Abstract
Aurora kinase A (AURKA) is located at 20q13, a region that is frequently amplified in gastric cancer. In this study, we have investigated the role of AURKA in regulating GSK-3β and β-catenin/TCF complex in gastric cancer cells. Our results demonstrate a significant increase in the phosphorylation of GSK-3β at Ser 9 following the over-expression of AURKA in AGS cells. The immunoprecipitation with antibodies specific for AURKA and GSK-3β indicated that the two proteins coexist in the same protein complex. The recombinant human AURKA protein phosphorylated the GSK-3β protein at Ser 9 in a concentration dependent manner, in vitro. The phosphorylation of β-catenin (Ser33/37/Thr41) by GSK-3β is known to target β-catenin towards degradation. In line with our findings, the increase in phospho-GSK-3β level was accompanied by a significant decrease in β-catenin phosphorylation (Ser33/37/Thr41) and accumulation of β-catenin protein. The knockdown of AURKA reversed the phosphorylation of GSK-3β and the β-catenin protein levels. The immunofluorescence analysis demonstrated co-localization of AURKA and GSK-3β proteins and a significant increase in the nuclear β-catenin levels in cells overexpressing AURKA. The β-catenin/TCF transcription activity was measured using the pTopFlash and its mutant pFopFlash luciferase reporter vectors. Indeed, AURKA over-expression led to a significant increase in the pTopFlash reporter activity, whereas kinase dead AURKA mutant (D274A) had no effect. Consistent with these findings, we detected a significant mRNA up-regulation of several direct targets of the β-catenin/TCF transcription complex (cyclin D1, c-MYC, c-MYC binding protein, CLDN1, FGF18, and VEGF), and a two-fold increase in the proliferation rate in AURKA over-expressing cells. We conclude that the AURKA/GSK-3β interaction plays an important role in regulating β-catenin, underscoring a novel oncogenic potential for AURKA in gastric tumorigenesis.
Keywords: AURKA, GSK-3β, β-catenin, gastric cancer
Introduction
Gastric cancers are characterized by poor response to therapy with unfavorable clinical outcome that reflects an inherent protective mechanism in these tumors against drug-induced apoptosis (Earle and Maroun, 1999; Jemal et al., 2006). Aurora kinase A (AURKA) is located in chromosome arm 20q13, a locus that is frequently amplified and overexpressed in gastric adenocarcinomas (El-Rifai et al., 2001; Varis et al., 2001). We have recently shown frequent over-expression of AURKA in adenocarcinomas of the stomach and esophagus (Dar et al., 2008). The AURKA gene encodes a centrosome associated cell cycle regulated serine/threonine kinase (Giet and Prigent, 1999). Cytological analysis revealed that over-expression of AURKA results in centrosome amplification, cytokinesis failure; thus, production of aneuploid cells (Zhou et al., 1998). In cancer cells, AURKA regulates several important proteins such as p53 (Dar et al., 2008; Katayama et al., 2004), AKT (Yang et al., 2006); and BRCA1 (Ouchi et al., 2004). The complete spectrum of molecular signaling mechanisms and biological functions that are regulated by AURKA are not currently fully understood.
β-catenin is a multifunctional protein that is both an integral component of adherens junctions and a pivotal member of the Wnt signal transduction pathway. Mutations and over-expression of β-catenin disrupt the function of E-cadherins. The increased level of β-catenin through mutations in either β-catenin or adenomatous polyposis coli (APC) has been suggested to be an important oncogenic step in the genesis of a number of malignancies and it is an early event in colorectal carcinogenesis (Damalas et al., 1999b; Morin et al., 1997; Polakis, 1999). Cytoplasmic accumulation of β-catenin results in its translocation to the nucleus where it associates with the members of TCF/LEF family of transcription factors. A large number of genes relevant for tumor formation and progression have been identified to be transcriptionally activated by the nuclear β-catenin/TCF complex (He et al., 1998; Shtutman et al., 1999; Tetsu and McCormick, 1999). Some of them are implicated in growth control and cell cycling (cyclin D1, c-MYC, c-Jun and fra1), others are relevant for cell survival (Id2, MDR1), and some are involved in tumor invasion and metastasis (Matrilysin, VEGF) (Crawford et al., 1999; Koh et al., 2000; Tetsu and McCormick, 1999; Yamada et al., 2000). Although APC mutations are infrequent in gastric carcinomas (Nakatsuru et al., 1992), accumulation and nuclear localization of β-catenin occurs in approximately one-third of gastric tumors (Clements et al., 2002).
The GSK-3β is a cytosolic protein that plays a critical role in determining the levels of β-catenin in the cells. The phosphorylation of β-catenin by the kinase active GSK-3β leads to ubiquitination and proteosomal degradation of β-catenin. In this way, β-catenin is restricted from the nucleus and prevented from interacting and activating the TCF/LEF transcription complex (Aberle et al., 1997; Orford et al., 1997). However, GSK-3β is rendered inactive after being phosphorylated at Ser 9, and thus, loses its ability to phosphorylate β-catenin leading to accumulation and nuclear localization of β-catenin (Giles et al., 2003; Kikuchi et al., 2006). In this study, we investigated the role of AURKA in regulating GSK-3β and the effects of this regulation on β-catenin in gastric cancer cells.
Results
AURKA over-expression regulates GSK-3β phosphorylation and β-catenin protein levels
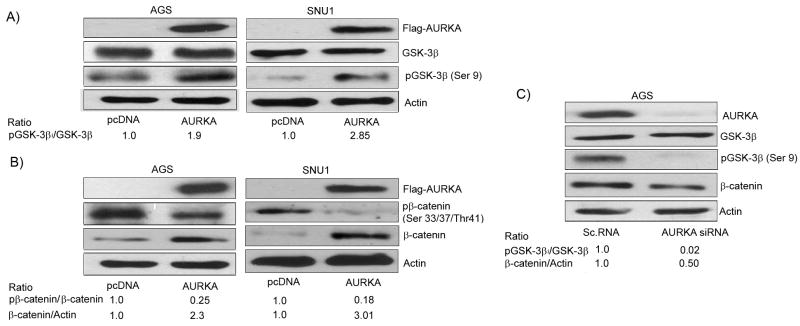
Over-expression of AURKA in AGS and SNU1 gastric cancer cells led to an up-regulation in the phosphorylation of GSK-3β (Ser 9) (Figure 1A). The GSK-3β is an important protein kinase that regulates the phosphorylation of β-catenin leading to its degradation. The phospho-GSK-3β is kinase inactive and cannot phosphorylate β-catenin, thus leading to a more stable β-catenin with its accumulation and subsequent nuclear localization which was detected as shown in Figure 4A. Consistent with our findings of increased phospho-GSK-3β in AURKA overexpressing cells, we detected a decrease in the phosphorylation level of β-catenin (Ser33/37/Thr41) together with an increase in its protein level (Figure 1B). The use of AURKA siRNA reversed these effects and led to a significant reduction in the level of phospho GSK-3β and a subsequent decrease in the β-catenin protein level (Figure 1C). These results suggested that AURKA can regulate the β-catenin protein levels in gastric cancer cells by regulating the phosphorylation of GSK-3β.
