL and inner ear dysfunction frequently target a common structure, the hair cell. The final common pathway for most forms of HL is hair cell injury or loss. Theoretically, and now experimentally, by targeting hair cells, supporting cells, or neurons, gene therapy can be used to alter the cellular microenvironment of the inner ear as well as change the cellular phenotype to protect, preserve, rescue, and even regenerate hair cells.
Before inner ear gene therapy becomes clinically applicable, however, numerous issues must be addressed, including vector safety and toxicity, method of viral delivery, ability to target the appropriate cell type, and ability to reproduce the endogenous expression pattern and expression level. Recently, Kesser et al.20 characterized adenovirus-driven expression of a reporter gene (green fluorescent protein [GFP]) and a functionally relevant gene (KCNQ4) into cultured human vestibular epithelia. This represents an important step toward demonstrating applicability of gene transfer to treat human inner ear disease states (see Studies in Human Tissue, below).
VECTORS USED IN INNER EAR GENE TRANSFER
Recombinant replication-deficient viruses including lentivirus,9 adenovirus,2,4,21 HSV-1,8,22 vaccinia virus,8 AAV,5 and a novel bovine adeno-associated viral vector23 have been shown to transfect varying cell types in the nonprimate mammalian inner ear. Each vector has its own profile with regard to the specific inner ear tissues it selectively transfects, stability of transfection, toxicity, genetic carrying capacity, ability to integrate into the host genome, and host inflammatory reaction (Table I).
TABLE I.
Viral Vectors: Advantages, Disadvantages, and Cellular Tropism
| Viral Vector | Advantages | Disadvantages | Cellular Tropism (based on expression of reporter genes) | References |
|---|---|---|---|---|
| Adenovirus | Easy to produce; large (7.5-10 kb) insert; cell division not required | Immunogenic; transient expression | Mesenchymal cells lining perilymphatic spaces; stria vascularis; fibrocytes of spiral ligament; supporting cells of OC; hair cells of OC and vestibular SGNs | 2, 4, 21, 48 |
| Adeno-associated virus | No human disease; stable expression; cell division not required | Difficult to produce; small (4-5 kb) insert; variable transfection efficiency | Fibrocytes of SL; Rm; supporting cells of OC SGNs; vestibular sensory hair cells and supporting cells | 5, 6, 36, 37 |
| Herpesvirus | Large (10-100 kb) insert; stable expression | Human disease; cytopathic | Mesenchymal cells lining perilymphatic spaces; fibrocytes of SL; Rm; supporting cells of OC SGNs; VGNs | 8, 22 |
| Lentivirus (retrovirus) | Stable expression | Insertional mutagenesis; low transfection efficiency; cell division required | SGNs; glia | 9 |
| Liposome | Easy to make; unlimited insert size; cell division not required nonpathogenic; nonimmunogenic | Transient expression; low transfection | Fibrocytes of SL; OHCs and SCs of OC; Rm; SGCs | 10 |
Adenovirus
Discovered in 1953 in human adenoid tissue, wild-type adenovirus is a linear 36 kbp dsDNA virus surrounded by an icosahedral capsid. Adenoviral infections in an immunocompetent patient include upper respiratory symptoms, conjunctivitis, and gastroenteritis. The virus infects a wide variety of organ systems, tissue types, and cells in humans, a distinct advantage for manipulation in the laboratory. Adenovirus does not require cell division for transfection, another significant advantage when attempting to transfect the postmitotic terminally differentiated cells of the mammalian inner ear. Intracellularly, adenovirus acts as an episome and does not integrate into the host genome, eliminating the risk of insertional mutagenesis at the expense of more transient expression.
Exogenous genes can be inserted into the adenoviral genome using a strategy of homologous recombination in bacteria, and high titers of virus are easily obtained.24 Adenovirus can carry a large genetic payload, with insertional genes as large as 10 kb. With the cytomegalovirus promoter, generally high rates of transgene expression can be achieved. Although studies using first generation adenoviral vectors showed substantial dose-dependent and time-dependent hair cell toxicity,4,21,25 more recent studies using a modified, multiply-deleted (E1-,E3-, pol- pTP-) adenovirus26 preserved cochlear function as measured by distortion-product otoacoustic emissions27 and by hair cell function26,28,29 without compromising viral infectivity.
Given the numerous candidate viral vectors that have been studied, adenovirus is a particularly attractive vector for inner ear gene transfer (for review, see Amalfitano30) because it has been shown to efficiently transfect a broad spectrum of cell types, including hair cells and supporting cells.26,27,31-34 More recently, adenovirus has been shown to transfer exogenous genetic material into both hair cells and supporting cells of cultured human vestibular epithelia, which raises its profile as a vehicle for gene transfer into the human inner ear (see Studies in Human Tissue, below).20
Adeno-Associated Virus
A 4.7 kbp single-stranded, nonpathogenic DNA parvovirus, AAV transfects pre- and postmitotic cells and does not require actively dividing cells for transfection. It does require adenovirus or HSV for autonomous replication. Unlike adenovirus, AAV incorporates into the host genome, resulting in stable, potentially long-term transgenerational expression. Integration into the host genome may be advantageous for driving long-term gene expression in the cochlea. AAV is safe and not associated with human disease or infection. Because it is a relatively small virus, AAV carries a smaller genetic “payload,” which limits the size of the gene construct that can be transferred into the host cell. When used as a vector, the replication and viral capsid genes are replaced by the transgene and its associated regulatory sequences. The total length of the insert cannot greatly exceed 4.7 kb, the length of the wild-type genome.35
AAV drives expression of genes in many cell types of the inner ear, including spiral ganglion cells, cells of the spiral limbus and spiral ligament,5,36 sensory and supporting cells of the cristae ampullaris and maculae of mammalian vestibular organs,37 and hair cells and supporting cells of murine cochlea explants.38 Stable expression for periods up to 24 weeks in the mammalian inner ear36 and 6 months in adult rat striatal neurons has been observed.39
Herpes Simplex Virus
A double-stranded 152 kbp DNA virus, HSV infects a broad range of cell types including epithelial cells, fibro-blasts, and neurons. The most commonly used vector is derived from HSV-1, and these HSV-1 vectors effectively deliver foreign genes to the postmitotic cells of the mammalian nervous system. With its large genome, HSV-1 can be engineered to carry single or multiple large genetic inserts. The vector can infect dividing or postmitotic cells, and its ability to assume a latent state in neurons represents a significant advantage that could potentially mediate stable, long-term transgene expression. A latent stage HSV-1 virus in a host neuronal cell is resistant to host immune surveillance. The virus does not incorporate into the host genome and exists as an episome in the neuronal nucleus, eliminating the possibility of insertional mutagenesis.
In the inner ear, HSV-1 vectors infect fibrocytes of the spiral ligament, mesenchymal and epithelial cells of Reissner’s membrane, Hensen’s cells and Deiter’s cells in the organ of Corti, and mesenchymal cells lining the scala vestibuli when injected via a micropipette inserted through the round window; hair cells were not transfected.8 Lack of hair cell transfection by HSV-1 was confirmed by Staecker et al.,7 who observed expression of the marker protein β-galactosidase in auditory and vestibular neurons, in cells in the stria vascularis, and in the supporting cells of the organ of Corti. Limitations of HSV-1 include difficult production, low infection efficiency, and its potential immunogenicity/cytotoxicity.40
Vaccinia Virus
Vaccinia virus, the live virus used in the smallpox vaccine, is a double stranded DNA poxvirus. Vaccinia can cause severe eczematoid reactions in certain individuals after vaccination but generally does not cause disease in humans.
