Abstract
TORDOFF, M.G., L.K. ALARCON AND M.P. LAWLER. Preferences of 14 rat strains for 17 taste compounds. PHYSIOL BEHAV 00(0) 000-000, 2008.--Two-bottle choice tests were used to assess the taste preferences of 8 male and 8 female rats from 3 outbred strains (SD, LE, WI) and 11 inbred strains (BN, BUF, COP, DA, Dahl-S, F344, FHH, LEW, Noble, PVG, SHR). Each rat received a series of 109 48-h tests with a choice between water and a “taste solution”. Four to eight concentrations of the following compounds were tested: NaCl, CaCl2, NH4Cl, KCl, MgCl2, saccharin, sucrose, ethanol, HCl, citric acid, quinine hydrochloride (QHCl), caffeine, denatonium, monosodium glutamate (MSG), Polycose, corn oil, and capsaicin. Strain differences (p<0.001) were observed in preferences for at least one concentration of all compounds tested except denatonium (p = 0.0015). There were also strain differences in the following ancillary measures: fungiform papillae number, water intake, food intake, and body weight. There were sex differences in food intake and body weight but no concerted sex differences in any of the other measures, including preferences for any taste solution. This comprehensive source of information can be used to guide the choice of appropriate rat strains and taste solution concentrations for future genetic studies.
Keywords: sweet, sour, salty, bitter, umami, calcium, oil, trigeminal, strain survey
Rats have been used in many experiments to understand the mechanisms underlying the selection of nutrients and taste compounds. The large majority of these studies have been focused on physiological and behavioral controls of ingestion but rarely on genetic ones. The genetic approach has been spearheaded by work in mice, and has led to several recent important advances, such as the identification of sweet, sour, bitter and umami receptors [reviews (4,10,76)]. One reason for this is that genetic and molecular tools are available for the mouse but not well-developed for the rat, although this is changing [e.g., (2,54,55,62,74)]. Another is that there are few appropriate taste phenotype data available for the rat. Genetic analyses in mice generally begin with the discovery of phenotypic differences among inbred strains but most studies aimed at understanding rat taste preferences have used outbred strains. There are a few comparisons of different rat strain preferences for sodium and ethanol (see below) but very limited data involving other taste compounds.
The purpose of this study was to provide comprehensive information about the taste preferences of rats that could be used as the basis for subsequent genetic analyses. To this end, we used two-bottle choice tests to compare the preferences for various concentrations of 17 commonly-used taste compounds among 3 outbred and 11 inbred rat strains.
METHODS
Choice of rat strains tested
Over 1000 rat strains have been bred for research of which about half are inbred (54,62). Several considerations went into the choice of the 14 strains tested here. The long-term goal of this work is to conduct genetic analyses, and so when we began this study in December 2005, we chose seven commercially-available inbred strains that formed the basis of a panel of single nucleotide polymorphisms (SNPs) being generated at Baylor College of Medicine (DA, Dahl, F344, FHH, LEW, PVG, and SHR; see Table 1 for full strain designations). This choice was unfortunate because the SNP data have not yet appeared in public databases. However, a European consortium has recently provided comprehensive SNP data based on 6 of these strains and 54 others (55,74). A second consideration was to provide maximum genetic diversity in order to capture the widest range of phenotypes. To do this, we consulted a rat phylogenetic tree based on microsatellite markers (77), which led us to include four additional inbred strains (BN, BUF, COP, and Noble), with also the consideration that these were commercially available. Finally, we included three outbred strains (SD, LE, WI). These have been used in much of the existing literature on rat taste preference research, and they allowed us to compare differences in variability between inbred and outbred strains.
Table 1.
List of rat strains tested, their sources, and the replication in which they were tested
| Our abb’n |
Full Strain Abbreviation |
Strain name | Inbred (I) or Outbred (O) |
Vendor and strain code |
Tested in squad |
|---|---|---|---|---|---|
| SD | Crl:CD(SD) | Sprague Dawley CD (IGS) | O | CR-001 | 2 |
| LE | Crl:LE | Long Evans | O | CR-006 | 2 |
| WI | Crl:WI | Wistar | O | CR-003 | 2 |
| BN | BN/SsNHsdMcwiCrl | Brown Norway | I | CR-327 | 2 |
| BUF | BUF/CrCrl | Buffalo | I | CR-281 | 2 |
| COP | COP/CrCrl | Copenhagen | I | CR-286 | 2 |
| DA | DA/OlaHsd | Dark Agouti | I | H | 1 |
| Dahl | Dahl SS/JrHsdMcwi | Dahl-S (salt-sensitive) | I | CR-320 | 1 |
| F344 | F344/NTac | Fischer 344 | I | T-F344 | 1 |
| FHH | FHH/EurMcwi | Fawn Hooded | I | CR-321 | 1a |
| LEW | LEW/SsNHsd | Lewis | I | H | 1 |
| Nob | Noble/CrCrl | Noble | I | CR-056 | 2 |
| PVG | PVG/OlaHsd | Piebald Virol Glaxo | I | H | 1 |
| SHR | SHR/NCrl | Spontaneously hypertensive | I | CR-007 | 1 |
Vendor: CR = Charles River, www.criver.com, MA; T = Taconic, www.taconic.com; H = Harlan, www.harlan.com. Harlan does not use strain codes. Squad 1 = tests began March 8th 2006, 1a = tests began March 22nd 2006; 2 = tests began January 16th 2007. For Crl:CD(SD), CD = Cesarean-derived, IGS = International Genetic Standard system of breeding.
When referring to the strains, we use the abbreviations shown in Table 1. When a list of strains is called for, we give the three outbred strains first then the 11 inbred strains, in alphabetical order.
Subjects and maintenance
The experiment was conducted in two almost identical squads, each involving 8 male and 8 female rats from 7 strains (Table 1). Rats were received from one of three commercial vendors (Table 1) when they were 47 – 59 days old and testing began when they were 54 – 66 days old. Due to an error by the one of the vendors, FHH rats arrived 2 wk late, so we tested these separately, 2-wk later than the first squad.
Male and female rats were housed in separate racks in the same vivarium, with an ambient temperature of 23°C and fluorescent illumination between 0600 – 1800 h. Each rat was housed alone in a 25 × 18 × 19 cm hanging cage, with stainless steel back and side walls and a mesh front wall and floor. Powdered AIN-76A diet was available from a 4-oz glass jar that was attached with a stainless-steel spring to the front wall. AIN-76A is a semisynthetic diet containing by weight: 20% protein (casein), 65% carbohydrate (sucrose and cornstarch), 5% fat (corn oil), and 10% fiber (cellulose), minerals and vitamins. It has an energy content of ~15.9 kJ/g, and concentrations of the following minerals relevant to this experiment (in g/kg diet): sodium, 1.02; potassium, 3.60; calcium, 5.20; magnesium, 0.51. The diet was purchased from Dyets Inc (Bethlehem, PA; catalog no. 100000; (23)]. Except during tests (see below), deionized water was available from a 300-mL glass bottle equipped with a neoprene stopper and a stainless steel sipper.
Food and at least one bottle of water were always available. Food cups and water bottles were refilled as needed. Cardboard sheets under the rats’ cages collected excrement and spillage, and were changed daily. The rats were given 7 days to adapt to their new housing before tests began.
Procedure
The rats received 17 series of two-bottle choice tests with 5 – 7 concentrations of each taste solution (Table 2). We use the terms “taste solution” and “taste preference” for convenience, with full knowledge that some of the compounds tested are not strictly solutions (e.g., corn oil emulsion) or tastes (e.g., capsaicin), and that fluid intakes in long-term two-bottle choice tests may involve postingestive, experiential, cognitive, and non-gustatory orosensory cues in addition to taste (see discussion).
Table 2.
Compounds tested and their sources
| Compound | Supplier | Catalogue number |
Batch number |
|---|---|---|---|
| NaCl | Sigma | S9625 | 104K0175 |
| CaCl2 | Sigma | C3881 | 125K0052 |
| NH4Cl | Sigma | A4514 | 085K0103 |
| KCl | Sigma | P4504 | 085K0061 |
| MgCl2 | Sigma | M8266 | 065K00881 |
| Saccharin | Sigma | S1002 | 075K0007* |
| Sucrose | Sigma | S9378 | 016K0119 |
| Ethanol | Pharmca | 06D22A-100 | 0604191 |
| HCl | Fluka | 84434 | EC.2315957 |
| Citric acid | Sigma | C7129 | 125K0173 |
| QHCl | Sigma | Q1125 | 085K2508 |
| Caffeine | Sigma | C0750 | 066K0085 |
| Denatonium | Sigma | D5765 | 054K1693 |
| MSG | USB | 16245 | 114966 |
| Polycose | Ross Nutrition | none | many |
| Corn oil | Crisco | none | many |
| Capsaicin | Pfaltz & Bauer | C02640 | 102001-6 |
Notes: Compounds are listed in the order they were tested.
Rats in 2nd batch tested with saccharin batch number 056K0146.
Ethanol was 190 proof ACS/USP Grade. QHCl = quinine hydrochloride, MSG = monosodium glutamate. Polycose was purchased in 12.3 oz cans from metromedicalonline.com. Crisco brand corn oil was purchased at a local Genuardi’s supermarket.
We chose concentrations based on previous work with rats and mice [e.g., (5,15,83,84)]. In general, concentrations were increased in half-log steps from just detectable to strongly avoided, although (a) for some compounds, quarter-log concentrations were tested in the range previous literature suggested a peak taste preference might be found, and (b) some nutrient compounds were tested using doubling concentrations (e.g., 1, 2, 4, 8%) to be consistent with previous literature. The choice of appropriate concentrations involved some guess-work because this information was not available for most of the strains.
Solutions were prepared within 24 h of when needed, except if this fell on the weekend, when they were prepared no more than 48 h in advance. Most solutions were made in 3-liter batches by stirring the appropriate quantity of taste compound into deionized water. Ethanol solutions were made by diluting 95% (190 proof) ethanol with the appropriate volume of deionized water (i.e., volume-by-volume). Because of solubility issues, concentrated capsaicin was initially dissolved in 1 mL of 95% ethanol and this was then dissolved in deionized water to produce the appropriate concentration. Corn oil was held in suspension with the addition of 0.3% soybean phosphatidylcholine and 0.2% xanthan gum. Fresh corn oil emulsions were provided every 24 h to reduce the possibility of separation, which was not a problem. The same concentrations of the suspension agents were added to the water bottle during tests with corn oil.
Each series consisted of (a) a 48-h test involving two bottles of water, (b) between 4 – 7 successive 48-h tests with a choice between water and ascending concentrations of a taste compound, and (c) a recovery or “wash out” period, with a single bottle of water available. The wash out period was 1 – 2 days after series involving nonnutrient taste solutions and 4 days after those involving the nutrient solutions sucrose, ethanol, and Polycose.
At the beginning of each test series, each rat was weighed (± 0.1 g) using a top-loading balance with a basket, and then returned to its cage. The rat’s regular water bottle was removed and two similar bottles were provided, with the spouts penetrating the front wall of the cage to rest with the tips 2 – 4 cm above the floor and 3 – 4 cm apart. The bottles were identical except for a 2 – 3 cm piece of colored tape stuck to the side of each bottle so that technical staff could identify them. For tests with a taste solution, the bottles were initially presented so that with the rat facing them, the water was on its left and taste solution on its right. The position of the two bottles was switched after 24 h. The bottles were weighed (± 0.1 g) before they were placed on the cage, when switched at 24 h, and when removed at 48 h.
Fluid intakes in grams were converted to milliliters with the assumption for all solutions that 1 mL = 1 g. Intakes from each bottle during 48-h tests were divided by two to obtain average daily intakes. Intakes from both bottles were summed to obtain total fluid intake. Solution preference was calculated as the ratio of solution intake/total fluid intake and expressed as a percentage.
Due to a technical error, rats in the second squad were not tested with 178 mM NH4Cl so data for this concentration from both squads were not used in analyses.
Body weights were measured at the beginning of each series and at the end of the experiment. Food intake was measured on three occasions, during tests with two bottles of water available. These were: (a) At the start of testing, when the rats were 7 wk old, (b) at the start of the saccharin series, when 21 wk old, and (c) at the start of the capsaicin series, when 46 wk old. To measure food intake, food cups were weighed (± 0.1 g) and fresh bedding sheets were placed under the rats’ cages. After 48 h, the food cups were reweighed. Spillage was collected from the bedding sheets with a paintbrush and weighed. Food intake was considered the change in weight of the food cup minus spillage (if any).
A few days after the last two-bottle choice test, the rats were killed by pentobarbital injection and their fat depots and major organs extracted as part of another experiment. Scissors were used to excise each rat’s tongue as far to the posterior as possible. The tongue was frozen at −86 C for several weeks. It was then defrosted, and stained with 1% methylene blue for 45 sec to aid visualization of taste papillae. A digital image was obtained using a Keyence VHX-500 digital microscope with 10x magnification. The image of the tongue was divided into anterior and posterior halves and each half was divided into quadrants to form a grid of eight sections, all as close to equally sized as possible (see on-line material). The fungiform papillae in each quadrant were counted. A fungiform papillae was recognized as a rounded projection on the surface of the tongue larger and less numerous than filliform papillae but smaller and typically farther anterior than vallate and circumvallate papillae. Papillae in contact with the anterior and left division lines of the quadrants were excluded from that quadrant and papillae in contact with the posterior and right were included.
RESULTS
The large number of groups and correspondingly large number of possible comparisons provides a challenge for statistical analysis and presentation. To provide some protection from Type II errors, we used a criterion for statistical significance of p < 0.001 unless otherwise noted.
Deaths
Each of the 14 strain groups initially comprised of 8 male and 8 female rats. However, one male FHH rat died 2 days after arrival and so was excluded from all data analyses. Of the remaining rats, the following died during the course of the experiment: 6 Dahl, 3 FHH, 2 SD, 1 LE, 1 BN, 1 DA, 1 LEW, 1 Noble, and 1 SHR. All WI, BUF, COP, F344, and PVG rats survived. The data of rats that died were included in analyses up to but excluding the test series in which they died. Most of the rats died toward the end of the experiment (3 died during tests with sucrose, 7 died during tests with Polycose). We did not attempt to determine the cause of death, although we note that the two strains with the highest fatality rates (Dahl and FHH) are models of hypertension.
Spillage
Previous work with SD rats suggests that spillage and evaporation during two-bottle tests is <0.5 mL/d (but see below). We did not attempt to correct for this source of error. However, we kept records of visible spillage. Occasionally during a 48-h test, a bottle fell off a cage, a stopper became dislodged, or there was lots of fluid on the cardboard sheet under a drinking spout. This could be due to a technical error (i.e., the drinking bottle stopper not firmly inserted) or to vigorous activity of the rat (i.e., gnawing at or playing with the drinking spout). The overall incidence of spillage was 0.55% (i.e., 0.60 spills/rat in 109 48-h tests). However, the SHR strain had much higher rates of spillage (3.95%) than did the other groups (all <0.69%). The incidence of spillage was too low to distinguish statistically whether some solutions were spilled more than others but MgCl2, ethanol and citric acid appeared to be the most frequently spilled.
