History and physical exam
A 57-year-old woman with primary antiphospholipid syndrome (APS) presented with acute onset fever, abdominal pain, and hypotension.
Her APS-related past medical history consisted of a cerebrovascular accident 14 years prior to admission. Two years prior to admission, she underwent renal transplant for hypertensive renal disease. She suffered transplant rejection within days due to renal thrombotic microangiopathy. The patient was subsequently maintained on peritoneal dialysis as well as warfarin. The patient had a persistently positive lupus anticoagulant (LA) test and high titer anticardiolipin antibody (aCL) IgG (>80 U). Past medical history also included gastrointestinal bleeding from a duodenal ulcer, hypertension, diabetes mellitus type II, and heparin-induced thrombocytopenia.
Two months prior to admission, the patient was hospitalized after becoming comatose. This was ultimately attributed to West Nile virus infection. Her course was complicated by deep-vein thrombosis, bilateral subdural hematomas, and bilateral adrenal infarctions. She was discharged to an acute rehabilitation facility but was readmitted with vancomycin-resistant enterococcus (VRE) bacteremia, methicillin-resistant Staphylococcus aureus peritonitis, and endocarditis with an aortic valve vegetation. She was briefly discharged to an acute rehabilitation center on antibiotics but then came in for the current admission with fever, abdominal pain, and altered mental status.
The patient had no alopecia, mucosal ulcers, rashes, chest pain, palpitations, orthopnea, paroxysmal nocturnal dyspnea, shortness of breath, cough, diarrhea, constipation, dysuria, or hematuria. She had no known drug allergies, and her family history was noncontributory. She lived in Brooklyn with her husband and was a retired real estate agent. She denied any smoking, alcohol, or illicit drug use. Her medications on admission included linezolid 600 mg by mouth (PO) twice daily (for VRE bacteremia), hydrocortisone 25 mg PO twice daily, Epogen 10,000 units subcutaneously weekly, magnesium oxide 400 mg PO twice daily, and warfarin 5 mg PO daily.
Significant physical examination findings were temperature of 39.9°C, blood pressure of 90/48 to 60/40 mmHg, heart rate of 106–116 bpm, and respiration rate of 20 breaths per minute. Oxygen saturation was 80% on room air. She was responsive only to pain, had a grade II systolic ejection murmur at the apex, distended and tender abdomen with hypoactive bowel sounds, and clean peritoneal dialysis catheter site. Her extremities were cool, mottled, and blanching with palpable radial pulses. She was intubated in the emergency department for hypotension and respiratory distress due to probable septic shock.
The patient was started on with broad-spectrum antibiotics (piperacillin/tazobactam 2.25 g intravenously (IV) every 8 h and linezolid 600 mg IV every 12 h), pressor support with phenylephrine and norepinephrine, and stress-dose corticosteroids (hydrocortisone succinate 50 mg IV every 6 h and fludrocortisone 50mcg IV daily). Initial significant laboratory showed the peritoneal fluid had white blood cell count of 6,350/μL and red blood cell count of 180/μL (Table 1). Peritoneal fluid culture was positive for Klebsiella pneumoniae; blood cultures were negative. Antibiotics were changed to meropenem 1 g IV every 12 h and amikacin 175 mg IV every 12 h. The peritoneal dialysis catheter was removed.
Table 1.
