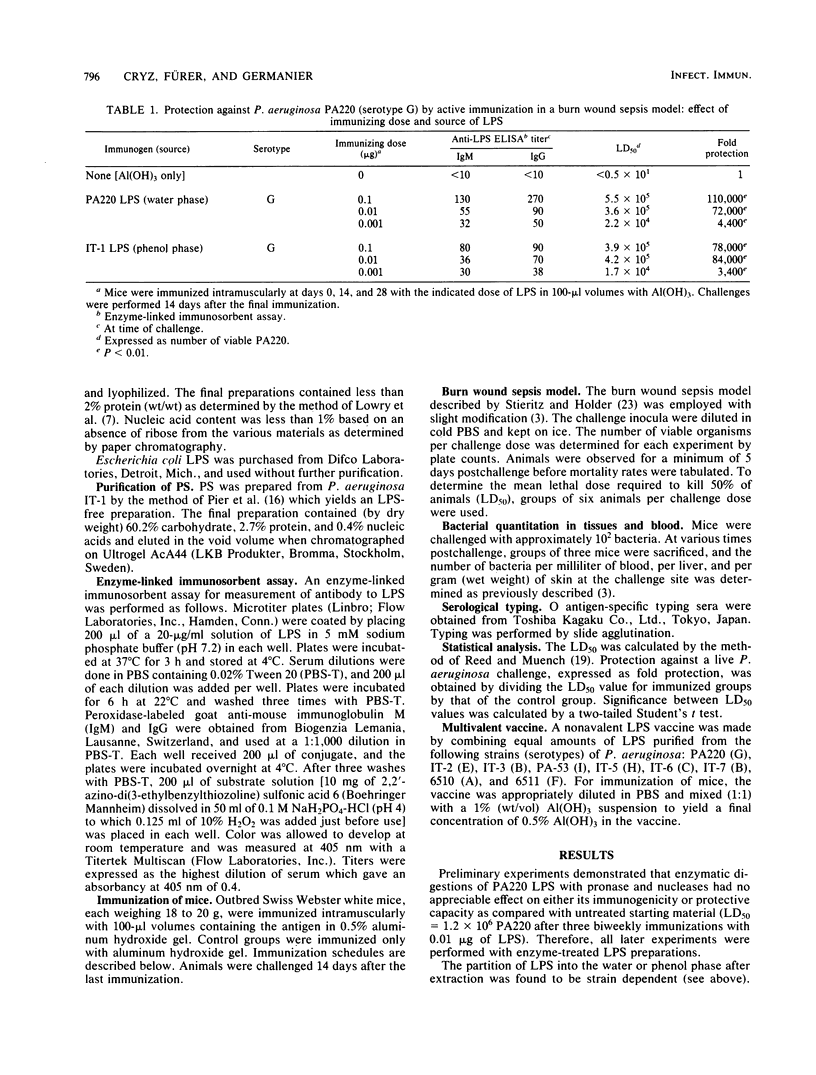
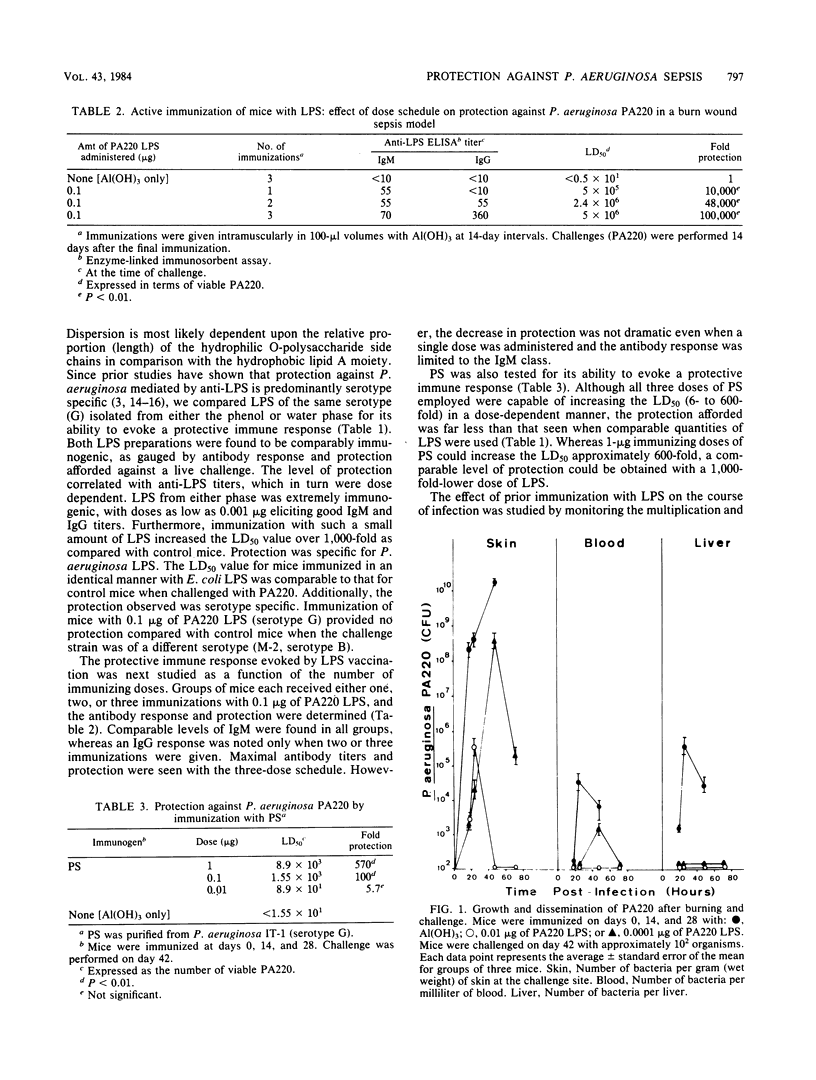
Abstract
A murine burn wound model was employed to evaluate the relative efficacy of purified Pseudomonas aeruginosa lipopolysaccharide (LPS) and high-molecular-weight polysaccharide as protective immunogens. LPS was found to be both highly immunogenic and protective. As little as three 0.001-microgram doses elicited good immunoglobulin M and G titers and increased the mean lethal dose more than 1,000-fold. The level of protection against a live challenge correlated with antibody titers and was found to be serotype specific. An immunizing regimen which evoked only an immunoglobulin M response was still found to offer substantial protection. Immunization with a high-molecular-weight polysaccharide was also found to be protective. However, approximately 1,000-fold more high-molecular-weight polysaccharide, as compared with LPS, was needed to protect mice to an equivalent degree. Immunization with LPS was found to promote bacterial clearance and prevent establishment of bacteremia. A multivalent LPS vaccine conferred high levels of protection (110- to 53,000-fold) against eight different challenge strains of various serotypes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjorn M. J., Iglewski B. H., Ives S. K., Sadoff J. C., Vasil M. L. Effect of iron on yields of exotoxin A in cultures of Pseudomonas aeruginosa PA-103. Infect Immun. 1978 Mar;19(3):785–791. doi: 10.1128/iai.19.3.785-791.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson A. B., Michael J. G. Biological Activities of Rabbit Immunoglobulin M and Immunoglobulin G Antibodies to Pseudomonas aeruginosa. Infect Immun. 1970 Oct;2(4):453–461. doi: 10.1128/iai.2.4.453-461.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Germanier R. Passive protection against Pseudomonas aeruginosa infection in an experimental leukopenic mouse model. Infect Immun. 1983 May;40(2):659–664. doi: 10.1128/iai.40.2.659-664.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Fürer E., Germanier R. Protection against Pseudomonas aeruginosa infection in a murine burn wound sepsis model by passive transfer of antitoxin A, antielastase, and antilipopolysaccharide. Infect Immun. 1983 Mar;39(3):1072–1079. doi: 10.1128/iai.39.3.1072-1079.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flick M. R., Cluff L. E. Pseudomonas bacteremia. Review of 108 cases. Am J Med. 1976 Apr;60(4):501–508. doi: 10.1016/0002-9343(76)90716-6. [DOI] [PubMed] [Google Scholar]
