Abstract
Bullous pemphigoid (BP) is a cutaneous autoimmune inflammatory disease associated with subepidermal blistering and autoantibodies against BP180, a transmembrane collagen and major component of the hemidesmosome. Numerous inflammatory cells infiltrate the upper dermis in BP. IgG autoantibodies in BP fix complement and target multiple BP180 epitopes that are highly clustered within a non-collagen linker domain, termed NC16A. Anti-BP180 antibodies induce BP in mice. In this study, we generated a humanized mouse strain, in which the murine BP180NC14A is replaced with the homologous human BP180NC16A epitope cluster region. We show that the humanized NC16A (NC16A+/+) mice injected with anti-BP180NC16A autoantibodies develop BP-like subepidermal blisters. The F(ab′)2 fragments of pathogenic IgG fail to activate complement cascade and are no longer pathogenic. The NC16A+/+ mice pretreated with mast cell activation blocker or depleting of complement or neutrophils become resistant to BP. These findings suggest that the humoral response in BP critically depends on innate immune system players.
Keywords: autoantibodies, basement membrane, humanized animal model, innate immunity, skin
1. Introduction
Bullous pemphigoid (BP) is a cutaneous autoimmune disease characterized by subepidermal blisters, a dermal inflammatory infiltrate, and in vivo deposition of autoantibodies and complement components along the basement membrane zone [1–3] BP autoantibodies avidly fix complement in vitro [4–7] These autoantibodies target two hemidesmosomal components: BP230 (also termed BPAG1), a member of the plakin protein family, and BP180 (also termed BPAG2, type XVII collagen) [3, 8–15] BP180 is a transmembrane homo-trimeric glycoprotein with a subunit MW of 180 kDa [3, 16, 17] Its C-terminal ectodomain consists of a long collagenous stretch interrupted and flanked by 16 non-collagen sequences [9] The membrane-proximal non-collagen linker domain (termed NC16A) harbors multiple epitopes recognized by BP autoantibodies [18, 19] Although the human BP180 shares high overall homology with the murine BP180, the NC16A domain is very poorly conserved in the murine protein (termed NC14A), resulting in a lack of immune-reactivity cross these two species [20]
A variety of cellular lineages have been identified in these inflammatory infiltrates, including eosinophils, neutrophils, lymphocytes, mast cells, and monocyte/macrophages [3, 21–24] Mast cells found in BP lesions exhibit morphological changes suggesting degranulation [24, 25] Lesional skin in BP patients exhibits several granular proteins derived from leukocytes, such as eosinophil cationic protein, eosinophil major basic protein, and neutrophil-derived myeloperoxidase [26] Various inflammatory mediators that can activate mast cells or leukocytes have been identified in lesional skin and/or blister fluids of BP patients, including C5a, eosinophilic/neutrophilic chemotactic factors, histamine, leukotrienes, and various cytokines (e.g. interleukin-1, -2, -5, -6, -8, tumor necrosis factors and interferon-γ) [26–33] Several proteinases are also found in BP blister fluid, including plasmin, collagenase, elastase, and 92-kD gelatinase [34–37]
The pathogenicity of anti-BP180 antibodies was initially demonstrated by IgG passive transfer experiment, in which rabbit antibodies against the mBP180NC14A domain, when injected into neonatal mice, induced a blistering skin phenotype that closely resembled human BP at the clinical, histological and immunopathological levels [20] More recently, Nishie et al showed that anti-BP180 autoantibodies from BP patients also induced BP in the humanized BP180 mice [38] Clinically, serum levels of BP autoantibodies to NC16A are correlated with the severity of disease [39–41] Thus, there is no doubt that anti-BP180 antibodies are able to induce BP. But, whether pathogenic anti-BP180 autoantibodies act alone or in combination with key innate immune system players to drive the disease remains unresolved.
Our present approach to this important question involved generating a humanized mouse strain in which the murine genomic region encoding the BP180NC14A domain was replaced with the homologous human BP180NC16A sequence. When injected into NC16A+/+ transgenic mice, affinity-purified anti-BP180NC16A autoantibodies from the sera of BP patients induced a BP-like disease. We then determined whether anti-BP180NC16A autoantibody-caused tissue damage at the BMZ requires complement, mast cells and neutrophils.