Vaccinia virus vectors transfect fibrocytes of the spiral ligament, mesenchymal and epithelial cells of Reissner’s membrane, inner and outer hair cells, mesothelial cells in the organ of Corti, and mesenchymal cells lining both the scala tympani and scala vestibuli.8 An inflammatory response characterized by a lymphocytic infiltrate was observed primarily in the basal two turns of the cochlea in animals injected with a vaccinia virus vector.8 Given the host inflammatory response to vaccinia, this vector has had limited use in studies of inner ear gene transfer.
Retrovirus and Lentivirus
Retroviruses are single stranded RNA viruses that use the viral reverse transcriptase to direct synthesis of a double stranded DNA molecule that enters the cell nucleus for incorporation into the host genome. This integration of viral DNA into the host genome requires cell division so that retroviral vectors can only integrate into the genome of dividing cells. Although retroviral vectors have generated significant interest in their ability to target rapidly dividing neoplastic cells,41,42 they are not considered efficient vectors for gene transfer into the postmitotic cells of the inner ear.
Lentivirus, derived from human immunodeficiency virus, is a retroviral vector that can infect nondividing cells.43 Lentiviral vectors infect a broad range of both dividing and nondividing cells, including the terminally differentiated neuron. The ability of lentiviral vectors to transfect postmitotic cells and to integrate into the host genome makes them potentially excellent candidates for long-term, stable protein expression in the inner ear, a significant advantage in the treatment of genetic HL.
Lentivirus transgene expression in the inner ear was restricted to cells along the periphery of the perilymphatic space and spiral ligament in vivo; however, spiral ganglion cells and glial cells were transfected in vitro, suggesting that the limited expression seen with lentivirus in vivo may reflect more its distribution and not its true ability to infect the variety of cells within the cochlea.9 Given its possibly limited cellular tropism in the inner ear, lentivirus may be a suitable vector for the secretion of neurotrophins and other protective factors as gene products into the perilymph (see Applications of Gene Therapy, below).
Cationic Liposomes
To avoid problems of immunogenicity, insertional mutagenesis, and viral vector-related disease or toxicity, vesicles composed of a bilayer of lipid molecules enclosing an aqueous volume have been complexed with DNA to produce cationic liposomes as drug or gene delivery vectors.44 Liposomes bind to the plasma membrane where DNA material is internalized. The bound DNA does not replicate or integrate into the host genome. These liposomes also have the advantage of an unlimited genetic carrying capacity and simplicity of production. Nevertheless, they have proven inefficient vectors to transfer exogenous DNA, with essentially no control over the target cell other than the site of vector injection.7
In the inner ear, cationic liposomes have been shown to deliver the reporter genes β-galactosidase and GFP to cells in the spiral ligament, spiral limbus, Reissner’s membrane, and spiral ganglia in the mouse,45 as well as outer hair cells and supporting cells in the organ of Corti in the guinea pig10; transgene expression persisted 14 days postinjection with no inflammatory response in the latter study. Despite their safety and ease of production, cationic liposomes will have to overcome problems of limited transgene expression time, cell targeting, and inefficient DNA transfer for these vectors to play a major role in inner ear gene transfer.
METHODS OF GENE DELIVERY
Efficient delivery of viral (or nonviral) vectors to the inner ear requires a technique that delivers an appropriate volume of vector with equal distribution throughout the cochlea or vestibular labyrinth while preserving hearing function and cochlear architecture. For human application, the technique must be safe with minimal risk to the patient. In cases in which gene delivery is used to transfer protective factors to the cochlea to minimize potential ototoxicity, the delivery method must not injure hair cells or disrupt the carefully balanced ionic gradients in the inner ear.
In the laboratory, the most common methods to deliver viral vectors into the inner ear in mammalian models include creation of a small cochleostomy in the basal turn of the cochlea with direct injection via a microcatheter,15,46,47 infusion with an osmotic mini-pump,5,6,25,36 or direct injection through the round window membrane.2,3,34 Table II presents the various experimental vector delivery methods and outlines their advantages and disadvantages.
TABLE II.
Advantages and Disadvantages of Vector Delivery Methods In Vivo
| Method | Advantages | Disadvantages | References |
|---|---|---|---|
| Cochleostomy with microcatheter injection | More efficient cochlear transduction; short infusion time | Hearing loss | 31, 47, 81 |
| Cochleostomy with osmotic mini-pump | More efficient cochlear transduction; allows longer-term infusion | Surgery more difficult; hearing loss | 82 |
| RWM injection | Preserves hearing; no inflammatory reaction | Less efficient cochlear transduction; less volume of vector delivered | 10, 47, 83 |
| Canalostomy (PSCC, SSCC) | Preserves hearing | Less efficient cochlear transduction | 31, 81 |
| Endolymphatic sac | Endolymphatic space transduction, including supporting cells in OC; vestibular end organs but no hair cells transfected | Less reliable cochlear transduction; hearing not tested | 49 |
| Intratympanic | Preserves hearing (theoretical); transfects cells in the middle ear | No inner ear transduction | 48 |
| Gelatin sponge with intact RWM | Easy, atraumatic, safe; preserves hearing; transfects cochlear cells through RWM | Vector-dependent: AAV did not transfect inner ear | 11 |
| Scala media | More reliable hair cell and supporting cell transduction | Hair cell injury around injection site; hearing loss | 32, 84 |
RWM = round window membrane; PSCC = posterior semicircular canal; SSCC = superior semicircular canal; OC = organ of Corti; AAV = adeno-associated virus.
For potential human application, gene delivery techniques that do not violate the round window or cochlea have been studied. Injection of vector through the tympanic membrane into the middle ear of the neonatal rat preserved auditory and vestibular function but did not result in reporter gene expression in the cochlea or vestibular system.48 Injection of an adenoviral vector via a canalostomy in the posterior semicircular canal of the mouse preserved hearing, but reporter gene expression was limited to the cells lining the perilymphatic space; cochleostomy injection improved expression of reporter gene in the sensory cells of the saccule and organ of Corti at the expense of significant threshold shifts on ABR testing.31 Viral inoculation in the guinea pig endolymphatic sac preserved hearing and resulted in reporter gene expression in the sac, the endolymphatic duct, the vestibular end organs (hair cells were not transfected), and in the cochlea (but not hair cells).49 Injection of an HSV-1 vector through a small opening in the utricle also preserved hearing while achieving efficient reporter gene expression.50
Gelatin sponge soaked with adenovirus and liposome, but not AAV, was successful in transfecting cochlear tissues through an intact round window membrane in the mouse with preservation of ABR thresholds.11 Low transfection rates of supporting cells and hair cells in the organ of Corti have tempered enthusiasm for these “cochlea-sparing” techniques. In an attempt to increase transfection efficiency, more recent studies report improved transfection of supporting cells and hair cells in the organ of Corti after inoculation through the scala media, at the expense of violating the stria vascularis and endolymphatic compartment.18,19,32
APPLICATIONS OF GENE THERAPY
Research in inner ear gene transfer is now moving from studies examining feasibility using simple vectors and reporter transgenes to studies exploring the safe delivery of potentially therapeutic genes to treat either acquired or genetic causes of HL. Hereditary hearing impairment genes whose dysfunction or mutation is associated with HL represent a potential subset of targets for gene therapy. Mammalian models of inner ear disease, including genetic HL and ototoxic deafening regimens, have provided numerous invaluable systems in which gene transfer has been and will continue to be investigated.
Gene therapy may be applied to four fundamental areas of inner ear dysfunction: 1) genetic HL with the goal of over-expression of the wild-type form of mutant deafness genes, 2) introduction of protective factors to enhance spiral ganglion cell survival after hair cell loss, 3) introduction of protective factors to enhance hair cell survival in the face of ototoxic insults (both chemical and noise induced), and 4) regeneration of functional hair cells by transdifferentiation of supporting cells into hair cells through the introduction of transcription factors.
