Abstract
Exposure of cells to Endoplasmic Reticulum (ER) stress leads to activation of phosphatidylinositol 3-kinase (PI3K)–Akt signaling pathway and transcriptional induction of the inhibitor of apoptosis family of proteins. One of the proximal effectors of the ER stress response, the PKR-like ER kinase (PERK), leads to cellular adaptation to stress by multiple mechanisms, including attenuation of protein synthesis, and transcriptional induction of pro-survival genes. While PERK activity leads to cellular adaptation to ER stress, we now demonstrate that PERK activity also inhibits the ER stress-induced apoptotic program through induction of cellular inhibitor of apoptosis (cIAP1 and cIAP2) proteins. This induction of IAPs occurs through both transcriptional and translational responses that are PERK-dependent. Reintroduction of cIAP1 or cIAP2 expression into PERK−/− MEFs during ER stress delays the early onset of ER stress-induced caspase activation and apoptosis observed in these cells. Furthermore, we demonstrate that activation of the PI3K-Akt pathway by ER stress is dependent on PERK, suggesting additional ways in which PERK activity protects cells from ER stress-induced apoptosis.
Keywords: PERK, ER Stress, Apoptosis, IAP
Introduction
The endoplasmic reticulum (ER) is the cellular site for synthesis, folding, and posttranslational modification of secreted and transmembrane proteins. Cellular stresses that impair the folding of proteins that traffic through the ER activate a signaling pathway commonly referred to as the Unfolded Protein Response (UPR), which coordinates transcriptional induction, translational attenuation, and ER-associated protein degradation (ERAD) allowing cells to adapt to and survive a given stress (Kaufman, 2004; Schroder and Kaufman, 2005). Stresses that activate the UPR include chemical inhibition of N-linked glycosylation, depletion of ER luminal Ca2+, or disruption of the oxidizing environment of the ER (Kaufman, 2004; Schroder and Kaufman, 2005). The physiological stresses of glucose restriction and hypoxia are potent stressors of the ER, and thus the proper function of the UPR is implicated in the progression of tumor growth (Bi et al., 2005; Jamora et al., 1996; Romero-Ramirez et al., 2004).
The proximal effectors of the UPR, or ER stress response, include the ER-resident transmembrane kinases Ire1 (α and β) and the PKR-like ER kinase (PERK), as well as the ER-resident transcription factor ATF6 (Cox et al., 1993; Harding et al., 1999; Haze et al., 1999; Shi et al., 1998; Tirasophon et al., 1998; Wang et al., 1998b). Activation of Ire1 and ATF6 results in the transcriptional induction of ER chaperones in an effort to remedy protein misfolding (Haze et al., 1999; Tirasophon et al., 1998). In contrast, PERK activation regulates cellular protein synthesis through phosphorylation of the eukaryotic translation initiation factor 2α (eIF2α) (Harding et al., 2000b; Harding et al., 1999). Phosphorylation of eIF2α results in a decrease in global protein synthesis, limiting the further influx of proteins into the lumen of the stressed ER. PERK also phosphorylates the transcription factor Nrf2, allowing the cell to regain redox homeostasis (Cullinan et al., 2003).
While one aspect of the UPR is cellular adaptation to acute stress, chronic stress results in the activation of apoptosis thereby preventing the propagation of damaged cells (Schroder and Kaufman, 2005). Initiation of ER stress-induced apoptosis has been demonstrated to occur through a variety of mechanisms including activation of BH3-only proteins and uptake of ER calcium into mitochondria, leading to mitochondrial outer membrane permeabilization and formation of the caspase-9-activating complex, the apoptosome (Deniaud et al., 2008; Di Sano et al., 2006; Li et al., 2006; Mathai et al., 2005; Morishima et al., 2004; Puthalakath et al., 2007; Reimertz et al., 2003; Shiraishi et al., 2006). Activation of the ER-localized caspase-12 (or human caspase-4) may also play a role in initiation of apoptosis during ER stress, activating an apoptotic pathway that has been demonstrated to be independent of mitochondrial release of cytochrome c and formation of the apoptosome (Hitomi et al., 2004; Katayama et al., 2004; Morishima et al., 2002; Nakagawa et al., 2000; Rao et al., 2002).
The mammalian Inhibitor of Apoptosis (IAP) gene family encodes proteins related to the prototypical baculoviral IAP that mediates host cell viability during infection (Crook et al., 1993; Salvesen and Duckett, 2002). Expression of IAPs endows cells with protection against a variety of apoptotic stimuli and IAP proteins, particularly cellular IAPs (cIAP1 and cIAP2), and the X-chromosome-linked IAP (XIAP) are expressed at high levels in many human malignancies (Clem et al., 2001; Orth and Dixit, 1997; Roy et al., 1997; Simons et al., 1999; Vucic and Fairbrother, 2007).
The expression of cIAP1, cIAP2, and XIAP has been demonstrated to be induced by the ER stress response, and this induction is important for cellular survival of stress (Hu et al., 2004; Warnakulasuriyarachchi et al., 2004). ER stress-induced expression of IAPs has been demonstrated to be dependent on the activity of the phosphatidylinositol 3-kinase (PI3K)–Akt signaling pathway as inhibition of this pathway prevents IAP accumulation and sensitizes cells to ER stress-induced apoptosis (Hu et al., 2004).
We have assessed the potential of PERK to regulate cellular survival via regulation of the accumulation of inhibitors of apoptosis. We demonstrate that the UPR-induced expression of IAPs occurs at both the level of transcription and translation and that both are PERK dependent processes. Reintroduction of IAPs delays the early onset of apoptosis exhibited by PERK deficient cells, highlighting the importance of IAP induction for the survival following ER stress. Additionally, we demonstrate that UPR-induced activation of the PI3K-Akt pathway is dependent on PERK highlighting a diversity of avenues through which PERK may affect cell fate. These findings reveal that PERK activity, in addition to promoting cellular adaptation to stress, actively inhibits the ER stress-induced apoptotic program, allowing for increased survival.
Results
UPR-induced IAP expression is a PERK-dependent process
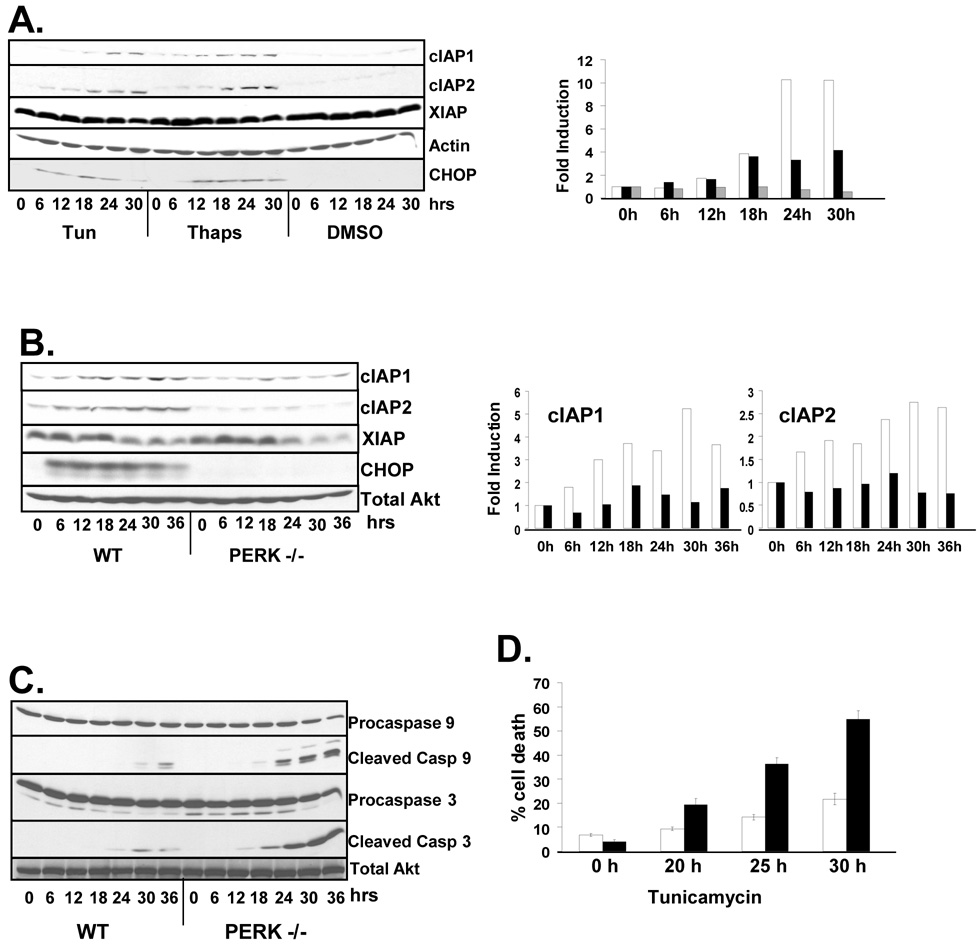
Previous work revealed accumulation of IAPs in tumor-derived cell lines treated with the ER stressors (Hu et al., 2004; Warnakulasuriyarachchi et al., 2004). To evaluate the capacity of the UPR to regulate expression of IAPs in non-transformed cell lines, and to determine if this induction is conserved across species, NIH-3T3 cells were treated with tunicamycin or thapsigargin, bona fide inducers of protein misfolding within the ER, or vehicle (DMSO) alone for up to 30 hours. Treatment of cells with tunicamycin or thapsigargin led to the accumulation cIAP1 and cIAP2 protein (Figure 1A). Induction of cIAP protein accumulation coincided with the induction of the ER stress inducible protein CHOP. In contrast, XIAP was highly expressed in NIH-3T3 cells and levels were slightly decreased by induction of the ER stress response (Figure 1A).