Initial laboratory tests
| Result | Normal values | |
|---|---|---|
| White blood cell | 5.5 | 4.0–10.0 × 109/μL |
| Hemoglobin | 8.7 | 12–16 g/dl |
| Hematocrit | 25.6 | 35–45% |
| Platelet | 145 | 150–350 × 10^9/μL |
| Prothrombin time | 20.9 | 11–13 s |
| International normalized ratio | 2.85 | 0.8–1.2 |
| Activated partial thromboplastin time | 58.7 | 20–35 s |
| Sodium | 137 | 135–145 mEq/l |
| Potassium | 3.4 | 3.5–5.1 mEq/l |
| Chloride | 98 | 98–106 mEq/l |
| Bicarbonate | 26 | 22–29 mEq/l |
| Basic urea nitrogen | 47 | 7–19 mg/dl |
| Creatinine | 6.4 | 0.6–1.2 mg/dl |
| Lactate dehydrogenase | 276 | 105–333 U/l |
| Lactate | 9.8 | 5.0–20.0 mg/dl |
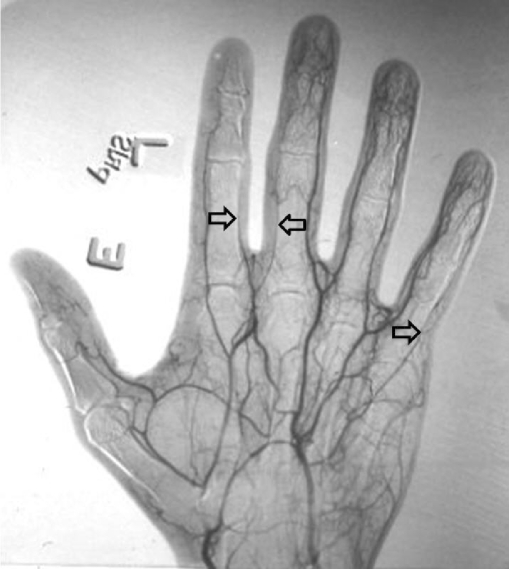
On the fourth day of admission, the patient developed nonblanching cyanosis, coolness, and swelling of the fingers of her right hand. The mottling was localized to the radial aspect of the right hand. Pulses were detected by color flow Doppler ultrasound, and computed tomography (CT) angiography of the upper extremity showed intact perfusion to the ulnar and radial branches of the hand (Fig. 1). By the fifth day of admission, the radial aspect of the right hand had become increasingly discolored, and the radial artery pulse was undetectable by Doppler studies. By the seventh day of admission, her hand appeared gangrenous, with erythema ascending to the distal third of the forearm, and she underwent hand amputation on the 12th day of admission.
Fig. 1.
Computed tomography angiography of right hand. Open arrows showing intact but diminished perfusion of distal radial and ulnar arteries, day 4 of hospitalization
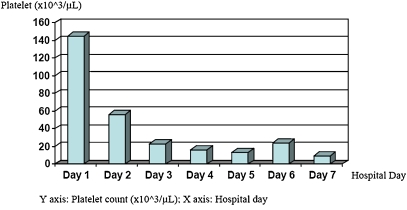
During the period leading up to her amputation, the patient also experienced worsening thrombocytopenia (Fig. 2), which was complicated by hematochezia on the sixth day. Coagulation profile showed elevated prothrombin time (24.4 s; normal range 11–15 s) and activated partial thromboplastin time (70.2 s; normal range 20–35 s), a positive d-dimer assay (870 ng/ml; normal range 0–300 ng/ml), and a normal fibrinogen level (386 mg/l; normal range 200–400 mg/dl). She was given massive platelet, blood, and fresh frozen plasma transfusions, and intravenous immunoglobulin (IVIG; 400 mg/kg daily) for 5 days and plasmapheresis. Anticoagulation, which was initially managed with argatroban, was temporarily held during the episodes of hematochezia (between days 6 and 14). She was treated with multiple rounds of therapeutic plasma exchange on days 7 to 9. The lower gastrointestinal bleeding slowly resolved by day 18, and anticoagulation with argatroban was resumed. On hospital day 27, the patient complained of decreased vision in both eyes. She was noted not to be making eye contact, and she was increasingly confused. A noncontrast head CT showed a new acute to subacute infarct in the right frontal lobe extending to the right frontal operculum. Rituximab 1,000 mg was given on hospital day 34. She subsequently developed a new right hemiplegia and complete cortical blindness. Noncontrast head CT showed bilateral evolving occipital infarcts along with the right frontal lobe infarct previously noted.