2. Materials and Methods
2.1. Patents, sera, and antibody purification
Serum samples were collected from three patients with active BP (BP1, BP2, and BP3). Rabbit anti-hBP180NC16A (R594) and rabbit anti-mBP180NC14A (R530) antibodies were generated by our laboratories as described previously [20] Briefly, New Zealand White rabbits were immunized with the GST-hBP180NC16A or GST-mNC14A fusion protein. IgG fractions from sera of the immunized rabbits were purified using a protein G Sepharose column (Sigma). BP180NC16A-specific autoantibodies from BP patients were purified using a BP180NC16A-glutathione Sepharose column. F(ab′)2 fragments of anti-BP180NC16A autoantibodies were generated by the pepsin digestion method [42]
2.2. Mice
To generate the humanized BP180NC16A mice (NC16A+/+), the murine BP180 genomic segment encoding the NC14A domain (exons 17 through 18) were replaced by the human NC16A genomic sequence (exons 18 through 19; see Figure 1). The targeting vector was electroporated into 129/Ola mouse ES cells. After the neo gene was excised from the targeted locus by Flp treatment, neo-minus cells were microinjected into C57BL/6J mouse blastocysts, which were then implanted into pseudopregnant recipients. High-chimera males were mated with B6 females to produce F1 heterozygotes (NC16A+/−). Crossing F1+/− produced F2 homozygotes expressing only mhBP180NC16A hybrid antigen (NC16A+/+). C57BL/6J mice were obtained from Jackson Laboratories. NC16A+/+ and C57BL/6J breeders were hosted at the University of North Carolina-Chapel Hill Animal Facility. Neonatal mice of NC16A+/+ and their littermates and C57BL/6J (24–48 h old with body weight between 1.4–1.6 g) were used for antibody passive transfer experiments. Animal care and animal experiments were in accordance with the Animal Care Committee at the University of North Carolina-Chapel Hill.
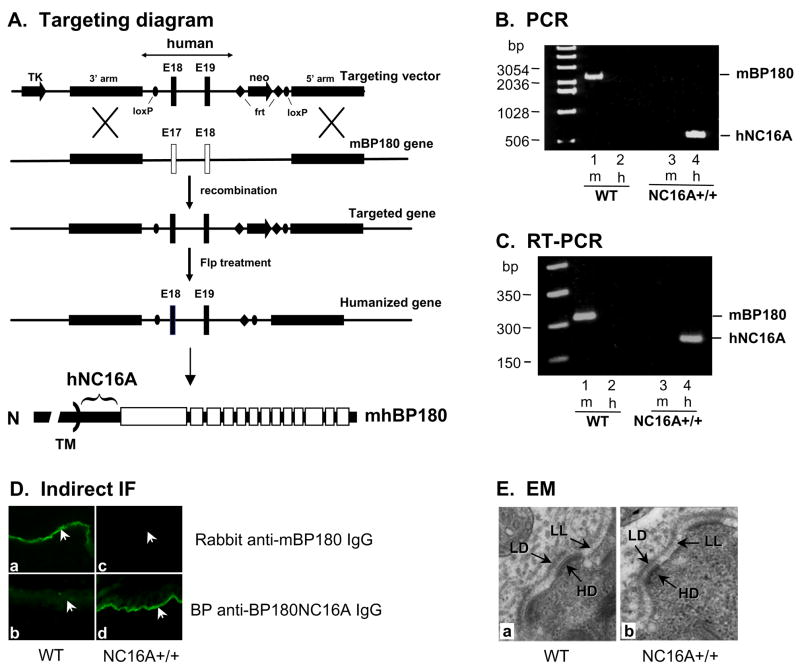
Figure 1. Generation of humanized BP180NC16A mice.
A The murine genomic segment encoding BP180 NC14A domain (exons 17 through 18) was replaced by the human NC16A genomic region (exons 18 through 19; black bars). TM, transmembrane. B. Tail DNA from wild-type (WT) and NC16A+/+ mice were PCR amplified with primer pairs specific for the mouse BP180 (m) or hBP180NC16A (h) sequence. A 2198-bp band corresponding to the mBP180 segment was obtained from WT (lane 1) but not in NC16A+/+ mice (Lane 3). A 571-bp stretch that contains only the human BP180 NC16A sequence was obtained from NC16A+/+ (lane 4) but not WT mice (Lane 2). C. Total RNA from the tails of WT and NC16A+/+ was assayed by RT-PCR with primer pairs specific for mouse BP180 or hBP180NC16A. A 331-bp band corresponding to mBP180 cDNA sequence was obtained from WT (lane 1) but not NC16A+/+ mice (lane 3), whereas a 191-bp band corresponding to hBP180NC16A cDNA was obtained from NC16A+/+ (lane 4) but not WT mice (Lane 2). D. Indirect IF showed that rabbit anti-mBP180NC14A stained the BMZ of WT (a) but not NC16A+/+ mouse skin (c), while anti-hBP180NC16A autoantibodies (BP1) stained the basement membrane zone of NC16A+/+ (d) but not WT mice (b). Arrow, basal keratinocytes. 100x magnification. E. Electron microscopic examination of WT (a) and NC16A+/+ (b) mouse skin showed no differences in the structure and distribution of hemidesmosomes. HD, hemidesmosome; LD, lamina densa; LL, lamina lucida. 30,000x magnification.