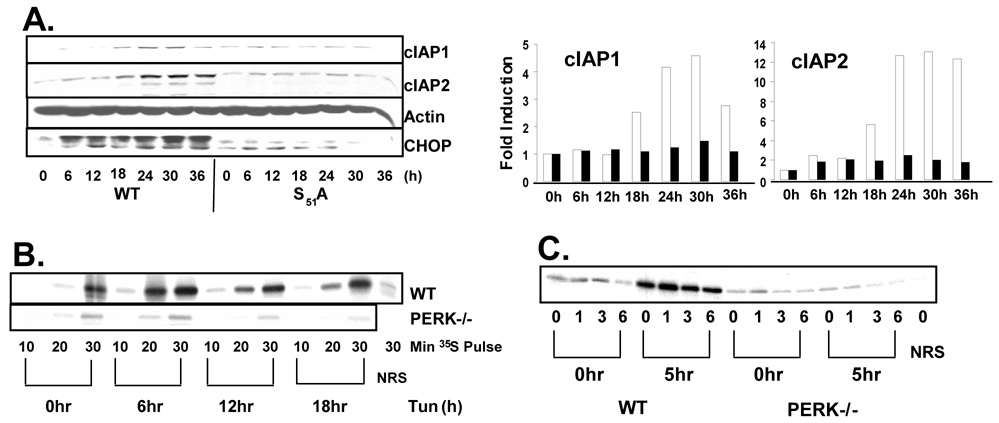
Figure 1. ER stress induces the expression of IAP proteins.
(A) NIH-3T3 or (B) wild-type and PERK −/− fibroblasts were treated with tunicamycin (2µg/mL), thapsigargin (50nM), or DMSO as a vehicle control as indicated. IAPs, CHOP and actin were visualized by western blot. Graphs represent fold induction of cIAP protein {(A) cIAP1 (white), cIAP2 (black), and XIAP (grey) indicate cIAP expression in tunicamycin treated cells; (B), wild-type (white) and PERK−/− (black) bars}. (C) Wild-type and PERK−/− fibroblasts were treated with tunicamycin for up to 36 hours and cleavage of caspases 3 and 9 were determined by western blot. (D) Wild-type and PERK−/− cells were treated with tunicamycin (5µg/mL) as indicated. Cells were re-suspended in PBS containing propidium iodide and analyzed by FACS to ascertain cell integrity.
Although PERK activity influences cellular adaptation to and survival of ER stress through multiple mechanisms (Cullinan et al., 2003; Harding et al., 2000b), we reasoned that the induction of IAP proteins might be one such PERK-dependent survival mechanism. To determine the role of PERK in the regulation of IAP expression following ER stress, wild-type and PERK−/− fibroblasts were treated with tunicamycin for up to 36 hours. The expression of cIAP1 and cIAP2 was induced in wild-type cells, while no such induction was detected in PERK−/− cells revealing PERK-dependent regulation (Figure 1B). Interestingly, the expression of XIAP was not induced by ER stress and in even decreased slightly, revealing that XIAP is not regulated in the same manner as cIAP1 and cIAP2.
Expression of cIAPs confers protection from a number of apoptosis-inducing stresses (Vucic and Fairbrother, 2007). To determine if the high levels of ER stress-dependent cIAP expression observed in wild-type cells corresponded with reduced apoptosis compared to PERK−/− fibroblasts, membranes were probed with antibodies specific for the unprocessed and processed forms of caspase 9 and caspase 3. Processing of caspase-9 and caspase-3 was accelerated in PERK−/− cells consistent with reduced expression of cIAPs (Figure 1C). The percentage of wild-type and PERK−/− cells undergoing apoptosis after tunicamycin treatment was determined by staining of cells with propidium iodide (PI). PERK−/− cells exhibited reduced viability compared to wild-type cells, correlating with increased sensitivity to ER stress (Figure 1D).
PERK and Akt regulate the expression of IAP mRNA
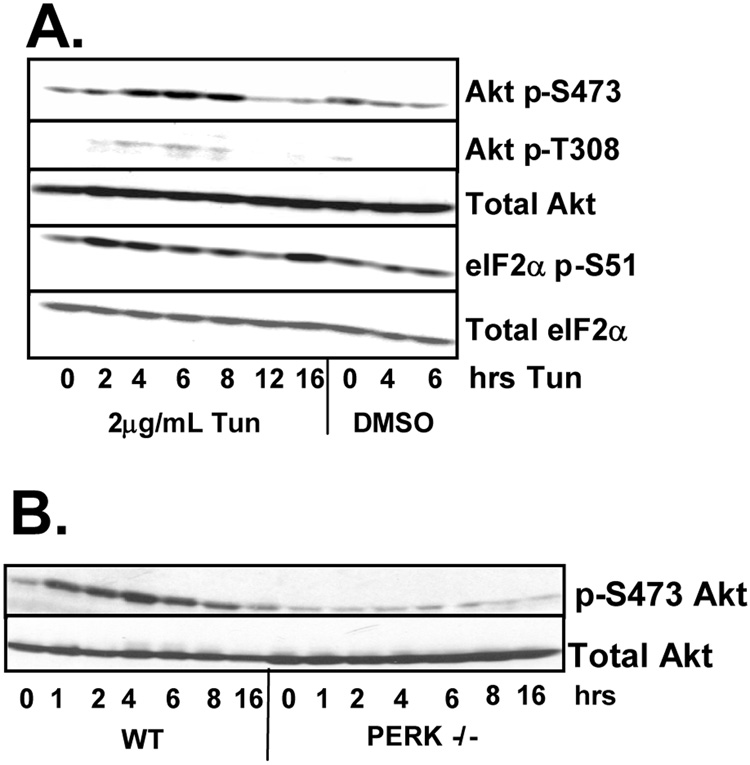
The phosphatidylinositol 3-kinase (PI3K)–Akt signaling pathway is activated by ER stress inducing agents (Hosoi et al., 2007; Hu et al., 2004; Kazemi et al., 2007) and Akt activation is implicated in the transcriptional induction of IAPs (Hu et al., 2004). Given that both PERK and Akt transduce cell survival signals, we initially determined whether PERK-dependent signals trigger Akt activation. NIH-3T3 cells treated with tunicamycin were harvested at the indicated intervals and Akt activation was determined by western analysis using antibodies specific for Akt phosphorylated at serine 473 or threonine 308. Tunicamycin triggered increased phosphorylation of both serine 473 and threonine 308 by 4 hours, consistent with Akt activation; phosphorylation declined by 8 hours (Figure 2A). Tunicamycin treatment also transiently induced the phosphorylation of p70 S6 kinase, a downstream effector of the Akt pathway, at threonine 389 (data not shown).
Figure 2. ER stress induces Akt activity in a PERK-dependent manner.
(A) NIH-3T3 cells or (B) wild type and PERK−/− fibroblasts were treated with 2µg/mL tunicamycin or DMSO as a vehicle control for the indicated intervals. Cell lysates were resolved by SDS-PAGE and membranes were probed with antibodies for phosphorylated and total Akt and eIF2α.
To determine whether UPR-dependent Akt activation requires PERK, lysates were prepared from either wild-type or PERK−/− fibroblasts that had been treated with tunicamycin. A robust induction of Akt phosphorylation was observed in wild-type cells, while no such induction was observed in PERK−/− cells (Figure 2B). The observed lack of Akt activation in PERK−/− cells was specific for ER stress as Akt remains subject to mitogen regulation in these cells (data not shown).