Figure 1. AURKA regulates the phosphorylation of GSK-3β.
A) AGS and SNU1 cells were transfected with Flag-tagged AURKA and control pcDNA vectors using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following manufacturer's instructions. The over-expression of AURKA in AGS and SNU1 cells up-regulated phosphorylation levels of GSK-3β (Ser 9). B) AURKA over-expression in AGS and SNU1 cells resulted in a decrease in the phospho-β-catenin (Ser33/37Thr41) with up-regulation of its protein level. C) Knockdown of endogenous AURKA by a specific siRNA oligonucleotide reduced the protein levels of phospho-GSK-3β (Ser 9) and β-catenin, thus validating the results of AURKA over-expression (panels A&B).
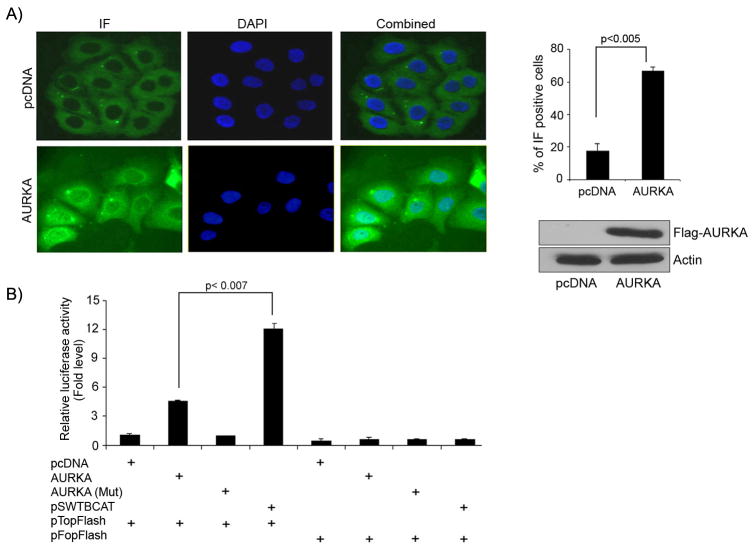
Figure 4. Nuclear accumulation of β-catenin and activation of the β-catenin/TCF complex.
A) Immunofluorescence (IF) assay for endogenous β-catenin indicates its nuclear accumulation in AGS cells stably over-expressing AURKA, as compared to control vector expressing cells. Two hundred cells were counted from AURKA and control vector for β-catenin IF. Seventy percent of AURKA over-expressing cells showed nuclear β-catenin staining (Bar graph). Western blot analysis demonstrates over-expression of AURKA in AGS cells. B) The luciferase reporter assay using pTopFlash and its mutant pFopFlash vectors was utilized to study the β-catenin TCF/LEF promoter activity. The pTopFlash reporter vector has 6-TCF binding sites, while pFopFlash has mutated TCF binding sites. AGS cells were co-transfected with different expression vectors as indicated. Over-expressing AURKA along with pTopFlash vector resulted in the induction of luciferase activity, indicating the transcription activity of the β-catenin/TCF complex. The kinase dead AURKA mutant (D274A) had no effect on pTopFlash activity. The wild type β-catenin expressing vector (pSWTBCAT) was used as a positive control. The over-expression of AURKA or its kinase dead mutant (D274A) with pFopFlash vectors had no effect on the luciferase activity. Luciferase activity was measured using a Dual luciferase reporter assay kit (Promega, Madison, WI) following manufactures' instructions. Results were averaged from three independent experiments and expressed as mean values (± standard deviation (SD)).
AURKA co-localizes and interacts with GSK-3β in AGS cells
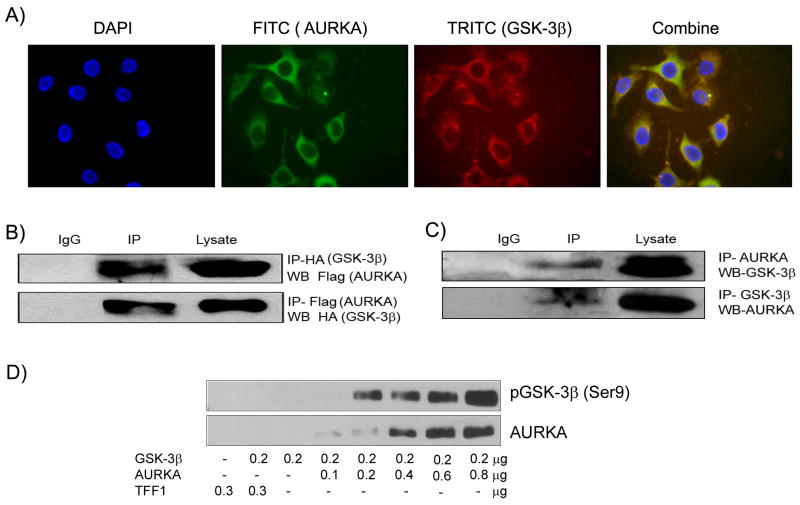
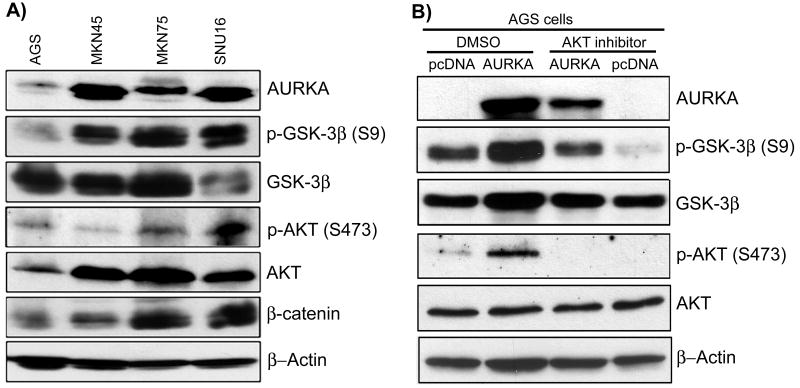
Immunofluorescence studies using AURKA and GSK-3β specific antibodies showed that both proteins co-localize in the cytoplasm in AGS gastric cancer cells (Figure 2A). We and others have shown cytosolic over-expression of AURKA in several malignancies such as gastric, esophageal, and lung cancer (Dar et al., 2008; Ogawa et al., 2008; Tong et al., 2004). In order to confirm the protein-protein interaction, two-way co-immunoprecipitations were performed for exogenously expressed AURKA and GSK-3β (Figure 2B) and for endogenously expressed AURKA and GSK-3β (Figure 2C). The results revealed co-existence of AURKA and GSK-3β in the same protein complex. Furthermore, the in vitro kinase assay demonstrated phosphorylation of GSK-3β by AURKA in a concentration dependent manner (Figure 2D). The TFF1 (trefoil factor 1) protein was used as a negative control. These results confirm that AURKA can directly bind to and phosphorylate GSK-3β. The inactivation of GSK-3β by phosphorylation can be mediated by the PI3-kinase/AKT survival signaling pathway (Mitsiades et al., 2004; Pap and Cooper, 1998; Sourbier et al., 2006). We have used AKT specific inhibitor in presence and absence of AURKA and demonstrated its efficacy in abrogating the phospho-AKT signals (Figure 3B). At the same time, the AGS cells overexpressing AURKA had approximately 5 fold higher signal for phospho-GSK-3β (S9) than the control cells in the presence of AKT inhibitor (Figure 3B). These findings together with the co-immunoprecipitation results (Figure 2B-C) and the in vitro recombinant kinase assay (Figure 2D) support the conclusion that AURKA directly can regulate the GSK-3β phosphorylation. However, the extent of GSK-3β phosphorylation in the presence of AKT inhibitor was less than in its absence. Therefore, it is possible that AURKA regulates GSK-3β phosphorylation through direct interaction as well as through an AURKA/AKT axis dependent mechanism.