Genetic HL
The development of mouse models of genetic HL has enabled researchers to characterize the cellular and molecular deficits involved in many of these genetic mutations. Perhaps more importantly, mouse models of genetic HL have provided a more clear understanding of normal cochlear physiology. Each of the over 75 different deafness genes identified, several associated with Usher’s syndrome and hair cell cytoarchitecture, represents a potential target for gene therapy (Table III).
TABLE III.
Few of the Potential Targets for Gene Therapy
| Gene Symbol | Gene Product | Function | Location | Mouse Model | Syndromic Locus | Nonsyndromic Locus | References |
|---|---|---|---|---|---|---|---|
| MYO7A | Myosin VIIa | Intracellular motor | 11q12.3 | Shaker-1 | USH1B | DFNA11, B2 | 85, 86 |
| USH1C | Harmonin | Scaffold protein | 11p15.1 | Deaf circler | USH1C | DFNB18 | 87 |
| CDH23 | Cadherin 23 | Intercellular adhesion | 10q21-q22 | Waltzer | USH1D | DFNB12 | 88, 89 |
| PCDH15 | Protocadherin 15 | Intercellular adhesion | 10q21 | Ames waltzer | USH1F | DFNB23 | 90, 91 |
| SANS | SANS | Scaffold protein | 17q24-q25 | Jackson circler | USH1G | 92 | |
| MYO6 | Myosin VI | Intracellular motor | 6q13 | Snell’s waltzer | DFNA22, B37 | 93, 94 | |
| KCNQ1 | KCNQ1 | Potassium channel | 11p15.5 | JLNS1 | 51 | ||
| KCNE1 | KCNE1 | Potassium channel | 21q22.1-q22.2 | JLNS2 | 52 | ||
| MYO15 | Myosin 15 | Intracellular motor | 17p11.2 | Shaker-2 | DFNB3 | 95, 96 |
For complete list, see Hereditary Hearing Loss Homepage, http://webhost.ua.ac.be/hhh.
Genetic defects in potassium channel proteins have also been implicated in hereditary HL. Jervell and Lange-Nielsen syndrome, a recessively inherited disorder, is characterized by congenital bilateral deafness and cardiac abnormalities. The cardiac abnormality is manifested as a prolonged QT interval, which can cause life-threatening cardiac arrhythmias and even sudden death. The underlying cardiac and otologic abnormalities have been attributed to defects in the K+ channel genes KCNQ1 and KCNE1.51,52 KCNQ1 is expressed on the apical surface of the marginal cells of the stria vascularis, in vestibular dark cells, and in cardiac myocytes. Defects in both K+ channels cause a loss of secretion of K+ by the stria vascularis and vestibular cells, causing eventual death of the hair cells (probably by K+ intoxication) with resultant deafness and vestibulopathy.
KCNQ4, another potassium channel gene, has been mapped to human chromosome 1p34, the locus for DFNA2.53 Whereas defects in KCNQ1 alter endolymph secretion, KCNQ4, localized to the basolateral surface of outer hair cells,54 has been implicated in outer hair cell degeneration by abolishing an outward potassium channel current causing chronic depolarization.29,55 These potassium channel genes may prove viable targets for gene therapy. In cultured human tissue, adenovirus drives expression of the reporter gene GFP and KCNQ4 in supporting cells and hair cells (see Studies in Human Tissue, below).20
Spiral Ganglion Cell Survival
When hair cells are lost from trauma, ototoxicity, or degeneration, trophic factors that maintain the survival and functional integrity of their corresponding spiral ganglion neurons (SGCs) are no longer secreted, and SGCs undergo secondary degeneration. SGC survival is critical for cochlear implant performance because the success of the implant depends on the presence of healthy SGCs. Neurotrophins NT-3 and brain-derived neurotrophic factor (BDNF) are critical paracrine regulating proteins involved in the development, maintenance, and survival of auditory and vestibular neurons and hair cells.56,57 Although direct cochlear infusion of BDNF or NT-3 via an osmotic mini-pump resulted in rescue of auditory neurons in the guinea pig treated with a combination aminoglycoside-diuretic deafening regimen,58 continuous infusion with a pump is impractical, cumbersome, and offers only a short-term solution.
Gene transfer of a vector-neurotrophin complex offers a potential means of promoting long-term SGC survival after hair cell loss. HSV-BDNF, AAV-BDNF, and adenovirus-BDNF have all been shown to enhance spiral ganglion cell survival in different mammalian (mouse, rat, and guinea pig) models of aminoglycoside-induced ototoxicity.14,15,17
Glial cell-line-derived neurotrophic factor (GDNF) is yet another paracrine factor shown to be important in the survival of inner ear auditory neurons.59,60 Gene therapy with an adenovirus-GDNF vector (Ad-GDNF) enhanced SGC survival in the guinea pig deafened by an aminoglycoside/diuretic regimen,16 and Ad-GDNF gene therapy plus electrical stimulation preserved SGCs significantly better than either treatment alone.61
Hair Cell Protection
The same trophic factors that enhance SGC survival have also been shown to protect hair cells, including NT-3,62 BDNF,62,63 and GDNF.64,65 Gene therapy using an adenoviral vector carrying the human GDNF gene protected vestibular hair cells and rescued cochlear hair cells and hearing function from gentamicin-induced ototoxicity.66 Co-inoculation of adenoviral vectors, one expressing GDNF and the other expressing transforming growth factor-β1, also protected hearing and hair cells in vivo from the gentamicin regimen.12
As the mechanisms of aminoglycoside induced ototoxicity are elucidated, new targets for gene therapy will be discovered that may allow new vector-therapeutic gene complexes to be designed. Antioxidant gene therapy using adenoviral vectors to over-express the antioxidant enzymes catalase and Mn superoxide dismutase afforded protection of hair cells and hearing when delivered 5 days before a deafening regimen of systemic kanamycin and ethacrynic acid.13
Antioxidant therapy may also target the underlying mechanisms of noise-induced HL. Iron chelators (deferoxamine, mannitol), free radical scavengers (mannitol, glutathione, ebselon), as well as neurotrophic factors have been shown to attenuate cochlear damage caused by acoustic trauma.62,65,67-70 As viral vectors are engineered to contain genes that express antioxidants, iron chelators, or free radical scavengers, another strategy for hair cell protection, in the face of noise-induced cochlear damage, will most likely be validated.
Hair Cell Regeneration
In the developing organism, hair cells and supporting cells arise from a common progenitor cell.71 In birds and reptiles, injured hair cells are replaced through the trans-differentiation of supporting cells into new hair cells.72,73 Mammalian hair cells, on the other hand, are terminally differentiated, and hair cell loss is irreversible. Many of the genes and factors that govern cell fate determination in the sensory epithelium of the inner ear have been elucidated. One such factor is Math1, a basic helix-loop-helix transcription factor, shown to be essential for the generation of inner ear hair cells.74 By cloning the Math1 gene into a plasmid vector and transfecting postnatal rat cochlea explants, Zheng and Gao75 induced over-expression of Math1 and observed ectopic hair cell production. These same researchers used an adenoviral vector with the human atonal homologue1 (Hath1) gene to generate a large number of hair cells in the sensory epithelium of cultured rat utricular maculae.76 In the same rat utricular culture system, new hair cells were also observed with adenovirus-mediated over-expression of Hath1 after aminoglycoside injury.76
Gene transfer with an adenoviral vector containing the Math1 insert has been shown to infect supporting cells and generate new hair cells after a deafening regimen in an adult guinea pig model18; the newly generated hair cells appear to be functional because Ad-Math1 infected guinea pigs demonstrated increased hair cell counts and lower thresholds on ABR relative to untreated, deafened controls.19 A similar experimental protocol was used in the murine labyrinth treated with an aminoglycoside regimen. These authors report recovery of the vestibular epithelium in vitro and recovery of vestibular function in vivo after infusion of an adenoviral vector containing the Math1 insert.77
STUDIES IN HUMAN TISSUE
To begin to examine the feasibility of gene therapy in humans, Kesser et al.20 developed a novel experimental preparation. Vestibular epithelia (ampullae of the semicircular canals and maculae of the utricle and saccule) were harvested from nine patients undergoing the translabyrinthine approach for vestibular schwannoma removal or labyrinthectomy for Ménière’s disease. Recombinant, replication-deficient adenoviral vectors that contained the sequence for the reporter gene GFP were bath applied at titers that ranged between 106 and 1010 infectious particles/mL and allowed to incubate for periods that ranged from 12 to 24 hours and cultured for up to 5 days. Transfection rates were calculated as percent of cells that expressed the GFP reporter gene and were as high as 47.5%.