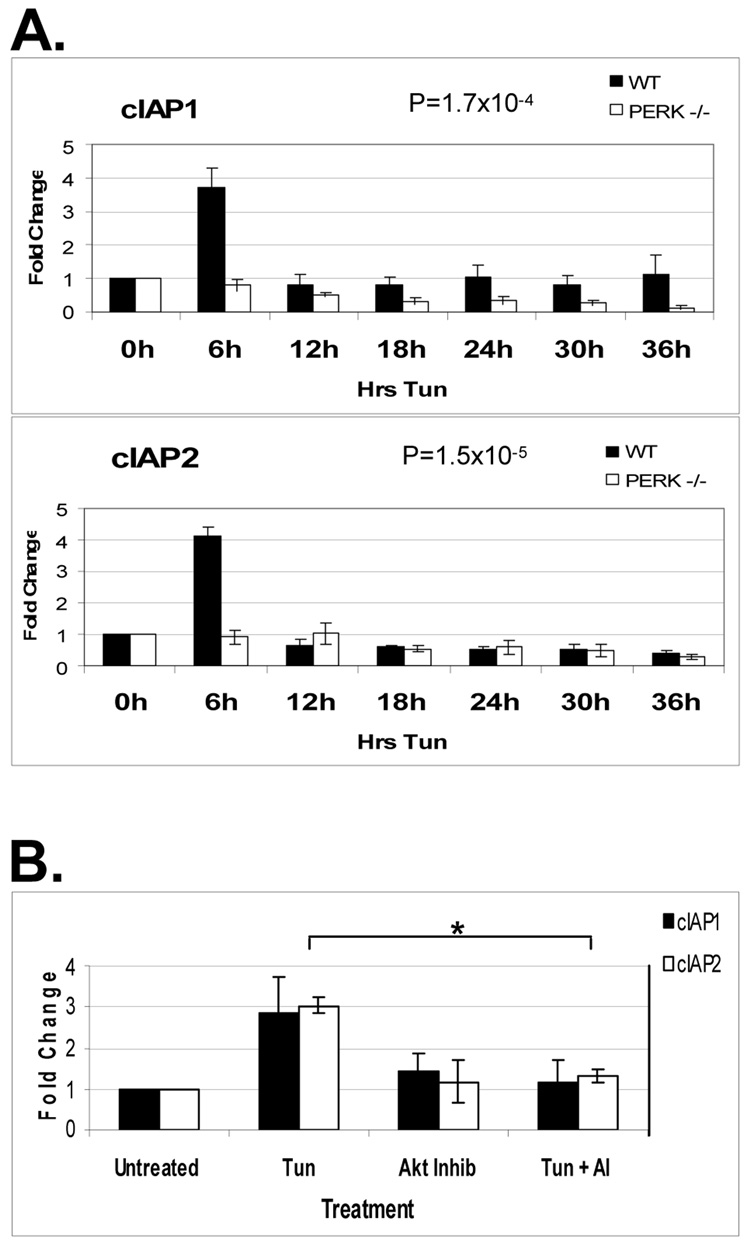
Akt activation is implicated in the regulation of IAP gene transcription (Hu et al., 2004). To determine whether PERK regulates IAP expression in an Akt-dependent manner, RNA was prepared from wild-type and PERK−/− MEFs treated with tunicamycin. mRNA was reverse transcribed and the resulting cDNA was subject to quantitative real-time PCR. Expression of cIAP1 and cIAP2 was transiently induced by tunicamycin treatment in a PERK-dependent manner (Figure 3A). This transient 3-fold increase in IAP mRNA was coincident with Akt activity in cells incurring ER stress. To directly test the importance of the PI3K-Akt pathway for UPR-induced cIAP transcription, WT fibroblasts were treated with tunicamycin, an Akt inhibitor, or both for 3 hours. mRNA was prepared from cellular lysates, reverse transcribed, and the resulting cDNA was subject to qRT-PCR. Pharmacological inhibition of Akt attenuated the tunicamycin-induced transcription of IAP mRNA (Figure 3B) indicating that PERK and Akt contribute to IAP mRNA accumulation.
Figure 3. ER stress induces the transcription of IAP mRNA in a PERK and Akt dependent manner.
WT or PERK−/− fibroblasts were treated with 2µg/mL tunicamycin (A) or tunicamycin, 5µM Akt inhibitor, or both (B) as indicated. Total RNA was collected from cells and reverse transcribed with the resulting cDNA being used as a template for quantitative RT-PCR. Graphs represent the average of (A) 5, or (B) 4 experiments with error bars representing +/− SEM. For (B), P=0.011239 for cIAP1 and 0.001206 for cIAP2.
IAP translation is induced by ER stress in a PERK-dependent manner
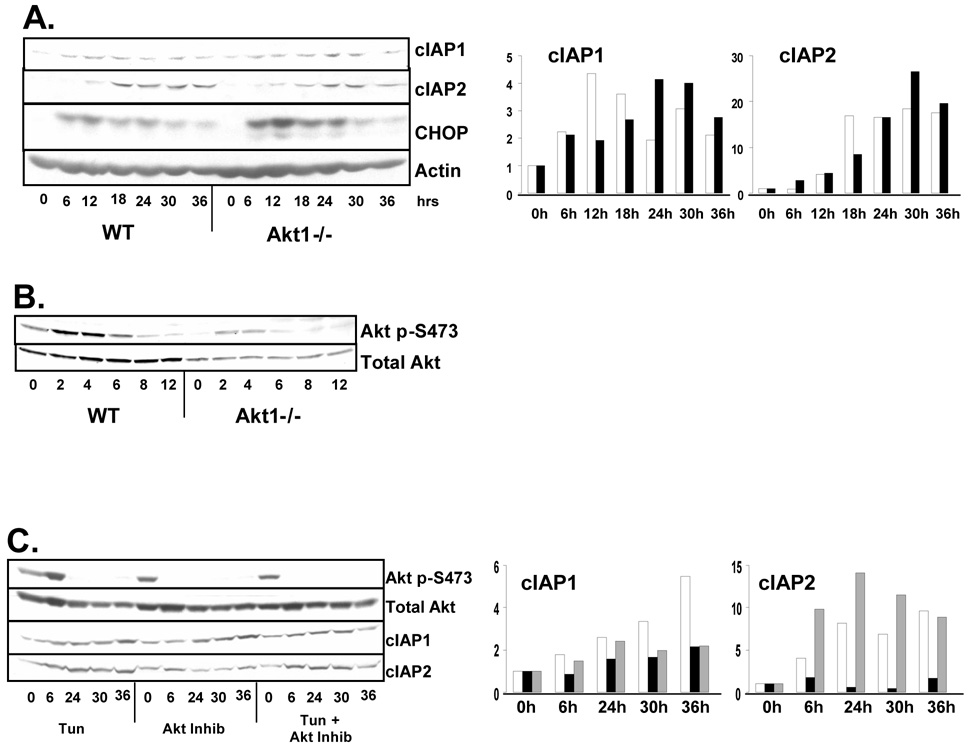
The above results reveal a role for Akt in the UPR-dependent accumulation of cIAP mRNA. We noted however that IAP proteins continue to accumulate at time points at which IAP mRNA levels are declining, suggesting additional levels of regulation. We therefore evaluated the contribution of PERK and Akt to the induction of IAP protein. Wild-type or Akt1−/− MEFs were challenged with tunicamycin and IAP levels were determined by western analysis. Strikingly, Akt1 deficiency did not prevent stress-induced accumulation of IAPs (Figure 4A). Although cIAP protein accumulation is not disrupted in Akt1−/− MEFs, it is possible that Akt isoforms Akt2 or Akt3 become activated by stress in the absence of Akt1, leading to IAP induction. The stress-mediated induction of a phosphorylated Akt isoform in Akt1−/− cells is evidence of this possibility (Figure 4B). We therefore used a small molecule inhibitor of Akt to inhibit all Akt isoforms. Wild-type MEFs were treated with tunicamycin, Akt inhibitor, or both. Coincident treatment of cells with tunicamycin and the Akt inhibitor did partially attenuated ER stress-induced accumulation of cIAP1 and cIAP2 protein; however this inhibition was not complete as cIAP protein was still induced upon tunicamycin treatment in the presence of the Akt inhibitor (Figure 4C). Similar results were observed in cells the PI3-kinase inhibitor LY294002 (data not shown). Thus, while Akt activation and induction of cIAP mRNA contribute to the expression of cIAP protein during ER stress, this transcriptional response is not sufficient for the maximal induction of cIAP protein. As PERK plays a crucial role in regulating cellular translation during stress, we next determined whether the continued accumulation of cIAPs reflected a PERK-dependent increase in translation.