Body weight
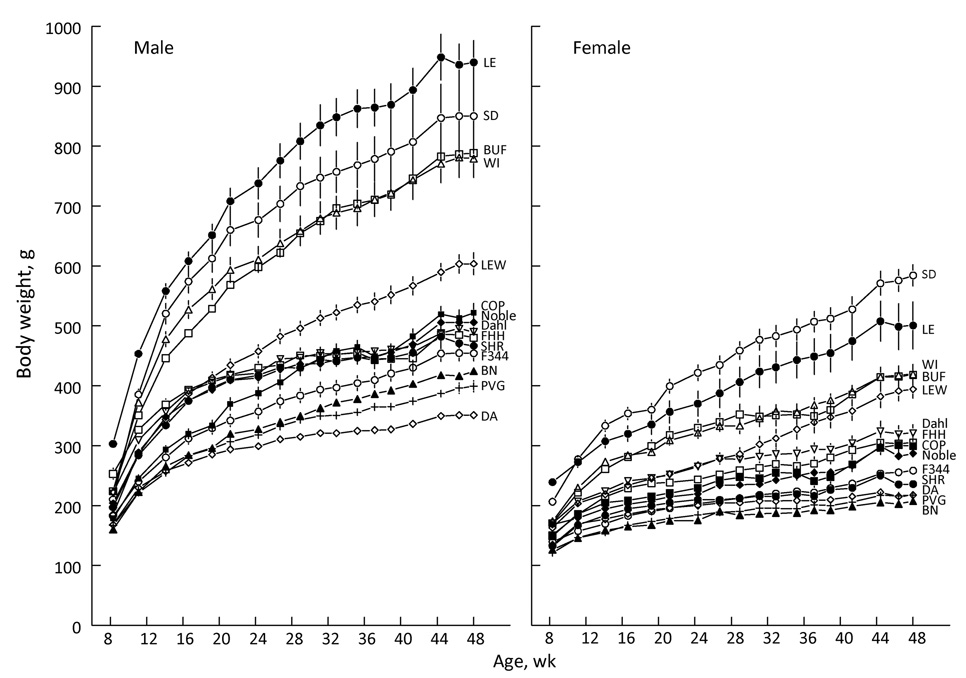
There were large differences between the strains in body weight with the pattern remaining more-or-less constant but the range diverging as the experiment progressed (Fig. 1). Because of the markedly higher body weights of males than females [e.g., at end of experiment, F(1,178) = 1129.2, p <0.00001], we analyzed the body weights of each sex separately. Figure 2 shows differences between the strains for each sex at the end of the experiment.
Figure 1. Body weights of male and female rats of 14 strains.
The rats were fed AIN-76A diet and had 17 series of two-bottle choice tests during this period.
Figure 2. Body weight (BW), food intake (FI) and water intake (WI) of male (left) and female (right) rats of 14 strains.
Top panels show BW at end of experiment. Intakes are average of three measurements collected at age 7, 21, and 46 wk. The middle and bottom panels show FI and WI adjusted for body weight. Each bar shows the mean ± SE for each strain (n = ~8). Strains sharing the same letters above the bars did not differ significantly (p<0.001). ANOVA results in each panel refer to effect of strain. Data from males and females were treated separately.
Food intake
Food intake was measured during three 48-h tests, and the results from each test were similar so, for simplicity, we averaged the three values obtained from each rat together for subsequent analyses. Males ate considerably more food than did females, F(1,195) = 133.9, p < 0.00001, and there was no Strain × Sex interaction, so we analyzed food intakes of each sex separately (see Fig. 2).
Water intake
Each of the 17 series of tests began with a 48-h choice between two bottles of water. Total intake from both bottles during these tests provided daily fluid intakes at approximately 2-wk intervals between age 8 – 46 wk. We conducted two analyses on water intakes. First, to allow comparison with food intakes and body weights, we analyzed water intakes during the three tests when food intake was measured (i.e., before the NaCl, saccharin, and capsaicin test series) using the same methods as for food intake (Fig. 2). Second, to analyze the complete set of data, we first conducted an omnibus ANOVA with factors of Strain, Sex, and Series. In this analysis, we omitted the SHR strain because only 3 SHR rats completed all 17 tests without spillage. This revealed strain differences, F(12,174) = 40.4, p < 0.00001, but no effect of Sex and no Strain × Sex interaction. Differences among the strains are shown in Figure 3 [based on averages for each strain, including SHR, F(13,209) = 29.6, p < 0.00001]. There was also a main effect of Test Time, F(16,2784) = 15.1, p<0.00001, and interactions of Test Time with Strain, F(192,2784) = 3.70, p<0.00001, and with Sex, F(16,2784) = 3.46, p < 0.00001. The most obvious source of these effects was low water intake on the first test relative to subsequent tests, but there were other more complex sources as well (Fig. 3).
Figure 3. Water intakes of rats during tests with two bottles of water to drink.
Within-subject and within-strain variability in water intakes and preferences
Since each rat was tested with two bottles of water 17 times, we could estimate both within-subject and within-strain variability. We used the standard error of the 17 observations around the mean water intake of each rat to assess within-subject variability. We used the standard error of each rat’s mean around the strain mean to assess within-strain variability. These two sources of variability were generally congruent; that is, individuals that differed the most from test-to-test also tended to belong to the strains with the most between-subject variation (r = 0.78; Table 3). Differences in within-subject variability were assessed using an ANOVA with factors of strain and sex. There were large and significant differences in variability between strains, F(13,195) = 16.0, p < 0.00001 (Table 3; Figure 3), but no sex differences. Regression analyses revealed strong positive associations between strain mean water intakes and variability (i.e., the more rats drank, the higher the variability; Table 3) indicating that most of the difference in variability among strains could be accounted for by differences in the mean volume consumed. Regressing variability of each rat against mean water intakes eliminated differences among the strains except for the SHR strain, which had higher variability than did the other 13 strains, F(13,195) = 9.45, p < 0.00001. In particular, once differences in intakes were accounted for, there was no difference in variability between outbred and inbred strains.
Table 3.
Mean intakes and variability during tests with two bottles of water to drink
| Strain | Mean water intake |
Within-strain standard error |
Within-subject standard error |
|---|---|---|---|
| SD | 28.5 | 2.4 | 2.1 |
| LE | 43.2 | 2.8 | 3.4 |
| WI | 50.7 | 3.7 | 2.8 |
| BN | 16.1 | 0.6 | 0.6 |
| BUF | 25.2 | 1.6 | 1.3 |
| COP | 41.8 | 2.7 | 3.9 |
| DA | 27.0 | 1.4 | 1.4 |
| Dahl | 34.1 | 1.2 | 2.8 |
| F344 | 17.4 | 0.6 | 1.3 |
| FHH | 49.5 | 2.1 | 3.7 |
| LEW | 15.8 | 0.5 | 1.2 |
| Nob | 47.7 | 2.7 | 3.9 |
| PVG | 29.9 | 1.6 | 1.7 |
| SHR | 43.4 | 3.5 | 6.5 |
Notes: Values are based on intakes of 15 or 16 rats/strain during 17 48-h two-bottle tests. Mean water intake = average daily intake from two water bottles over all 17 tests, mL/d. Within-strain standard error = standard error of the mean water intakes (i.e., variation of average intake of each subject around the strain mean). Within-subject standard error = mean standard error of the 17 tests of each subject (i.e., average variation of each subject from trialto-trial). Correlation between the two measures of variability, r = 0.78; intake vs. within-strain variability, r = 0.87; intake vs. within-subject variability, r = 0.78.
We conducted informal analyses on the within-strain variability of taste preferences for several compounds and in each case found more-or-less the same pattern of results as was obtained with water intakes. A complicating factor was that, in general, the closer the mean preference of a group was to indifference (50%) the larger was the within-strain variation (see on-line material). If this source of variation was taken into account then the SHR strain usually had more variable preferences than did the other 13 strains, which did not differ in variability from each other. Because of space constraints we do not present formal analyses that lead to these generalizations but variability in preferences can be assessed by inspection of the standard errors displayed in figures or directly from supplemental data available on-line.
Relationship between body weight, food intake, and water intake
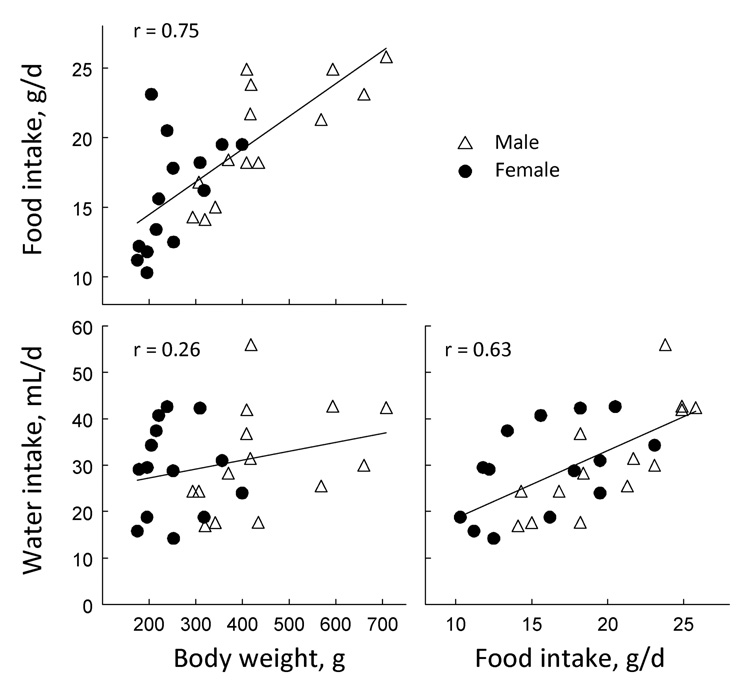
There are inconsistencies in prior reports due to food and fluid intakes being expressed as absolute values or in relation to body weight [see e.g., (6,86)]. To determine whether adjusting for body weight influenced strain differences, the analyses of food and water intake (during the three food intake tests) were repeated using intakes divided by body weights (i.e., grams eaten per kilogram body weight, or milliliters drunk per kilogram body weight). These adjustments appeared to reduce the strain variation associated with food intake but not water intake (Fig. 2). To provide a more direct test, we compared the body weights of each strain and sex with corresponding average food and water intakes during the three tests when food intake was measured. There were moderately strong correlations between food intake and body weight (r = 0.75, p < 0.00001), and between food intake and water intake (r = 0.63, p = 0.0003; Fig. 4). However, the correlation between body weight and water intake was much weaker and not statistically significant (r = 0.26, p = 0.181; Fig. 4). This implies that body weight can account for less than 7% of the variation in water intake, so we judged it inappropriate to adjust fluid intakes for body weight.
Figure 4. Scatter plots showing the relationship between body weight, food intake, and water intake in male and female rats of 14 strains.
Each symbol is the mean of ~8 rats of the same strain and sex. Food and water intake = average food intake from three trials when rats had two bottles of water available at age 8, 24, and 46 wk. Body weight = weight at 24 wk. Regression lines for both sexes combined are shown but these were very similar to those for each sex separately.
Number of fungiform taste papillae
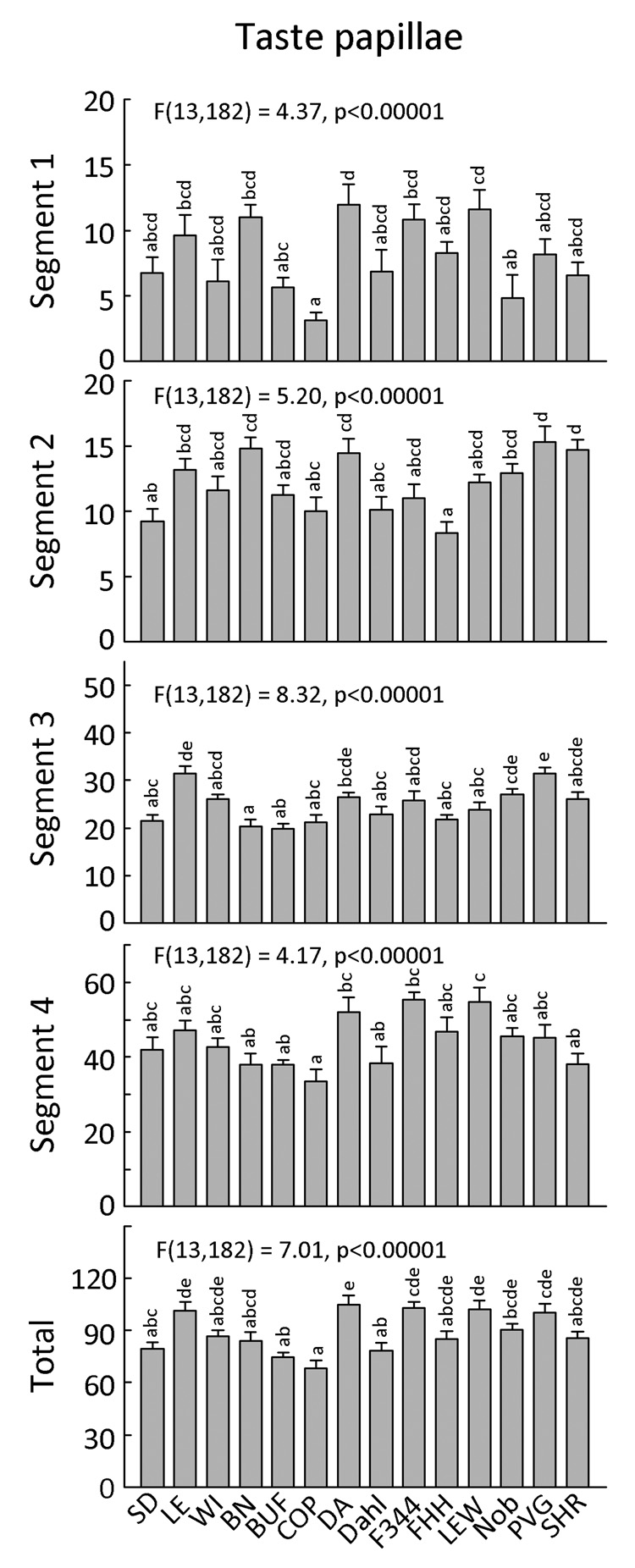
There were no concerted differences in the number of papillae on the left and right side of the tongue so analyses were conducted based on 4 segments from the base (Segment 1) to tip (Segment 4), with the left and right sides combined. There was a significant three-way interaction between Strain, Sex, and Segment, F(39,504) = 2.201, p = 00007. Analyses of each of the four segments separately revealed that the effect involving sex was confined to the anterior segment and this did not reach the criterion for significance used throughout the rest of this study [strain × sex interaction, F(13,168) = 2.63, p = 0.0022]. LSD post hoc tests showed that in the anterior segment, there were more papillae in SD male than SD female rats (52 ± 6 vs. 35 ± 2, p = 0.0077), and less papillae in Dahl male than Dahl female rats (29 ± 3 vs. 48 ± 5, p = 0.0121). There were no other sex differences in papillae number.
Based on the total number of papillae over all four segments of the tongue, there were significant differences among the strains, F(13,168) = 7.30, p < 0.00001, and no significant differences among the sexes, F(1,168) = 0.90, p = 0.3447, or Strain × Sex interaction, F(13,168) = 1.92, p = 0.0307. Nine strains belonged to the group with the most papillae and eight strains belonged to the group with the least papillae (3 strains belonged to both groups; Fig. 5). The distribution of high- and low-counts of papillae was similar across the four segments, although minor differences were present (Fig. 5; individual data are available on-line).
Figure 5. Number of fungiform taste papillae on tongue of 14 strains of rats.
Separate counts for four segments are shown, from its base (Segment 1) to tip (Segment 4); the total number of papillae is given in the bottom panel. Each bar shows the mean ± SE for each strain with males and females combined. Strains sharing the same letters above the bars did not differ significantly (p<0.001). ANOVA results in each panel refer to effect of strain.
We generated a correlation matrix to identify relationships between the number of fungiform papillae and preferences for each concentration of each of the 17 compounds tested. There were no correlations significant at the p<0.001 level, although three approached this level of significance: There were negative correlations between the total number of fungiform papillae and preferences for 3.16 mM HCl (r = −0.73), 0.0316 mM QHCl (r = −0.71) and 0.1 mM QHCl (r = −0.70).