2.3 Antibody passive transfer and animal evaluation
A 50 μl aliquot of sterile IgG (control IgG, BP IgG, R594, or R530) in PBS was administered to neonatal mice by i.d. or i.p. injection [20] The mouse skin was examined 24 h after injection. The extent of cutaneous disease was scored as follows: “(−)”, no detectable skin disease; “1+”, mild erythematous reaction with no evidence of the “epidermal detachment” sign; “2+”, intense erythema and “epidermal detachment” sign involving 10–50% of the epidermis in localized areas; and “3+”, intense erythema with frank “epidermal detachment” sign involving more than 50% of the epidermis.
After clinical examination, the animals were sacrificed, and skin and serum specimens were obtained. The skin sections were used for routine histological examination by light microscopy (H/E staining) to localize the lesional site and neutrophil infiltration, and by toluidine blue staining to quantify mast cells and mast cell degranulation. Direct IF assays were done to detect deposition of human or rabbit IgG and mouse C3 at the basement membrane zone. Myeloperoxidase (MPO) enzymatic assay was performed to quantify the neutrophil accumulation at the skin injection site as described below. The sera of injected animals were tested by indirect IF techniques to determine the titers of anti-BP180 antibodies using humanized NC16A mouse skin cryosections as substrate. Direct and indirect IF studies were performed as previously described [20] using commercially available FITC-conjugated goat anti-human and goat anti-rabbit IgG (Kirkeggard & Perry Laboratories Inc.). Monospecific goat anti-mouse C3 IgG was purchased from Cappel Laboratories.
2.4. Detection of infiltrating cells and MPO assay
Infiltrating neutrophils in the antibody-injected skin were detected by indirect IF using an anti-mouse myeloperoxidase (MPO) antibody (Cell Sciences). Infiltrating eosinophils were identified by an eosinophil-specific histochemical staining [43] For quantification of neutrophil accumulation in the skin, tissue MPO activity in skin sites of the injected animals was assayed as described [44, 45], using purified MPO as standard. MPO content was expressed as relative MPO activity (OD460nm reading/mg protein of the mouse skin injected with pathogenic anti-mBP180 IgG minus OD460nm reading/mg protein of the mouse skin injected with control IgG). Protein concentrations were determined by the Bio-Rad dye-binding assay using BSA as a standard.
2.5. Mast cell degranulation
Mast cells and mast cell degranulation in skin samples were quantified by examination of toluidine blue stained sections [46, 47] Total number of mast cells was counted and classified as degranulated (>10% of the granules exhibiting fusion or discharge) or normal in five fields under a light microscope. The results were expressed as percentage of mast cells degranulating (number of degranulating mast cells/total number of mast cells/field).
2.6. In vivo inhibition of mast cell degranulation, complement depletion, and neutrophil depletion
To block mast cell degranulation, neonatal NC16A+/+ mice were injected i.d. with cromolyn sodium (Sigma, St. Louis, MO) (10 μg/g body weight), a mast cell degranulation inhibitor [46] To deplete complement, neonatal NC16A+/+ mice were pretreated with cobra venom factor (3 units/100 μl PBS, i.p.) [42] To deplete circulating neutrophils, neonatal NC16A+/+ mice were pretreated with a polyclonal rabbit anti-murine neutrophil antibody, AI-A31140, which selectively depletes mouse neutrophils in vivo (Accurate Chemical & Scientific Corp.) [45] The cromolyn-treated mice were then injected i.d with anti-BP180NC16A autoantibodies 90 min later [46] The complement- and neutrophil-depleted mice were injected i.d with anti-BP180NC16A autoantibodies 12 h later [42, 45] All mice were examined 24 h after pathogenic antibody injection.
2.7. Electron microscopic analysis of BMZ and hemidesmosome
Skin samples were dissected from the injected sites of test and control animals, and immersed in a fixative of 2% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.2. The tissue blocks were post-fixed with OsO4 and then dehydrated with a series of graded ethanol. Thin sections were contrasted with uranyl acetate and lead citrate and examined with a Hitachi-600 transmission electron microscope at 75 KV [46]
2.8. Statistical analysis
The data are expressed as mean ± SEM and were analyzed using the Student’s t-test. A p value less than 0.05 was considered significant.
3. Results
3.1. Humanized BP180NC16A mice are phenotypically normal
To directly test the hypothesis that anti-BP180NC16A autoantibodies in BP patients are pathogenic and to establish a novel animal model to study immunopathology of BP with patient materials, we created a humanized mouse strain that expresses a hybrid BP180 protein containing the immunodominant human BP180NC16A domain. NC16A+/+ mice developed normally and were fertile. No skin abnormalities at the gross, microscopic and ultrastructural levels were seen up to 12 months of age. PCR and RT-PCR analyses of NC16A+/+ mouse tails detected the hBP180NC16A sequence (Figure 1B and 1C). Indirect IF showed correct expression of hBP180NC16A in basal keratinocytes (Figure 1D). Electron microscopic analysis of the skin revealed no abnormalities in the structure or distribution of hemidesmosome in basal keratinocytes (Figure 1E). The NC16A+/+ mice also reproduced normally with compatible frequency and size of litters as controls. These results showed that the replacement of mBP180NC14A with hBP180NC16A did not impair functions of BP180 protein in the skin.