Figure 2. AURKA co-localizes and interacts with GSK-3β.
A) Immunofluorescence assay demonstrates co-localization of AURKA (green) and GSK-3β (red) in AGS cells. B) Co-immunoprecipitation (CO-IP) of GSK-3β and AURKA. AGS cells were co-transfected with Flag-AURKA and HA-GSK3β plasmids for 48hrs. For AURKA and GSK-3β lysates were subjected to IP with Flag and HA antibodies followed by immunoblotting with HA and Flag antibodies (Cell signaling, Boston, MA) to detect AURKA and GSK-3β, respectively. The results indicate their presence in the same protein complex. C) The CO-IP studies were performed for endogenous AURKA and GSK-3β and demonstrated similar findings. D) In vitro kinase assay was performed using recombinant AURKA and GSK-3β proteins followed by immunoblotting and detection with phospho-GSK-3β Ser9. GSK-3β concentration was kept constant with an increasing concentration of AURKA as indicated. A concentration dependent increase in the phosphorylation GSK-3β was observed. TFF1 (trefoil factor 1) protein was used as a negative control.
Figure 3. AURKA, AKT and GSK-3β expression in gastric cancer cells.
A) Western blot analysis of AURKA, AKT, p-AKT, GSK-3β, p-GSK-3β, and β-catenin in AGS, MKN45, MKN75, and SNU16 gastric cancer cell lines. The levels of p-GSK-3β (S9) and β-catenin correlated with the expression level of AURKA. The MKN45 cells demonstrated high levels of AURKA and p-GSK-3β and β-catenin, despite a relatively low level of p-AKT (S473). B) Western blot analysis of AGS cells following treatment with DMSO as a control (left panel) or AKT inhibitor VIII (right panel). AGS cells overexpressing AURKA showed an increase in the phosphorylation of AKT (S473) and GSK-3β (S9) as compared to pcDNA3 empty vector control cells. The treatment of AGS cells with AKT inhibitor VIII (1μM) for 24 hours led to a significant downregulation of phospho-AKT (S473) in AURKA expressing AGS cells (right panel). However, GSK-3β phosphorylation (S9) was 5 fold higher in AURKA overexpressing cells than in pcDNA3 empty vector control cells, in presence of AKT inhibitor VIII.
The interaction between AURKA and GSK-3β leads to accumulation and nuclear translocation of β-catenin with activation of the β-catenin/TCF complex
The aforementioned results indicated that AURKA binds to and regulates GSK-3β phosphorylation in gastric cancer cells. Consistent with our findings of increased phospho-GSK-3β in AURKA overexpressing cells, we detected a decrease in the phosphorylation level of β-catenin (Ser33/37/Thr41) together with an increase in its protein level (Figure 1B). Accumulation of β-catenin is a major step that promotes its nuclear localization and activates its oncogenic properties. The immunofluorescence assay on AGS cells stably over-expressing AURKA demonstrated a remarkable increase in the nuclear β-catenin, as compared to control cells (Figure 4A). Several reports have indicated that the oncogenic functions of β-catenin are mediated by its interaction with members of the TCF/LEF family of transcription factors in the nucleus (Behrens et al., 1996; Miller et al., 1999). We utilized the pTopFlash/ pFopFlash luciferase vectors as a measure of β-catenin/TCF complex activity. AURKA over-expression led to a 5-fold induction of the luciferase activity of pTopFlash luciferase vector but, as expected, had no effect on the pFopFlash luciferase vector (Figure 4B). Conversely, expressing kinase dead AURKA-mutant (D274A) did not induce luciferase activity when transfected along with pTopFlash or pFopFlash vectors; thus, indicating that the induced luciferase activity in cells transfected with pTopFlash vector is due to over-expression of kinase active AURKA. As expected, the β-catenin transfection (positive control) led to induction of pTopFlash, but not pFopFlash luciferase activity. Taken together, our results as shown in Figures 1 to 3 indicate the AURKA interaction with GSK-3β regulates the β-catenin/TCF transcription activity. We have confirmed this finding by measuring the transcript level of several direct targets of the β-catenin/TCF complex. The qRT-PCR indicated up-regulation of cyclin D1, c-MYC, c-MYC binding protein, CLDN1, FGF-18, and VEGF in AURKA overexpressing cells as compared to control (Figure 5A). Using cyclin D1 as an example of these targets, we further confirmed the induction of its promoter in AURKA overexpressing cells (Figure 5B) and demonstrated the increase in its protein level in AGS and SNU1 cells (Figure 5C). Cyclin D1 plays an important role in cell cycle and proliferation. Indeed, AGS cells stably expressing AURKA showed >2.5-fold increase in their proliferation rate as compared to control vector cells, as determined by BrdU incorporation assay (Figure 6A). The cell cycle analysis demonstrated a doubling in the S-phase in AGS (from 10% to 18%) and RIE (from 6.5% to 16%) cells over-expressing AURKA as compared to control vector expressing cells (Figure 6B&C). Taken together, these results indicate that the AURKA/GSK-3β interaction results in a functional signaling outcome that is manifested by an increase in the β-catenin protein, activation of the β-catenin/TCF transcription complex, and increase in the cell proliferation rate. Other oncogenic consequences of the AURKA/GSK-3β interaction and nuclear accumulation and activation of β-catenin remain to be elucidated in further studies.