Taste solution intakes and preferences
We conducted parallel analyses on each of the four dependent variables involved in two-bottle choice tests: taste solution intake, water intake, total fluid intake, and taste solution preference. As a first step, we used omnibus three-way analyses of variance with factors of Strain, Sex, and Concentration (i.e., 14 strains × 2 sexes × 5 – 8 concentrations) for each taste solution series. All analyses revealed highly significant main effects and interactions involving the Concentration factor but these proved uninteresting except to verify that, for all 17 taste solution series, we chose ranges of concentrations that influenced intakes and preferences. These analyses were complicated by two factors. First, there was sometimes heterogeneity of variance due to basement or ceiling effects at the highest concentrations. Second, missing data were difficult to deal with. The within-subject design necessitated dropping from the entire test series a subject that spilled even once. This led to very few SHR rats in some analyses (see Spillage, above). To avoid these problems and to simplify, we conducted discrete two-way ANOVAs with factors of Strain and Sex for each of the 109 tests of each concentration of each taste solution.
The results of the analyses involving taste solution intakes, water intakes, and total intakes are given in supplemental material on-line but can be summarized easily. Of the 109 analyses of each type conducted, nearly all had significant strain differences (106 involving taste solution intake, 97 involving water intake, and all 109 involving total intake), there were very few Strain × Sex interactions (6 involving taste solution intake, none involving water intake, and 8 involving total intake), and very few had significant main effects of sex (10 involving taste solution intake, 5 involving water intake, and 20 involving total intake).
The analyses of taste solution preference scores were a little more complex. As expected, there were no significant preferences displayed in any of the 17 tests involving a choice between two bottles of water. There were significant strain differences for all concentrations of NaCl, citric acid, MSG, corn oil and capsaicin, and most concentrations of CaCl2, NH4Cl, KCl, saccharin, sucrose, ethanol, HCl, QHCl, and caffeine. There were also strain differences at 31.6 mM MgCl2 and 32% Polycose but not at other concentrations of these compounds. The only compound for which preferences were not affected by strain at any concentration was denatonium although the effect of strain on preferences for 0.0316 mM denatonium was close to significance; F(13,193) = 2.73, p = 0.0015. None of the 109 analyses involved significant Strain × Sex interactions and only two involved significant Sex differences (for 100 mM saccharin and 316 mM MSG; in each case males had higher preference scores than did females).
Given the almost complete lack of sex differences in preference scores, to determine which strains differed from each other, we conducted post hoc LSD tests based on strain means with both sexes combined. Like the ANOVAs, these tests used a criterion for significance of p < 0.001. The results of these ANOVAs and differences between strains for each concentration of each taste compound are given in Figure 6 – Figure 22. Note that in these figures, most tables and subsequent descriptions of the results, compounds believed to have similar taste qualities are grouped together rather than in the order they were tested, which is given in Table 2. This considerably simplifies description.
Figure 6. Two-bottle intake and preference for 7 concentrations of NaCl by 14 strains of rats.
Each bar shows the mean ± SE for each strain with males and females combined. Strains sharing the same letters above the bars did not differ significantly (p<0.001). ANOVA results in each panel refer to effect of strain; not shown are ANOVA results for preferences for 3.16 mM NaCl, F(13,209) = 4.25, p<0.00001; 10 mM NaCl, F(13,201) = 3.82, p=0.00002; 31.6 mM NaCl, F(13,207) = 4.25, p=0.00008; 100 mM NaCl, F(13,207) = 3.03, p<0.00042; and 178 mM NaCl, F(13,209) = 8.66, p<0.00001.
Figure 22. Two-bottle intake and preference for 4 concentrations of capsaicin by 14 strains of rats.
Each bar shows the mean ± SE for each strain with males and females combined. Strains sharing the same letters above the bars did not differ significantly (p<0.001). ANOVA results in each panel refer to effect of strain.
Behavioral preference and avoidance thresholds
Behavioral preference and avoidance thresholds, originally called “taste thresholds” by Richter and Campbell (67), are the lowest concentration at which rats demonstrate a discrimination between water and a taste compound in a choice test. To assess this, we used one-sample t-tests to determine whether strain mean preferences at each concentration of each taste solution differed significantly from indifference (i.e., 50% preference). Table 4 shows concentrations that were significantly preferred and Table 5 shows the lowest concentrations that were avoided by each strain.
Table 4.
Ranges of preferred concentrations of taste solutions by strain
| Solution | SD | LE | WI | BN | BUF | COP | DA | Dahl | F344 | FHH | LEW | Noble | PVG | SHR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NaCl | 10 – 178 | 3.16 – 178 | 3.16 – 178 | 3.16 – 178 | 3.16 – 100 | 10 – 178 | 3.16 – 178 | 10 – 178 | 3.16 – 100 | 3.16 – 178 | 3.16 – 178 | 10 – 100 | 3.16 – 100 | 3.16 – 178 |
| NH4Cl | 3.16, 31.6 | - | - | - | - | - | - | 31.6 | - | - | - | - | 3.16, 31.6 | 31.6 |
| KCl | 31.6 | - | - | - | - | - | - | - | - | - | - | - | 31.6 | 31.6 |
| CaCl2 | 0.316 | 0.316 | - | - | - | 0.316 | 10 – 31.6 | - | - | - | - | - | - | - |
| MgCl2 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - |
| Saccharin | 0.316 – 31.6 | 0.316 – 31.6 | 0.316 – 10 | 3.16 – 31.6 | 0.316 – 31.6 | 1 – 10 | 0.316 – 10 | 0.316 – 31.6 | 3.16 – 31.6 | 0.316 – 100 | 10 – 31.6 | 1 – 10 | 0.316 – 31.6 | 3.16 – 31.6 |
| Sucrose | 10 – 100 | 10 – 100 | 17.8 – 100 | 17.8 – 100 | 10 – 100 | 17.8 – 100 | 10 – 100 | 10 – 100 | 10 – 100 | 17.8 – 100 | 10 – 100 | 17.8 – 100 | 10 – 100 | 17.8 – 100 |
| Polycose | 0.5 – 32% | 0.5 – 16% | 1 – 32% | 0.5 – 32% | 1 – 32% | 0.5 – 32% | 0.5 – 32% | 0.5 – 32% | 0.5 – 32% | 0.5 – 32% | 1 – 32% | 0.5 – 32% | 0.5 – 32% | 0.5 – 32% |
| HCl | 0.1 – 0.316 | 0.316 | - | - | - | - | 0.1 | 1 | - | - | - | - | 0.1 – 1 | - |
| MSG | 10 – 100 | 10, 100 | - | - | 3.16 – 100 | 3.16 – 100 | 3.16 – 316 | 10 – 100 | - | 3.16 – 100 | 31.6 –316 | 1 – 100 | 1 – 100 | 10 – 316 |
| Ethanol | 1 – 2% | 1 – 4% | 1 – 2% | 1 – 2% | 1% | 1, 4% | 2% | 2% | - | 4% | 1 – 2% | 1 – 4% | 1 – 4% | - |
| Corn oil | 2 – 8% | - | 1 – 8% | - | - | 1 – 4% | 2 – 4 % | - | 2 – 4% | 1 – 2% | - | - | 1 – 4% | 1 – 4% |
Notes: values are in either mM or %. dash = no concentration was preferred. A taste solution was considered preferred if the strain mean preference was significantly greater than 50% according to a one-sample t-test, with p < 0.001. Compounds not shown in the table were not preferred by any strain at any concentration. Some strains preferred the highest concentrations tested of saccharin, sucrose and Polycose so these strains may have extended preference ranges.
Table 5.
Lowest concentration of taste solutions avoided by each strain
| Solution | SD | LE | WI | BN | BUF | COP | DA | Dahl | F344 | FHH | LEW | Noble | PVG | SHR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NaCl, mM | 562 | 316 | 316 | 316 | 316 | 562 | 562 | 316 | 316 | 562 | 562 | 316 | 316 | 562 |
| NH4Cl, mM | 316 | 316 | 316 | 100 | 316 | 316 | 316 | 316 | 316 | 316 | 316 | 100 | 316 | 316 |
| KCl, mM | 316 | 316 | 100 | 100 | 316 | 316 | 316 | 316 | 316 | 316 | 316 | 316 | 316 | 316 |
| CaCl2, mM | 100 | 100 | 10 | 10 | 100 | 100 | 100 | 100 | 100 | 3.16 | 100 | 31.6 | 100 | 100 |
| MgCl2, mM | 100 | 100 | 10 | 100 | 31.6 | 100 | 100 | 31.6 | 10 | 100 | 31.6 | 31.6 | 100 | 31.6 |
| Saccharin, mM | - | 100 | 100 | 100 | - | 100 | - | - | 100 | - | - | 100 | 100 | - |
| HCl, mM | 10 | 3.16 | 3.16 | 3.16 | 10 | 10 | 3.16 | - | 3.16 | - | 3.16 | 3.16 | 3.16 | 3.16 |
| Citric acid, mM | 3.16 | 3.16 | 3.16 | 3.16 | 10 | 3.16 | 3.16 | 10 | 3.16 | 10 | 3.16 | 3.16 | 10 | 10 |
| QHCl, mM | 0.0316 | 0.01 | 0.01 | 0.01 | 0.1 | 0.316 | 0.0316 | 0.1 | 0.0316 | 0.1 | 0.01 | 0.0316 | 0.01 | 0.01 |
| Caffeine, mM | 10 | 10 | 1 | 1 | 3.16 | 10 | 10 | 3.16 | 10 | 31.6 | 10 | 10 | 10 | 10 |
| Denatonium, mM | 0.0316 | 0.1 | 0.0316 | 0.01 | 0.1 | 0.0316 | 0.1 | 0.1 | 0.1 | 0.1 | 0.0316 | 0.1 | 0.0316 | 0.0316 |
| MSG, mM | - | - | 316 | - | - | - | - | - | - | - | - | 316 | - | - |
| Corn oil, mM | - | 16% | - | - | 16% | - | 16% | - | - | - | - | - | - | - |
| Ethanol, % | 12% | 8% | 12% | 8% | 12% | 12% | 12% | 12% | 12% | - | 12% | 8% | 12% | - |
| Capsaicin, µM | 3.16 | 3.16 | 1 | 1 | 10 | 3.16 | 3.16 | 1 | 1 | 3.16 | 31.6 | 1 | 1 | 10 |
Notes: dash = no concentration was avoided. A taste solution was considered avoided if the strain mean preference was significantly lower than 50% according to a one-sample t-test, with p < 0.001. Sucrose and Polycose (not shown in the table) were not avoided by any strain at any concentration.
Correlation among preferences for various taste compounds
Correlations among preferences for different taste compounds can provide information about common mechanisms, including those underlying similar taste qualities. To examine this, we generated the complete 109 × 109 correlation matrix based on strain mean preferences for each concentration of each taste compound (see on-line supplemental material). We caution that this analysis is based on only 14 strain means, so it lacks statistical power; all correlations given below are significant at p<0.01, but few meet the p<0.001 (rcritical = 0.78) criterion used throughout the rest of the paper. We consider them as descriptive rather than hypothesis tests. We attempted several descriptive methods including cluster analysis and multidimensional scaling but the resulting graphical representations were too complex to provide much insight (see online materials). The greatest problem was that strong correlations between taste compounds did not occur at all concentrations, and that different concentrations correlated highly for different pairs of taste compounds. Nevertheless, we believe useful insights can be gained by judicious cherry-picking of the highest correlations between various concentrations of different taste compounds.
Not surprisingly, there were many strong correlations between adjacent concentrations of the same compound (e.g., the correlation between 31.6 mM vs. 100 mM NaCl preference was r = 0.83). Generally, the greater the difference between the concentrations, the weaker the correlation. There were also the expected high correlations between compounds with similar taste qualities to humans. Examples include salty (e.g., 316 mM NaCl vs. 316 mM MSG, r = 0.89; 316 mM NaCl vs. 100 mM KCl, r = 0.72), sour (e.g., 0.316 mM HCl vs. 0.1 mM citric acid, r = 0.76), bitter (e.g., 1 mM QHCl vs. 10 mM caffeine, r = 0.75; 0.0316 mM QHCl vs. 0.1 mM denatonium, r = 0.80), and sweet (e.g., 0.316 mM saccharin vs. 31.6 mM sucrose, r = 0.72). In contrast, correlations between exemplars of the four primary taste qualities were generally very low, although an exception was between sour and bitter (e.g., 3.16 mM HCl vs. 0.01 mM QHCl, r = 0.75; 31.6 mM citric acid vs. 1 mM QHCl, r = 0.82). Preferences for the only umami taste compound tested, MSG, correlated highly with preferences for several compounds. In addition to the correlation with NaCl preference (see above) there were correlations with sweet compounds (10 mM MSG vs. 0.1 mM saccharin, r = 0.83; 31.6 mM MSG vs. 31.6 mM sucrose, r = 0.80), which is consistent with taste generalization data (39,93). There were also correlations of MSG preference with compounds that had no obvious relationship to MSG or each other (e.g., 3.16 mM MSG vs. 31.6 mM CaCl2, r = 0.70; 31.6 mM MSG vs. 100 mM NH4Cl, r = 0.75; 10 mM MSG vs. 4% ethanol, r = 0.84; 31.6 mM MSG vs. 0.1 mM HCl, r = 0.84; 31.6 mM MSG vs. 0.01 mM denatonium, r = 0.79).
Consistent with human psychophysics and mouse studies, there were correlations between the non-sodium monovalent chlorides (e.g., 100 mM NH4Cl and 100 mM KCl, r = 0.80), between the divalent chlorides (e.g., 31.6 mM CaCl2 vs. 10 mM MgCl2, r = 0.67), and between exemplars of these two categories (e.g., 31.6 mM CaCl2 vs. 100 mM NH4Cl, r = 0.75).
There was a strong relationship between preferences for some concentrations of ethanol and some concentrations of saccharin (e.g., 0.1 mM saccharin and 4% ethanol, r = 0.79; 1 mM saccharin and 4% ethanol, r = 0.74, 100 mM saccharin and 8% ethanol, r = 0.77). Correlations between preferences for sucrose and ethanol were lower, presumably because sucrose preferences were constrained by ceiling effects. The relationship between preferences for ethanol and sweetness is consistent with other work [see (21,40,57)].
Preferences for sweet compounds were related to preferences for Polycose (e.g., 1% Polycose vs. 3.16 mM saccharin, r = 0.73; 1% Polycose vs. 56.2 mM sucrose, r = 0.70) and ethanol (e.g., 0.1 mM saccharin vs. 4% ethanol, r = 0.79). There were high negative correlations between preferences for ethanol and bitter compounds (e.g., 2% ethanol vs. 0.1 mM QHCl, r = −0.84; 2% ethanol vs. 10 mM caffeine, r = −0.81), and concentration-dependent correlations between preferences for ethanol and citric acid (8% ethanol vs. 3.16 mM citric acid, r = 0.66; 2% ethanol and 31.6 mM citric acid, r = −0.73). There were negative correlations between preferences for sweeteners and both CaCl2 (178 mM CaCl2 vs. 31.6 mM sucrose, r = −0.69) and capsaicin (e.g., 31.6 µM capsaicin and 1 mM saccharin, r = −0.71).
Results and discussion of preferences for specific taste compounds
This section describes the results found with individual taste compounds. It would be tedious to describe every strain difference for all 109 tests so these are depicted in Figure 6 – Figure 22. In the text, we report (a) which concentrations of each taste compound are most informative with respect to revealing strain differences, and (b) which strains are likely to capture the most genetic variability responsible for strain differences. To this end, we list strains with the lowest and highest preferences at the taste solution concentration that produced the largest strain differences (based on the F-statistic of the ANOVA). Table 6 provides the results of an alternative approach. The preference of each strain for each concentration of each compound was ranked from 1 to 14. These ranks were then summed, and the resulting sums ranked to get an overall ranking of preference for each compound. In general but not always, the conclusions drawn by the two approaches are complimentary.