Figure 4. Akt activation is not required for the ER stress-induced induction of IAP expression.
(A and B) Wild-type or Akt1−/− fibroblasts were treated with tunicamycin for the indicated intervals. Graphs represent fold increase in cIAP protein. For (A), wild-type (white) and Akt1−/− (black). (C) Wild-type fibroblasts were treated with tunicamycin (white), Akt inhibitor (black), or both (grey) as indicated. Cell lysates were resolved by SDS-PAGE and membranes were probed with antibodies for cIAP1, cIAP2, CHOP. As a surrogate for Akt activity, western analysis for total Akt and Akt phosphorylated on S473 was performed.
Although PERK-mediated phosphorylation of eIF2α generally inhibits protein translation, translation of some transcripts, such as that of ATF4, are paradoxically induced by eIF2α phosphorylation (Fernandez et al., 2002a; Harding et al., 2000a; Vattem and Wek, 2004). To determine if ER stress-mediated induction of IAPs is dependent on eIF2α phosphorylation, WT fibroblasts and fibroblasts harboring a homozygous knock-in allele of eIF2α S51A were treated with tunicamycin for up to 36 hrs. Cellular lysates were resolved by SDS-PAGE and IAP levels were determined by Western blot. ER stress-induced IAP accumulation is completely dependent on phosphorylation of eIF2α as no IAP induction was detected in S51A cells (Figure 5A).
Figure 5. IAP mRNA is selectively translated during ER stress.
(A) Wild-type and eIF2α S51A fibroblasts were treated with tunicamycin as indicated. Cell lysates were resolved by SDS-PAGE and IAP levels were determined by immunoblot. Graphs represent fold increase in cIAP protein. Wild-type (white) and S51A (black). (B) Wild-type and PERK−/− fibroblasts were treated with tunicamycin or left untreated. Cells were pulsed with 35S methionine during the last 10, 20, or 30 min of treatment and cIAP2 was immunoprecipitated from cell lysates and observed by autoradiograph. (C) Wild-type and PERK−/− fibroblasts were treated with tunicamycin for 5 hours or left untreated. Cells were then pulsed with 35S-mentionine for 30 min followed by chase in cold methionine for the indicated intervals. cIAP2 was immunoprecipitated from cell lysates and observed by autoradiograph. NRS lanes represent immunoprecipitation with Normal Rabbit Serum.
To determine if the PERK and eIF2α dependent induction of cIAP proteins during ER stress reflected increased protein translation, wild type and PERK−/− fibroblasts were treated with tunicamycin for up to 18 hours or left untreated. Cells were then pulse-labeled with 35S methionine for 10, 20, or 30 minutes, and cIAP2 was immunoprecipitated from cellular lysates and viewed by autoradiograph. Treatment of wild-type cells with tunicamycin lead to an induction of 35S incorporation into cIAP2 protein. This induction did not occur in PERK−/− fibroblasts (Figure 5B).
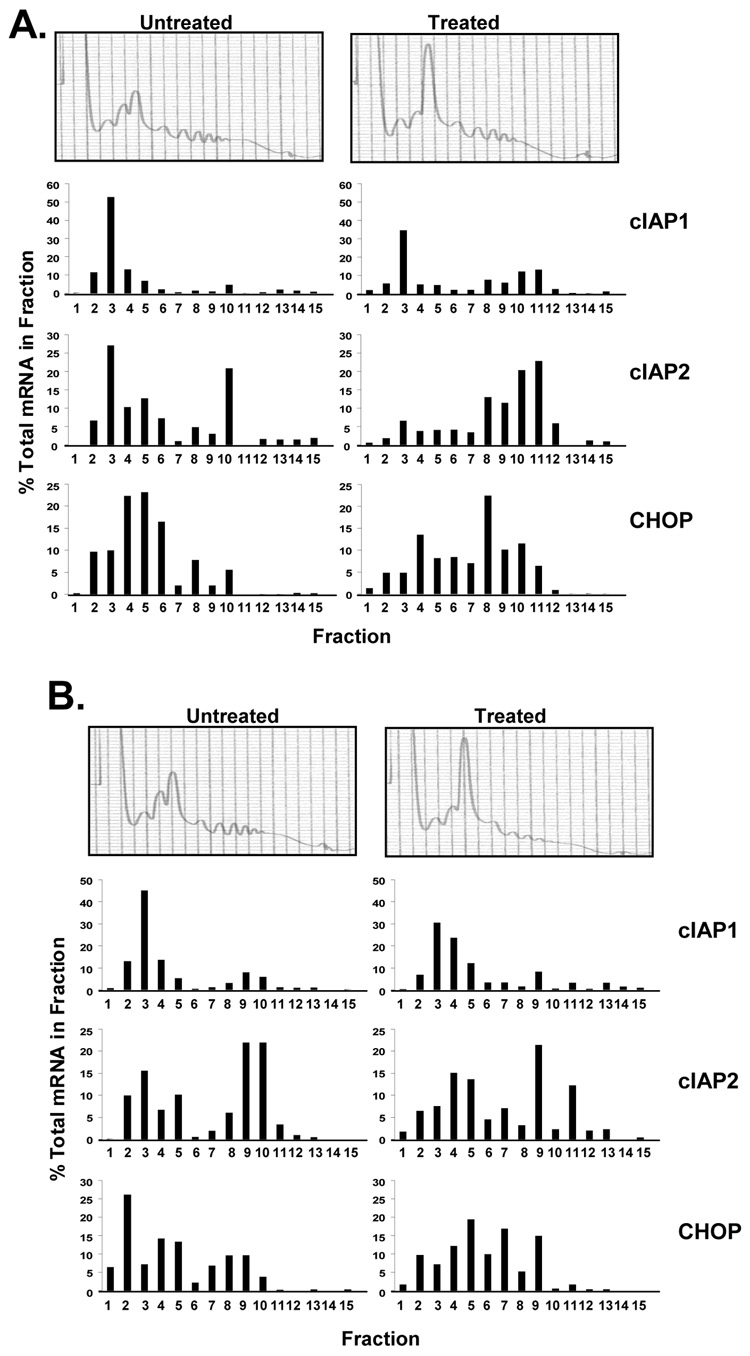
To confirm the pulse-labeling analysis, we analyzed cIAP translation by polysome profile. Wild type and PERK−/− fibroblasts were treated with tunicamycin for 12 hours or left untreated. Cellular lysates were fractionated by sucrose gradient ultracentrifugation for polysome analysis. RNA was collected from each fraction, reverse transcribed, and subject to quantitative real-time PCR. Tunicamycin treatment led to the accumulation of cIAP1 and cIAP2 mRNA in the actively translated, polysome fractions (Figure 6A). This preferential translation of cIAP message was PERK-dependent as PERK−/− cells exhibited no shift in polysome accumulation of cIAP mRNA (Figure 6B).
Figure 6. ER stress induces the accumulation of cIAP mRNA on polysomes.
(A) Wild-type and (B) PERK−/− fibroblasts were treated with tunicamycin for 12 hours or left untreated and cell lysates were fractionated by sucrose gradient ultracentrifugation. The absorbance profile (A254nm) of each gradient is shown. RNA was extracted from each fraction and the level of cIAP1, cIAP2, and CHOP mRNA was assessed by quantitative RT-PCR. Data is represented as the percent of each mRNA present in an individual fraction.
cIAP1 and cIAP2 contain RING finger domains, and are E3 ubiquitin ligases capable of autoubiquitination and ubiquitination of several binding proteins (Liston et al., 2003; Salvesen and Duckett, 2002). We therefore used pulse-chase analysis to determine if IAP stability is affected by ER stress. WT and PERK−/− cells were treated with tunicamycin for 5 hours or left untreated, followed by pulse with 35S methionine and subsequent chase. Autoradiography revealed no ER stress-induced alterations in cIAP2 half-life (Figure 5C).