3.2. Humanized BP180NC16A mice injected with anti-BP180NC16A autoantibodies develop BP
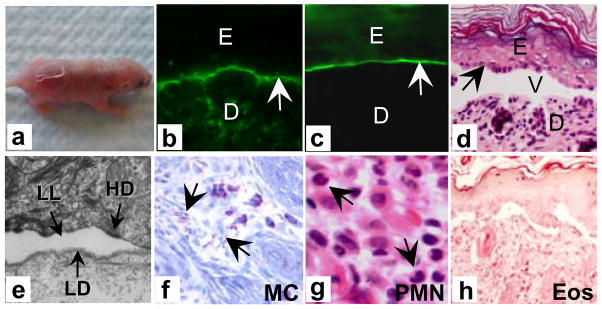
To test the pathogenicity of anti-human BP180 antibodies, anti-BP180NC16A autoantibodies or rabbit anti-hBP180NC16A IgG (R594) were injected intradermally (i.d.) or intraperitoneally (i.p.) into neonatal NC16A+/+ mice. The injected animals developed skin lesions at 24 h post injection (Figure 2 and Table 1). Direct IF of skin from the diseased mice showed in situ deposition of human IgG (Figure 2b) and murine C3 (Figure 2c) at the basement membrane zone. Histologically, the skin blisters were subepidermal (Figure 2d), and electron microscopic analysis revealed that the lesional site was at the lamina lucida (Figure 2e), exactly the same location as human BP. The diseased skin contained degranulated dermal mast cells (Figure 2f) and a predominant neutrophil infiltrate (Figure 2g), but no eosinophils (Figure 2h). NC16A+/+ mice injected with control human IgG showed no skin lesions (Table 1). Rabbit anti-mBP180NC14A IgG, which is pathogenic in wild-type mice, was no longer pathogenic in the NC16A+/+ mice (Table 1).
Figure 2. Anti-BP180NC16A autoantibodies induce subepidermal blisters in the humanized NC16A mice.
Neonatal NC16A+/+ mice injected i.d. with BP180NC16A-specific autoantibodies (BP1, 0.29 mg/g body weight) developed clinical blistering (a). Direct IF showed deposition of human IgG (b) and murine C3 (d) at the basement membrane zone. H/E staining showed dermal-epidermal separation (d). Electron microscopic analysis revealed a split at the lamina lucida (e). Examination of toluidine blue stained skin sections revealed degranulating mast cells (f). H/E staining showed infiltrating neutrophils in the upper dermis (g). 400x magnification. Eosinophil-specific histochemical staining of the skin failed to identify eosinophils in the dermis (dark-brown staining for eosinophils) (h). NC16A+/+ mice injected with normal human IgG or rabbit anti-mBP180 IgG showed no skin lesions (Table 1). E, epidermis. D, dermis. V, vesicle. arrow, basal keratinocytes. 100x magnification for b–c and i. 400x magnification for g and h. 30,000x magnification for f.
Table I.