Table 6.
Rankings of taste compound preferences for each strain
| Compound | SD | LE | WI | BN | BUF | COP | DA | Dahl | F344 | FHH | LEW | Nob | PVG | SHR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NaCl | 5 | 11 | 9 | 13 | 12 | 4 | 1 | 6 | 8 | 3 | 2 | 14 | 10 | 7 |
| NH4Cl | 1 | 4 | 6 | 13 | 12 | 9 | 6 | 4 | 14 | 8 | 9 | 9 | 2 | 3 |
| KCl | 5 | 3 | 12 | 12 | 11 | 8 | 9 | 3 | 2 | 10 | 7 | 14 | 5 | 1 |
| CaCl2 | 1 | 2 | 14 | 13 | 5 | 2 | 4 | 8 | 7 | 12 | 8 | 10 | 6 | 10 |
| MgCl2 | 2 | 3 | 14 | 7 | 7 | 4 | 5 | 10 | 13 | 9 | 11 | 12 | 1 | 5 |
| Saccharin | 3 | 6 | 7 | 7 | 2 | 10 | 9 | 5 | 13 | 1 | 14 | 12 | 4 | 10 |
| Sucrose | 3 | 9 | 8 | 14 | 2 | 10 | 5 | 4 | 6 | 7 | 12 | 11 | 1 | 13 |
| Polycose | 1 | 14 | 11 | 6 | 2 | 8 | 3 | 13 | 7 | 10 | 9 | 5 | 4 | 12 |
| HCl | 1 | 7 | 13 | 14 | 6 | 9 | 8 | 4 | 11 | 2 | 12 | 10 | 3 | 5 |
| Citric acid | 7 | 8 | 14 | 10 | 4 | 3 | 13 | 4 | 11 | 1 | 9 | 12 | 4 | 2 |
| QHCl | 3 | 7 | 14 | 10 | 4 | 1 | 12 | 5 | 6 | 2 | 11 | 8 | 13 | 8 |
| Caffeine | 11 | 12 | 14 | 13 | 8 | 4 | 6 | 10 | 1 | 2 | 5 | 6 | 9 | 3 |
| Denatonium | 8 | 10 | 14 | 13 | 5 | 2 | 12 | 1 | 6 | 4 | 6 | 3 | 9 | 11 |
| MSG | 3 | 11 | 12 | 14 | 5 | 7 | 1 | 9 | 13 | 6 | 10 | 4 | 2 | 7 |
| Corn oil | 5 | 14 | 3 | 12 | 10 | 6 | 8 | 13 | 4 | 9 | 7 | 11 | 2 | 1 |
| Ethanol | 3 | 5 | 8 | 13 | 1 | 11 | 6 | 8 | 14 | 2 | 11 | 8 | 4 | 7 |
| Capsaicin | 11 | 7 | 14 | 5 | 4 | 8 | 10 | 12 | 5 | 3 | 1 | 13 | 9 | 2 |
| Average rank | 4.3 | 7.8 | 11.0 | 11.1 | 5.9 | 6.2 | 6.9 | 7.0 | 8.3 | 5.4 | 8.5 | 9.5 | 5.2 | 6.3 |
Notes: Values are ranks, with 1 being the strain with the highest preference and 14 being the strain with the lowest preference. Each value is the rank of the sum of the ranks for each of the 4–8 concentrations tested. In cases where the sum for two strains was tied both strains are given the same rank.
Each of the following subsections includes literature citations to previous studies in which rat strain preferences were compared, and where possible these are integrated with the present results. The literature involving rat preferences for most compounds is sparse so each section begins with a citation to pertinent mouse strain surveys, which provide an introduction to this literature.
NaCl (Fig. 6)
There have been two large surveys of sodium preferences in mice (5,86) but this is the first large survey in rats. The largest strain differences in preference were present at the 178 mM and 316 mM NaCl concentrations. With access to 316 mM NaCl, the DA, LEW and SHR rats had the highest preferences and 10 strains formed a group with the lowest preferences. The high NaCl preference of the SHR strain is well documented [e.g., (11,13,16,22,25,27,29,44,50,92)]. The DA strain has not been tested with hypertonic NaCl previously but has been categorized as having normal NaCl preference based on tests with 150 mM NaCl (22). The observation of high NaCl preferences in the LEW strain is apparently a novel finding.
There is a well-known inverted U-shaped function relating sodium concentration to preference, with a peak at about 150 mM [e.g., (66)]. In our study, the function was shaped more like an inverted J, with most strains showing strong preferences for all hypotonic NaCl concentrations tested (i.e., 3.16, 10, 31.6, and 100 mM NaCl; Table 4). Ten strains showed preferences relative to water for 3.16 mM NaCl (the lowest concentration tested) and all 14 strains showed preferences for 10 mM NaCl. This behavioral preference threshold is very close to the concentration observed in early-domesticated rats by Richter [~9 mM; (66)] but lower than those observed by Fregly and Rowland in SD rats (30 mM NaCl) or in LE or Dahl rats, which did not have a preference threshold (31,32,70). Differences in the maintenance diet the animals were fed may be responsible for the discrepancies (Richter used a McCallum diet, Fregly and Rowland used Purina Chow, we used AIN-76A diet) although the preference threshold did not track diet sodium content [3.9, ~3.9 g, and 1.02 g Na+/kg diet respectively; see also (30)].
The F344 strain is considered to be a low-NaCl preferring strain relative to the SD or WI strains (51,52,68,73). Consistent with this, in the present study, the F344 strain had lower intakes of 100 – 316 mM NaCl relative to the SD and WI strains. However, it also tended to have lower water intakes and consequently its NaCl preferences did not differ from the SD and WI strains at any concentration. It was ranked 8th overall in NaCl preference. In our study, the WI and SD strains, which have been used for their “normal” NaCl preferences, were always in the lowest group of strains (except for the SD strain at 178 mM NaCl). Once again, we suspect that the type of maintenance diet may be responsible for the discrepancy with previous literature.
Consistent with our results, the BN strain was considered a low-sodium drinking strain based on the results of short-term tests (78). Other rat strains that have been used in comparisons of NaCl preference include the Brattleboro (22), BUF (51), DA (22), Dahl-R and Dahl-S (1,26,70,92), Genetically Hypertensive [GHR (22,44)], Holtzman (13), Low- and High-saccharin intake [LoS and HiS (20)], Munich-Wistar (51), Smirk (22), and Wistar-Furth (69).
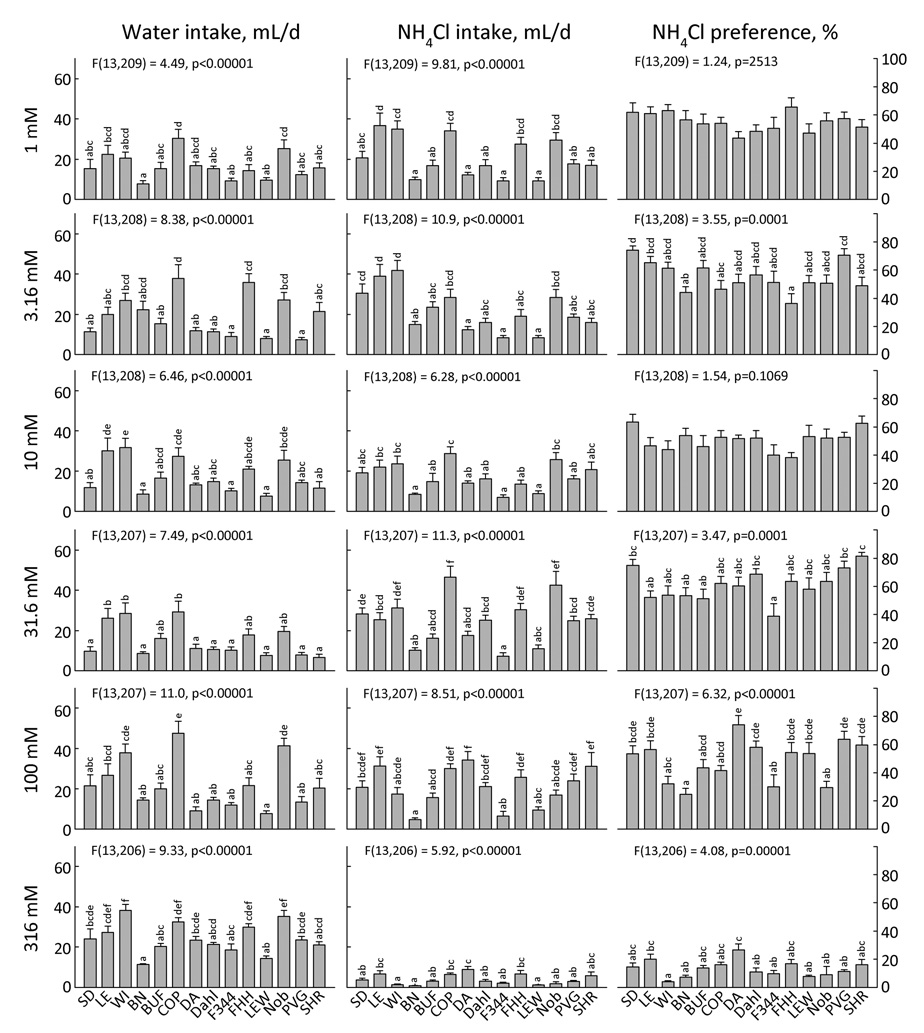
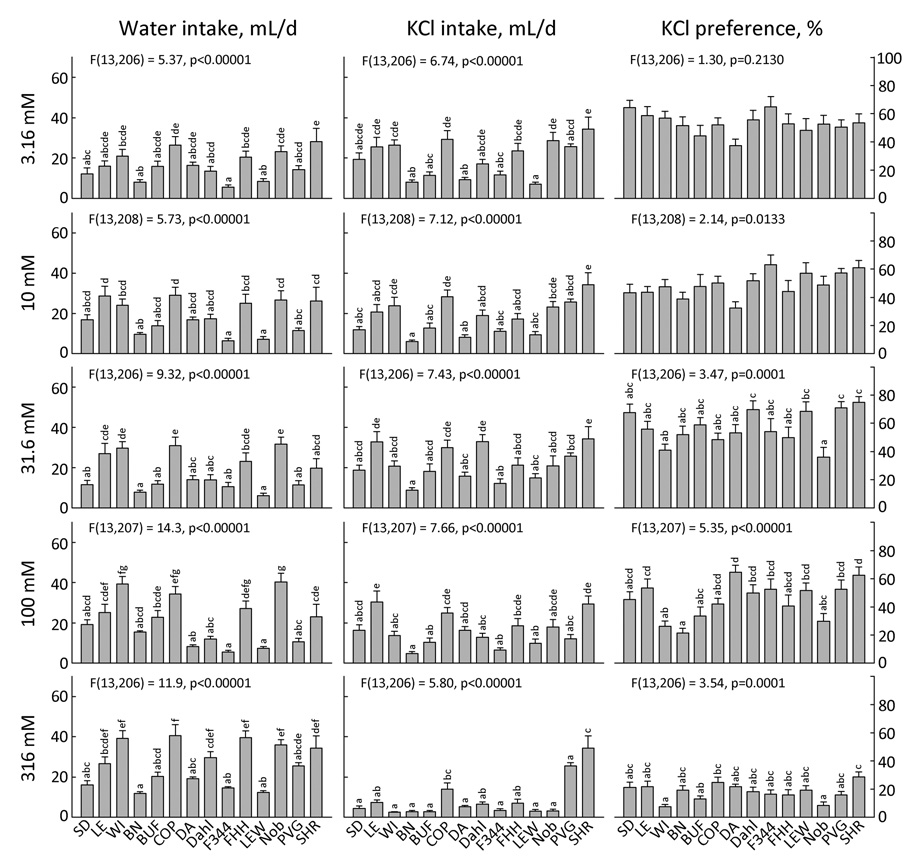
NH4Cl and KCl (Fig. 7 and 8)
Figure 7. Two-bottle intake and preference for 6 concentrations of NH4Cl by 14 strains of rats.
Each bar shows the mean ± SE for each strain with males and females combined. Strains sharing the same letters above the bars did not differ significantly (p<0.001). ANOVA results in each panel refer to effect of strain.
Figure 8. Two-bottle intake and preference for 5 concentrations of KCl by 14 strains of rats.
Each bar shows the mean ± SE for each strain with males and females combined. Strains sharing the same letters above the bars did not differ significantly (p<0.001). ANOVA results in each panel refer to effect of strain.
There is survey of NH4Cl and KCl preferences in 28 mouse strains (5) but to our knowledge, there have been no previous rat strain comparisons of preferences for NH4Cl or KCl. Confirming previous work with SD rats (81), some strains showed an inverted U-shaped concentration-preference function in response to NH4Cl (SD, Dahl, PVG, and SHR) and KCl (SD, PVG, and SHR; Table 4). The largest strain differences were observed at the 100 mM concentration of both NH4Cl and KCl, and the pattern of responses was similar (r = 0.80, see above). For both 100 mM NH4Cl and 100 mM KCl, the group with the highest preferences included the SD, LE, DA, Dahl, FHH, LEW, PVG and SHR strains. The group with the lowest preferences included the WI, BN, BUF, COP, and Noble strains with the F344 also having low NH4Cl preferences and the SD and FHH strains also having low KCl preferences).
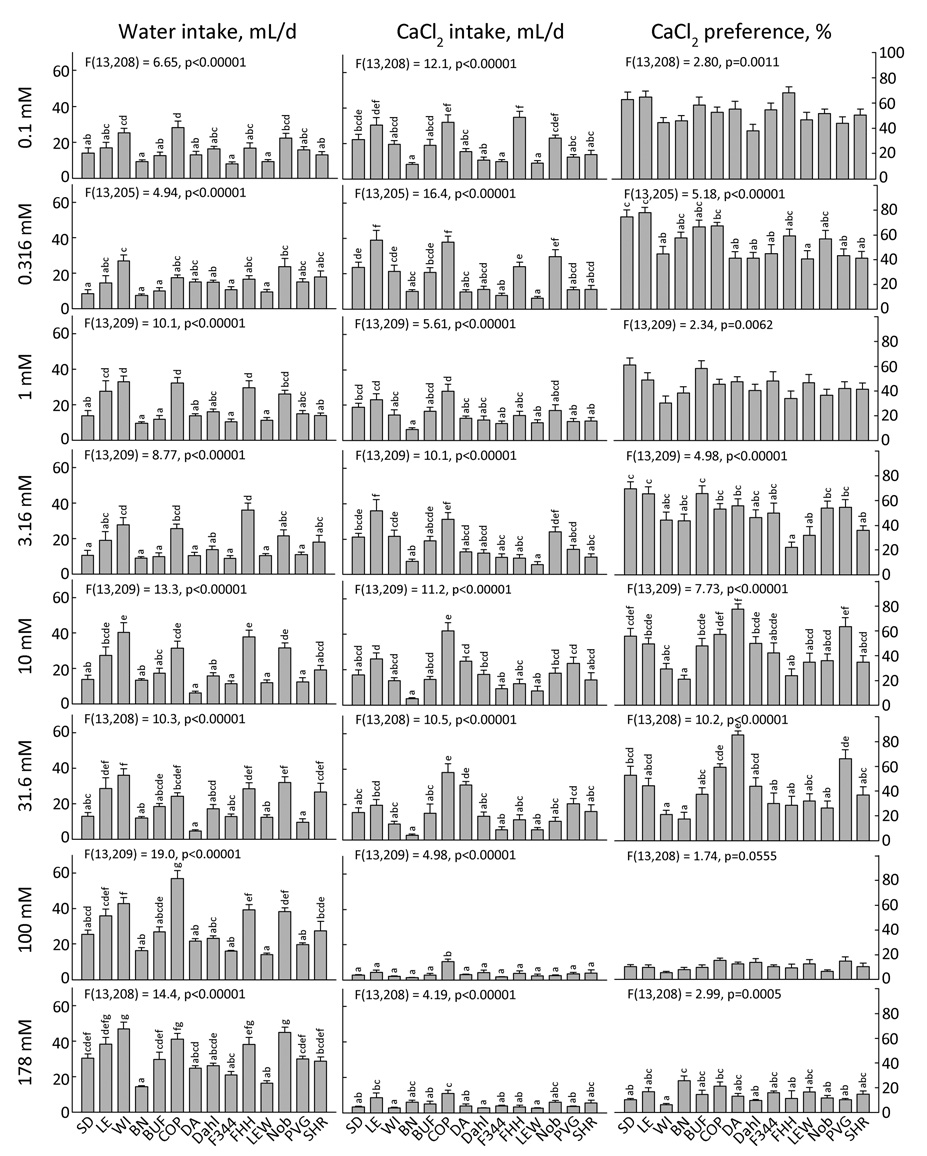
CaCl2 and MgCl2 (Fig. 9 and 10)
Figure 9. Two-bottle intake and preference for CaCl2 by 14 strains of rats.