Fig. 2.
Platelet trend during hospitalization (initial 7 days)
The patient continued to receive anticoagulation with intravenous argatroban plus high-dose corticosteroids and received the second dose of the rituximab (1,000 mg) 15 days after the first dose. The platelet count stabilized, there was no further bleeding or thrombosis, and the patient was discharged to a subacute rehabilitation facility.
Pathological discussion: right hand
The amputated hand and forearm showed extensive hemorrhagic necrosis with mottling and focal sloughing of the skin and edema and pallor of the deep subcutaneous tissue and muscle.
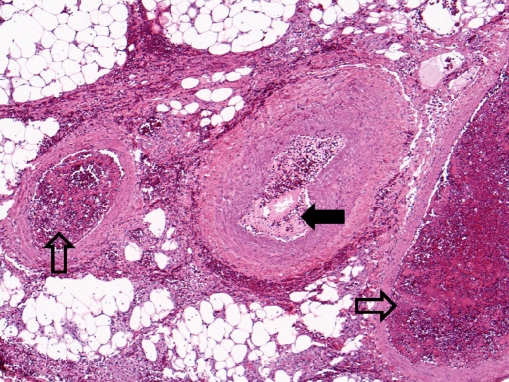
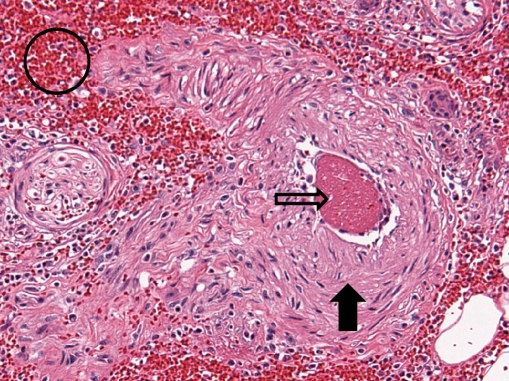
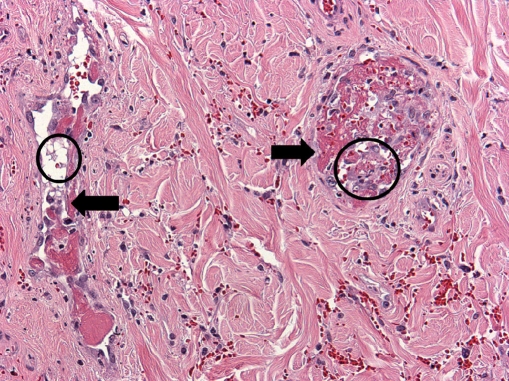
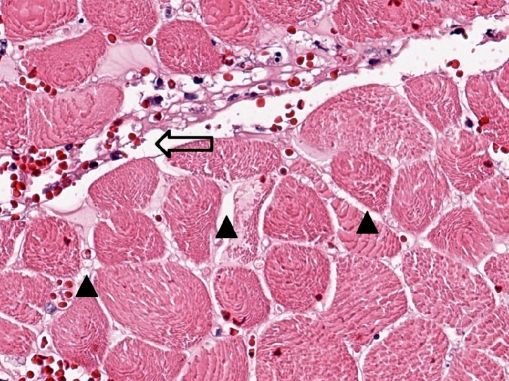
Microscopic examination showed subcutaneous bullous edema with hemorrhagic necrosis associated with intense acute inflammation that was most intense in the subcutaneous tissues. Many arteries and veins of varying sizes showed partial or complete occlusion by fibrin thrombi (Figs. 3 and 4), some of which were recanalized to varying degrees (Fig. 5). Small veins and capillary beds were dilated, and the adjacent tissues showed interstitial hemorrhage. Muscle and all other tissues sampled were edematous and at least focally necrotic, but most intramuscular vessels, including capillaries (Fig. 6), were dilated and not collapsed, although also focally occluded by fibrinous thrombi. The dilation of these vessels indicated occlusion in the venous drainage system of the affected tissues.