Both first generation (Ad1-GFP-Q4 and Ad.19/Ad.VgRxR) and second generation adenoviral vectors (Ad2-GFP-Q4) were shown to transfect hair cells and supporting cells in the cultured human vestibular tissue, as demonstrated by expression of the reporter gene GFP (Fig. 1). There were no statistically significant differences between transfection rates of the three adenoviral vectors studied, between the otolithic organs and the semicircular canals, and in inoculation times. Transfection rates were found to be titer and time dependent, with a titer of 2.7 × 107 infectious particles/mL corresponding to a transfection rate of 50% and a steady state transfection rate of 26.5% achieved with a time constant of 6.5 hours.
Fig. 1.
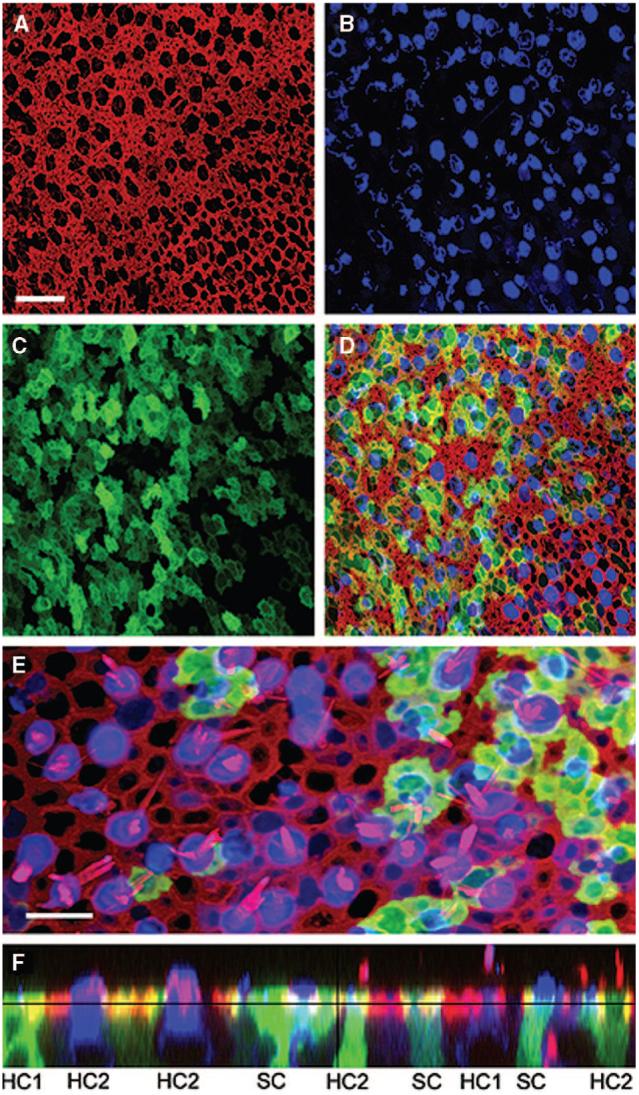
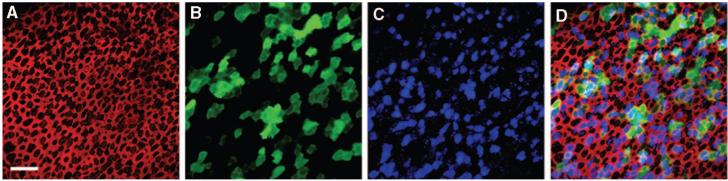
Confocal images of a human utricle harvested from patient 2. The explant was exposed to 107 viral particles/mL of a second generation, replication-deficient adenoviral vector containing the green fluorescent protein (GFP) gene insert for 15 hours and maintained in culture for 48 hours. The explant was then fixed and stained with Alexa Fluor 546-conjugated phalloidin and an antibody to myosin VIIa and an Alexa Fluor 633-conjugated secondary antibody. Scale bar = 20 μm and applies to A to D, which all show the same image field. (A) A stack of 12 images taken just beneath the apical surface of the epithelium was collapsed to show the cell bodies (B) and the subapical actin (red) that rings each hair cell and supporting cell. (B) Myosin VIIa immunoreactivity (blue) localizes sensory hair cell bodies. (C) Green fluorescence revealed that both hair cells and supporting cells were transfected by the vector and expressed GFP. (D) Merge of A to D. (E) Higher magnification overlay view from a different region of the same epithelium shown in panels A to D. To generate the image a stack of 13 images focused at the hair bundle level was collapsed (red=actin) and overlaid atop a collapsed stack of 10 images focused at the cell body level from the same image field (blue=myosin VIIa; green=GFP). Scale bar=10 μm. (F) A stack of 19 images projected to reveal a cross-sectional view of the sensory epithelium. Both type I (HC1) and type II hair cells (HC2) and supporting cells were GFP-positive. Myosin VIIa-positive hair cells are also evident. Same color code as for panels A to E. Reprinted from Kesser et al.20
One adenoviral vector (Ad2-GFP-Q4) was engineered to contain the potassium channel gene KCNQ4. In the human vestibular epithelia, this vector drove expression of not only GFP but also the KCNQ4 potassium channel protein. Although some of the KCNQ4 expression seen in the images represents endogenous expression (Fig. 2), the high rate of colocalization of KCNQ4 with GFP (79% of cells) argues for at least some exogenous virally expressed KCNQ4 (Fig. 3).
Fig. 2.
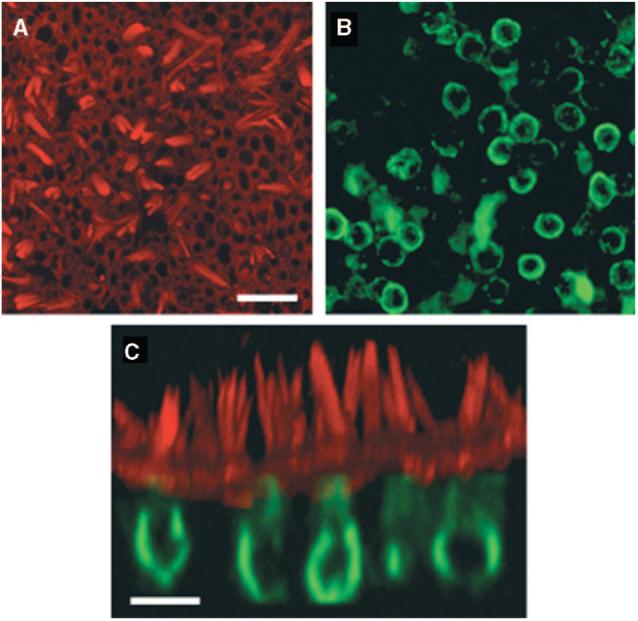
Confocal images of a saccule harvested from patient 1. The explant was cultured for 24 hours, fixed, and stained with Alexa Fluor 546 phalloidin (red) and endogenous KCNQ4 (green). (A) View from just above the apical surface of the epithelium reveals the actin-rich hair bundles. Scale bar = 25 μm and applies to A and B. (B) An image focused at the cell body level revealed the endogenous pattern of KCNQ4 expression. (C) Cross-sectional projection of the saccule generated from a stack of 43 images focused every 1.0 μm. The actin and KCNQ4 channels were merged. Scale bar = 10 μm. Reprinted from Kesser et al.20
Fig. 3.