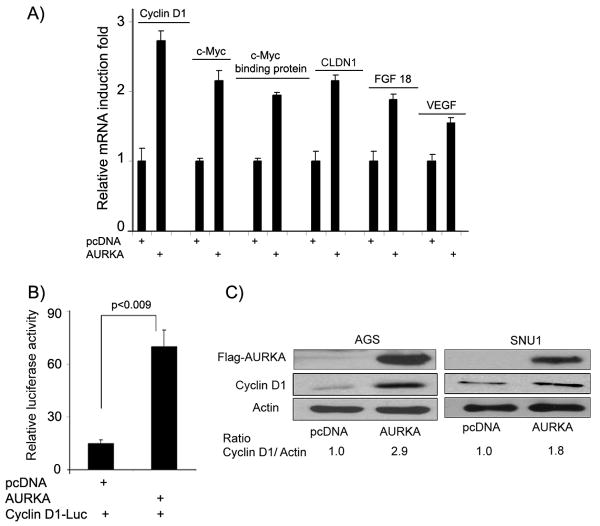
Figure 5. Upregulation of the β-catenin downstream targets.
A) Quantitative real time-PCR demonstrates up-regulation of several direct downstream transcriptional targets of β-catenin/TCF complex in AGS cells overexpressing AURKA as compared to empty vector control. The mRNA expression levels of cyclin D1, c-MYC, c-MYC binding protein, FGF18, CLDN1, and VEGF were normalized to HPRT1 mRNA expression. For comparison, the expression in empty vector control cells was set to 1. B) The luciferase assay using the cyclin-D1-luc reporter indicated induction (4.75-fold) of cyclin D1 transcriptional activity in AURKA over-expressing cells. C) Immunoblot analysis indicated up-regulation in the total protein level of cyclin D1 in AGS and SNU1 cells upon over-expressing AURKA. Flag-antibody was used to detect exogenously expressed AURKA.
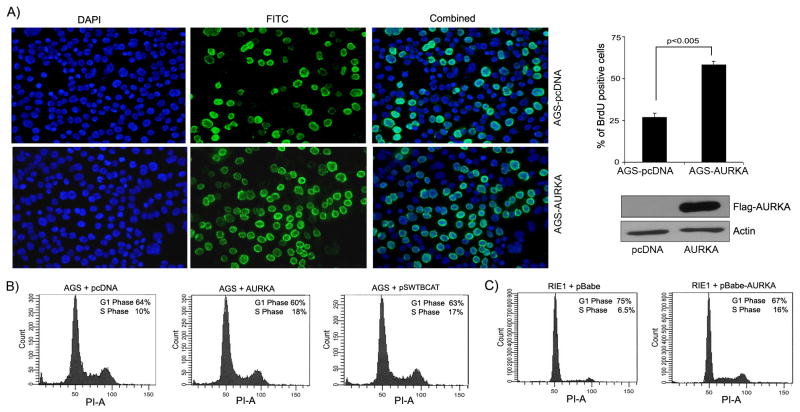
Figure 6. AURKA over-expression enhances cell proliferation of gastric cancer cells.
A) AGS cells stably expressing AURKA showed enhancement in the cell proliferation as evidenced by the 5-Bromo-2′-deoxy-uridine (BrdU) incorporation assay. BrdU assay was performed by using the BrdU labeling and detection kit I (Roche Applied Science, Indianapolis, IN) following the manufacturer's protocol. Quantification of BrdU positive stained cells indicated ≥2.5 fold increase in the cell proliferation of AGS cells expressing AURKA compared to control vector expressing cells (Bar graph). The results represent the average of three independent stable clones. Western blot demonstrates the over-expression of AGS cells stably overexpressing AURKA. B) Cell cycle analysis of AGS cells over-expressing AURKA indicated an almost doubling of the S-phase (10% to 18%) as compared to control vector expressing cells. Transfection of AGS cells with β-catenin expressing vector (pSWTBCAT) was used as a positive control. C) Primary gastrointestinal epithelial RIE-1 cells transduced with AURKA retroviruses showed more proliferation than cells transduced with control retroviruses as determined by an increase from 6.5 to 16% in the S-phase of cell cycle.
Discussion
Gastric cancers are characterized by poor response to therapy with unfavorable clinical outcome that reflects an inherent protective mechanism in these tumors against drug-induced apoptosis (Earle and Maroun, 1999; Jemal et al., 2006). The critical molecular alterations and signaling pathways that drive gastric tumorigenesis remain largely uncharacterized. In this study, we have investigated the role of AURKA in this devastating disease. AURKA is located at chromosome 20q and is frequently amplified and overexpressed in gastric cancer (Dar et al., 2008; El-Rifai et al., 2001; Kamada et al., 2004; Varis et al., 2001). We and others have shown AURKA over-expression in a wide range of primary tumors including adenocarcinomas of the breast, colorectal, pancreatic, ovarian, esophageal, gastric and bladder cancers (Bischoff et al., 1998; Giet et al., 2005; Hu et al., 2005; Kamada et al., 2004; Tanaka et al., 1999; Zhou et al., 1998).
In this study, we have demonstrated that AURKA interacts with GSK-3β and directly binds to and phosphorylates GSK-3β at Ser 9. The GSK-3β has been previously identified as a key downstream target of the PI3-kinase/AKT survival signaling pathway (Mitsiades et al., 2004; Pap and Cooper, 1998; Sourbier et al., 2006). We have used AKT specific inhibitor in presence and absence of AURKA and our results demonstrated a high level of phospho-GSK-3β (S9) in the presence of AKT inhibitor in AURKA overexpressing cells, supporting our notion that AURKA can directly regulates GSK-3β. It is worth mentioning that we and others have shown that AURKA can phosphorylate and activate AKT (Dar et al., 2008; Yang et al., 2006). Therefore, it is possible that AURKA regulates GSK-3β phosphorylation through direct interaction and/or through AURKA/AKT axis dependent mechanism.