Fig. 3.
Amputated hand specimen in low-power photomicrograph showing thrombosis of the two veins (open arrows) accompanying a medium-sized muscular artery (closed arrow) that is patent (×4, H&E)
Fig. 4.
Amputated hand specimen in intermediate power photomicrograph of a small muscular artery (closed arrow) showing recent occlusion by a fibrin clot (open arrow) without inflammation of the vessel wall but with hematoma (open circle) in the surrounding tissue (×20, H&E)
Fig. 5.
Amputated hand specimen in intermediate-power photomicrograph of two dermal veins (arrows) showing endothelial organization and recanalization of fibrin thrombi (open circles; ×20, H&E)
Fig. 6.
Amputated hand specimen in high-power photomicrograph of necrotic skeletal muscle showing interstitial edema (arrow heads) and patency of the capillary network (open arrow; ×40, H&E)
The pathologic findings indicated fibrinous thrombotic occlusion of all types of arteries and veins to variable degrees with variable degrees of recanalization. The findings were indicative of a widespread continuing clotting disorder of subacute onset, consistent with the vasculopathy of disseminated thrombosis seen in APS.
Clinical discussion
This APS patient presented with acute abdominal pain and hypotension due to Klebsiella peritonitis and septic shock. The hospital course was complicated by the acute infarction of the right hand, acute to subacute infarction of the right frontal lobe of the brain, and later, bilateral occipital lobes, associated with thrombocytopenia, and hematochezia.
The multi-organ involvement and rapid progression of disease raises the possibility that this patient had the accelerated variant of APS, better known as catastrophic APS (CAPS). The characteristic features of CAPS are: (a) rapid onset thrombosis (usually within 1 week) resulting in multiple-organ dysfunction syndrome (MODS), (b) pathological evidence of small-vessel thrombosis, (c) evidence of systemic inflammatory response syndrome (SIRS), and (d) high risk of unusual organ involvement [1]. Based on the international consensus of the classification criteria for CAPS, this case can be classified as “probable CAPS” with thrombocytopenia (Table 2) [1]. Although the thrombotic events occurred within 3 weeks (the ischemia and necrosis of the hand was noted between days 4 and 7 of hospital stay, and change in mental status reflecting frontal lobe infarct occurred on day 28), it was clear that these events (including the deep-vein thrombosis and bilateral adrenal infarcts that had occurred 2 months prior) were all part of a continuous thrombotic process. Furthermore, the rapid and diffuse small-vessel involvement that was noted on pathological analysis of the amputated hand specimen supports the diagnosis that this patient suffered a CAPS-like phenomenon rather than classic APS, which is usually limited to single venous or arterial medium-to-large vessel occlusions.
Table 2.