ER stress-induced IAP expression delays the onset of ER stress-induced apoptosis
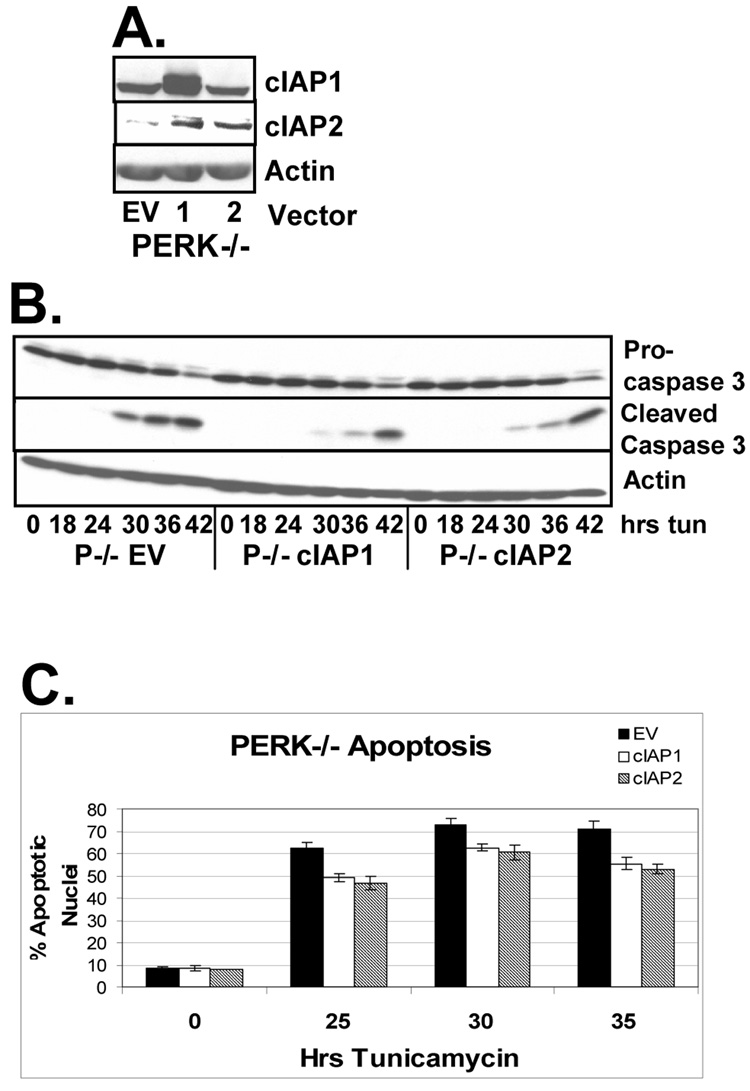
To determine whether reintroduction of IAP expression could rescue PERK deficient cells from accelerated, ER stress-induced apoptosis, PERK−/− fibroblasts were infected with retrovirus expressing cIAP1, cIAP2, or empty vector (Figure 7A). It is of interest to note that expression of cIAP1 resulted in increased accumulation of cIAP2. Cell lines were treated with tunicamycin and cleavage of the executioner caspase, caspase 3, was assessed by western analysis. Reintroduction of cIAPs into PERK−/− cells attenuated the onset of caspase cleavage in these cells (Figure 7B). To determine if the delayed caspase cleavage corresponded with delayed onset of apoptosis, nuclei were scored for apoptotic morphology. PERK−/− cells expressing cIAP1, cIAP2, or empty vector were grown on cover glass and treated with tunicamycin for up to 35 hours. Cells were stained with DAPI and nuclear morphology was assessed. Reintroduction of cIAP1 and cIAP2 into PERK−/− cell lines inhibited the accumulation of cells with apoptotic nuclear morphology (Figure 7C). No anti-apoptotic effect of IAP expression was observed in PERK wild-type cells, however ER stress-induced translational repression in these cells prevented the exogenous expression of IAPs upon treatment with tunicamycin (data not shown).
Figure 7. Reintroduction of IAPs into PERK−/− delays the onset of apoptosis upon induction of the ER stress response.
(A) PERK−/− fibroblasts were infected with retrovirus expressing empty vector (lane 1), cIAP1 (lane 2), or cIAP2 (lane 3). (B) Cell lines were treated with tunicamycin for the indicated intervals. Cell lysates were resolved by SDS-PAGE and caspase cleavage was determined by immunoblot. (C) PERK −/− cell lines expressing empty vector, cIAP1, or cIAP2 were grown on coverglass and treated with tunicamycin for the indicated intervals. Cells were stained with DAPI and apoptosis was determined by scoring nuclear morphology.
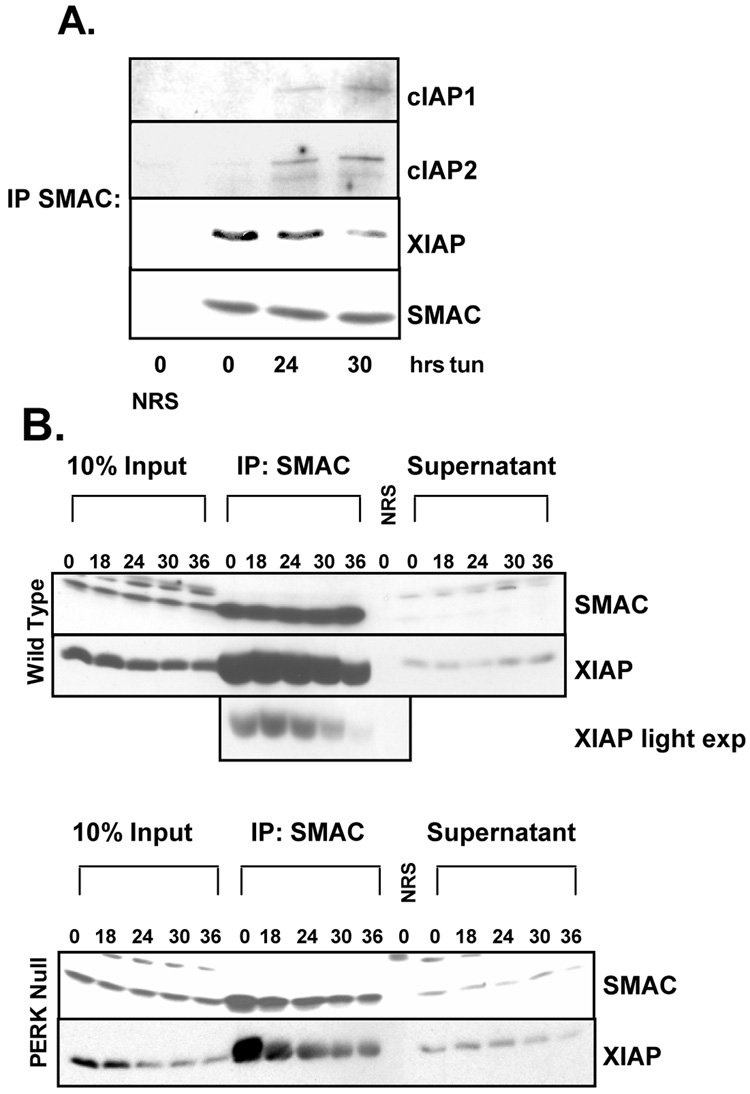
Although expression of cIAPs is closely associated with protection from apoptosis, the mechanism by which cIAPs mediate their protective effect remains unclear. It is postulated that the role cIAPs in apoptotic suppression lies in sequestration of the IAP inhibitor SMAC/DIABLO from XIAP, allowing XIAP to remain active (Eckelman and Salvesen, 2006). To the hypothesis that induction of cIAP expression during ER stress allows for the accumulation of SMAC-free XIAP, wild type cells were treated with tunicamycin for up to 36 hours and SMAC was immunoprecipitated from cellular lysates. Levels of SMAC-associated IAPs were determined by Western blot. Tunicamycin treatment increased SMAC-associated cIAP1 and cIAP2, and a corresponding decrease in SMAC-associated XIAP (Figure 8A). This replacement of SMAC associated XIAP with cIAPs corresponded with an increase of soluble XIAP (supernatant), suggesting that cIAPs function during ER stress to free XIAP from SMAC-mediated inhibition (Figure 8B). An ER stress-induced reduction of SMAC-associated XIAP was also noted in PERK−/− cells, however, total cellular XIAP substantially decreased over the same time course (Figure 8B). There was no observed increase in SMAC-free XIAP upon induction of ER stress in PERK−/− cells (Figure 8B).
Figure 8. ER stress-induced cIAP expression prevents SMAC binding to XIAP.
(A) Wild-type and cells were treated with tunicamycin for the indicated intervals. SMAC was immunoprecipitated from sonicated cell lysates, and precipitates were resolved by SDS-PAGE. (B) Wild-type and PERK−/− cells were treated with tunicamycin for the indicated intervals. SMAC was immunoprecipitated from sonicated cell lysates of resolved by SDS-PAGE along with 100µg of whole cell lystate was used as input control, and 100µg of supernatant from immunoprecipitates. Membranes were probed with antibodies specific for cIAP1, cIAP2, XIAP, and SMAC. NRS lanes represent immunoprecipitation with Normal Rabbit Serum.