Each bar shows the mean ± SE for each strain with males and females combined. Strains sharing the same letters above the bars did not differ significantly (p<0.001). ANOVA results in each panel refer to effect of strain.
Figure 10. Two-bottle intake and preference for 5 concentrations of MgCl2 by 14 strains of rats.
Each bar shows the mean ± SE for each strain with males and females combined. Strains sharing the same letters above the bars did not differ significantly (p<0.001). ANOVA results in each panel refer to effect of strain.
There are 28- and 40-strain surveys of calcium preferences in mice (5,85), but the only previous rat strain comparisons of calcium preferences are confined to the WKY and SHR strains (24,80), and there is no work in either species on magnesium preferences. As was the case with the monovalent chlorides, some strains showed an inverted U-shaped concentration-preference function in response to CaCl2 and MgCl2. Four strains preferred low concentrations of CaCl2 to water (SD, LE, COP, DA) and the PVG strain preferred 1 mM MgCl2 to water (Table 4). Peak preferences were at lower concentrations than for the monovalent chlorides [confirming (81)]. There was a large range in the rejection threshold for both divalent chlorides. For CaCl2, the FHH strain significantly avoided concentrations as low as 3.16 mM CaCl2 whereas the majority of strains were indifferent to concentrations up to 100 mM CaCl2. For MgCl2, the rejection threshold was 10 mM for the WI and F344 strains but 31.6 or 100 mM for the other 12 strains (Table 5). For both divalent chlorides, the largest strain differences were captured at the 31.6 mM concentration, and there was a fairly similar pattern of response (r = 0.57). The COP, DA, and PVG strains had the highest CaCl2 and MgCl2 preferences although for MgCl2 the SD, LE, BN, and F344 strains were also included in this group. Ranked lowest were the WI, BN, FHH, LEW, Noble PVG and SHR strains with the BUF, COP, Dahl and F344 strains also joining the lowest MgCl2 preference group.
The finding that the SHR strain had relatively low CaCl2 preferences is surprising because two reports suggest this strain has higher preferences for a range of concentrations of CaCl2 and calcium lactate than does the Wistar Kyoto (WKY) strain (24,80). It may be that the WKY strain has very low CaCl2 preferences rather than the SHR strain that has high preferences.
Saccharin, sucrose and Polycose (Fig. 11, 12, and 13)
Figure 11. Two-bottle intake and preference for 7 concentrations of saccharin by 14 strains of rats.
Each bar shows the mean ± SE for each strain with males and females combined. Strains sharing the same letters above the bars did not differ significantly (p<0.001). ANOVA results in each panel refer to effect of strain. Results for 1 mM saccharin preference, F(13,204) = 5.11, p<0.00001; 3.16 mM saccharin preference, F(13,207) = 4.07, p<0.00001 ; 31.6 mM saccharin preference, F(13,205) = 4.01, p < 0.00001.
Figure 12. Two-bottle intake and preference for 5 concentrations of sucrose by 14 strains of rats.
Each bar shows the mean ± SE for each strain with males and females combined. Strains sharing the same letters above the bars did not differ significantly (p<0.001). ANOVA results in each panel refer to effect of strain. Results for 17.8 mM sucrose preference, F(13,204) =4.94, p<0.00001; 31.6 mM sucrose preference, F(13,207) = 6.06, p<0.00001; 56.2 mM sucrose preference, F(13,205) = 3.40, p = 0.0001, 100 mM sucrose preference, F(13,205) = 1.43, p = 0.1479.
Figure 13. Two-bottle intake and preference for 7 concentrations of Polycose by 14 strains of rats.
Each bar shows the mean ± SE for each strain with males and females combined. Strains sharing the same letters above the bars did not differ significantly (p<0.001). ANOVA results in each panel refer to effect of strain. Results for Polycose preferences: 0.5%, F(13,196) = 2.40, p = 0.0051; 1%, F(13,196) = 2.18, p = 0.0117; 2%, F(13,195) = 1.14, p = 0.3310; 4%, F(13,195) = 0.77, p = 0.6918; 8%, F(13,194) = 1.59, p = 0.0905; 16%, F(13,193) = 2.64, p = 0.0021; 32%, F(13,193) = 6.98, p < 0.00001.
Large surveys of mouse strain preferences for saccharin and sucrose are available [(45,63) see also (43)], but there have been no concerted rat strain surveys of sweet or carbohydrate preferences. In our study, all strains showed strong preferences for nearly all concentrations of saccharin, sucrose and Polycose. Depending on the strain, the threshold at which preferences were significantly above indifference was between 0.316 – 3.16 mM saccharin, 10 – 17.8 mM sucrose, and 0.5 – 1% Polycose (for some strains, this was the lowest concentration tested). At concentrations above threshold, all strains strongly preferred sucrose and Polycose to water. Indeed, most strains drank virtually all their fluid from the sucrose or Polycose bottle. There were also very high preferences for moderate concentrations of saccharin, but some strains had less high preferences for the highest concentrations, and 7 strains avoided 100 mM saccharin, perhaps because of its bitter component [review; (19)].
Because all strains had very high preferences for moderate concentrations of saccharin and nearly all concentrations of sucrose and Polycose there was very little range in preference and so few strain differences (Fig. 11, 12, and 13). Despite the restricted range, there were several significant correlations between strain mean preferences for the three compounds (0.316 mM saccharin vs. 17.8, 31.6, 56.2, and 100 mM sucrose, 1 mM saccharin vs. 17.8 mM sucrose, 3.16 mM saccharin vs. 0.5% and 1% Polycose, 56.2 mM sucrose vs. 1% Polycose]. Based on intakes, it was clear that the FHH strain was an outlying, avid consumer of saccharin, sucrose and Polycose [see also (36)]. Consistent with this, the FHH was the only strain that preferred 100 mM saccharin to water. The SHR strain had unremarkable intakes of low concentrations of the sweeteners but notably high intakes of high concentrations of the sweeteners and all concentrations of Polycose.
Our results are consistent with other reports that LE and SD rats do not differ in 0.25% (~12 mM) saccharin preference (32) and that WI, BUF or F344 rats do not differ in 0.25 – 32% sucrose preference (51). Other strains that have been tested with sweeteners and/or Polycose include the HiS and LoS (20), and Munich-Wistar (51) strains.
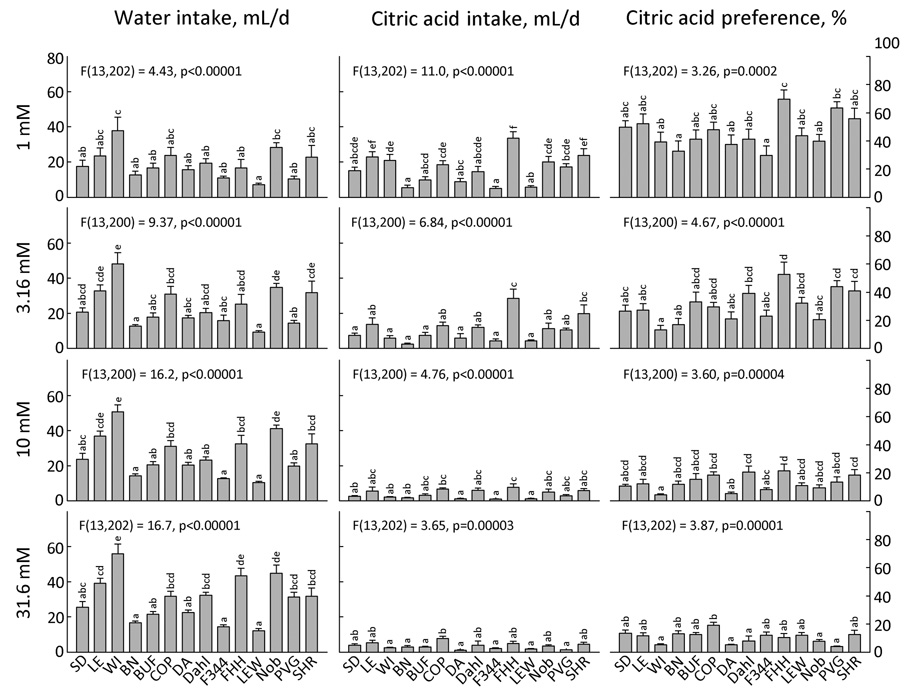
HCl and citric acid (Fig. 14 and 15)
Figure 14. Two-bottle intake and preference for 5 concentrations of HCl by 14 strains of rats.
Each bar shows the mean ± SE for each strain with males and females combined. Strains sharing the same letters above the bars did not differ significantly (p<0.001). ANOVA results in each panel refer to effect of strain.
Figure 15. Two-bottle intake and preference for 4 concentrations of citric acid by 14 strains of rats.
Each bar shows the mean ± SE for each strain with males and females combined. Strains sharing the same letters above the bars did not differ significantly (p<0.001). ANOVA results in each panel refer to effect of strain.
We do not know of a comprehensive survey of mouse strain acid preferences although in one study, intake of 3 mM HCl was measured in 10 strains in 6-h one-bottle tests (43). We are also unaware of any concerted attempts to compare rat strain responses to acids. In our study, there were significant associations between strain mean preferences for HCl and citric acid (1 mM HCl vs. 3.16 mM citric acid, r = 0.69, 10 mM HCl vs. 3.16 mM citric acid, r = 0.75; 10 mM HCl vs. 10 mM citric acid, r = 0.81) suggesting common mechanisms underlie the response to both acids. The most informative concentrations were 1 mM HCl and 3.16 mM citric acid (Fig. 14 and 15). At these concentrations the PVG strain had notably high preferences and the WI, COP, LEW and Noble strains had notably low preferences for HCl; the FHH strain had the highest preferences and the WI strain the lowest preferences for citric acid.
Five strains preferred at least one concentration of HCl to water (SD, LE, DA, Dahl, PVG) but none found citric acid preferable to water (the FHH strain came close, i.e., p = 0.0084). The threshold for avoidance was 3.16 or 10 mM HCl and 3.16 or 10 mM citric acid for every strain, except for the Dahl and FHH strains which did not significantly avoid any concentration of HCl.
Consistent with our results, there was no difference between SD and LE rats in preference for 3 mM HCl (32), and no differences among WI, BUF and F344 rats in preferences for 0.006 – 0.05% (0.31 – 2.6 mM) citric acid (51). The WI, BUF and F344 rats had lower preferences than did Munich-Wistar rats (51). Other strains tested with acids include the HiS and LoS strains, which did not differ in citric acid preference (20)].
QHCl, caffeine, and denatonium (Fig. 16, 17 and 18)
Figure 16. Two-bottle intake and preference for 5 concentrations of quinine hydrochloride (QHCl) by 14 strains of rats.
Each bar shows the mean ± SE for each strain with males and females combined. Strains sharing the same letters above the bars did not differ significantly (p<0.001). ANOVA results in each panel refer to effect of strain.
Figure 17. Two-bottle intake and preference for 4 concentrations of caffeine by 14 strains of rats.
Each bar shows the mean ± SE for each strain with males and females combined. Strains sharing the same letters above the bars did not differ significantly (p<0.001). ANOVA results in each panel refer to effect of strain.
Figure 18. Two-bottle intake and preference for 4 concentrations of denatonium by 14 strains of rats.
Each bar shows the mean ± SE for each strain with males and females combined. Strains sharing the same letters above the bars did not differ significantly (p<0.001). ANOVA results in each panel refer to effect of strain.
Several surveys of mouse strain preferences for bitter compounds have been conducted [e.g., (48,49)] and a recent 10-strain survey involved 6-h one-bottle tests (43). There have also been a smattering of previous studies investigating taste preferences for bitter compounds in rats (see below). One issue is whether bitter taste compounds have a unitary bitter taste. Based on correlations in preferences among the strains there was evidence for similar patterns of response to QHCl and caffeine (0.1 mM QHCl vs. 10 mM caffeine, r = 0.75; 1 mM QHCl vs. 10 mM caffeine, r = 0.75) and between QHCl and denatonium (0.0316 mM QHCl vs. 0.1 mM denatonium, r = 0.80, 0.0316 mM QHCl vs. 0.316 mM denatonium, r = 0.74; 0.316 mM QHCl vs. 0.316 mM denatonium, r = 0.69, 1 mM QHCl vs. 0.316 mM denatonium, r = 0.69) but only a weak, nonsignificant positive relationship between preferences for any concentration of caffeine and any concentration of denatonium (all r’s < 0.55). In other words, preferences for QHCl were related to preferences for caffeine and denatonium but preferences for caffeine and denatonium were unrelated to each other.
None of the 14 strains preferred any concentration of the three bitter compounds to water, which is surprising in view of reports that LE rats drink more sucrose octaacetate than water in one-bottle tests (34) and that FHH rats prefer low concentrations of cycloheximide (79) and quinine (36) to water. There were large differences in avoidance thresholds among the strains. The FHH strain was outlying in that it had the highest avoidance threshold for caffeine and it was also a member of the groups with the highest avoidance thresholds for the other two bitter compounds. In contrast, the BN strain was outlying in that it had the lowest avoidance threshold of all strains for denatonium and was also a member of the groups with lowest avoidance threshold for QHCl and caffeine.
The largest strain differences in preference for bitter compounds were observed with 0.0316 mM QHCl and 1 mM caffeine. For 0.0316 mM QHCl, ten strains comprised the group with the lowest preferences and the BUF, COP, Dahl, and FHH strains comprised the group with the highest preferences (the SHR strain had preferences intermediate between the extremes and also high variability so they did not differ from either the highest or the lowest responders). For 1 mM caffeine, there were 10 strains in the group with highest preferences; the WI, BN and Noble stains had the lowest preferences. There were no significant differences among the strains in preference for any concentration of denatonium, although results with 0.0316 mM denatonium bordered on significance (p = 0.0015). The muted effect was not because the concentrations were undetectable or unduly disliked by all strains (Fig. 18).
Rats drank so little of the 0.316 mM QHCl, 1 mM QHCl and 31.6 mM caffeine solutions that these concentrations were not informative. We are not the first to use too high concentrations of bitter compounds in rat taste preference studies. Preference for 0.25% quinine solution did not differ among 7 strains of rats in one study (57) or in a comparison of LEW, WIS and WKY rats in another (37) but, in each case, intakes of this concentration of quinine were essentially zero (i.e., <1 mL/4 days).