International classification criteria for catastrophic antiphospholipid syndrome [1]
| 1) Evidence of involvement of three or more organs, systems, and/or tissuesa |
| 2) Development of manifestations simultaneously or in less than 1 week |
| 3) Confirmation by histopathology of small-vessel occlusion in at least one organ or tissueb |
| 4) Laboratory confirmation of the presence of aPL (LA and/or aCL and /or anti-B2 GPI antibodies)c |
| Definite CAPS |
| →All 4 criteria |
| Probable CAPS |
| → All 4 criteria, except for involvement of only 2 organs, systems, and/or tissues |
| → All 4 criteria, except for the absence of laboratory confirmation at least 6 weeks apart attributable to the early death of a patient never tested for aPL before CAPS |
| → 1, 2, and 4 |
| → 1, 3, and 4 and the development of a third event in more than 1 week but less than 1 month, despite anticoagulation treatment |
aUsually, clinical evidence of vessel occlusions, confirmed by imaging techniques. Renal involvement is defined by 50% increase in serum creatinine, severe systemic hypertension (>180/100 mmHg), and/or proteinuria (>500 mg/24 h)
bFor histopathological confirmation, significant evidence of thrombosis must be present, although vasculitis may coexist occasionally
cIf the patient had not been previously diagnosed as having APS, the laboratory confirmation requires that presence of aPL must be detected on two or more occasions at least 6 weeks apart (not necessarily at the time of the event)
A “trigger” factor often plays an important role in the development of thrombosis in antiphospholipid antibody (aPL)-positive patients [2]. A cohort analysis of 250 patients in the international web-based CAPS registry [25] identified numerous precipitating factors for CAPS, including infection (22%), trauma (14%), anticoagulation withdrawal (7%), malignancies (7%), obstetric complications (6%), and interestingly, 40% without any identifiable triggers [3]. Given that a significant portion of CAPS patients has identifiable triggers, the accepted model of CAPS is the “double- or triple-hit hypothesis”. That is, thrombosis occurs in aPL-positive patients when other non-aPL prothrombotic risk factors also exist [4, 5]. Interestingly, in addition to having aPL, hypertension, diabetes, and major infections, this patient also had a history of heparin-induced thrombocytopenia (HIT). The presence of anti-heparin platelet factor 4 (anti-HPF4) may represent an additional prothrombotic risk factor for APS. Alpert et al. demonstrated an association between anti-HPF4 and APS, in particular with increased incidence of arterial thrombosis, in systemic lupus erythematosus patients who are both anti-HPF4 and aPL-positive [6].
Thrombocytopenia compounded the intravascular thrombotic complications in this patient. The patient’s platelet count decreased from 145,000/μL to as low as 6/μL (Fig. 2) over 7 days, during which time she developed a lower gastrointestinal bleed and a necrotic right hand. The thrombocytopenia was likely a reflection of widespread thrombosis due to CAPS (platelet consumption), sepsis (with disseminated intravascular coagulation (DIC)), or both. The differential diagnosis also included an antibiotic side effect or a self-perpetuating platelet consumption process in the necrotic hand.
The prevalence of thrombocytopenia is 20% in APS and 60% in CAPS [7]; thrombocytopenia in aPL-positive patients does not protect against thrombosis and can be associated with more severe disease and an increased risk of APS-related events. The severe thrombocytopenia in CAPS is in contrast to the milder thrombocytopenia (50,000/μL to 140,000/μL) typically seen in typical APS [7].
Thrombosis, thrombocytopenia, coagulopathy, and hemorrhage can have multiple causes. It is often difficult to distinguish among severe sepsis, disseminated intravascular coagulation, and CAPS in a given patient [8]. Features of DIC, such as thromboembolism and bleeding, resulting in MODS can be present in about one fifth of CAPS patients [9]. Certain laboratory data may help distinguish between the two syndromes; in CAPS, the prothrombin time is not usually elevated, and fibrinogen is not decreased as they are in DIC, and persistently and significantly elevated antiphospholipid antibody (aPL) levels strongly support a CAPS-like picture [5].
This patient experienced hematochezia on the sixth day of admission, in the setting of full anticoagulation and worsening thrombocytopenia (Fig. 2). Anticoagulation had to be held for 9 days. Bleeding in the midst of CAPS is a disaster of the highest order; thrombotic risks increase when anticoagulation is held, as well as when fresh frozen plasma and platelets are given.
Early diagnosis and aggressive treatment with first-line therapies of anticoagulation and high-dose corticosteroids are essential in the management of CAPS. The classification of “definite CAPS” or “probable CAPS” should play no role in the management of CAPS patients since the purpose of the “probable CAPS” definition is simply to allow initiation of treatment as soon as the CAPS diagnosis is suspected. The amputation of this patient’s necrotic right hand was indicated to remove a site of refractory thrombosis and a potential source of the patient’s worsening thrombocytopenia. CAPS patients have been reported to improve after the amputation of an infarcted extremity [10].