BP autoantibodies in humanized BP180NC16A mice
| Strain | Treatment | Antibody dose(mg/g body weight) | Number of mice | Mean disease activity score |
|---|---|---|---|---|
| WT | R530 IgG | 2.64 | 6 | 2.92 ± 0.20 |
| R594 IgG | 3.00 | 3 | 0.00 ± 0.00 | |
| BP1 anti-NC16A | 0.29 | 3 | 0.00 ± 0.00 | |
| BP2 anti-NC16A | 0.17 | 3 | 0.00 ± 0.00 | |
| BP3 anti-NC16A | 0.20 | 3 | 0.00 ± 0.00 | |
| R530 IgG | 2.64 | 6 | 0.00 ± 0.00 | |
| NC16A+/+ | NH IgG | 0.29 | 6 | 0.00 ± 0.00 |
| R594 IgG | 3.00 | 6 | 2.83 ± 0.26 | |
| BP1 anti-NC16A | 0.29 | 15 | 2.33 ± 0.24 | |
| BP2 anti-NC16A | 0.17 | 6 | 2.17 ± 0.25 | |
| BP3 anti-NC16A | 0.20 | 6 | 2.08 ± 0.38 | |
| BP1 anti-NC16A F(ab′)2 | 0.29 | 6 | 0.58 ± 0.15 | |
| BP1 anti-NC16A + CVF | 0.29 | 6 | 0.42 ± 0.15 | |
| BP1 anti-NC16A + Cromolyn | 0.29 | 6 | 0.33 ± 0.11 | |
| BP1 anti-NC16A + AI-A31140 | 0.29 | 6 | 0.25 ± 0.11 | |
| BP1 anti-NC16A + NR IgG | 0.29 | 6 | 2.00 ± 0.00 |
Neonatal wild-type littermates and humanized mice (NC16A+/+) were injected i.d. with rabbit anti-mBP180 IgG R530, rabbit anti-hBP180NC16A IgG R594, BP180NC16A-specific autoantibodies from patients with BP (BP1, BP2, BP3), or with F(ab′)2 fragments of anti-BP180NC16A. To deplete complement, mice were pretreated with an i.p. injection of cobra venom factor (CVF, 3 units/mouse). To inhibit mast cell degranulation, mice were pretreated with an i.d. injection of cromolyn sodium (10 μg/g body weight). To deplete neutrophils, mice were pretreated with an i.p. injection of neutrophil-depleting antibody AI-A31140 (100 μl/g body weight). The cromolyn-treated mice and complement- and neutrophil-depleted mice were given pathogenic anti-BP180NC16A autoantibodies at 90 min and 12 h later, respectively. The animals were examined clinically 24 h after IgG injection and disease activity was scored and averaged in each group (Mean disease score ± SEM).
3.3. Anti-BP180NC16A autoantibody-induced BP depends on complement activation, mast cell degranulation, and neutrophil infiltration
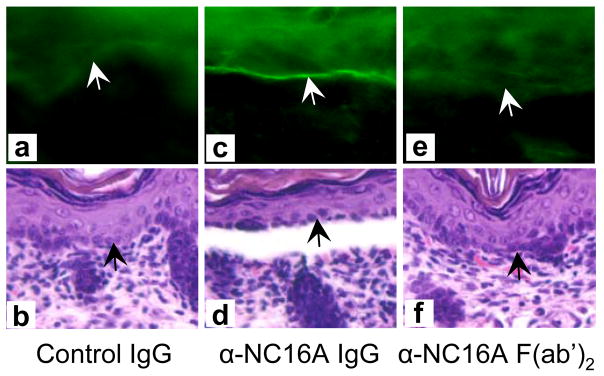
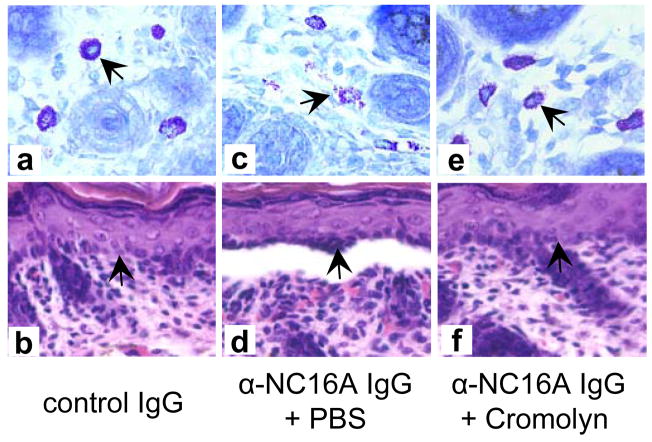
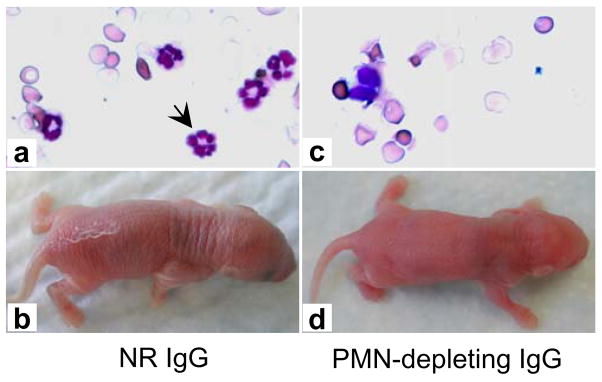
Intact pathogenic anti-BP180NC16A IgG autoantibodies activated complement and induced subsequent blistering in the NC16A+/+ mice (Figures 3c and 3d). In contrast, F(ab′)2 fragments of pathogenic anti-BP180NC16A antibodies were able to bind to their target, but failed to activate the complement cascade as evidenced by the lack of C3 deposition at the basement membrane zone (Figure 3e) and induced no skin disease (Figure 3f and Table 1). In addition, the NC16A+/+ mice, pretreated with cobra venom factor to deplete complement, became resistant to the pathogenic effects of anti-BP180NC16A autoantibodies (Table 1). The NC16A+/+ mice injected with pathogenic anti-BP180NC16A autoantibodies showed extensive mast cell degranulation and developed BP (Figures 4c and 4d). In contrast, pretreatment of NC16A+/+ mice with cromolyn, an inhibitor of mast cell activation, inhibited anti-BP180NC16A antibody-induced mast cell degranulation (Figure 4e) and subsequent blistering (Figure 4f). Pathogenic anti-BP180NC16A autoantibodies induced BP in the NC16A+/+ mice pretreated with control rabbit antibody (Figures 5a and 5b). In contrast, anti-BP180NC16A antibodies were no longer pathogenic in neutrophil-deficient NC16A+/+ mice pretreated with neutrophil-depleting rat antibody (Figures 5c and 5d).