Consistent with our results with 0.1 mM QHCl and 10 mM caffeine, FHH rats had lower avoidances than did WI or LEW rats for a wide range of quinine concentrations (36), and lower avoidances of high concentrations of cycloheximide and phenylthiocarbamide than did LE or WI rats (79). WI, BUF and F344 rats did not differ in quinine sulfate preference (0.0001% – 0.0009%) but these strains avoided some concentrations less than did Munich-Wistar rats (51). Brattleboro rats avoided high concentrations of quinine sulfate (20 – 50 mg/L) more than did LE rats (34).
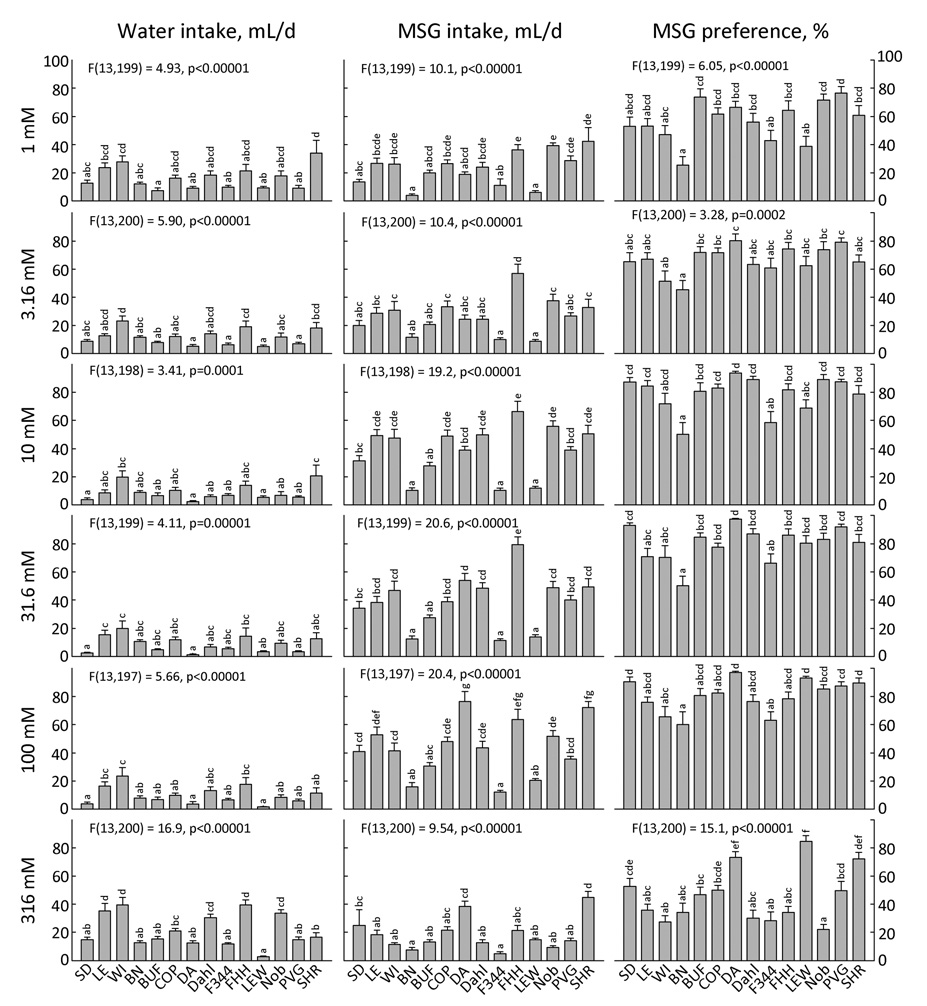
Monosodium glutamate (Fig. 19)
Figure 19. Two-bottle intake and preference for 6 concentrations of monosodium glutamate (MSG) by 14 strains of rats.
Each bar shows the mean ± SE for each strain with males and females combined. Strains sharing the same letters above the bars did not differ significantly (p<0.001). ANOVA results in each panel refer to effect of strain. Results for 10 mM MSG preference, F(13,198) = 5.94, p<0.00001; 31.6 mM MSG preference, F(13,199) = 6.47, p<0.00001; 100 mM MSG preference, F(13,197) = 5.67, p<0.00001.
Monosodium glutamate (MSG) is the compound most commonly used as an exemplar of umami taste. The only published survey of mouse strain responses to umami involved 6-h one-bottle tests (43). However, a previous survey using 12-h one-bottle tests to assess consumption of 13 concentrations of MSG involved 14 rat strains, of which 7 were the same or similar to those used here [SD, LE, WI, BN, F344, LEW and SHR; additional strains were Donryu, Hairless, LEA, LEC, WKY and SD×LEA F1; (42)]. Unfortunately, comparison with the current study is precluded because of differences in the methods and presentation of the results.
We found that MSG yielded the largest strain differences in preference of all 17 taste compounds tested. Eleven strains preferred at least one concentration of MSG to water but the range of preferred concentrations differed among the strains and the WI, BN and F344 strains did not prefer any concentration of MSG to water (Table 4). The largest strain differences in preference were obtained with 316 mM MSG, the highest concentration tested (Fig. 19). The DA, LEW, and SHR strains had the highest preferences and each member of this group drank significantly more 316 mM MSG than water. In contrast, the WI and Noble strains significantly avoided 316 mM MSG and along with 5 other strains were members of the group with the lowest preferences.
Corn Oil (Fig. 20)
Figure 20. Two-bottle intake and preference for 5 concentrations of corn oil by 14 strains of rats.
Each bar shows the mean ± SE for each strain with males and females combined. Strains sharing the same letters above the bars did not differ significantly (p<0.001). ANOVA results in each panel refer to effect of strain. Results for 1% corn oil preference, F(13,192) = 6.49, p<0.00001; 2% corn oil preference, F(13,192) = 4.33, p<0.00001; 4% corn oil preference, F(13,189) = 6.06, p<0.00001. Note that corn oil was held in suspension with 0.3% soybean phosphatidylcholine and 0.2% xanthan gum, and these were also added to the “water” bottle.
There is a survey of oil (intralipid) preferences in 11 strains of mice (46) but no previous strain survey of rats. We found that 8 strains preferred at least one concentration of corn oil; the other 6 strains did not prefer any corn oil emulsion significantly more than the suspension agent mixture (Table 4). Three strains (LE, BUF and DA) significantly avoided the highest (16%) concentration of corn oil (Table 5).
The 16% corn oil emulsion supported the largest strain differences in preference. Even so, at this concentration there were no particularly outstanding strains with respect to corn oil preference. There were 9 strains in the group with the highest preferences and 8 strains in the group with the lowest preferences (the BN, COP, and Noble strains were in both the high-and low-preferring groups).
Differences in fat preference between Osborne-Mendel and S5B/Pl) rat (56) have been exploited by Gilbertson and colleagues (35) to compare fat taste transduction and the enhancement of saccharin preference by fat. Based on these two strains, Gilbertson et al have argued that there is an inverse relationship between sensitivity to fat taste and preference, which is related to body weight. In our results, there were nonsignificant negative correlations between preferences for corn oil and body weight (the strongest correlation was preference for 4% corn oil vs. body weight, r = −0.36, p = 0.2061) and no significant difference in body weight between those strains that preferred corn oil to its suspension agent mixture and those that did not. Thus, we saw no simple relationship between fat preference and body weight.
Ethanol (Fig. 21)
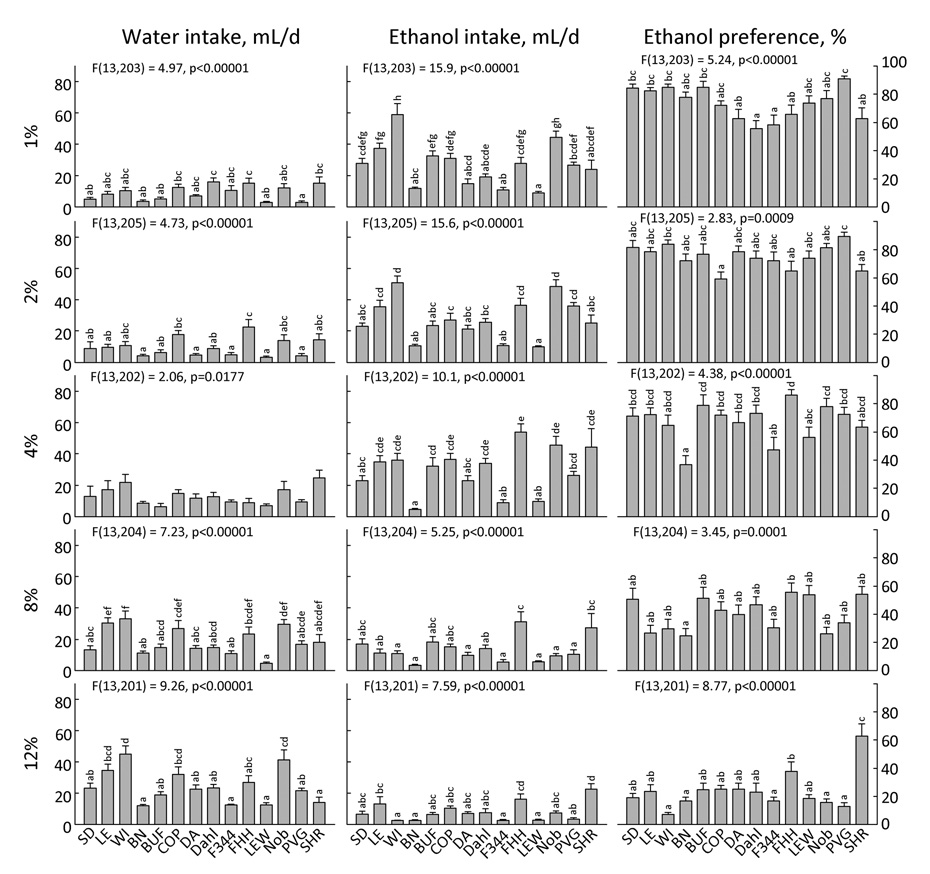
Figure 21. Two-bottle intake and preference for 5 concentrations of ethanol by 14 strains of rats.
Each bar shows the mean ± SE for each strain with males and females combined. Strains sharing the same letters above the bars did not differ significantly (p<0.001). ANOVA results in each panel refer to effect of strain. Ethanol concentrations (shown at left) are in percent volume/volume.
A recent study compares the ethanol preferences of 22 inbred mouse strains (94). In contrast to most of the other taste compounds tested in this project, there has been considerable interest in rat strain differences in ethanol preference. Several strains have been bred to selectively prefer or avoid ethanol [e.g., High Alcohol Drinking (HAD) and Low Alcohol Drinking (LAD), Alcohol-Preferring (P) and Nonpreferring (NP), Alcohol Accepting (AA) and Nonaccepting (ANA) see (57)]. Other strains have been selectively bred for other traits but differ in ethanol preferences [e.g., Flinders Sensitive and Resistant Lines (FSL and FRL), Maudsley Reactive and Nonreactive (59), Floripa high (H) and low (L) (17), Tryon maze-bright and maze-dull (3)]. Some of the more “general use” strains including several of those used here have also been tested for ethanol preference [e.g., LEW (17,37), Dahl, SHR, Wistar Kyoto Hyperactive (WKHA), and WKY (12,17,37,41)].
In our study, all strains except the F344 and SHR preferred at least one low (1 – 4%) ethanol concentration to water and all except the FHH and SHR avoided high (8 or 12%) concentrations. Strain differences appeared to be concentration-specific. For example, the BN strain had notably low preferences for 4% ethanol but not for other ethanol concentrations. Similarly, the SHR strain had notably high preferences for 12% ethanol but not for other concentrations.
The FHH strain has been intensively investigated as an ethanol-preferring strain, with the ACI or SD strains often used as a low-preferring controls [e.g., (3,18,37,60)]. An important consideration is that we tested the FHH/Har substrain, which does not have as high ethanol preferences as the FHH/Wjd sub-strain (58). Even so, the FHH strain was always in the highest ethanol-preferring group, had notably high intakes of 8% ethanol (Fig. 21), and was ranked 2nd highest in ethanol preference overall (BUF was highest).
Capsaicin (Fig. 22)
Capsaicin is often used as an exemplar of a compound that activates the trigeminal system. There is a report of the response of 10 strains of mice during one-bottle access to various concentrations of capsaicin (33) but no previous rat strain comparisons of preferences for capsaicin or other trigeminal stimuli. In our study, no strain preferred any concentration of capsaicin to water. However, six strains significantly avoided the lowest (1 µM) concentration we tested so, in retrospect, it would have been useful to have tested lower concentrations of capsaicin. The LEW strain had a higher rejection threshold than did the other 13 strains. It was also ranked highest in capsaicin preference, and along with the BUF and SHR strains it had the highest preferences for 3.16 µM capsaicin, the concentration supporting the greatest strain differences. The remaining 11 strains all had equivalently low preferences at this concentration.
GENERAL DISCUSSION
This study provides the results of a comprehensive series of over 24,000 two-bottle choice tests, involving consumption of 17 taste compounds by male and female rats from 14 strains. The strains were chosen to provide as much genetic diversity as possible and the success of this was reflected in the wide range of taste preferences observed in nearly every test conducted. There were strain differences in preferences that were significant at greater than the p<0.001 level for 16 of the 17 compounds tested, and even for the exception, denatonium, there was a strain effect of p = 0.0015 for one concentration. Apparently, the genome of the laboratory rat is sufficiently diverse that it can influence preferences for exemplars of all basic taste qualities (sweet, sour, salty and bitter), several putative basic taste qualities (MSG-umami, calcium-mineral, corn oil-fat, Polycose-starch) and compounds with a trigeminal component (ethanol, capsaicin).
Most strains did not show a general sensitivity or insensitivity to all taste compounds although there were at least three exceptions. The SD strain had the 1st, 2nd or 3rd highest preference for 10 out of the 17 compounds tested and had no particularly low rankings (Table 6). In contrast, the BN and WI strains were ranked in the bottom three or four strains for 11 compounds. These results suggest that there may be a general responsiveness to taste compounds in some strains. This could be related to neophobia or a general aguesia. An interesting possibility is that the general proclivity to drink taste solutions is an inverse function of the avidity for water (since all tests involved a choice with water). Perhaps SD rats have generally high taste solution preferences because they do not much like the taste of water, and conversely WI and BN strains have generally low taste solution preferences because they find water more acceptable than do other strains.
One impetus for conducting this study was to compare differences in variability between inbred and outbred strains. Using water intakes to assess this, we found that variability was strongly related to intake; that is, rats with high intakes also had highly variable intakes. With the exception of the SHR strain, which had much higher variability than all the other strains, inbred and outbred strains were equally variable. This was unexpected given the greater genetic variability of the outbred than inbred strains. Perhaps the greater genetic range of the outbred strains is insubstantial in comparison to inter-strain differences. Alternatively, uncontrolled environmental factors may have greater effects on inbred than outbred strains.
There have been many reports that preferences for taste compounds are higher in female than male rats [e.g., with NaCl (14,28,75), CaCl2 (64,65,71), saccharin (53,89), quinine (53) but see (12,38,72,90)]. Thus, a puzzling aspect of the present results was that there were only two sex differences in taste preferences (with 100 mM saccharin and 316 mM MSG) out of 1526 comparisons. One explanation for the discrepancy might be that in the present work, sex differences were subtle relative to strain differences, and thus they were obscured by this larger source of variability or lost due to the unusually stringent criterion for significance used to protect against Type II errors. However, this seems unlikely because there were no concerted trends in preference, either between strains or across taste compounds (see on-line material). We have also observed few sex differences in the preferences of mice. For example, out of a total of 40 mouse strains, 32 had no sex difference in sodium preference and 29 had no sex difference in calcium preference (82,85,86). Thus, sex differences in taste preferences are the exception rather than the rule in our laboratory. There are two procedural details that are shared by our rat and mouse studies but uncommon in other studies. First, most investigators conduct two-bottle choice tests with taste solutions dissolved in tap water whereas we use deionized water for all tests, as well as the maintenance fluid. Second, to our knowledge, all two-bottle choice tests by other investigators have involved animals fed chow but we feed our subjects a semisynthetic diet. Rodents fed chow drink considerably more fluid than those fed semisynthetic diets (82) so we wonder if sex differences in preference are related in some way to this diet-stimulated water intake. It is noteworthy that that chow contains uncontrolled levels of phytoestrogens that could potentially influence sex-related behaviors (91). Additional analysis is warranted.