Stacks of confocal images taken just beneath the apical surface were used to generate images of the saccule harvested from a second patient undergoing labyrinthectomy for Ménière’s disease (patient 7). The explant culture was exposed to 2.3 × 107 viral particles/mL of a second generation, replication-deficient adenovirus containing both the green fluorescent protein (GFP) and wild-type KCNQ4 gene inserts for 24 hours, fixed, and stained with Alex Fluor 546 phalloidin (red) and KCNQ4 (blue) and GFP (green). The scale bar represents 20 μm and applies to all images. The same field and focal planes are shown in A to D. (A) An image just beneath the apical surface of the epithelium shows the actin that rings each cell. (B) One hundred three GFP-positive cells are evident in this field. (C) The KCNQ4 antibody labeled both endogenous hair cell KCNQ4 and exogenous virally expressed KCNQ4. (D) Merge of A to C shows colocalization of GFP and KCNQ4 in 78.6% of the cells. Reprinted from Kesser et al.20
By demonstrating that human vestibular sensory epithelia, including hair cells and supporting cells, can be kept viable in culture as long as 5 days (and possibly longer because longer culture times were not tested), these experiments establish a model system for the study of potential therapeutic agents to treat inner ear dysfunction. Patients undergoing labyrinthectomy for vestibular schwannoma resection or for Ménière’s disease are potential tissue donors to study such reagents. Both healthy and diseased tissue can be assayed with this experimental protocol; adenoviral vectors transfected hair cells and supporting cells of aged, adult patients with both vestibular schwannoma, whose sensory epithelium is presumably normal, and Ménière’s disease, whose vestibular epithelia are most likely diseased.
Adenovirus drove expression not only of the reporter gene GFP but also of the deafness gene KCNQ4, as demonstrated by the significantly higher expression rates of KCNQ4 in GFP-positive cells. Although the transgenic potassium channel’s function is unknown, over-expression of wild-type transgenes is one strategy for overcoming dominant-negative mutations. Human vestibular explants harvested from mature human vestibular end organs at the time of labyrinthectomy and maintained in organotypic culture provide a unique opportunity to study gene transfer agents as well as other pharmacologic agents designed to treat inner ear disorders. This in vitro model system may be instrumental in the translation of agents from the laboratory to clinical trials to treat patients with HL or balance disorders.
CONCLUSIONS
Viral-mediated gene transfer into the inner ear has evolved over the last decade from simple vectors with single reporter gene inserts to document feasibility and cellular tropism to the engineering of more complex vectors with varying and multiple transgenes to treat the spectrum of insults to the inner ear. Experiments demonstrating gene transfer in human vestibular sensory epithelia offer proof of principle that mature, even aged, and in some cases, diseased, human inner ear tissue is a suitable target for gene transfer agents and that adenovirus is a promising vehicle for gene transfer in humans. Challenges for the future of inner ear gene therapy include refining vectors to improve transfection efficiency and cell targeting, refining methods of delivery to minimize trauma to the inner ear while ensuring widespread transfection of the whole cochlea or inner ear,78 and discovering new genes whose replacement or whose silencing may restore function.79
Applications of transgene therapy, including correction of genetic HL, protection of hair cells and spiral ganglion cells to prevent HL or rescue hearing, and hair cell regeneration to restore hearing, will be refined over the next several years with improvements in methods of delivery and distribution within the inner ear, targeting of specific inner ear cell-types with the use of specialized promoter sequences and viral serotypes, and engineering of vectors to provide stable, safe, long-term transgene expression. With the discovery that human vestibular epithelia can be harvested, cultured, and transfected with a recombinant, replication-deficient adenovirus, human hair cells can now be accessed and examined with various experimental manipulations. This platform can be used to test viral vectors and their transgenes, neurotrophic factors, cell-signaling proteins, transcription factors, stem cell candidates, and other pharmacologic agents designed to treat human inner ear dysfunction. As our knowledge of hair cell function and the factors that injure hair cells deepens, new targets and new transgenes will likely be identified, and new strategies for curing HL and balance dysfunction will likely emerge.
Acknowledgments
Supported by a Triological Society Career Development Award to Dr. Kesser and NIH/NIDCD Grant #DC005439 to Dr. Holt and the Virginia Lion’s Hearing Foundation.
Footnotes
Presented at the Combined Otolaryngology Spring Meetings, San Diego, California, U.S.A., April 26, 2007.
BIBLIOGRAPHY
- 1.Lalwani AK, Castelein CM. Cracking the auditory genetic code: nonsyndromic hereditary hearing impairment. Am J Otol. 1999;20:115–132. [PubMed] [Google Scholar]
- 2.Raphael Y, Frisancho JC, Roessler BJ. Adenoviral-mediated gene transfer into guinea pig cochlear cells in vivo. Neurosci Lett. 1996;207:137–141. doi: 10.1016/0304-3940(96)12499-x. [DOI] [PubMed] [Google Scholar]
- 3.Weiss MA, Frisancho JC, Roessler BJ, Raphael Y. Viral-mediated gene transfer in the cochlea. Int J Devel Neurosci. 1997;15:577–583. doi: 10.1016/s0736-5748(96)00112-8. [DOI] [PubMed] [Google Scholar]
- 4.Dazert Transfection of neonatal rat cochlear cells in vitro with an adenovirus vector. Int J Devel Neurosci. 1997;15:595–600. doi: 10.1016/s0736-5748(96)00114-1. [DOI] [PubMed] [Google Scholar]
- 5.Lalwani AK, Walsh BJ, Reilly PG, Muzyczka N, Mhatre AN. Development of in vivo gene therapy for hearing disorders: introduction of adeno-associated virus into the cochlea of the guinea pig. Gene Ther. 1996;3:588–592. [PubMed] [Google Scholar]
- 6.Lalwani AK, Han JJ, Walsh BJ, Zolotukhin S, Muzyczka N, Mhatre AN. Green fluorescent protein as a reporter for gene transfer studies in the cochlea. Hear Res. 1997;114:139–147. doi: 10.1016/s0378-5955(97)00151-2. [DOI] [PubMed] [Google Scholar]
- 7.Staecker H, Li D, O’Malley BW, Van de Water TR. Gene expression in the mammalian cochlea: A study of multiple vector systems. Acta Otolaryngol. 2001;121:157–163. doi: 10.1080/000164801300043307. [DOI] [PubMed] [Google Scholar]