GSK-3β is a major protein kinase that regulates the phosphorylation of β-catenin in the cytoplasm, targeting it to degradation (Giles et al., 2007; Kikuchi et al., 2006). The inactivation of GSK-3β by phosphorylation has been shown to reduce the ubiquitination of β-catenin, resulting in nuclear accumulation and transcriptional activity of β-catenin (Giles et al., 2003; Kikuchi et al., 2006). Indeed, we detected a decrease in the phosphorylation level of β-catenin (Ser33/37/Thr41) together with a significant up-regulation in the total β-catenin protein levels following over-expression of AURKA in AGS and SNU1 cells. These results suggested that down-regulation in the phosphorylation of β-catenin (Ser 33/37/Thr41) has ultimately led to an accumulation of β-catenin in AURKA over-expressing cells. We have validated our results using siRNA knockdown of AURKA and demonstrated a significant decrease of phosphorylated GSK-3β (Ser 9) and β-catenin protein levels.
Up-regulation of β-catenin and its nuclear accumulation has been reported to play a major role in gastric cancer (Clements et al., 2002; Morin et al., 1997; Washington et al., 1998). Accumulation of the β-catenin protein is attributed to either mutation of APC or β-catenin itself. One of the most widely observed mechanism for regulating β-catenin in cancer cells involves mutations in adenomatous polyposis coli (APC) gene that is an important oncogenic step in the genesis of a number of malignancies and it is an early event in colorectal carcinogenesis (Damalas et al., 1999a; Morin et al., 1997; Polakis, 1999). Mutations in β-catenin have been identified in many human tumors, including hepatocellular carcinoma (Terris et al., 1999), colorectal cancer (Sparks et al., 1998), ovarian cancer (Gamallo et al., 1999) and prostate cancer (Voeller et al., 1998). Although APC mutations are rare in gastric carcinomas (Nakatsuru et al., 1992), accumulation and nuclear localization of β-catenin occur in approximately one-third of gastric tumors (Clements et al., 2002). Therefore, the AURKA/GSK-3β axis may explain some of these findings.
Several reports have indicated that the oncogenic functions of β-catenin are mediated by the nuclear fraction due to its interaction with the members of the TCF/LEF family of transcription factors. The β-catenin/TCF transcription complex regulates the expression of several genes that are involved in human carcinogenesis such as cyclin D1, c-MYC (Crawford et al., 1999; He et al., 1998; Hojilla et al., 2007; Pennica et al., 1998; Shtutman et al., 1999). Indeed, our results demonstrate upregulation and nuclear localization of β-catenin. The AURKA over-expression increased the pTopFlash luciferase activity whereas the kinase dead AURKA-mutant (D274A) did not induce this activity, confirming the role of the kinase active AURKA. Furthermore, we demonstrated the mRNA upregulation of several direct transcription targets of the β-catenin/TCF complex; Cyclin D1, cMYC, cMYC binding protein, CLDN1, FGF18 and VEGF (A list of targets of β-catenin/TCF is available at http://www.stanford.edu/∼rnusse/pathways/targets.html, with specific references for each of them). Taken together, these results indicate that over-expression of AURKA leads to an increase in β-catenin protein level with induction of its nuclear translocation and activation of the β-catenin/TCF transcriptional activity.
Cyclin D1 is a transcription target of the β-catenin/TCF complex that plays an important role in cell proliferation, tumor progression and metastasis (Wang et al., 2004; Zhang et al., 1993). We have demonstrated induction of its promoter activity following AURKA over-expression. The AGS cells stably expressing AURKA showed more than two-fold increase in their proliferation rate as compared to control vector cells and demonstrated an almost doubling in the S-phase of cells over-expressing AURKA as compared to control vector expressing cells. The increase in β-catenin level promotes neoplastic conversion and plays distinct roles in uncontrolled progression into the cell cycle through the G1 phase (Sherr and Roberts, 1999; Shtutman et al., 1999; Tetsu and McCormick, 1999). Our results suggest that AURKA/GSK-3β axis, by regulating β-catenin, may play a critical role in this process in gastric cancer cells.
In summary, our results demonstrate that AURKA regulates the GSK-3β phosphorylation and activity leading to accumulation and activation of the β-catenin/TCF complex in gastric cancers. The AURKA/GSK-3β axis underscores a novel angle for the role of AURKA in cancer where other biological and cell signaling consequences of this axis remain to be elucidated in further studies.
Materials and Methods
Cell culture, vectors, small-interfering RNA, and transfection
AGS, SNU1, SNU16, MKN45, MKN75, and RIE-1 cells were cultured in F-12 (HAM) or Dulbecco's modified eagle medium (DMEM) together with 10% fetal bovine serum (Invitrogen Life Technologies, Carlsbad, CA) at 37°C in an atmosphere containing 5% CO2. AGS, SNU1, and SNU16 cells were obtained from American Tissue Culture Collection (ATCC, Manassas, VA). MKN45 cells were obtained from the German Collection of Microorganisms and Cell Cultures (Braunschweig Germany). The MKN75 cells were a gift from Dr. Barbara Schneider and the RIE-1 cells were obtained from Dr. Robert J. Coffey, Jr. (Vanderbilt University, Nashville, TN). The expression plasmid for AURKA was generated by PCR amplification of the full-length coding sequence of AURKA and cloned in frame into pcDNA3.1 (Invitrogen Life Technologies). A synthetic Flag-tag sequence was added at the N-terminus of AURKA. Cloning of AURKA was confirmed by sequencing and restriction enzyme digestion. pSWTBCAT plasmid (wild type β-catenin cloned into p-shuttle backbone vector from Clontech, Mount View, CA) was a kind gift from Dr. Andy Lowy (University of California, San Diego). Retroviral expression constructs, pBabe-puro, containing either the full length of AURKA coding sequence (pBabe-AURKA) or a kinase-dead AURKA mutant (D274A) and HA-GSK-3β pcDNA were purchased from Addgene (Cambridge, MA). Validated small-interfering RNA (siRNA) oligonucleotides specific for AURKA and negative control siRNA were purchased from Ambion (Austin, TX). Transient transfections were performed by using Fugene-6 (Roche, Indianapolis, IN), and Lipofectamine (Invitrogen Life Technologies) following the manufacturers' protocols. Recombinant active GSK-3β and AURKA proteins were purchased from BioVision (Mountain View, CA) and Cell Sciences (Canton, MA), respectively. AKT inhibitor VIII was purchased from Calbiochem (Gibbstown, NJ) and AGS cells over-expressing AURKA or control vector were treated with 1 μM of AKT inhibitor VIII for 24hrs.