Figure 3. Pathogenicity of anti-BP180NC16A autoantibodies in the NC16A+/+ mice requires complement activation.
Neonatal NC16A+/+ mice injected with anti-BP180NC16A intact antibodies (BP1, 0.29 mg/g body weight) developed subepidermal blistering with C3 deposition at the basement membrane zone (c and d). In contrast, F(ab′)2 fragments (0.29 mg/g body weight) of the pathogenic antibodies failed to induce skin lesions or C3 deposition (e and d). NC16A+/+ mice injected with normal human IgG (NH IgG, 0.29 mg/g body weight) showed no skin lesions and complement activation (a and b). Arrow, basal keratinocytes. 100x magnification.
Figure 4. Anti-BP180NC16A autoantibody-induced BP depends on mast cell degranulation.
Normal human IgG (0.29 mg/g body weight), when injected i.d. into NC16A+/+ mice, induced minimal mast cell degranulation (a) and no skin lesions (b). NC16A+/+ mice injected i.d. with pathogenic anti-BP180NC16A autoantibodies (BP1, 0.29 mg/g body weight) showed extensive mast cell degranulation (c) and subepidermal blistering (d). In contrast, NC16A+/+ mice pretreated with the mast cell degranulation inhibitor, cromolyn sodium (10 μg/g body weight), and then injected i.d. with pathogenic antibodies (BP1, 0.29 mg/g body weight) exhibited minimal mast cell degranulation (e) and no skin lesions (f).
Figure 5. Neutrophils are required for subepidermal blistering triggered by anti-BP180NC16A autoantibodies.
Neonatal NC16A+/+ mice pretreated with normal control rabbit IgG (NR IgG, 100 μl/g body weight, i.p.) followed by an i.d. injection with pathogenic antibodies (BP1, 0.29 mg/g body weight) had normal numbers of neutrophils in circulation (a) and developed subepidermal blistering (b). In contrast, the mice depleted of neutrophils by i.p. injection of AI-A31140 (100 μl/g body weight) and then injected i.d. with pathogenic antibodies (BP1, 0.29 mg/g body weight) showed >90% reduction in circulating neutrophils (c) and developed no skin lesions (d). Arrow, neutrophil.
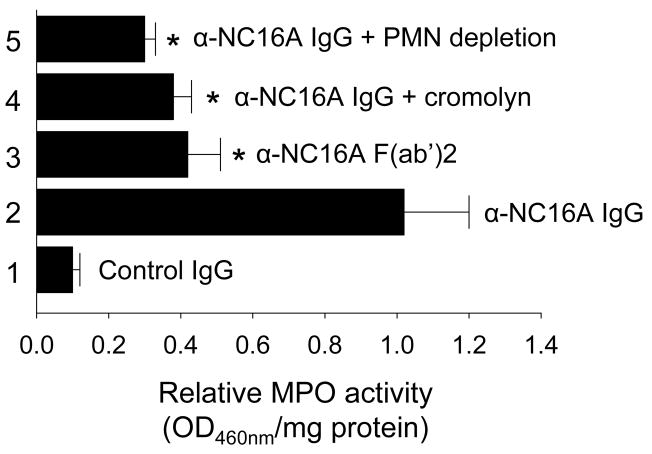
MPO activity assays revealed a drastic reduction in neutrophil infiltration in NC16A+/+ mice injected with anti-BP180NC16A F(ab′)2 fragments, anti-BP180NC16A IgG plus cromolyn, or anti-BP180NC16A IgG plus neutrophil-depleting antibody (Figure 6). Taken together, these results demonstrate that subepidermal blistering triggered by pathogenic anti-BP180NC16A antibodies in NC16A+/+ mice requires complement activation, mast cell activation, and neutrophil recruitment.
Figure 6. Blocking complement activation and mast cell degranulation impairs skin neutrophil infiltration in the pathogenic antibody-induced NC16A+/+ mice.