- 8.Derby ML, Sena-Esteves M, Breakefield XO, Corey DP. Gene transfer into the mammalian inner ear using HSV-1 and vaccinia virus vectors. Hear Res. 1999;134:1–8. doi: 10.1016/s0378-5955(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 9.Han JJ, Mhatre AN, Wareing M, et al. Transgene expression in the guinea pig cochlea mediated by a lentivirus-derived gene transfer vector. Hum Gene Ther. 1999;10:1867–1873. doi: 10.1089/10430349950017545. [DOI] [PubMed] [Google Scholar]
- 10.Wareing M, Mhatre AN, Pettis R, et al. Cationic liposome mediated transgene expression in the guinea pig cochlea. Hear Res. 1999;128:61–69. doi: 10.1016/s0378-5955(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 11.Jero J, Mhatre AN, Tseng CJ, et al. Cochlear gene delivery through an intact round window membrane in mouse. Hum Gene Ther. 2001;12:539–548. doi: 10.1089/104303401300042465. [DOI] [PubMed] [Google Scholar]
- 12.Kawamoto K, Yagi M, Stover T, Kanzaki S, Raphael Y. Hearing and hair cells are protected by adenoviral gene therapy with TGF-beta1 and GDNF. Mol Ther. 2003;7:484–492. doi: 10.1016/s1525-0016(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 13.Kawamoto K, Sha SH, Minoda R, et al. Antioxidant gene therapy can protect hearing and hair cells from ototoxicity. Mol Ther. 2004;9:173–181. doi: 10.1016/j.ymthe.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 14.Lalwani AK, Han JJ, Castelein CM, Carvalho GJ, Mhatre AN. In vitro and in vivo assessment of the ability of adeno-associated virus-brain-derived neurotrophic factor to enhance spiral ganglion cell survival following ototoxic insult. Laryngoscope. 2002;112:1325–1334. doi: 10.1097/00005537-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Staecker H, Gabaizadeh R, Federoff H, Van de Water TR. Brain-derived neurotrophic factor gene therapy prevents spiral ganglion degeneration after hair cell loss. Otolaryngol Head Neck Surg. 1998;119:7–13. doi: 10.1016/S0194-5998(98)70194-9. [DOI] [PubMed] [Google Scholar]
- 16.Yagi M, Kanzaki S, Kawamoto K, et al. Spiral ganglion neurons are protected from degeneration by GDNF gene therapy. J Assoc Res Otolaryngol. 2000;1:315–325. doi: 10.1007/s101620010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakaizumi T, Kawamoto K, Minoda R, Raphael Y. Adenovirus-mediated expression of brain-derived neurotrophic factor protects spiral ganglion neurons from ototoxic damage. Audiol Neurootol. 2004;9:135–143. doi: 10.1159/000077264. [DOI] [PubMed] [Google Scholar]
- 18.Kawamoto K, Ishimoto S, Minoda R, Brough de, Raphael Y. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci. 2003;23:4395–4400. doi: 10.1523/JNEUROSCI.23-11-04395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Izumikawa M, Minoda R, Kawamoto K, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals [see comment] Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- 20.Kesser BW, Hashisaki GT, Fletcher K, Eppard H, Holt JR. An in vitro model system to study gene therapy in the human inner ear. Gene Ther. 2007;14:1121–1131. doi: 10.1038/sj.gt.3302980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holt JR, Johns DC, Wang S, et al. Functional expression of exogenous proteins in mammalian sensory hair cells infected with adenoviral vectors. J Neurophysiol. 1999;81:1881–1888. doi: 10.1152/jn.1999.81.4.1881. [DOI] [PubMed] [Google Scholar]
- 22.Geschwind MD, Hartnick CJ, Liu W, Amat J, Van de Water TR, Federoff HJ. Defective HSV-1 vector expressing BDNF in auditory ganglia elicits neurite outgrowth: model for treatment of neuron loss following cochlear degeneration. Hum Gene Ther. 1996;7:173–182. doi: 10.1089/hum.1996.7.2-173. [DOI] [PubMed] [Google Scholar]
- 23.Di Pasquale G, Rzadzinska A, Schneider ME, Bossis I, Chiorini JA, Kachar B. A novel bovine virus efficiently transduces inner ear neuroepithelial cells. Mol Ther. 2005;11:849–855. doi: 10.1016/j.ymthe.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 24.He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B. A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA. 1998;95:2509–2514. doi: 10.1073/pnas.95.5.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luebke AE, Foster PK, Muller CD, Peel AL. Cochlear function and transgene expression in the guinea pig cochlea, using adenovirus- and adeno-associated virus-directed gene transfer. Hum Gene Ther. 2001;12:773–781. doi: 10.1089/104303401750148702. [DOI] [PubMed] [Google Scholar]
- 26.Holt JR. Viral-mediated gene transfer to study the molecular physiology of the mammalian inner ear. Audiol Neurootol. 2002;7:157–160. doi: 10.1159/000058302. [DOI] [PubMed] [Google Scholar]
- 27.Luebke AE, Steiger JD, Hodges BL, Amalfitano A. A modified adenovirus can transfect cochlear hair cells in vivo without compromising cochlear function. Gene Ther. 2001;8:789–794. doi: 10.1038/sj.gt.3301445. [DOI] [PubMed] [Google Scholar]
- 28.Corey DP, Garcia-Anoveros J, Holt JR, et al. TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells [see comment] Nature. 2004;432:723–730. doi: 10.1038/nature03066. [DOI] [PubMed] [Google Scholar]
- 29.Holt JR, Stauffer EA, Abraham D, Geleoc GS. Dominant-negative inhibition of M-like potassium conductances in hair cells of the mouse inner ear. J Neurosci. 2007;27:8940–8951. doi: 10.1523/JNEUROSCI.2085-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amalfitano A. Utilization of adenovirus vectors for multiple gene transfer applications [Review, 95 refs] Methods (Duluth) 2004;33:173–178. doi: 10.1016/j.ymeth.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 31.Kawamoto K, Oh SH, Kanzaki S, Brown N, Raphael Y. The functional and structural outcome of inner ear gene transfer via the vestibular and cochlear fluids in mice. Mol Ther. 2001;4:575–585. doi: 10.1006/mthe.2001.0490. [DOI] [PubMed] [Google Scholar]
- 32.Ishimoto S, Kawamoto K, Kanzaki S, Raphael Y. Gene transfer into supporting cells of the organ of Corti. Hear Res. 2002;173:187–197. doi: 10.1016/s0378-5955(02)00579-8. [DOI] [PubMed] [Google Scholar]
- 33.Yamasoba T, Suzuki M, Kondo K. Transgene expression in mature guinea pig cochlear cells in vitro. Neurosci Lett. 2002;335:13–16. doi: 10.1016/s0304-3940(02)01139-4. [DOI] [PubMed] [Google Scholar]
- 34.Han D, Yu Z, Fan E, et al. Morphology of auditory hair cells in guinea pig cochlea after transgene expression. Hear Res. 2004;190:25–30. doi: 10.1016/S0378-5955(04)00020-6. [DOI] [PubMed] [Google Scholar]
- 35.Smith AE. Viral vectors in gene therapy. Annu Rev Microbiol. 1995;49:807–838. doi: 10.1146/annurev.mi.49.100195.004111. [DOI] [PubMed] [Google Scholar]
- 36.Lalwani A, Walsh B, Reilly P, et al. Long-term in vivo cochlear transgene expression mediated by recombinant adeno-associated virus. Gene Ther. 1998;5:277–281. doi: 10.1038/sj.gt.3300573. [DOI] [PubMed] [Google Scholar]
- 37.Lalwani AK, Walsh BJ, Carvalho GJ, Muzyczka N, Mhatre AN. Expression of adeno-associated virus integrated transgene within the mammalian vestibular organs. Am J Otol. 1998;19:390–395. [PubMed] [Google Scholar]