Luciferase assays
The TCF-responsive luciferase construct pTopFlash, its mutant pFopFlash (Upstate Biotechnology, Waltham, MA), and cyclin D1-Luc (Tetsu and McCormick, 1999) were used to study the promoter activity. The pTopFlash vector has six TCF binding sites, each three in the opposite direction, while pFopFlash, a negative control, has mutant TCF binding sites. The luciferase activity was determined using a dual-luciferase reporter assay kit (Promega, Madison, WI). The relative luciferase activity was determined after 48hrs of transfection and normalized to protein concentration. Results are average of three independent experiments and expressed as mean values (± standard deviation (SD)).
Fluorescence-activated cell sorting (FACS)
Cell-cycle analysis was performed using flow cytometry. Cells were synchronized by culture in the presence of 1% fetal bovine serum for 24 hours, followed by 10% fetal bovine serum. Cells were trypsinized, washed twice with 1× ice-cold phosphate buffer saline (PBS), and then re-suspended in 0.2 mL ice-cold PBS. Cells were then fixed in 1mL ice-cold 70% ethanol and incubated for 1 hour at 4°C. Cells were centrifuged and re-suspended in 1mL PBS and treated with propidium iodide (50 μg/mL) and RNase (1 μg/mL) for 30 minutes at 37°C and analyzed by BD LSR III flow cytometer (BD Biosciences, San Jose, CA). Data were analyzed by BD FACS Diva software.
Western blot analysis
Cell lysates were prepared in 1× PBS buffer containing 1× Halt protease inhibitor cocktail and 1× Halt phosphatase inhibitor cocktail (Pierce, Rockford, IL), centrifuged at 3500 rpm for 10 minutes at 4°C. Protein concentration was measured using a Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA). Proteins (10-15 μg) from each sample were subjected to SDS-PAGE and transferred onto nitro-cellulose membrane. Target proteins were detected by using specific antibodies against AURKA (TransGenic Inc., Japan); β-actin, β-catenin, phospho-β-catenin (Ser33/37/Thr41), GSK-3β, phospho-GSK3β (Ser 9), cyclin D1, AKT, pAKT(S473), HA-tag, and Flag (Cell Signaling Technology, Boston, MA).
5-Bromo-2′-deoxy-uridine (BrdU) labeling and detection
BrdU labeling was performed using the BrdU labeling and detection kit I (Roche Applied Science, Indianapolis, IN) following the manufacturer's protocol. In brief, cells were grown in an 8-well chamber and BrdU labeling medium was added to cells and incubated at 37°C for 3 hours. Cells were washed once with 1× PBS followed by ethanol fixation for 20 minutes at -20°C. After washing cells, anti-BrdU reagent was added for 30 minutes at 37°C. For fluorescence detection, anti-mouse Ig-fluorescein was added and incubated for 30 minutes. Cells were visualized under an Olympus BX51 fluorescence microscope (Olympus Co., Tokyo, Japan).
Immunoprecipitation
5×106 cells were centrifuged for 5 minutes at 1500 rpm. After discarding the supernatant, cell pellet was re-suspended in RIPA buffer. Samples were sonicated 3 times for 10 seconds and centrifuged for 20 minutes at maximum speed (13000 rpm, 4°C). Supernatants were collected into new tubes and 50μL of protein G-Agarose were added to samples and incubated at 4°C for 10 minutes. Samples were then centrifuged for 15 minutes and divided into three tubes. Specific and non-specific antibodies were added to their respective tubes containing protein samples and incubated on ice for 15 minutes. 50μL of protein G-Agarose beads were added to each tube and incubated on a rotating platform at 4°C overnight. Samples were centrifuged for 1 minute and washed 8 times with RIPA buffer. 20μL of 2× protein loading buffer were added to each sample and boiled for 5 minutes. Samples were resolved by SDS/PAGE and subjected to western blotting.
Quantitative real-time RT-PCR
Total RNA was isolated using the RNeasy mini kit (Qiagen, Valencia, CA), and single-stranded cDNA was subsequently synthesized using the Advantage™ RT-for-PCR Kit (Clontech, Palo Alto, CA). Gene-specific primers for cyclin D1, c-MYC, c-MYC binding protein, CLDN1, FGF18, VEGF, and HPRT1 were designed using the online software, Primer 3 (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi). The forward and the reverse primers were designed to span two different exons. All primers were purchased from GeneLink (Hawthorne, NY) and their sequences are available from the authors upon request. Quantitative real-time RT-PCR (qRT-PCR) was performed using an iCycler (Bio-Rad, Hercules, CA) with a threshold cycle number determined with the use of iCycler software version 3.0. Reactions were performed in triplicate and threshold cycle numbers were averaged. The results were normalized to HPRT1 as described earlier (El-Rifai et al., 2002). The fold change in all samples was calculated according to the formula 2(Rt-Et)/2(Rn-En), as described previously (El-Rifai et al., 2002).
In vitro kinase assay
The in vitro kinase assay was performed using active recombinant AURKA (Cell sciences, Canton, MA) and recombinant GSK-3β (BioVision, Mountain View, CA) following the manufacturer's protocol. Briefly, reaction was carried out in 20μL of assay buffer (50mM HEPES, pH7.4 + 3mM Mgcl2 + 3mM Mncl2 + 1mM DTT + 3μM Na-orthovanadate + 0.5mM ATP) containing a constant concentration of 0.2 μg of GSK-3β and increasing concentrations from 0.1 to 0.8 μg of human recombinant AURKA. The TFF1 (Trefoil factor 1) protein (Abnova, Taipei City, Taiwan) was used as a negative control since it is not a phospho protein and is not known to interact with AURKA or GSK-3β. Reaction mixtures were incubated at 30°C for 30 minutes. 6μL of 2× protein loading buffer was added to each sample and boiled for 5 minutes. Samples were resolved by SDS/PAGE and subjected to western blotting using specific antibodies for AURKA and pGSK-3β Ser 9.