Discussion
The activation of PERK following oxygen or glucose restriction, contributes to cell adaptation and are thus implicated in the progression maintenance of tumors (Fels and Koumenis, 2006). Because cells deficient for PERK are acutely sensitive to ER stress, an understanding of the pro-survival mechanisms initiated by PERK activity may reveal therapeutic targets for cancer as well as other UPR-based pathologies. The pro-survival mechanisms that have been described downstream of PERK are adaptive programs that reduce the cytotoxic consequences of the stress that a cell incurs. The current work demonstrates that in addition to the adaptive pathways downstream of PERK, once a cell has reached a critical level of stress, PERK also mediates cell survival through the selective induction of the IAP family proteins. ER stress-induced expression of cIAPs coincides with reduced activation of caspases, suggesting an inhibitory effect of IAPs on the ER stress-induced apoptotic program. Our results confirm previously reported data that suggests IAPs are transcriptionally induced in an Akt-dependent manner (Hu et al., 2004). Our data demonstrate that IAP mRNA is transiently induced early during the ER stress response. This induction coincides temporally with ER stress-induced Akt activity and is attenuated by Akt inhibition. While the specific transcription factors downstream of Akt have not been identified, NFκB remains a likely candidate. Akt promotes NFκB activity by phosphorylating and activating IκB kinase, leading to NFκB nuclear translocation and transcription of its target genes, which include cIAP1 and cIAP2 (Bellacosa et al., 2004; Debatin and Krammer, 2004). While ER stress can induce NFκB transcriptional activity in a PERK-dependent manner (Deng et al., 2004; Jiang et al., 2003), the role of Akt activity in ER stress-induced NFκB activation has not been addressed.
Importantly, while IAP transcription does increase following ER stress, our results indicate that increased expression is not necessary for IAP induction. Inhibition of Akt effectively inhibited IAP mRNA accumulation, but did not inhibit protein accumulation suggesting an alternative mechanism contributes to IAP protein accumulation during ER stress. Indeed, our data demonstrates that IAP mRNA is preferentially translated during the ER stress response in a PERK and eIF2α-dependent manner. While phosphorylation of eIF2α is associated with attenuation of protein synthesis, translation of specific transcripts is induced by eIF2α phosphorylation. The mRNAs of the transcription factor ATF4 (Harding et al., 2000a) and the cationic amino acid transporter Cat-1 (Fernandez et al., 2002b) contain upstream open reading frames (uORFs) in their 5’-untranslated regions (5’UTR) that repress translation under basal conditions, but allow for efficient translation when eIF2α is phosphorylated (Schroder and Kaufman, 2005). The 5’UTRs of both human and mouse cIAP1 and cIAP2 contain uORFs, suggesting a potential regulatory mechanism.
Alternatively, translational regulation of IAPs could reflect the presence of putative internal ribosome entry sites (IRES). IRES elements have been reported for cIAP1 (Warnakulasuriyarachchi et al., 2004) mRNA, suggesting additional mechanisms by which cells upregulate IAPs during ER stress. IRES elements in the cIAP1 mRNA may explain why, in our hands, inhibition of Akt alone upregulates cIAP1 protein expression.
Although reintroduction of cIAP expression into PERK−/− cells during ER stress only partially rescues these cells from apoptosis, it is important to note that PERK mediates cellular adaptation to ER stress through multiple mechanisms. We thus did not expect to completely rescue PERK−/− cells from ER stress-induced apoptosis by reintroducing only one mechanism by which PERK mediates survival.
Interestingly, the expression of the most potent IAP, XIAP is not induced by ER stress. XIAP directly inhibits caspases-3, -7, and –9 (Deveraux et al., 1997; Scott et al., 2005; Shiozaki et al., 2003). Although not direct caspase inhibitors, cIAPs are believed function as modulators of XIAP activity through the sequestration of SMAC (Eckelman and Salvesen, 2006). Our data is consistent with this hypothesis as ER stress-induced expression of cIAP proteins leads to a reduction of XIAP in SMAC immunoprecipitates. Thus, while XIAP protein levels are not induced by ER stress, there is a net increase XIAP through PERK-dependent cIAP induction. The importance of XIAP activity for survival of ER stress is highlighted by the fact that knockdown of XIAP dramatically sensitizes cells to tunicamycin treatment (Hu et al., 2004); our unpublished results).
The activation of Akt may also directly affect XIAP activity during ER stress as Akt has been demonstrated to phosphorylate and thus stabilize XIAP protein (Dan et al., 2004). The transient activation of Akt during ER stress may thus regulate cellular levels of XIAP protein, and possibly contribute the loss of XIAP protein observed at later hours of ER stress.
Another possible and intriguing role for cIAP1 and cIAP2 in ER stress may lie in the modulation of death receptor signaling. Signaling downstream of TNFα can be either pro-survival or pro-apoptotic and cIAPs are crucial for pro-survival signaling through the TNFα receptor (Wang et al., 1998a). Autocrine TNFα pro-apoptotic signaling can occur during ER stress through IRE1 activity (Hu et al., 2006). However, the role of cIAP1 and cIAP2 in ER stress-induced TNFα signaling has not been addressed. While we failed to detect a change of TNFα levels in the media of fibroblasts treated with tunicamycin (unpublished data), the critical role of cIAPs in protecting tumor cells from TNFα-induced apoptosis is becoming increasingly clear (Bertrand et al., 2008; Petersen et al., 2007; Varfolomeev et al., 2007; Vince et al., 2007). Given these observations, PERK inhibition, as a therapeutic strategy may not only eliminate the ability of cells to adapt to the tumor microenvironment, but also induce the extrinsic apoptotic pathway in tumor cells that excrete TNFα. Future experiments will be required to determine the role of PERK in maintaining tumor cIAP levels and viability in vivo.
A striking finding is that ER stress-mediated activation of Akt is completely dependent on PERK. The precise mechanism whereby Akt is activated during ER stress is under current investigation. We and others have observed that this activation is dependent on PI3-kinase activity as ER stress-induced Akt phosphorylation is ablated by the presence of LY294002 (Hosoi et al., 2007; Hu et al., 2004),our unpublished results. We have also observed induction of PI3K activity in in vitro kinase assays after prior treatment of cells with tunicamycin (our unpublished data). This activation of PI3K is independent of extracellular growth factor signaling, as Akt becomes activated by ER stress in the absence of serum (Hu et al., 2004); unpublished data).
A recent report suggests that activation of PI3K occurs downstream of the eIF2α kinase PKR, suggesting that PI3K and Akt activation is a property of all eIF2α kinases (Kazemi et al., 2007). Elucidation of the mechanism by which eIF2α kinases activate PI3-kinase is of great interest as signaling pathways downstream of PI3K bring to light a myriad of new, unexplored mechanisms by which cells respond to stress.
Materials and Methods
Cell Culture and Materials
Cell lines were maintained in MEF Media (Dulbecco’s Modified Eagle’s Medium (Cellgro) supplemented with 10% fetal bovine serum (Gemini), Penicillin/Streptomycin (Cellgro), nonessential amino acids (Gibco), L-glutamine (Cellgro), and β-mercaptoethanol (Gibco). eIF2α S51A homozygous knock-in murine embryonic fibroblasts (MEFs) were a gift from Donalyn Scheuner and Randal Kaufman (Scheuner et al., 2001). Akt1−/− MEFs were a gift of Morris Birnbaum (Cho et al., 2001). All MEF cell lines were immortalized via a standard 3T9 passage protocol (Todaro, 1963). Transfections were performed using LipofectAMINE Plus (Invitrogen) according to the manufacturer’s instructions. Tunicamycin was purchased from Sigma, and Akt Inhibitor VIII was purchased from EMD Biosciences. For overexpression experiments, mouse fibroblasts were infected with retroviral supernatants as previously described (Brewer and Diehl, 2000). Forty-Eight hours post-infection, cells were treated as indicated and harvested for analysis.
Protein Analysis
For direct Western blot analysis, cells were lysed in EBC buffer (50 mM TrisHCl pH 8.0, 120 mM NaCl, 1mM EDTA, 0.5% Igapel, 10 U/ml aprotinin, 5µg/ml leupeptin, 10 mM β-glycerolphosphate, 4mM NaF, 1mM PMSF.) Total protein (100 µg) was resolved on denaturing polyacrylamide gels, transferred to nitrocellulose membranes or PVDF (Millipore), and blotted with the indicated primary antibodies: cIAP1 (Cell Signaling), cIAP2 (Chemicon), XIAP (BD Biosciences), β-Actin (Sigma), CHOP (Santa Cruz), caspase 3 (Cell Signaling), caspase 9 (Cell Signaling), Akt total, pS473, and pT308 (Cell Signaling), eIF2α total and pS51 (Biosource), p70S6 kinase total and pT389 (Cell Signaling). Sites of antibody binding were visualized by enhanced chemiluminescence detection (Perkin-Elmer).