- 38.Stone IM, Lurie DI, Kelley MW, Poulsen DJ. Adeno-associated virus-mediated gene transfer to hair cells and support cells of the murine cochlea. Mol Ther. 2005;11:843–848. doi: 10.1016/j.ymthe.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Kaplitt MG, Leone P, Samulski RJ, et al. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 40.Lalwani AK, Mhatre AN. Cochlear gene therapy [Review, 26 refs] Ear Hear. 2003;24:342–348. doi: 10.1097/01.AUD.0000079798.24346.35. [DOI] [PubMed] [Google Scholar]
- 41.Palu G, Parolin C, Takeuchi Y, Pizzato M. Progress with retroviral gene vectors. Rev Med Virol. 2000;10:185–202. doi: 10.1002/(sici)1099-1654(200005/06)10:3<185::aid-rmv285>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 42.Gunzburg WH, Salmons B. Development of retroviral vectors as safe, targeted gene delivery systems. J Mol Med. 1996;74:171–182. doi: 10.1007/BF00204747. [DOI] [PubMed] [Google Scholar]
- 43.Ailles LE, Naldini L. HIV-1-derived lentiviral vectors. Curr Top Microbiol Immunol. 2002;261:31–52. doi: 10.1007/978-3-642-56114-6_2. [DOI] [PubMed] [Google Scholar]
- 44.Leventis R, Silvius JR. Interactions of mammalian cells with lipid dispersions containing novel metabolizable cationic amphiphiles. Biochim Biophys Acta. 1990;1023:124–132. doi: 10.1016/0005-2736(90)90017-i. [DOI] [PubMed] [Google Scholar]
- 45.Jero J, Tseng CJ, Mhatre AN, Lalwani AK. A surgical approach appropriate for targeted cochlear gene therapy in the mouse. Hear Res. 2001;151:106–114. doi: 10.1016/s0378-5955(00)00216-1. [DOI] [PubMed] [Google Scholar]
- 46.Stover T, Yagi M, Raphael Y. Transduction of the contralateral ear after adenovirus-mediated cochlear gene transfer. Gene Ther. 2000;7:377–383. doi: 10.1038/sj.gt.3301108. [DOI] [PubMed] [Google Scholar]
- 47.Stover T, Yagi M, Raphael Y. Cochlear gene transfer: round window versus cochleostomy inoculation. Hear Res. 1999;136:124–130. doi: 10.1016/s0378-5955(99)00115-x. [DOI] [PubMed] [Google Scholar]
- 48.Dazert S, Aletsee C, Brors D, Gravel C, Sendtner M, Ryan A. In vivo adenoviral transduction of the neonatal rat cochlea and middle ear. Hear Res. 2001;151:30–40. doi: 10.1016/s0378-5955(00)00189-1. [DOI] [PubMed] [Google Scholar]
- 49.Yamasoba T, Yagi M, Roessler BJ, Miller JM, Raphael Y. Inner ear transgene expression after adenoviral vector inoculation in the endolymphatic sac. Hum Gene Ther. 1999;10:769–774. doi: 10.1089/10430349950018526. [DOI] [PubMed] [Google Scholar]
- 50.Praetorius M, Knipper M, Schick B, et al. A novel vestibular approach for gene transfer into the inner ear. Audiol Neurootol. 2002;7:324–334. doi: 10.1159/000066157. [DOI] [PubMed] [Google Scholar]
- 51.Neyroud N, Tesson F, Denjoy I, et al. A novel mutation in the potassium channel gene KVLQT1 causes the Jervell and Lange-Nielsen cardioauditory syndrome [see comment] Nat Genet. 1997;15:186–189. doi: 10.1038/ng0297-186. [DOI] [PubMed] [Google Scholar]
- 52.Schulze-Bahr E, Wang Q, Wedekind H, et al. KCNE1 mutations cause Jervell and Lange-Nielsen syndrome. Nat Genet. 1997;17:267–268. doi: 10.1038/ng1197-267. [DOI] [PubMed] [Google Scholar]
- 53.Kubisch C, Schroeder BC, Friedrich T, et al. KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell. 1999;96:437–446. doi: 10.1016/s0092-8674(00)80556-5. [DOI] [PubMed] [Google Scholar]
- 54.Kharkovets T, Hardelin JP, Safieddine S, et al. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway [see comment] Proc Natl Acad Sci USA. 2000;97:4333–4338. doi: 10.1073/pnas.97.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kharkovets T, Dedek K, Maier H, et al. Mice with altered KCNQ4 K+ channels implicate sensory outer hair cells in human progressive deafness. EMBO J. 2006;25:642–652. doi: 10.1038/sj.emboj.7600951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ernfors P, Van de Water T, Loring J, Jaenisch R. Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron. 1995;15:739. doi: 10.1016/0896-6273(95)90263-5. erratum. [DOI] [PubMed] [Google Scholar]; Neuron. 1995;14:1153–1164. doi: 10.1016/0896-6273(95)90263-5. [DOI] [PubMed] [Google Scholar]
- 57.Morrison RS, Kinoshita Y, Johnson MD, Ghatan S, Ho JT, Garden G. Neuronal survival and cell death signaling pathways. Adv Exp Med Biol. 2002;513:41–86. doi: 10.1007/978-1-4615-0123-7_2. [DOI] [PubMed] [Google Scholar]
- 58.Staecker H, Kopke R, Malgrange B, Lefebvre P, Van de Water TR. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. Neuroreport. 1996;7:889–894. doi: 10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- 59.Ylikoski J, Pirvola U, Virkkala J, et al. Guinea pig auditory neurons are protected by glial cell line-derived growth factor from degeneration after noise trauma. Hear Res. 1998;124:17–26. doi: 10.1016/s0378-5955(98)00095-1. [DOI] [PubMed] [Google Scholar]
- 60.Kuang R, Hever G, Zajic G, et al. Glial cell line-derived neurotrophic factor. Potential for otoprotection. Ann NY Acad Sci. 1999;884:270–291. doi: 10.1111/j.1749-6632.1999.tb08648.x. [DOI] [PubMed] [Google Scholar]
- 61.Kanzaki S, Stover T, Kawamoto K, et al. Glial cell line-derived neurotrophic factor and chronic electrical stimulation prevent VIII cranial nerve degeneration following denervation. J Comp Neurol. 2002;454:350–360. doi: 10.1002/cne.10480. [DOI] [PubMed] [Google Scholar]
- 62.Shoji F, Miller AL, Mitchell A, Yamasoba T, Altschuler RA, Miller JM. Differential protective effects of neurotrophins in the attenuation of noise-induced hair cell loss. Hear Res. 2000;146:134–142. doi: 10.1016/s0378-5955(00)00106-4. [DOI] [PubMed] [Google Scholar]
- 63.Lopez I, Honrubia V, Lee S, et al. The protective effect of brain-derived neurotrophic factor after gentamicin ototoxicity. Am J Otol. 1999;20:317–324. [PubMed] [Google Scholar]
- 64.Keithley EM, Ma CL, Ryan AF, Louis JC, Magal E. GDNF protects the cochlea against noise damage. Neuroreport. 1998;9:2183–2187. doi: 10.1097/00001756-199807130-00007. [DOI] [PubMed] [Google Scholar]
- 65.Shoji F, Yamasoba T, Magal E, Dolan DF, Altschuler RA, Miller JM. Glial cell line-derived neurotrophic factor has a dose dependent influence on noise-induced hearing loss in the guinea pig cochlea. Hear Res. 2000;142:41–5. doi: 10.1016/s0378-5955(00)00007-1. [DOI] [PubMed] [Google Scholar]
- 66.Suzuki M, Yagi M, Brown JN, Miller AL, Miller JM, Raphael Y. Effect of transgenic GDNF expression on gentamicin-induced cochlear and vestibular toxicity. Gene Ther. 2000;7:1046–1054. doi: 10.1038/sj.gt.3301180. [DOI] [PubMed] [Google Scholar]
- 67.Yamasoba T, Schacht J, Shoji F, Miller JM. Attenuation of cochlear damage from noise trauma by an iron chelator, a free radical scavenger and glial cell line-derived neurotrophic factor in vivo. Brain Res. 1999;815:317–325. doi: 10.1016/s0006-8993(98)01100-7. [DOI] [PubMed] [Google Scholar]