The strains differed substantially in the number of fungiform papillae; at the extremes, the DA strain had 54% more papillae than did the COP strain. There was no obvious relationship between a strain’s taste preferences and the number of fungiform papillae it had, although there was a tendency for strains with the most fungiform papillae to have the greatest avoidance of some concentrations of HCl and QHCl. This conclusion must be treated cautiously because the number of fungiform papillae may change over the life span of the rat, and our measurements were made only at the end of experiment, when the rats were ~1 yr old.
Although the primary focus of this study was on taste preferences, incidental data on water intake, food intake and body weight were collected. There are many reports of rat strain differences in body weight [e.g., (61)] but our study was unusual in that the maintenance diet was rigorously formulated (AIN-76A). Relative to typical laboratory chows this diet has a slightly higher energy density (15.9 vs. ~13 kJ/g), roughly equivalent fat content (~12% of total energy in each case), less protein (20% vs. ~29% of total energy), and more carbohydrate (68% vs. ~59% of total energy) with the largest difference being that most of the carbohydrate in AIN-76A is provided as sucrose whereas that in chow is provided as starch. Based on comparisons made in mice (82,87), the AIN-76A diet appears to be more “obesigenic” than chow but not nearly as much as the semisynthetic high fat diets typically used to produce obesity. We found marked strain and sex differences in body weight that became larger as the rats aged. Body weight may have been influenced by providing the rats with taste solutions to drink, some of which had known postingestive effects, although if so these were too small to notably influence the regular, progressive growth of any strain (Fig. 1).
Food and water intakes also differed among strains. It is not surprising that big rats eat and drink more than do little rats, and differences in body weight could account for most of the variation in food intake (the SHR strain was an exception). However, this was not true for water intake, implying that this behavior is regulated by controls that are not simply a reflection of body size. Indeed, even within strains, there was clearly little relationship between water intake and body weight. Whereas body weight progressively increased over the experiment and males were much heavier than females (Fig. 1), water intake remained more-or-less constant as animals aged and there were no concerted sex differences (if anything, females drank more than did males; Fig. 3). A practical implication of this is that it is generally inappropriate to adjust fluid intakes by body weight (i.e., mL/kg) in order to compare rat strains [see (6,86) additional discussion based on similar work in the mouse]. Unfortunately, this is a widespread practice, particularly in studies involving ethanol consumption.
The primary measure used here is the taste preference score, which is the ratio of taste solution intake to total intake. This has the advantage of allowing meaningful comparisons among stains with disparate total fluid intakes. However, by virtue of being ratios, preference scores are nominal (rather than interval) values and although they approximate interval values in the mid-range of the scale, they can fail at the extremes (i.e., when preferences are <15% or >85%). This was most likely the case with some concentrations of NaCl, saccharin, sucrose, Polycose and MSG. For these, there were only small differences among groups in preference scores despite large differences in solution intakes that did not simply reflect body size or daily fluid intakes. Ways to reduce this problem with strongly preferred taste solutions include arcsine transformation of all preference scores, or expressing consumption in relation to average daily fluid intakes (e.g., solution intake/water intake during a test with only water available × 100). Ultimately, the most appropriate measure of consumption will depend upon the questions being asked.
Limitations
The results of this study provide a reasonable first resource for choosing rat strains for taste preference studies, but there are a number of limitations that should be considered. First, like any comprehensive strain survey, a very large number of comparisons are involved, and this raises the chance of statistical errors. We used a criterion for significance of p<0.001, which is 50 times more conservative than the more typical p<0.05 used in smaller studies but it still was unlikely to protect from all Type II errors. Conversely, by using this conservative criterion, it is very likely that some relatively subtle strain differences were missed (i.e., Type I errors). It will require replication from different laboratories in order to determine the validity of our results.
A limitation of this study is that rats were tested sequentially with many different taste compounds so consumption during tests conducted late in the experiment may have been influenced by prior experience with other taste solutions. The existence of these carry-over effects has been demonstrated in long-term taste preference tests for some taste solutions, particularly sodium [e.g., (7–9,32,47)] but their generality and persistence is unknown. We did two things to remediate carry-over effects. First, in each taste series we presented the taste solution in progressively increasing concentrations, with the idea that any carry-over effects from drinking low taste solution concentrations would be negligible in the face of the higher intensity of the solution given next in the series. We considered this preferable to presenting concentrations in counterbalanced order, which has the disadvantages of (a) increasing the opportunity for an aversive experience to precede a neutral one, and (b) increasing within-strain variability and thus decreasing the opportunity to observe genetic effects. Second, we included days with only water to drink between each test series. In most cases, this was one or two days with a single bottle of water but after tests with nutrient solutions this was extended to 4 days with a single bottle. These wash-out periods were actually even longer because each test series also began with a 2-day test with two water bottles. In the mouse, carry-over effects have been observed to influence strain mean preferences but not the relative ranking of the strains [e.g., (7,8)]. We suspect the same is likely to prove true for rats as well, but it will require independent replication to be sure.
The whole series of preference tests required over 40 wk to conduct so maturational and other age-related effects may be a concern. We assumed this would not be an issue based on work in two strains of mice that suggests taste preferences are stable between 8 – 100 wk old (82). However, some rat strains had notably low water intakes at 8 wk and so may not have had stable “adult” intakes until they were older (see Fig. 3). It would be prudent to avoid this problem in future work by testing rats beginning at age 10 wk or older.
From the point of view of choosing strains for taste preference studies, there are reasons to avoid two of the strains we tested. One, the Dahl strain had a high fatality rate over the 40-wk duration study, although this would not be a problem for shorter studies. More seriously, the SHR strain is hyperactive, particularly in novel conditions [review, (88)], and in our hands, SHR rats were particularly prone to dislodge drinking spouts leading to high rates of spillage. Because of this, the number of subjects contributing to averages was frequently lower than for other strains, and there is a possibility that a component of the “intake” of this strain was actually due to spillage. Interpretation of the results obtained from the SHR strain deserves particular scrutiny and future studies should include accommodation to prevent the high spillage of this strain.
We consider this study a broad-brush approach to paint a big picture of taste preferences in rats and, undoubtedly, some details will have been missed and others misplaced. We trust that the limitations of this study will be considered by investigators using the data to inform their own study designs. We note that the limitations do not detract from the main conclusion, which is that rats show tremendous diversity in taste preferences. The data provided here are a foundation for choosing rat strains that can be exploited in order to understand the genetic and physiological bases of this diversity.
Supplementary Material
Acknowledgements
We thank Dr. Mary Shimoyama and other members of the RGD rat forum for advice choosing the strains tested here. Stacy Hultine and Stephanie Craw provided excellent technical assistance. Supported by NIH grant DK-46791.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abrams M, DeFriez AIC, Tosteson DC, Landis EM. Self-selection of salt solutions and water by normal and hypertensive rats. Am. J. Physiol. 1949;156:233–241. doi: 10.1152/ajplegacy.1949.156.2.233. [DOI] [PubMed] [Google Scholar]
- 2.Aitman T. co-signatories), e. a. a. a. Progress and prospects in rat genetics: a community view. Nature Genetics. doi: 10.1038/ng.147. in press. [DOI] [PubMed] [Google Scholar]
- 3.Amit Z, Smith BR. Differential ethanol intake in Tryon maze-bright and Tryon maze-dull rats: implications for the validity of the animal model of selectively bred rats for high ethanol consumption. Psychopharmacology (Berl) 1992;108:136–140. doi: 10.1007/BF02245298. [DOI] [PubMed] [Google Scholar]
- 4.Bachmanov AA, Beauchamp GK. Taste receptor genes. Annu Rev Nutr. 2007;27:389–414. doi: 10.1146/annurev.nutr.26.061505.111329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachmanov AA, Beauchamp GK, Tordoff MG. Voluntary consumption of NaCl, KCl, CaCl2 and NH4Cl solutions by 28 mouse strains. Behav. Genetics. 2002;32:445–457. doi: 10.1023/a:1020832327983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav. Genetics. 2002;32:435–443. doi: 10.1023/a:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachmanov AA, Schlager G, Tordoff MG, Beauchamp GK. Consumption of electrolytes and quinine by mouse strains with different blood pressures. Physiol. Behav. 1998;64:323–330. doi: 10.1016/s0031-9384(98)00069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bachmanov AA, Tordoff MG, Beauchamp GK. Voluntary sodium chloride consumption by mice: differences among five inbred strains. Behav. Genetics. 1998;28:117–124. doi: 10.1023/a:1021471924143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachmanov AA, Tordoff MG, Beauchamp GK. Intake of umami-tasting solutions by mice: a genetic analysis. J. Nutr. 2000;130 supplement:935S–941S. doi: 10.1093/jn/130.4.935S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boughter JD, Jr., Bachmanov AA. Behavioral genetics and taste. BMC Neurosci. 2007;8 Suppl 3:S3. doi: 10.1186/1471-2202-8-S3-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourjeili N, Turner M, Stinner J, Ely D. Sympathetic nervous system influences salt appetite in four strains of rats. Physiol Behav. 1995;58:437–443. doi: 10.1016/0031-9384(95)00077-v. [DOI] [PubMed] [Google Scholar]
- 12.Cailhol S, Mormede P. Sex and strain differences in ethanol drinking: effects of gonadectomy. Alcohol Clin Exp Res. 2001;25:594–599. [PubMed] [Google Scholar]
- 13.Catalanotto F, Schecter PJ, Henkin RI. Preference for NaCl in the spontaneously hypertensive rat. Life Sci. 1972;11:557–564. doi: 10.1016/0024-3205(72)90190-7. [DOI] [PubMed] [Google Scholar]
- 14.Chow SY, Sakai RR, Witcher JA, Adler NT, Epstein AN. Sex and sodium intake in the rat. Behav. Neurosci. 1992;106:172–180. doi: 10.1037//0735-7044.106.1.172. [DOI] [PubMed] [Google Scholar]
- 15.Coldwell SE, Tordoff MG. Acceptance of minerals and other compounds by calcium-deprived rats: 24-h tests. Am. J. Physiol. 1996;271:R1–R10. doi: 10.1152/ajpregu.1996.271.1.R1. [DOI] [PubMed] [Google Scholar]
- 16.Cook VI, Moe KE. The early ontogeny of sodium appetite in spontaneously hypertensive vs. normotensive rats. Physiol. Behav. 1989;46:1003–1007. doi: 10.1016/0031-9384(89)90205-9. [DOI] [PubMed] [Google Scholar]
- 17.Da Silva GE, Ramos A, Takahashi RN. Comparison of voluntary ethanol intake by two pairs of rat lines used as genetic models of anxiety. Braz J Med Biol Res. 2004;37:1511–1517. doi: 10.1590/s0100-879x2004001000010. [DOI] [PubMed] [Google Scholar]
- 18.Daoust M, Compagnon P, Legrand E, Boucly P. Ethanol intake and 3H-serotonin uptake I: A study in Fawn-Hooded rats. Life Sci. 1991;48:1969–1976. doi: 10.1016/0024-3205(91)90230-9. [DOI] [PubMed] [Google Scholar]
- 19.Dess NK. Saccharin's aversive taste in rats: evidence and implications. Neurosci Biobehav Rev. 1993;17:359–372. doi: 10.1016/s0149-7634(05)80113-7. [DOI] [PubMed] [Google Scholar]
- 20.Dess NK. Responses to basic taste qualities in rats selectively bred for high versus low saccharin intake. Physiol Behav. 2000;69:247–257. doi: 10.1016/s0031-9384(99)00246-2. [DOI] [PubMed] [Google Scholar]
- 21.Dess NK, Badia-Elder NE, Thiele TE, Kiefer SW, Blizard DA. Ethanol consumption in rats selectively bred for differential saccharin intake. Alcohol. 1998;16:275–278. doi: 10.1016/s0741-8329(98)00010-x. [DOI] [PubMed] [Google Scholar]
- 22.Di Nicolantonio R, Mendelsohn FAO, Hutchinson JS. Sodium chloride preference of genetically hypertensive and normotensive rats. Am. J. Physiol. 1983;245:R38–R44. doi: 10.1152/ajpregu.1983.245.1.R38. [DOI] [PubMed] [Google Scholar]
- 23.Dyets Inc. 100000 AIN-76A Purified rodent diet. 2003 http://www.dyets.com/100000.htm.