Because this patient had a history of HIT, she was treated with argatroban, a direct thrombin inhibitor, rather than heparin, although there is no published data on the use of argatroban in aPL-positive patients. This patient also required other second-line therapies for CAPS including IVIG and plasmapheresis and received the third-line agent, rituximab, because of ongoing thrombosis, severe thrombocytopenia, and a lack of clinical response to the previous interventions. Of note, there are no data demonstrating superiority of plasma exchange over IVIG, or vice versa, in the treatment of CAPS [11]. However, IVIG has been shown to effect short-term neutralization of pathological autoantibodies, as well as long-term suppression of aCL titers by inactivating idiotype-bearing B cell clones [12]. IVIG may also be beneficial for its antibacterial and antiviral effects, especially in CAPS patients who have an identifiable infectious trigger [13]. Uthman et al. compiled a review of studies that have shown the beneficial effects of plasma exchange through the removal of pathogenic IgG aCL and anti-β2-glycoprotein-I [aβ2-GPI] antibodies, complement factors, and inflammatory cytokines including interleukin (IL)-1, IL-6, and tumor necrosis factor-α (TNF-α) [14]. Rituximab has been successfully used in patients with CAPS and has resulted in rapid improvement of severe thrombocytopenia in some cases [15, 16]. However, there are only limited numbers of anecdotal reports on the use of rituximab in patients with primary, secondary, and catastrophic APS.
Immunologic discussion
Antiphospholipid syndrome is characterized by vascular thromboses and/or pregnancy morbidity with aPL (positive LA test, aCL, and/or aβ2-GPI antibodies) [17]. This family of autoantibodies is directed against plasma proteins that bind to negatively charged membrane phospholipids. Proposed mechanisms for aPL-mediated thrombosis include: (a) aPL-induced endothelial cell and monocyte activation resulting in the expression of adhesion molecules, (b) aPL-induced platelet activation by binding to phospholipid-bound annexins on the platelet membranes resulting in adhesion and thrombosis [18], (c) aPL interferance with the protein C and S pathway and inhibition of protein C activation (thereby creating an acquired activated protein C resistance) and the tissue factor pathway inhibitor by impairing phospholipid-mediated auto-activation of factor XII [5], and (d) aPL-induced increase in tissue factor (a physiologic trigger for coagulation) expression on monocytes [5, 19].
The mechanism of aPL-related thrombocytopenia also remains unclear; one of the proposed mechanisms is immune-mediated antibodies against glycoprotein IIb/IIIa complex and/or glycoprotein Ib/IX [7]. Godeau et al. showed a high prevalence of platelet-specific glycoprotein antibodies (gpIIb/IIIa, gpIa/IIa, gpIbIX, gpIV, and CD9) in 73% (11/15) of APS patients with thrombocytopenia compared to only 10% (1/10) of patients in the control group (p < 0.01) [20]. Antibodies other than aPL, such as specific antiplatelet glycoprotein autoantibodies, may also be responsible for the thrombocytopenia seen in APS patients, though they do not completely exclude the possibility of aPL participating in the pathogenesis of platelet dysfunction. In CAPS patients, thrombocytopenia is most likely multifactorial.
The exact pathophysiology of CAPS is also to be determined; in addition to the potential mechanisms of aPL-induced thrombosis discussed above, proposed mechanisms for CAPS suggest that thrombotic events are primarily responsible for propagating ongoing thrombosis. Similar to DIC, the initial thrombosis may act as a nidus for perpetuating further thrombosis, in a process which Kitchens has termed “thrombotic storm” during which fibrinolysis decreases via increased tissue plasminogen activator inhibitor type-1 levels [21, 22]. Progressive microthromboses occur as coagulation activation products increase in the circulation, and consumption of natural anticoagulant proteins, such as protein C, protein S, and antithrombin III, is accelerated [21]. Furthermore, massive cytokine release from an infectious etiology and/or necrotic tissues in CAPS patients contributes to SIRS, which can lead to septic shock and further disruption of the coagulation cascade.