Immunofluorescence Assay
AGS cells (3×103 per well) were plated in 8-well plate chamber for 24 hours. Cells were washed with 1× PBS and fixed with acetone-methanol (1:1) mixture for 15 minutes at -20°C. Cells were then washed twice with 1× PBS, followed by blocking with 10% normal goat serum blocking solution (Zymed Laboratories, Carlsbad CA) for 20 minutes at room temperature in a humidified chamber. Cells were hybridized with the specific primary antibodies against AURKA, β-catenin or GSK-3β (Cell Signaling) diluted in 1× PBS (1:400) for 2 hours at room temperature in a humidified chamber. Cells were washed three times in 1× PBS and hybridized with fluorescently conjugated secondary antibody (1:1000) (Jackson Immuno Research, West Grove, PA) for 45 minutes at room temperature in a humidified chamber. AURKA and β-catenin were detected by fluorescein isothiocyanate (FITC) green fluorescence and GSK-3β was by tetramethylrhodamine isothiocyanate (TRITC) (red fluorescence). Cells were washed in 1× PBS, mounted with Vectashield/ DAPI (Vector Laboratories, Burlingame CA) and visualized by an Olympus BX51 fluorescence microscope (Olympus Co., Tokyo, Japan). At least 200 cells were counted from each experiment.
Acknowledgments
This study was supported by a pilot fund from the National Cancer Institute GI SPORE grant CA95103 and the Vanderbilt-Ingram Cancer Center support funds. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or Vanderbilt University Medical Center.
References
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. beta-catenin is a target for the ubiquitin-proteasome pathway. Embo J. 1997;16:3797–804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–42. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. Embo J. 1998;17:3052–65. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements WM, Wang J, Sarnaik A, Kim OJ, MacDonald J, Fenoglio-Preiser C, et al. beta-Catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res. 2002;62:3503–6. [PubMed] [Google Scholar]
- Crawford HC, Fingleton BM, Rudolph-Owen LA, Goss KJ, Rubinfeld B, Polakis P, et al. The metalloproteinase matrilysin is a target of beta-catenin transactivation in intestinal tumors. Oncogene. 1999;18:2883–91. doi: 10.1038/sj.onc.1202627. [DOI] [PubMed] [Google Scholar]
- Damalas A, Ben-Ze'ev A, Simcha I, Shtutman M, Leal JF, Zhurinsky J, et al. Excess beta-catenin promotes accumulation of transcriptionally active p53. Embo J. 1999a;18:3054–63. doi: 10.1093/emboj/18.11.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damalas A, Ben-Ze'ev A, Simcha I, Shtutman M, Leal JF, Zhurinsky J, et al. Excess beta-catenin promotes accumulation of transcriptionally active p53. Embo J. 1999b;18:3054–63. doi: 10.1093/emboj/18.11.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dar AA, Zaika A, Piazuelo MB, Correa P, Koyama T, Belkhiri A, et al. Frequent overexpression of Aurora Kinase A in upper gastrointestinal adenocarcinomas correlates with potent antiapoptotic functions. Cancer. 2008;112:1688–98. doi: 10.1002/cncr.23371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earle CC, Maroun JA. Adjuvant chemotherapy after curative resection for gastric cancer in non-Asian patients: revisiting a meta-analysis of randomised trials. Eur J Cancer. 1999;35:1059–64. doi: 10.1016/s0959-8049(99)00076-3. [DOI] [PubMed] [Google Scholar]
- El-Rifai W, Frierson HJ, Moskaluk C, Harper J, Petroni G, Bissonette E, et al. Genetic Differences between adenocarcinomas arising in Barrett's esophagus and gastric mucosa. Gastroenterology. 2001;121:592–598. doi: 10.1053/gast.2001.27215. [DOI] [PubMed] [Google Scholar]
- El-Rifai W, Moskaluk CA, Abdrabbo MK, Harper J, Yoshida C, Riggins GJ, et al. Gastric cancers overexpress S100A calcium-binding proteins. Cancer Res. 2002;62:6823–6. [PubMed] [Google Scholar]
- Gamallo C, Palacios J, Moreno G, Calvo de Mora J, Suarez A, Armas A. beta-catenin expression pattern in stage I and II ovarian carcinomas : relationship with beta-catenin gene mutations, clinicopathological features, and clinical outcome. Am J Pathol. 1999;155:527–36. doi: 10.1016/s0002-9440(10)65148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giet R, Petretti C, Prigent C. Aurora kinases, aneuploidy and cancer, a coincidence or a real link? Trends Cell Biol. 2005;15:241–50. doi: 10.1016/j.tcb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Giet R, Prigent C. Aurora/Ipl1p-related kinases, a new oncogenic family of mitotic serine-threonine kinases. J Cell Sci. 1999;112(Pt 21):3591–601. doi: 10.1242/jcs.112.21.3591. [DOI] [PubMed] [Google Scholar]
- Giles FJ, Cortes J, Jones D, Bergstrom D, Kantarjian H, Freedman SJ. MK-0457, a novel kinase inhibitor, is active in patients with chronic myeloid leukemia or acute lymphocytic leukemia with the T315I BCR-ABL mutation. Blood. 2007;109:500–2. doi: 10.1182/blood-2006-05-025049. [DOI] [PubMed] [Google Scholar]
- Giles RH, van Es JH, Clevers H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim Biophys Acta. 2003;1653:1–24. doi: 10.1016/s0304-419x(03)00005-2. [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Hojilla CV, Kim I, Kassiri Z, Fata JE, Fang H, Khokha R. Metalloproteinase axes increase beta-catenin signaling in primary mouse mammary epithelial cells lacking TIMP3. J Cell Sci. 2007;120:1050–60. doi: 10.1242/jcs.003335. [DOI] [PubMed] [Google Scholar]
- Hu W, Kavanagh JJ, Deaver M, Johnston DA, Freedman RS, Verschraegen CF, et al. Frequent overexpression of STK15/Aurora-A/BTAK and chromosomal instability in tumorigenic cell cultures derived from human ovarian cancer. Oncol Res. 2005;15:49–57. doi: 10.3727/096504005775082101. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- Kamada K, Yamada Y, Hirao T, Fujimoto H, Takahama Y, Ueno M, et al. Amplification/overexpression of Aurora-A in human gastric carcinoma: potential role in differentiated type gastric carcinogenesis. Oncol Rep. 2004;12:593–9. [PubMed] [Google Scholar]