- 24.Ferrell F, Dreith AZ. Calcium appetite, blood pressure and electrolytes in spontaneously hypertensive rats. Physiol. Behav. 1986;37:337–343. doi: 10.1016/0031-9384(86)90243-x. [DOI] [PubMed] [Google Scholar]
- 25.Ferrell F, Gray SD. Longitudinal study of salt preferences in normotensive and hypertensive rats. Hypertension. 1985;7:326–332. [PubMed] [Google Scholar]
- 26.Ferrell F, Lanou A, Gray SD. Salt level in weaning diet affects saline preference and fluid intake in Dahl rats. Hypertension. 1986;8:1021–1026. doi: 10.1161/01.hyp.8.11.1021. [DOI] [PubMed] [Google Scholar]
- 27.Flynn FW, Culver B, Newton SV. Salt intake by normotensive and spontaneously hypertensive rats: two-bottle and lick rate analyses. Physiol Behav. 2003;78:689–696. doi: 10.1016/s0031-9384(03)00062-3. [DOI] [PubMed] [Google Scholar]
- 28.Flynn FW, Schulkin J, Havens M. Sex differences in salt preference and taste reactivity in rats. Brain Res. Bull. 1993;32:91–95. doi: 10.1016/0361-9230(93)90061-f. [DOI] [PubMed] [Google Scholar]
- 29.Fregly MJ. NaCl intake and preference threshold of spontaneously hypertensive rats. Proc. Soc. Exp. Biol. Med. 1975;149:915–920. doi: 10.3181/00379727-149-38926. [DOI] [PubMed] [Google Scholar]
- 30.Fregly MJ, Harper JM, Radford EP. Regulation of sodium chloride intake by rats. Am. J. Physiol. 1965;209:287–292. doi: 10.1152/ajplegacy.1965.209.2.287. [DOI] [PubMed] [Google Scholar]
- 31.Fregly MJ, Rowland NE. Role of the renin-angiotensin-aldosterone system in NaCl appetite of rats. Am. J. Physiol. 1985;248:R1–R11. doi: 10.1152/ajpregu.1985.248.1.R1. [DOI] [PubMed] [Google Scholar]
- 32.Fregly MJ, Rowland NE. Comparison of preference thresholds for NaCl solution in rats of the Sprague-Dawley and Long-Evans strains. Physiol. Behav. 1992;51:915–918. doi: 10.1016/0031-9384(92)90070-i. [DOI] [PubMed] [Google Scholar]
- 33.Furuse T, Blizard DA, Moriwaki K, Miura Y, Yagasaki K, Shiroishi T, Koide T. Genetic diversity underlying capsaicin intake in the Mishima battery of mouse strains. Brain Res Bull. 2002;57:49–55. doi: 10.1016/s0361-9230(01)00636-0. [DOI] [PubMed] [Google Scholar]
- 34.Gartside IB, Laycock JF. Increased aversion to bitter-tasting fluids in the Brattleboro rat. Physiol Behav. 1987;39:571–577. doi: 10.1016/0031-9384(87)90155-7. [DOI] [PubMed] [Google Scholar]
- 35.Gilbertson TA, Liu L, Kim I, Burks CA, Hansen DR. Fatty acid responses in taste cells from obesity-prone and -resistant rats. Physiol Behav. 2005;86:681–690. doi: 10.1016/j.physbeh.2005.08.057. [DOI] [PubMed] [Google Scholar]
- 36.Goodwin FL, Amit Z. Relative taste thresholds for ethanol, saccharin, and quinine solutions in three strains of rats nonselected for ethanol: a comparative study. Exp Clin Psychopharmacol. 2000;8:216–224. doi: 10.1037//1064-1297.8.2.216. [DOI] [PubMed] [Google Scholar]
- 37.Goodwin FL, Bergeron N, Amit Z. Differences in the consumption of ethanol and flavored solutions in three strains of rats. Pharmacol Biochem Behav. 2000;65:357–362. doi: 10.1016/s0091-3057(99)00222-1. [DOI] [PubMed] [Google Scholar]
- 38.Heppner CC, Kemble ED, Cox WM. Effects of food deprivation on caffeine consumption in male and female rats. Pharmacol Biochem Behav. 1986;24:1555–1559. doi: 10.1016/0091-3057(86)90484-3. [DOI] [PubMed] [Google Scholar]
- 39.Heyer BR, Taylor-Burds CC, Mitzelfelt JD, Delay ER. Monosodium glutamate and sweet taste: discrimination between the tastes of sweet stimuli and glutamate in rats. Chem Senses. 2004;29:721–729. doi: 10.1093/chemse/bjh081. [DOI] [PubMed] [Google Scholar]
- 40.Kampov-Polevoy AB, Garbutt JC, Janowsky DS. Association between preference for sweets and excessive alcohol intake: a review of animal and human studies. Alcohol Alcohol. 1999;34:386–395. doi: 10.1093/alcalc/34.3.386. [DOI] [PubMed] [Google Scholar]
- 41.Khanna JM, Kalant H, Chau AK, Sharma H. Initial sensitivity, acute tolerance and alcohol consumption in four inbred strains of rats. Psychopharmacology (Berl) 1990;101:390–395. doi: 10.1007/BF02244059. [DOI] [PubMed] [Google Scholar]
- 42.Kondoh T, Mori M, Ono T, Torii K. Mechanisms of umami taste preference and aversion in rats. J Nutr. 2000;130:966S–970S. doi: 10.1093/jn/130.4.966S. [DOI] [PubMed] [Google Scholar]
- 43.Kotlus BS, Blizard DA. Measuring gustatory variation in mice: a short-term fluid-intake test. Physiol Behav. 1998;64:37–47. doi: 10.1016/s0031-9384(98)00016-x. [DOI] [PubMed] [Google Scholar]
- 44.Ledingham JM, Simpson FO, Hamada M. Salt appetite, body sodium, handling of a NaCl load, renin, and aldosterone in genetically and spontaneously hypertensive rats. J Cardiovasc Pharmacol. 1990;16 Suppl 7:S6–S8. [PubMed] [Google Scholar]
- 45.Lewis SR, Ahmed S, Dym C, Khaimova E, Kest B, Bodnar RJ. Inbred mouse strain survey of sucrose intake. Physiol Behav. 2005;85:546–556. doi: 10.1016/j.physbeh.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 46.Lewis SR, Dym C, Chai C, Singh A, Kest B, Bodnar RJ. Genetic variance contributes to ingestive processes: a survey of eleven inbred mouse strains for fat (Intralipid) intake. Physiol Behav. 2007;90:82–94. doi: 10.1016/j.physbeh.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 47.London RM, Snowdon CT, Smithana JM. Early experience with sour and bitter solutions increases subsequent ingestion. Physiol Behav. 1979;22:1149–1155. doi: 10.1016/0031-9384(79)90270-1. [DOI] [PubMed] [Google Scholar]
- 48.Lush IE. The genetics of tasting in mice. I. Sucrose octaacetate. Genet Res. 1981;38:93–95. doi: 10.1017/s0016672300020425. [DOI] [PubMed] [Google Scholar]
- 49.Lush IE. The genetics of tasting in mice. III Quinine. Genetic. Res. 1984;44:151–160. doi: 10.1017/s0016672300026355. [DOI] [PubMed] [Google Scholar]
- 50.McConnell SD, Henkin RI. NaCl preference in spontaneously hypertensive rats: age and blood pressure effects. Am. J. Physiol. 1973;225:624–627. doi: 10.1152/ajplegacy.1973.225.3.624. [DOI] [PubMed] [Google Scholar]
- 51.Midkiff EE, Fitts DA, Simpson JB, Bernstein IL. Absence of sodium chloride preference in Fischer-344 rats. Am. J. Physiol. 1985;249:R438–R442. doi: 10.1152/ajpregu.1985.249.4.R438. [DOI] [PubMed] [Google Scholar]
- 52.Muntzel M, Lacour B, Pouzet B, Geelen G, Drueke T. Physiological correlates of attenuated salt appetite in Fischer 344 rats. Physiol Behav. 1994;55:77–82. doi: 10.1016/0031-9384(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 53.Nance DM, Gorski RA, Panksepp J. Neural and hormonal determinants of sex differences in food intake and body weight. In: Novin D, Wyrwicka W, Bray G, editors. Hunger: basic mechanisms and clinical implications. New York: Raven Press; 1976. pp. 257–271. [Google Scholar]
- 54.National Bio Resource Project for the Rat (NBRP) National Bio Resource Project for the Rat (NBRP) 2007 http://www.anim.med.kyoto-u.ac.jp/NBR/home.htm.
- 55.Nijman IJ, Kuipers S, Verheul M, Guryev V, Cuppen E. A genome-wide SNP panel for mapping and association studies in the rat. BMC Genomics. 2008;9:95. doi: 10.1186/1471-2164-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Okada S, York DA, Bray GA, Mei J, Erlanson-Albertsson C. Differential inhibition of fat intake in two strains of rat by the peptide enterostatin. Am J Physiol. 1992;262:R1111–R1116. doi: 10.1152/ajpregu.1992.262.6.R1111. [DOI] [PubMed] [Google Scholar]
- 57.Overstreet D, Kampov-Polevoy A, Rezvani A, Murelle L, Halikas J, Janowsky D. Saccharin intake predicts alcohol intake in genetically heterogenous rats as well as different rat strains. Alcohol Clin. Exp. Res. 1993;17:366–369. doi: 10.1111/j.1530-0277.1993.tb00777.x. [DOI] [PubMed] [Google Scholar]
- 58.Overstreet DH, Rezvani AH. Behavioral differences between two inbred strains of Fawn-Hooded rat: a model of serotonin dysfunction. Psychopharmacology (Berl) 1996;128:328–330. doi: 10.1007/s002130050141. [DOI] [PubMed] [Google Scholar]
- 59.Overstreet DH, Rezvani AH, Janowsky DS. Genetic animal models of depression and ethanol preference provide support for cholinergic and serotonergic involvement in depression and alcoholism. Biol Psychiatry. 1992;31:919–936. doi: 10.1016/0006-3223(92)90118-j. [DOI] [PubMed] [Google Scholar]
- 60.Overstreet DH, Rezvani AH, Parsian A. Behavioural features of alcohol-preferring rats: focus on inbred strains. Alcohol Alcohol. 1999;34:378–385. doi: 10.1093/alcalc/34.3.378. [DOI] [PubMed] [Google Scholar]
- 61.Poiley SM. Growth tables for 66 strains and stocks of laboratory animals. Lab. Anim. Sci. 1972;22:759–779. [PubMed] [Google Scholar]
- 62.Rat genome database. Rat genome database. 2007 http://rgd.mcw.edu/ [Google Scholar]
- 63.Reed DR, Li S, Li X, Huang L, Tordoff MG, Starling-Roney R, Taniguchi K, West DB, Ohmen JD, Beauchamp GK, Bachmanov AA. Polymorphisms in the taste receptor gene (Tas1r3) region are associated with saccharin preference in 30 mouse strains. J Neurosci. 2004;24:938–946. doi: 10.1523/JNEUROSCI.1374-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reilly JJ, Epstein AN, Schulkin J. Hormonal control of calcium ingestion: The effects of neonatal gonadectomy on the calcium ingestion of male and female rats. SSIB. 1992;53 [Google Scholar]
- 65.Reilly JJ, Nardozzi J, Schulkin J. The ingestion of calcium in multiparous and virgin female rats. Brain Res. Bull. 1995;37:301–303. doi: 10.1016/0361-9230(95)00036-e. [DOI] [PubMed] [Google Scholar]
- 66.Richter CP. Salt taste thresholds of normal and adrenalectomized rats. Endocrinol. 1939;24:367–371. [Google Scholar]
- 67.Richter CP, Campbell KH. Taste thresholds and taste preferences of rats for five common sugars. Journal of Nutrition. 1940;20:31–46. [Google Scholar]
- 68.Rowland NE. Ontogeny of preference and aversion to salt in Fischer 344 rats and Syrian hamsters. Develop. Psychobiol. 1991;24:211–218. doi: 10.1002/dev.420240306. [DOI] [PubMed] [Google Scholar]
- 69.Rowland NE. NaCl appetite in two strains of rat reported to be resistant to mineralocorticoid-induced hypertension. Physiol Behav. 1998;64:49–56. doi: 10.1016/s0031-9384(98)00018-3. [DOI] [PubMed] [Google Scholar]
- 70.Rowland NE, Fregly MJ. Thirst and sodium appetite in Dahl rats. Physiol. Behav. 1990;47:331–335. doi: 10.1016/0031-9384(90)90151-s. [DOI] [PubMed] [Google Scholar]
- 71.Schulkin J. The ingestion of calcium in female and male rats. Psychobiol. 1991;19:262–264. [Google Scholar]
- 72.Sclafani A, Hertwig H, Vigorito M, Feigin MB. Sex differences in polysaccharide and sugar preferences in rats. Neurosci Biobehav Rev. 1987;11:241–251. doi: 10.1016/s0149-7634(87)80032-5. [DOI] [PubMed] [Google Scholar]
- 73.Sollars SI, Bernstein IL. Gustatory deafferentation and desalivation: effects on NaCl preference of Fischer 344 rats. Am J Physiol. 1994;266:R510–R517. doi: 10.1152/ajpregu.1994.266.2.R510. [DOI] [PubMed] [Google Scholar]
- 74.STAR. STAR-RATS-STAR: A SNP and haplotype map for the rat. 2008 http://www.snp-star.eu/ [Google Scholar]
- 75.Stricker EM, Thiels E, Verbalis JG. Sodium appetite in rats after prolonged dietary sodium deprivation: a sexually dimorphic phenomenon. Am J Physiol. 1991;260:R1082–R1088. doi: 10.1152/ajpregu.1991.260.6.R1082. [DOI] [PubMed] [Google Scholar]
- 76.Sugita M. Taste perception and coding in the periphery. Cell Mol Life Sci. 2006;63:2000–2015. doi: 10.1007/s00018-006-6100-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thomas MA, Chen CF, Jensen-Seaman MI, Tonellato PJ, Twigger SN. Phylogenetics of rat inbred strains. Mamm Genome. 2003;14:61–64. doi: 10.1007/s00335-002-2204-5. [DOI] [PubMed] [Google Scholar]
- 78.Thunhorst RL, Johnson AK. Thirst and salt appetite responses in young and old Brown Norway rats. Am J Physiol Regul Integr Comp Physiol. 2003;284:R317–R327. doi: 10.1152/ajpregu.00368.2002. [DOI] [PubMed] [Google Scholar]
- 79.Tobach E, Bellin JS, Das DK. Differences in bitter taste perception in three strains of rats. Behav. Genetics. 1974;4:405–410. doi: 10.1007/BF01066160. [DOI] [PubMed] [Google Scholar]
- 80.Tordoff MG. Influence of dietary calcium on sodium and calcium intake of spontaneously hypertensive rats. Am. J. Physiol. 1992;262:R370–R381. doi: 10.1152/ajpregu.1992.262.3.R370. [DOI] [PubMed] [Google Scholar]
- 81.Tordoff MG. Voluntary intake of calcium and other minerals by rats. Am. J. Physiol. 1994;267:R470–R475. doi: 10.1152/ajpregu.1994.267.2.R470. [DOI] [PubMed] [Google Scholar]
- 82.Tordoff MG. Taste solution preferences of C57BL/6J and 129X1/SvJ mice: Influence of age, sex, and diet. Chem. Senses. 2007;32:655–671. doi: 10.1093/chemse/bjm034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tordoff MG, Bachmanov AA. Monell mouse taste phenotyping project. Monell Chemical Senses Center. 2001 www.monell.org/MMTPP.
- 84.Tordoff MG, Bachmanov AA. Survey of calcium and sodium intake and metabolism with bone and body composition data (MPD:103) Mouse Phenome Project. 2002 http://phenome.jax.org/pub-cgi/phenome/mpdcgi?rtn=projects/details&id=103. [Google Scholar]
- 85.Tordoff MG, Bachmanov AA, Reed DR. Forty mouse strain survey of voluntary calcium intake, blood calcium, and bone mineral content. Physiol. Behav. 2007;91:632–643. doi: 10.1016/j.physbeh.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tordoff MG, Bachmanov AA, Reed DR. Forty mouse strain survey of water and sodium intake. Physiol. Behav. 2007;91:620–631. doi: 10.1016/j.physbeh.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tordoff MG, Pilchak DM, Williams JA, McDaniel AH, Bachmanov AA. The maintenance diets of C57BL/6J and 129X1/SvJ mice influence their taste solution preferences: Implications for large-scale phenotyping projects. J. Nutr. 2002;132:2288–2297. doi: 10.1093/jn/132.8.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tucker DC, Johnson AK. Behavioral correlates of spontaneous hypertension. Neurosci Biobehav Rev. 1981;5:463–471. doi: 10.1016/0149-7634(81)90016-6. [DOI] [PubMed] [Google Scholar]
- 89.Valenstein ES, Kakolewski JW, Cox VC. Sex differences in taste preference for glucose and saccharin solutions. Science. 1967;156:942–943. doi: 10.1126/science.156.3777.942. [DOI] [PubMed] [Google Scholar]
- 90.Vataeva LA, Vershinina EA, Kassil VG. Sex dimorphism in saccharin consumption in rat ontogenesis. Effect of age of weaning. Journal of Evolutionary Biochemistry and Physiology. 2001;37:75–63. [Google Scholar]
- 91.Wang H, Tranguch S, Xie H, Hanley G, Das SK, Dey SK. Variation in commercial rodent diets induces disparate molecular and physiological changes in the mouse uterus. Proc Natl Acad Sci U S A. 2005;102:9960–9965. doi: 10.1073/pnas.0501632102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wolf G, Dahl LK, Miller NE. Voluntary sodium chloride intake of two strains of rats with opposite genetic susceptibility to experimental hypertension. Proc. Soc. Exp. Biol. Med. 1965;120:301–305. doi: 10.3181/00379727-120-30517. [DOI] [PubMed] [Google Scholar]
- 93.Yamamoto T, Matsuo R, Fujimoto Y, Fukunaga I, Miyasaka A, Imoto T. Electrophysiological and behavioral studies on the taste of umami substances in the rat. Physiol. Behav. 1991;49:919–925. doi: 10.1016/0031-9384(91)90204-2. [DOI] [PubMed] [Google Scholar]
- 94.Yoneyama N, Crabbe JC, Ford MM, Murillo A, Finn DA. Voluntary ethanol consumption in 22 inbred mouse strains. Alcohol. 2008 doi: 10.1016/j.alcohol.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.