Finally, CAPS resembles severe sepsis, with features of microvascular thrombosis, multi-organ dysfunction, and vascular dysfunction, and infection is the most common known trigger of CAPS [23]. Both CAPS and sepsis can result in SIRS, which is defined as an inflammatory reaction to injury, infection, or other stressors, and diagnosed when ≥2 or the following exist: temperature >38°C or <36°C; heart rate >90 bpm; respiratory rate >20 breaths per minute or PaCO2 <32 mmHg; white blood cell count >12,000 cells/mm3, or >10% immature (band) forms [23]. In severe sepsis, there is endotoxin-induced endothelial cell injury that results in vascular dysfunction and a pro-coagulant state. Mediators involved in sepsis include TNF-α, IL-1, IL-12, IF-8, macrophage inflammatory protein, and lipid mediators such as prostaglandins and leukotrienes. Similarly, in CAPS, aPL-induced activation of endothelium results in widespread inflammation and elevated TNF-α, IL-I, and IL-6 levels [24]. Ultimately, microvascular thrombosis occurs in both CAPS and sepsis due to disturbance of coagulation and fibinolysis factors.
Conclusion
This case provides numerous important perspectives on CAPS, including both thrombotic and nonthrombotic (thrombocytopenia or bleeding) complications. Although CAPS patients suffer significant morbidity and mortality due to multiple-organ thromboses, nonthrombotic complications also factor into prognostic considerations. Further understanding of the pathogenesis of CAPS and its similarities to sepsis will aid in the development of targeted therapies to decrease the overwhelming devastating complications and mortality rate. Early recognition and aggressive treatment of CAPS is also critical to improving patient outcomes.
Acknowledgments
The authors would like to thank Dr. Maria DeSancho and Dr. Daluiski for their presentations and discussions during the clinical pathology conference.
Footnotes
A Clinical Pathology Conference held by the Department of Rheumatology at Hospital for Special Surgery
References
- 1.Asherson RA, Cervera R, de Groot PG et al (2003) Catastrophic Antiphospholipid Syndrome Registry Project Group. Catastrosphic antiphospholipid syndrome: international consensus statement on classification criteria and treatment guidelines. Lupus 12:530–534 [DOI] [PubMed]
- 2.Rand JH (2002) Molecular pathogenesis of antiphospholipid syndrome. Circ Res 90:29–37 [DOI] [PubMed]
- 3.Cervera R, Asherson RA (2006) Catastrophic antiphospholipid syndrome. J Pathophy Haem Thrombosis 181–186 [DOI] [PubMed]
- 4.Erkan D, Yazici Y, Peterson MG et al (2002) A cross-sectional study of clinical thrombotic risk factors and preventive treatments in antiphospholipid syndrome. Rheumatology 41:924–929 [DOI] [PubMed]
- 5.Vero SK, Asherson RA, Erkan D (2006) Catastrophic antiphospholipid syndrome. J Intensive Care Med 21:144–159 [DOI] [PubMed]
- 6.Alpert D, Mandl LA, Erkan D et al (2008) Anti-heparin platelet factor 4 antibodies in systemic lupus erythaematosus are associated with IgM antiphospholipid antibodies and the antiphospholipid syndrome. Ann Rheum Dis 67:395–401 [DOI] [PubMed]
- 7.Galli M, Finazzi G, Barbui T (1996) Thrombocytopenia in the antiphospholipid syndrome. Brit J Haem 93:1–5 [DOI] [PubMed]