For SMAC immunoprecipitation, cells were lysed in Triton X-100 lysis buffer (20mM Tris pH 7.5, 150mM NaCl, 10% Glycerol, 2mM EDTA, 1% Triton X-100, 10 U/ml aprotinin, 5µg/ml leupeptin, 10 mM β-glycerolphosphate, 4mM NaF, 1mM PMSF) and briefly sonicated. SMAC was immunoprecipitated from 1mg of cellular lysate using a rabbit polyclonal antisera (Novus Biologicals).
Real Time RT-PCR
For detection of cIAP1 and cIAP2 mRNA and 18S rRNA by reverse transcription (RT)-PCR, total RNA was extracted from fibroblasts using TRIzol (Invitrogen). RT reactions were performed using Superscript II RT (Invitrogen) using 5µg RNA and random hexomers following the manufacturer’s instructions. cIAP1 was amplified using primers (sense, 5’-gaagaaaatgctgaccctacaga-3’; anti-sense 5’-gctcatcatgacgacatctttc-3’), cIAP2 was amplified using primers (sense, 5’-cgatgcagaagacgagatga-3’; anti-sense 5’-tttgttcttccggattagtgc-3’), and 18S rRNA was amplified as a control using primers (sense, 5’-aaatcagttatggttcctttggtc-3’; anti-sense 5’-gctctagaattaccacagttatccaa-3’). ΔΔCT analysis was performed in an Applied Biosystems 7900HT Sequence Detection System with amplification quantified with SYBR green.
Biosynthetic Labeling
Subconfluent cells were treated with either 2 µg/ml tunicamycin for the indicated intervals, or left untreated, and shifted to methionine/cystine-free DMEM (Sigma) for the final 30 minutes of treatment. Cells were pulsed with medium (+/− tunicamycin) containing 150 µCi/ml trans-35S-label for the indicated intervals and lysed in NP40 Lysis Buffer (50mM TrisHCl pH 7.5, 1% Igapel, 0.5% deoxycholate, 150mM NaCl, 10 U/ml aprotinin, 5µg/ml leupeptin, 10mM β-glycerolphosphate, 4mM NaF, 1mM PMSF). cIAP2 was immunoprecipitated from whole cell lysates, resolved on a denaturing polyacrylamide gel and visualized by autoradiography.
For pulse-chase analysis, cells were treated with tunicamycin and starved of methionine/cysteine as before. Cells were then pulsed in medium (+/− tunicamycin) containing 150 µCi/ml trans-35S-label for 30 minutes, washed with PBS and chased for the indicated interval in medium (+/− tunicamycin) supplemented with 200µM cold methionine.
Polysome Analysis
Polysome analysis was carried out as described previously (Ji et al., 2003). Briefly, wild type and PERK−/− fibroblasts were treated with tunicamycin for 12 hours or left untreated, and subsequently incubated with cycloheximide (100 µg/ml) for 15 min prior to harvesting. Cells were lysed in TMK100 lysis buffer (10 mm Tris-HCl, pH 7.4, 5 mm MgCl2, 100 mm KCl, 2 mm dithiothreitol, 1% Triton X-100, and 100 units of RNase inhibitor (Promega) per ml in diethyl pyrocarbonate-treated water) for 5 min. Nuclei were cleared, supernatants were loaded on top of 10 to 50% linear sucrose gradients containing 100 mm KCl, 5 mm MgCl2, 2 mm dithiothreitol, and 20 mm HEPES, pH 7.4. Gradients were ultracentrifuged at 38,000 rpm for 90 min at 4 °C (Beckman SW41 rotor). 750 µl fractions were collected into microcentrifuge tubes containing 70 µl of 10% SDS, and the gradient profile was monitored via UV absorbance at 254 nm with a UA-5 detector (ISCO, Lincoln, NE). Each sample was digested with 8 µl of protease K (20 mg/ml) solution at 37 °C for 30 min and stored at −80 °C before RNA extraction with phenol and chloroform.
Apoptosis Detection
For propidium iodide staining, cells were treated with 5µg/mL Tunicamycin for the indicated intervals. Cells were trypsinized and resuspended in PBS. Propidium iodide was added to a concentration of 2.5 µg/mL. PI fluorescence was measured with a Becton Dickinson FACSCalibur
For DAPI staining, cells proliferating on glass coverslips were treated with 2µg/mL Tunicamycin for the indicated intervals. Cells were fixed in 1:1 Methanol:Acetone, rehydrated in PBS, and mounted in ProLong Gold antifade reagent with DAPI (Invitrogen). A minimum of 1000 nuclei per cell line was scored using a Nikon E800 fitted with appropriate filters.
ACKNOWLEDGEMENTS
We would like to thank M. Romero for technical assistance and X. Yang for providing IAP expression vectors. This work was supported by a grant from the National Institutes of Health F32CA1238252 (EBM); P01 CA104838, a Leukemia & Lymphoma Scholar award and the Abramson Family Cancer Research Institute (JAD).
References
- Bellacosa A, Testa JR, Moore R, Larue L. A portrait of AKT kinases: human cancer and animal models depict a family with strong individualities. Cancer Biol Ther. 2004;3:268–275. doi: 10.4161/cbt.3.3.703. [DOI] [PubMed] [Google Scholar]
- Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Bi M, Naczki C, Koritzinsky M, Fels D, Blais J, Hu N, et al. ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. Embo J. 2005;24:3470–3481. doi: 10.1038/sj.emboj.7600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JW, Diehl JA. PERK mediates cell-cycle exit during the mammalian unfolded protein response. Proc Natl Acad Sci U S A. 2000;97:12625–12630. doi: 10.1073/pnas.220247197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- Clem RJ, Sheu TT, Richter BW, He WW, Thornberry NA, Duckett CS, et al. c-IAP1 is cleaved by caspases to produce a proapoptotic C-terminal fragment. J Biol Chem. 2001;276:7602–7608. doi: 10.1074/jbc.M010259200. [DOI] [PubMed] [Google Scholar]
- Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73:1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- Crook NE, Clem RJ, Miller LK. An apoptosis-inhibiting baculovirus gene with a zinc finger-like motif. J Virol. 1993;67:2168–2174. doi: 10.1128/jvi.67.4.2168-2174.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA. Nrf2 Is a Direct PERK Substrate and Effector of PERK-Dependent Cell Survival. Mol Cell Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan HC, Sun M, Kaneko S, Feldman RI, Nicosia SV, Wang HG, et al. Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP) J Biol Chem. 2004;279:5405–5412. doi: 10.1074/jbc.M312044200. [DOI] [PubMed] [Google Scholar]
- Debatin KM, Krammer PH. Death receptors in chemotherapy and cancer. Oncogene. 2004;23:2950–2966. doi: 10.1038/sj.onc.1207558. [DOI] [PubMed] [Google Scholar]
- Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, et al. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol. 2004;24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniaud A, Sharaf el dein O, Maillier E, Poncet D, Kroemer G, Lemaire C, et al. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27:285–299. doi: 10.1038/sj.onc.1210638. [DOI] [PubMed] [Google Scholar]
- Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–304. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- Di Sano F, Ferraro E, Tufi R, Achsel T, Piacentini M, Cecconi F. Endoplasmic reticulum stress induces apoptosis by an apoptosome-dependent but caspase 12-independent mechanism. J Biol Chem. 2006;281:2693–2700. doi: 10.1074/jbc.M509110200. [DOI] [PubMed] [Google Scholar]
- Eckelman BP, Salvesen GS. The human anti-apoptotic proteins cIAP1 and cIAP2 bind but do not inhibit caspases. J Biol Chem. 2006;281:3254–3260. doi: 10.1074/jbc.M510863200. [DOI] [PubMed] [Google Scholar]
- Fels DR, Koumenis C. The PERK/eIF2alpha/ATF4 module of the UPR in hypoxia resistance and tumor growth. Cancer Biol Ther. 2006;5:723–728. doi: 10.4161/cbt.5.7.2967. [DOI] [PubMed] [Google Scholar]
- Fernandez J, Bode B, Koromilas A, Diehl JA, Krukovets I, Snider MD, et al. Translation mediated by the internal ribosome entry site of the cat-1 mRNA is regulated by glucose availability in a PERK kinase-dependent manner. J Biol Chem. 2002a;277:11780–11787. doi: 10.1074/jbc.M110778200. [DOI] [PubMed] [Google Scholar]
- Fernandez J, Yaman I, Sarnow P, Snider MD, Hatzoglou M. Regulation of internal ribosomal entry site-mediated translation by phosphorylation of the translation initiation factor eIF2alpha. J Biol Chem. 2002b;277:19198–19205. doi: 10.1074/jbc.M201052200. [DOI] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000a;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell. 2000b;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- Haze K, Yoshida H, Yanagi H, Yura T, Mori K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell. 1999;10:3787–3799. doi: 10.1091/mbc.10.11.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, et al. Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J Cell Biol. 2004;165:347–356. doi: 10.1083/jcb.200310015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi T, Hyoda K, Okuma Y, Nomura Y, Ozawa K. Akt up- and down-regulation in response to endoplasmic reticulum stress. Brain Res. 2007;1152:27–31. doi: 10.1016/j.brainres.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Hu P, Han Z, Couvillon AD, Exton JH. Critical role of endogenous Akt/IAPs and MEK1/ERK pathways in counteracting endoplasmic reticulum stress-induced cell death. J Biol Chem. 2004;279:49420–49429. doi: 10.1074/jbc.M407700200. [DOI] [PubMed] [Google Scholar]
- Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH. Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol. 2006;26:3071–3084. doi: 10.1128/MCB.26.8.3071-3084.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamora C, Dennert G, Lee AS. Inhibition of tumor progression by suppression of stress protein GRP78/BiP induction in fibrosarcoma B/C10ME. Proc Natl Acad Sci U S A. 1996;93:7690–7694. doi: 10.1073/pnas.93.15.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X, Kong J, Liebhaber SA. In vivo association of the stability control protein alphaCP with actively translating mRNAs. Mol Cell Biol. 2003;23:899–907. doi: 10.1128/MCB.23.3.899-907.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HY, Wek SA, McGrath BC, Scheuner D, Kaufman RJ, Cavener DR, et al. Phosphorylation of the alpha subunit of eukaryotic initiation factor 2 is required for activation of NF-kappaB in response to diverse cellular stresses. Mol Cell Biol. 2003;23:5651–5663. doi: 10.1128/MCB.23.16.5651-5663.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama T, Imaizumi K, Manabe T, Hitomi J, Kudo T, Tohyama M. Induction of neuronal death by ER stress in Alzheimer's disease. J Chem Neuroanat. 2004;28:67–78. doi: 10.1016/j.jchemneu.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Kaufman RJ. Regulation of mRNA translation by protein folding in the endoplasmic reticulum. Trends Biochem Sci. 2004;29:152–158. doi: 10.1016/j.tibs.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Kazemi S, Mounir Z, Baltzis D, Raven JF, Wang S, Krishnamoorthy JL, et al. A novel function of eIF2alpha kinases as inducers of the phosphoinositide-3 kinase signaling pathway. Mol Biol Cell. 2007;18:3635–3644. doi: 10.1091/mbc.E07-01-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lee B, Lee AS. Endoplasmic reticulum stress-induced apoptosis: multiple pathways and activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53. J Biol Chem. 2006;281:7260–7270. doi: 10.1074/jbc.M509868200. [DOI] [PubMed] [Google Scholar]
- Liston P, Fong WG, Korneluk RG. The inhibitors of apoptosis: there is more to life than Bcl2. Oncogene. 2003;22:8568–8580. doi: 10.1038/sj.onc.1207101. [DOI] [PubMed] [Google Scholar]
- Mathai JP, Germain M, Shore GC. BH3-only BIK regulates BAX,BAK-dependent release of Ca2+ from endoplasmic reticulum stores and mitochondrial apoptosis during stress-induced cell death. J Biol Chem. 2005;280:23829–23836. doi: 10.1074/jbc.M500800200. [DOI] [PubMed] [Google Scholar]
- Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y. An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem. 2002;277:34287–34294. doi: 10.1074/jbc.M204973200. [DOI] [PubMed] [Google Scholar]
- Morishima N, Nakanishi K, Tsuchiya K, Shibata T, Seiwa E. Translocation of Bim to the endoplasmic reticulum (ER) mediates ER stress signaling for activation of caspase-12 during ER stress-induced apoptosis. J Biol Chem. 2004;279:50375–50381. doi: 10.1074/jbc.M408493200. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- Orth K, Dixit VM. Bik and Bak induce apoptosis downstream of CrmA but upstream of inhibitor of apoptosis. J Biol Chem. 1997;272:8841–8844. doi: 10.1074/jbc.272.14.8841. [DOI] [PubMed] [Google Scholar]
- Petersen SL, Wang L, Yalcin-Chin A, Li L, Peyton M, Minna J, et al. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 2007;12:445–456. doi: 10.1016/j.ccr.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthalakath H, O'Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, et al. ER stress triggers apoptosis by activating BH3-only protein Bim. Cell. 2007;129:1337–1349. doi: 10.1016/j.cell.2007.04.027. [DOI] [PubMed] [Google Scholar]
- Rao RV, Castro-Obregon S, Frankowski H, Schuler M, Stoka V, del Rio G, et al. Coupling endoplasmic reticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway. J Biol Chem. 2002;277:21836–21842. doi: 10.1074/jbc.M202726200. [DOI] [PubMed] [Google Scholar]
- Reimertz C, Kogel D, Rami A, Chittenden T, Prehn JH. Gene expression during ER stress-induced apoptosis in neurons: induction of the BH3-only protein Bbc3/PUMA and activation of the mitochondrial apoptosis pathway. J Cell Biol. 2003;162:587–597. doi: 10.1083/jcb.200305149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Ramirez L, Cao H, Nelson D, Hammond E, Lee AH, Yoshida H, et al. XBP1 is essential for survival under hypoxic conditions and is required for tumor growth. Cancer Res. 2004;64:5943–5947. doi: 10.1158/0008-5472.CAN-04-1606. [DOI] [PubMed] [Google Scholar]
- Roy N, Deveraux QL, Takahashi R, Salvesen GS, Reed JC. The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. Embo J. 1997;16:6914–6925. doi: 10.1093/emboj/16.23.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen GS, Duckett CS. IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol. 2002;3:401–410. doi: 10.1038/nrm830. [DOI] [PubMed] [Google Scholar]
- Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- Scott FL, Denault JB, Riedl SJ, Shin H, Renatus M, Salvesen GS. XIAP inhibits caspase-3 and -7 using two binding sites: evolutionarily conserved mechanism of IAPs. Embo J. 2005;24:645–655. doi: 10.1038/sj.emboj.7600544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Vattem KM, Sood R, An J, Liang J, Stramm L, et al. Identification and characterization of pancreatic eukaryotic initiation factor 2 alpha-subunit kinase, PEK, involved in translational control. Mol Cell Biol. 1998;18:7499–7509. doi: 10.1128/mcb.18.12.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozaki EN, Chai J, Rigotti DJ, Riedl SJ, Li P, Srinivasula SM, et al. Mechanism of XIAP-mediated inhibition of caspase-9. Mol Cell. 2003;11:519–527. doi: 10.1016/s1097-2765(03)00054-6. [DOI] [PubMed] [Google Scholar]
- Shiraishi H, Okamoto H, Yoshimura A, Yoshida H. ER stress-induced apoptosis and caspase-12 activation occurs downstream of mitochondrial apoptosis involving Apaf-1. J Cell Sci. 2006;119:3958–3966. doi: 10.1242/jcs.03160. [DOI] [PubMed] [Google Scholar]
- Simons M, Beinroth S, Gleichmann M, Liston P, Korneluk RG, MacKenzie AE, et al. Adenovirus-mediated gene transfer of inhibitors of apoptosis protein delays apoptosis in cerebellar granule neurons. J Neurochem. 1999;72:292–301. doi: 10.1046/j.1471-4159.1999.0720292.x. [DOI] [PubMed] [Google Scholar]
- Tirasophon W, Welihinda AA, Kaufman RJ. A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev. 1998;12:1812–1824. doi: 10.1101/gad.12.12.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro GJaG H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. Journal of Cellular Biology. 1963;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci U S A. 2004;101:11269–11274. doi: 10.1073/pnas.0400541101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- Vucic D, Fairbrother WJ. The inhibitor of apoptosis proteins as therapeutic targets in cancer. Clin Cancer Res. 2007;13:5995–6000. doi: 10.1158/1078-0432.CCR-07-0729. [DOI] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998a;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- Wang XZ, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, Ron D. Cloning of mammalian Ire1 reveals diversity in the ER stress responses. Embo J. 1998b;17:5708–5717. doi: 10.1093/emboj/17.19.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnakulasuriyarachchi D, Cerquozzi S, Cheung HH, Holcik M. Translational induction of the inhibitor of apoptosis protein HIAP2 during endoplasmic reticulum stress attenuates cell death and is mediated via an inducible internal ribosome entry site element. J Biol Chem. 2004;279:17148–17157. doi: 10.1074/jbc.M308737200. [DOI] [PubMed] [Google Scholar]