NC16A+/+ mice injected i.d. with pathogenic anti-BP180NC16A antibodies (BP1, 0.29 mg/g body weight; HG1, 0.17 mg/g body weight) showed extensive neutrophil infiltration (bar 2). In contrast, NC16A+/+ mice injected i.d with F(ab′)2 fragments of anti-BP180NC16A (BP1, 0.29 mg/g body weight) (bar 3) showed a dramatically lower levels of neutrophil infiltration. Also showing a significant reduction in neutrophil infiltration were mice injected with pathogenic anti-BP180NC16A IgG following pretreatment with either the mast cell degranulation inhibitor cromolyn sodium (10 μg/g body weight) (bar 4) or the neutrophil-depleting antibody (AI-A31140, 100 μl/g body weight) (bar 5) n=6. *p< 0.01.
4. Discussion
Since human and murine BP180 antigens are not immune cross-reactive, a humanized mouse strain expressing the BP immunodominant epitopes, BP180NC16A domain, is needed to study the humoral immune response in BP disease immunopathology. In this work, we created such a mouse strain (NC16A+/+) and demonstrated that anti-BP180NC16A autoantibodies in BP are indeed pathogenic. These findings are in agreement with a recent report by Nishie et al [38], in which anti-BP180 autoantibodies induce BP in mice humanized with whole BP180 instead of only the NC16A domain.
The NC16A humanized mouse model also sheds light on the structure/function of BP180. This transmembrane collagen is a key component of the junctional adhesion complex, or hemidesmosome, and is thought to function in cell-matrix adhesion [3, 48] The homologous human BP180NC16A and murine BP180NC14A domains are not cross-reactive immunologically and exhibit only 59% identity and 71% similarity, compared with the relatively high overall homology exhibited by the human and murine BP180 proteins (81% identity, 86% similarity) [3] Nevertheless, the humanized NC16A mice are phenotypically normal and develop normal hemidesmosomes at both the microscopic and ultrastuctural levels. Our findings suggest that domain-specific replacement of mBP180NC14A with hBP180NC16A does not impair the expression and function of this protein in vivo, indicating that this model system might well prove useful in future investigations into the biological roles of BP180. In addition, domain-specific humanization could be a useful approach for a human protein that may lead to a dominant negative phenotype when introduced into mice as a whole molecule.
We previously showed that an intact complement cascade, degranulation of resident mast cells, and activation of infiltrating neutrophils are required in the dermal-epidermal separation induced by experimentally generated rabbit anti-mBP180 antibodies [42, 45, 46, 49] While the rabbit anti-mBP180 IgG passive transfer model has provided invaluable insight to the key steps in BP disease development, it has been debated whether the rabbit anti-mBP180 IgG faithfully mirrors effector functions of pathogenic anti-BP180 autoantibodies since rabbit IgG is a single isotype while the BP autoantibodies are a mixture of IgG1, IgG3 and IgG4. Our present study offers us direct evidence that the key innate immune system players, complement and inflammatory cells, also play a critical role in BP autoantibody-mediated subepidermal blistering. Involvement of these same components in the immuno-pathogenic response to BP autoantibodies has also been supported by the results of previous clinical and in vitro studies. Intact and degranulating mast cells, eosinophils, and neutrophils are seen in the dermis, suggesting these cells have been activated locally [24] In vitro dermal-epidermal junction separation of human skin sections induced by human BP autoantibodies depends on complement and leukocytes (predominantly neutrophils) [50, 51] Human BP blister fluids contain high levels of both neutrophil elastase and gelatinase B, but BP180 degradation by blister fluid depends on neutrophil elastase activity [52, 53] Thus, the present findings provide important information concerning potential therapeutic targets for BP.
While human BP and humanized murine BP closely mimic each other at the clinical, histological, and immunological levels, the IgG passive transfer models do not reflect the large number of eosinophils typically found in the inflammatory infiltrate of human BP lesional skin. Therefore, the question of the role of eosinophils in BP cannot be addressed in our humanized BP180NC16A mouse model.
In conclusion, this study provides direct evidence that anti-BP180NC16A autoantibodies are pathogenic and their tissue injury activity depends on innate immune responses. This novel experimental mouse model will be useful for dissecting the immunopathology of BP, and will aid in the future investigation of some important questions which could not be addressed before. These include the role of different isotypes and IgG subclasses of anti-BP180 autoantibodies since BP180-specific IgA, IgE, IgG1, IgG3, and IgG4 are present in BP [39, 54, 55] This humanized mouse strain may also provide an in vivo system to elucidate the pathogenic mechanisms of other subepidermal blistering diseases with an autoimmune response to BP180, such as herpes gestationis, mucous membrane pemphigoid, linear IgA bullous dermatosis, and lichen planus pemphigoides [18, 56–58]
Acknowledgments
We thank Dr. Pamela Groben for routine histology, Shiliang Wang and Sarah Rice for their excellent technical assistance, and Eric Bankaitis for assistance of manuscript preparation. We are indebted to Dr. Lowell Goldsmith and Lisa Leighty for their critical reading of the manuscript. This work was supported in part by U.S. Public Health Service NIH grants AI40768 and AI61430 (Z. L.), AR052109 and AR053313 (N.L.), AR32599 and AR32081 (L.A.D.).