- Holder I. A., Wheeler R., Montie T. C. Flagellar preparations from Pseudomonas aeruginosa: animal protection studies. Infect Immun. 1982 Jan;35(1):276–280. doi: 10.1128/iai.35.1.276-280.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Okada K., Kawaharajo K., Kasai T., Homma J. Y. Effects of somatic component of Pseudomonas aeruginosa on protective immunity in experimental mouse burn infection. Jpn J Exp Med. 1980 Feb;50(1):53–61. [PubMed] [Google Scholar]
- Pavlovskis O. R., Edman D. C., Leppla S. H., Wretlind B., Lewis L. R., Martin K. E. Protection against experimental Pseudomonas aeruginosa infection in mice by active immunization with exotoxin A toxoids. Infect Immun. 1981 May;32(2):681–689. doi: 10.1128/iai.32.2.681-689.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington J. E., Hickey W. F., Blackwood L. L., Arnaut M. A. Active immunization with lipopolysaccharide Pseudomonas antigen for chronic Pseudomonas bronchopneumonia in guinea pigs. J Clin Invest. 1981 Nov;68(5):1140–1148. doi: 10.1172/JCI110358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington J. E., Kuchmy D. Mechanism for pulmonary protection by lipopolysaccharide pseudomonas vaccine. J Infect Dis. 1980 Aug;142(2):191–198. doi: 10.1093/infdis/142.2.191. [DOI] [PubMed] [Google Scholar]
- Pennington J. E. Lipopolysaccharide pseudomonas vaccine: efficacy against pulmonary infection with Pseudomonas aeruginosa. J Infect Dis. 1979 Jul;140(1):73–80. doi: 10.1093/infdis/140.1.73. [DOI] [PubMed] [Google Scholar]
- Pier G. B. Cross-protection by Pseudomonas aeruginosa polysaccharides. Infect Immun. 1982 Dec;38(3):1117–1122. doi: 10.1128/iai.38.3.1117-1122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B. Safety and immunogenicity of high molecular weight polysaccharide vaccine from immunotype 1 Pseudomonas aeruginosa. J Clin Invest. 1982 Feb;69(2):303–308. doi: 10.1172/JCI110453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Sidberry H. F., Sadoff J. C. High-molecular-weight polysaccharide antigen from Pseudomonas aeruginosa immunotype 2. Infect Immun. 1981 Nov;34(2):461–468. doi: 10.1128/iai.34.2.461-468.