- Katayama H, Sasai K, Kawai H, Yuan ZM, Bondaruk J, Suzuki F, et al. Phosphorylation by aurora kinase A induces Mdm2-mediated destabilization and inhibition of p53. Nat Genet. 2004;36:55–62. doi: 10.1038/ng1279. [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Kishida S, Yamamoto H. Regulation of Wnt signaling by protein-protein interaction and post-translational modifications. Exp Mol Med. 2006;38:1–10. doi: 10.1038/emm.2006.1. [DOI] [PubMed] [Google Scholar]
- Koh TJ, Bulitta CJ, Fleming JV, Dockray GJ, Varro A, Wang TC. Gastrin is a target of the beta-catenin/TCF-4 growth-signaling pathway in a model of intestinal polyposis. J Clin Invest. 2000;106:533–9. doi: 10.1172/JCI9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JR, Hocking AM, Brown JD, Moon RT. Mechanism and function of signal transduction by the Wnt/beta-catenin and Wnt/Ca2+ pathways. Oncogene. 1999;18:7860–72. doi: 10.1038/sj.onc.1203245. [DOI] [PubMed] [Google Scholar]
- Mitsiades CS, Mitsiades N, Koutsilieris M. The Akt pathway: molecular targets for anti-cancer drug development. Curr Cancer Drug Targets. 2004;4:235–56. doi: 10.2174/1568009043333032. [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–90. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- Nakatsuru S, Yanagisawa A, Ichii S, Tahara E, Kato Y, Nakamura Y, et al. Somatic mutation of the APC gene in gastric cancer: frequent mutations in very well differentiated adenocarcinoma and signet-ring cell carcinoma. Hum Mol Genet. 1992;1:559–63. doi: 10.1093/hmg/1.8.559. [DOI] [PubMed] [Google Scholar]
- Ogawa E, Takenaka K, Katakura H, Adachi M, Otake Y, Toda Y, et al. Perimembrane Aurora-A expression is a significant prognostic factor in correlation with proliferative activity in non-small-cell lung cancer (NSCLC) Ann Surg Oncol. 2008;15:547–54. doi: 10.1245/s10434-007-9653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orford K, Crockett C, Jensen JP, Weissman AM, Byers SW. Serine phosphorylation-regulated ubiquitination and degradation of beta-catenin. J Biol Chem. 1997;272:24735–8. doi: 10.1074/jbc.272.40.24735. [DOI] [PubMed] [Google Scholar]
- Ouchi M, Fujiuchi N, Sasai K, Katayama H, Minamishima YA, Ongusaha PP, et al. BRCA1 phosphorylation by Aurora-A in the regulation of G2 to M transition. J Biol Chem. 2004;279:19643–8. doi: 10.1074/jbc.M311780200. [DOI] [PubMed] [Google Scholar]
- Pap M, Cooper GM. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-Kinase/Akt cell survival pathway. J Biol Chem. 1998;273:19929–32. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- Pennica D, Swanson TA, Welsh JW, Roy MA, Lawrence DA, Lee J, et al. WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc Natl Acad Sci U S A. 1998;95:14717–22. doi: 10.1073/pnas.95.25.14717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakis P. The oncogenic activation of beta-catenin. Curr Opin Genet Dev. 1999;9:15–21. doi: 10.1016/s0959-437x(99)80003-3. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D'Amico M, Pestell R, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96:5522–7. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourbier C, Lindner V, Lang H, Agouni A, Schordan E, Danilin S, et al. The Phosphoinositide 3-Kinase/Akt Pathway: A New Target in Human Renal Cell Carcinoma Therapy. Cancer Res. 2006;66:5130–5142. doi: 10.1158/0008-5472.CAN-05-1469. [DOI] [PubMed] [Google Scholar]
- Sparks AB, Morin PJ, Vogelstein B, Kinzler KW. Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer. Cancer Res. 1998;58:1130–4. [PubMed] [Google Scholar]
- Tanaka T, Kimura M, Matsunaga K, Fukada D, Mori H, Okano Y. Centrosomal kinase AIK1 is overexpressed in invasive ductal carcinoma of the breast. Cancer Res. 1999;59:2041–4. [PubMed] [Google Scholar]
- Terris B, Pineau P, Bregeaud L, Valla D, Belghiti J, Tiollais P, et al. Close correlation between beta-catenin gene alterations and nuclear accumulation of the protein in human hepatocellular carcinomas. Oncogene. 1999;18:6583–8. doi: 10.1038/sj.onc.1203051. [DOI] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–6. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- Tong T, Zhong Y, Kong J, Dong L, Song Y, Fu M, et al. Overexpression of Aurora-A contributes to malignant development of human esophageal squamous cell carcinoma. Clin Cancer Res. 2004;10:7304–10. doi: 10.1158/1078-0432.CCR-04-0806. [DOI] [PubMed] [Google Scholar]
- Varis A, Puolakkainen P, Savolainen H, Kokkola A, Salo J, Nieminen O, et al. DNA copy number profiling in esophageal Barrett adenocarcinoma: comparison with gastric adenocarcinoma and esophageal squamous cell carcinoma. Cancer Genet Cytogenet. 2001;127:53–8. doi: 10.1016/s0165-4608(00)00423-4. [DOI] [PubMed] [Google Scholar]
- Voeller HJ, Truica CI, Gelmann EP. Beta-catenin mutations in human prostate cancer. Cancer Res. 1998;58:2520–3. [PubMed] [Google Scholar]
- Wang C, Li Z, Fu M, Bouras T, Pestell RG. Signal transduction mediated by cyclin D1: from mitogens to cell proliferation: a molecular target with therapeutic potential. Cancer Treat Res. 2004;119:217–37. doi: 10.1007/1-4020-7847-1_11. [DOI] [PubMed] [Google Scholar]
- Washington K, Chiappori A, Hamilton K, Shyr Y, Blanke C, Johnson D, et al. Expression of beta-catenin, alpha-catenin, and E-cadherin in Barrett's esophagus and esophageal adenocarcinomas [In Process Citation] Mod Pathol. 1998;11:805–13. [PubMed] [Google Scholar]
- Yamada T, Takaoka AS, Naishiro Y, Hayashi R, Maruyama K, Maesawa C, et al. Transactivation of the multidrug resistance 1 gene by T-cell factor 4/beta-catenin complex in early colorectal carcinogenesis. Cancer Res. 2000;60:4761–6. [PubMed] [Google Scholar]
- Yang H, He L, Kruk P, Nicosia SV, Cheng JQ. Aurora-A induces cell survival and chemoresistance by activation of Akt through a p53-dependent manner in ovarian cancer cells. Int J Cancer. 2006;119:2304–12. doi: 10.1002/ijc.22154. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, Jiang W, Chen CJ, Lee CS, Kahn SM, Santella RM, et al. Amplification and overexpression of cyclin D1 in human hepatocellular carcinoma. Biochem Biophys Res Commun. 1993;196:1010–6. doi: 10.1006/bbrc.1993.2350. [DOI] [PubMed] [Google Scholar]
- Zhou H, Kuang J, Zhong L, Kuo WL, Gray JW, Sahin A, et al. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20:189–93. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]