- 8.Erkan D, Cervera R, Asherson RA (2003) Catastrophic antiphospholipid syndrome: where do we stand? Arthritis Rheum 48(12):3320–3327 [DOI] [PubMed]
- 9.Cervera R, Font J, Gomez-Puerta JA et al (2005) Validation of the preliminary criteria for the classification of catastrophic antiphospholipid syndrome. Ann Rheum Dis 64:1205–1209 [DOI] [PMC free article] [PubMed]
- 10.Amital H, Levy Y, Davidson C et al (2001) Catastrophic antiphospholipid syndrome: remission following leg amputation in 2 cases. Semin Arthritis Rheum 31(2):127–132 [DOI] [PubMed]
- 11.Erkan D, Asherson RA, Cervera R et al (2004) Plasma exchange or intravenous immunoglobulins in catastrophic antiphospholipid syndrome. In: 7th International Lupus Congress Abstract Book. New York: 15; 265–268
- 12.Sherer Y, Levy Y, Shoenfeld Y (2000) Intravenous immunoglobulin therapy of antiphospholipid syndrome. Rheumatology 39:421–426 [DOI] [PubMed]
- 13.Bayry J, Lacroix-Desmazes S, Kazatchkine MD et al (2004) Intravenous immunoglobulin for infectious disease: back to the pre-antibiotic and passive prophylaxis era? Trends Pharmacol Sci 25:306–310 [DOI] [PMC free article] [PubMed]
- 14.Uthman I, Shamseddine A, Taher A (2005) The role of therapeutic plasma exchange in the catastrophic antiphospholipid syndrome. Transfus Apher Sci 33:11–17 [DOI] [PubMed]
- 15.Ehrsemann S, Arkfeld D, Shinada S et al (2004) A novel therapeutic approach for catastrophic antiphospholipid syndrome when conventional therapy with anticoagulants and steroids were unsuccessful [abstract]. Ann Rheum Dis 64(suppl):FR10278
- 16.Erre GL, Pardini S, Faedda R et al (2008) Effect of riuximab on clinical and laboratory features of antiphospholipid syndrome: a case report and a review of literature. Lupus 17:50–55 [DOI] [PubMed]
- 17.Miyakis S, Lockshin MD, Atsumi T et al (2006) International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 4(2):295–306 [DOI] [PubMed]
- 18.Pierangeli S, Chen P, Gonzalez E (2006) Antiphospholipid antibodies and the antiphospholipid syndrome: an update on treatment and pathogenic mechanisms. Curr Opin Hematol 13:366–375 [DOI] [PubMed]
- 19.Wolberg AS, Roubey RA (2004) Mechanisms of autoantibody-induced monocyte tissue factor expression. Thromb Res 114:391–396 [DOI] [PubMed]
- 20.Godeau B, Piette J, Fromont P et al (1997) Specific antiplatelet glycoprotein autoantibodies are associated with the thrombocytopenia of primary antiphospholipid syndrome. Brit J of Haem 98:873–879 [DOI] [PubMed]
- 21.Kitchens CS (1998) Thrombotic storm: when thrombosis begets thrombosis. Am J Med 104:381–385 [DOI] [PubMed]
- 22.Espinosa G, Cervera R, Asherson R (2007) Catastrophic antiphospholipid syndrome and sepsis: a common link? JRheum 34:923 [PubMed]
- 23.Erkan D (2006) Therapeutic and prognostic considerations in catastrophic antiphospholipid syndrome. Autoimmun Rev 6:98–103 [DOI] [PubMed]
- 24.Burcoglu-O’Ral A, Erkan D, Asherson RA (2002) Treatment of catastrophic antiphospholipid syndrome with defibrotide, a proposed vascular endothelial cell modulator. J Rheumatol 29:2006–2011 [PubMed]
- 25.Registry of the “European forum on antiphospholipid antibodies” for patients with the “catastrophic” antiphospholipid syndrome. http://www.med.ub.es/MIMMUN/FORUM/CAPS.HTM (accessed on August 8th, 2008)