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Sidberry H. F., Sadoff J. C. Protective immunity induced in mice by immunization with high-molecular-weight polysaccharide from Pseudomonas aeruginosa. Infect Immun. 1978 Dec;22(3):919–925. doi: 10.1128/iai.22.3.919-925.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack M., Young L. S. Protective activity of antibodies to exotoxin A and lipopolysaccharide at the onset of Pseudomonas aeruginosa septicemia in man. J Clin Invest. 1979 Feb;63(2):276–286. doi: 10.1172/JCI109300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruitt B. A., Jr Infections caused by Pseudomonas species in patients with burns and in other surgical patients. J Infect Dis. 1974 Nov;130 (Suppl)(0):S8–13. doi: 10.1093/infdis/130.supplement.s8. [DOI] [PubMed] [Google Scholar]
- Pseudomonas aeruginosa infections: persisting problems and current research to find new therapies. Ann Intern Med. 1975 Jun;82(6):819–831. doi: 10.7326/0003-4819-82-6-819. [DOI] [PubMed] [Google Scholar]
- Schimpff S. C., Greene W. H., Young V. M., Wiernik P. H. Pseudomonas septicemia: incidence, epidemiology, prevention and therapy in patients with advanced cancer. Eur J Cancer. 1973 Jun;9(6):449–455. doi: 10.1016/0014-2964(73)90110-2. [DOI] [PubMed] [Google Scholar]
- Stieritz D. D., Holder I. A. Experimental studies of the pathogenesis of infections due to Pseudomonas aeruginosa: description of a burned mouse model. J Infect Dis. 1975 Jun;131(6):688–691. doi: 10.1093/infdis/131.6.688. [DOI] [PubMed] [Google Scholar]
- Walker H. L., McLeod C. G., Jr, Leppla S. H., Mason A. D., Jr Evaluation of Pseudomonas aeruginosa toxin A in experimental rat burn wound sepsis. Infect Immun. 1979 Sep;25(3):828–830. doi: 10.1128/iai.25.3.828-830.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L. S., Armstrong D. Human immunity to Pseudomonas aeruginosa. I. In-vitro interaction of bacteria, polymorphonuclear leukocytes, and serum factors. J Infect Dis. 1972 Sep;126(3):257–276. doi: 10.1093/infdis/126.3.257. [DOI] [PubMed] [Google Scholar]
- Young L. S. Human immunity to Pseudomonas aeruginosa. II. Relationship between heat-stable opsonins and type-specific lipopolysaccharides. J Infect Dis. 1972 Sep;126(3):277–287. doi: 10.1093/infdis/126.3.277. [DOI] [PubMed] [Google Scholar]