- 68.Ohinata Y, Yamasoba T, Schacht J, Miller JM. Glutathione limits noise-induced hearing loss. Hear Res. 2000;146:28–34. doi: 10.1016/s0378-5955(00)00096-4. [DOI] [PubMed] [Google Scholar]
- 69.Pourbakht A, Yamasoba T. Ebselen attenuates cochlear damage caused by acoustic trauma. Hear Res. 2003;181:100–10. doi: 10.1016/s0378-5955(03)00178-3. [DOI] [PubMed] [Google Scholar]
- 70.Yamasoba T, Pourbakht A, Sakamoto T, Suzuki M. Ebselen prevents noise-induced excitotoxicity and temporary threshold shift. Neurosci Lett. 2005;380:234–238. doi: 10.1016/j.neulet.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 71.Fekete DM, Muthukumar S, Karagogeos D. Hair cells and supporting cells share a common progenitor in the avian inner ear. J Neurosci. 1998;18:7811–7821. doi: 10.1523/JNEUROSCI.18-19-07811.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cotanche DA. Structural recovery from sound and aminoglycoside damage in the avian cochlea. Audiol Neurootol. 1999;4:271–285. doi: 10.1159/000013852. [DOI] [PubMed] [Google Scholar]
- 73.Adler HJ, Raphael Y. New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear. Neurosci Lett. 1996;210:73. doi: 10.1016/0304-3940(96)12367-3. erratum. 24. [DOI] [PubMed] [Google Scholar]; Neurosci Lett. 1996;205:17–20. doi: 10.1016/0304-3940(96)12367-3. [DOI] [PubMed] [Google Scholar]
- 74.Bermingham NA, Hassan BA, Price SD, et al. Math1: an essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- 75.Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- 76.Shou J, Zheng JL, Gao WQ. Robust generation of new hair cells in the mature mammalian inner ear by adenoviral expression of Hath1. Mol Cell Neurosci. 2003;23:169–179. doi: 10.1016/s1044-7431(03)00066-6. [DOI] [PubMed] [Google Scholar]
- 77.Staecker H, Praetorius M, Baker K, Brough DE. Vestibular hair cell regeneration and restoration of balance function induced by math1 gene transfer. Otol Neurotol. 2007;28:223–231. doi: 10.1097/MAO.0b013e31802b3225. [DOI] [PubMed] [Google Scholar]
- 78.Sugahara K, Shimogori H, Okuda T, Takemoto T, Yamashita H. Novel method for homogeneous gene transfer to the inner ear. Acta Otolaryngol Suppl. 2004:19–22. doi: 10.1080/03655230410017607. [DOI] [PubMed] [Google Scholar]
- 79.Kanzaki S, Beyer L, Karolyi IJ, et al. Transgene correction maintains normal cochlear structure and function in 6-month-old Myo15a mutant mice. Hear Res. 2006;214:37–40. doi: 10.1016/j.heares.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 80.Staecker H, Brough DE, Praetorius M, Baker K. Drug delivery to the inner ear using gene therapy [Review, 64 refs] Otolaryngol Clin North Am. 2004;37:1091–1108. doi: 10.1016/j.otc.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 81.Praetorius M, Baker K, Weich CM, Plinkert PK, Staecker H. Hearing preservation after inner ear gene therapy: the effect of vector and surgical approach. ORL J Otorhinolaryngol Relat Spec. 2003;65:211–214. doi: 10.1159/000073117. [DOI] [PubMed] [Google Scholar]
- 82.Carvalho GJ, Lalwani AK. The effect of cochleostomy and intracochlear infusion on auditory brain stem response threshold in the guinea pig. Am J Otol. 1999;20:87–90. [PubMed] [Google Scholar]
- 83.Kho ST, Pettis RM, Mhatre AN, Lalwani AK. Cochlear microinjection and its effects upon auditory function in the guinea pig. Eur Arch Otorhinolaryngol. 2000;257:469–472. doi: 10.1007/s004050000280. [DOI] [PubMed] [Google Scholar]
- 84.Swiderski DL, Johnson M, Raphael Y. A new surgical approach for injections into the guinea pig cochlea [Abstract 283]; From the Abstracts of the Twenty-Ninth Annual Mid-winter Research Meeting; Association for Research in Otolaryngology. 2006. [Google Scholar]
- 85.Self T, Mahony M, Fleming J, Walsh J, Brown SD, Steel KP. Shaker-1 mutations reveal roles for myosin VIIA in both development and function of cochlear hair cells. Development. 1998;125:557–566. doi: 10.1242/dev.125.4.557. [DOI] [PubMed] [Google Scholar]
- 86.Weil D, Blanchard S, Kaplan J, et al. Defective myosin VIIA gene responsible for Usher syndrome type 1B. Nature. 1995;374:60–61. doi: 10.1038/374060a0. [DOI] [PubMed] [Google Scholar]
- 87.Verpy E, Leibovici M, Zwaenepoel I, et al. A defect in harmonin, a PDZ domain-containing protein expressed in the inner ear sensory hair cells, underlies Usher syndrome type 1C [see comment] Nat Genet. 2000;26:51–55. doi: 10.1038/79171. [DOI] [PubMed] [Google Scholar]
- 88.Di Palma F, Holme RH, Bryda EC, et al. Mutations in Cdh23, encoding a new type of cadherin, cause stereocilia disorganization in waltzer, the mouse model for Usher syndrome type 1D. Nat Genet. 2001;27:103–107. doi: 10.1038/83660. [DOI] [PubMed] [Google Scholar]
- 89.Bork JM, Peters LM, Riazuddin S, et al. Usher syndrome 1D and nonsyndromic autosomal recessive deafness DFNB12 are caused by allelic mutations of the novel cadherin-like gene CDH23. Am J Hum Genet. 2001;68:26–37. doi: 10.1086/316954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Alagramam KN, Murcia CL, Kwon HY, Pawlowski KS, Wright CG, Woychik RP. The mouse Ames waltzer hearing-loss mutant is caused by mutation of Pcdh15, a novel protocadherin gene. Nat Genet. 2001;27:99–102. doi: 10.1038/83837. [DOI] [PubMed] [Google Scholar]
- 91.Ahmed ZM, Riazuddin S, Ahmad J, et al. PCDH15 is expressed in the neurosensory epithelium of the eye and ear and mutant alleles are responsible for both USH1F and DFNB23. Hum Mol Genet. 2003;12:3215–3223. doi: 10.1093/hmg/ddg358. [DOI] [PubMed] [Google Scholar]
- 92.Weil D, El-Amraoui A, Masmoudi S, et al. Usher syndrome type I G (USH1G) is caused by mutations in the gene encoding SANS, a protein that associates with the USH1C protein, harmonin. Hum Mol Genet. 2003;12:463–471. doi: 10.1093/hmg/ddg051. [DOI] [PubMed] [Google Scholar]
- 93.Ahmed ZM, Morell RJ, Riazuddin S, et al. Mutations of MYO6 are associated with recessive deafness, DFNB37. Am J Hum Genet. 2003;72:1315–1322. doi: 10.1086/375122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Melchionda S, Ahituv N, Bisceglia L, et al. MYO6, the human homologue of the gene responsible for deafness in Snell’s waltzer mice, is mutated in autosomal dominant nonsyndromic hearing loss. Am J Hum Genet. 2001;69:635–640. doi: 10.1086/323156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Probst FJ, Fridell RA, Raphael Y, et al. Correction of deafness in shaker-2 mice by an unconventional myosin in a BAC transgene [see comment] Science. 1998;280:1444–1447. doi: 10.1126/science.280.5368.1444. [DOI] [PubMed] [Google Scholar]
- 96.Wang A, Liang Y, Fridell RA, et al. Association of unconventional myosin MYO15 mutations with human nonsyndromic deafness DFNB3 [see comment] Science. 1998;280:1447–1451. doi: 10.1126/science.280.5368.1447. [DOI] [PubMed] [Google Scholar]