Abbreviations
- BP
bullous pemphigoid
- hBP180
human BP180
- mBP180
murine BP180 antigen
- MPO
myeloperoxidase
- NC16A+/+ mice
humanized NC16A mice
Footnotes
Disclosures: The authors have no financial conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jordon RE, Beutner EH, Witebsky E, Blumental G, Hale WL, Lever WF. Basement zone antibodies in bullous pemphigoid. JAMA. 1967;200:751–6. [PubMed] [Google Scholar]
- 2.Lever WF. Pemphigus. Medicine (Baltimore) 1953;32:1–123. doi: 10.1097/00005792-195302000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Stanley JR. Bullous Pemphigoid. In: IM, AZF, Eisen KW, Austen KF, Goldsmith LA, Katz SI, editors. Fitzpatrick’s Dermatology in General Medicine. McGraw-Hill; New York: 1999. pp. 666–71. [Google Scholar]
- 4.Jordon RE, Nordby JM, Milstein H. The complement system in bullous pemphigoid. III Fixation of C1q and C4 by pemphigoid antibody. J Lab Clin Med. 1975;86:733–40. [PubMed] [Google Scholar]
- 5.Jordon RE, Schroeter AL, Good RA, Day NK. The complement system in bullous pemphigoid. II. Immunofluorescent evidence for both classical and alternate-pathway activation. Clin Immunol Immunopathol. 1975;3:307–14. doi: 10.1016/0090-1229(75)90017-3. [DOI] [PubMed] [Google Scholar]
- 6.Katz SI, Hertz KC, Yaoita H. Herpes gestationis. Immunopathology and characterization of the HG factor. J Clin Invest. 1976;57:1434–41. doi: 10.1172/JCI108413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Provost TT, Tomasi TB., Jr Evidence for complement activation via the alternate pathway in skin diseases, I. Herpes gestationis, systemic lupus erythematosus, and bullous pemphigoid. J Clin Invest. 1973;52:1779–87. doi: 10.1172/JCI107359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaz LA, Ratrie H, 3rd, Saunders WS, Futamura S, Squiquera HL, Anhalt GJ, Giudice GJ. Isolation of a human epidermal cDNA corresponding to the 180-kD autoantigen recognized by bullous pemphigoid and herpes gestationis sera. Immunolocalization of this protein to the hemidesmosome. J Clin Invest. 1990;86:1088–94. doi: 10.1172/JCI114812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giudice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol. 1992;99:243–50. doi: 10.1111/1523-1747.ep12616580. [DOI] [PubMed] [Google Scholar]
- 10.Hopkinson SB, Riddelle KS, Jones JC. Cytoplasmic domain of the 180-kD bullous pemphigoid antigen, a hemidesmosomal component: molecular and cell biologic characterization. J Invest Dermatol. 1992;99:264–70. doi: 10.1111/1523-1747.ep12616615. [DOI] [PubMed] [Google Scholar]
- 11.Nishizawa Y, Uematsu J, Owaribe K. HD4, a 180 kDa bullous pemphigoid antigen, is a major transmembrane glycoprotein of the hemidesmosome. J Biochem. 1993;113:493–501. doi: 10.1093/oxfordjournals.jbchem.a124072. [DOI] [PubMed] [Google Scholar]
- 12.Sawamura D, Li K, Chu ML, Uitto J. Human bullous pemphigoid antigen (BPAG1). Amino acid sequences deduced from cloned cDNAs predict biologically important peptide segments and protein domains. J Biol Chem. 1991;266:17784–90. [PubMed] [Google Scholar]
- 13.Stanley JR, Hawley-Nelson P, Yuspa SH, Shevach EM, Katz SI. Characterization of bullous pemphigoid antigen: a unique basement membrane protein of stratified squamous epithelia. Cell. 1981;24:897–903. doi: 10.1016/0092-8674(81)90115-x. [DOI] [PubMed] [Google Scholar]
- 14.Stanley JR, Tanaka T, Mueller S, Klaus-Kovtun V, Roop D. Isolation of complementary DNA for bullous pemphigoid antigen by use of patients’ autoantibodies. J Clin Invest. 1988;82:1864–70. doi: 10.1172/JCI113803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanaka T, Parry DA, Klaus-Kovtun V, Steinert PM, Stanley JR. Comparison of molecularly cloned bullous pemphigoid antigen to desmoplakin I confirms that they define a new family of cell adhesion junction plaque proteins. J Biol Chem. 1991;266:12555–9. [PubMed] [Google Scholar]
- 16.Balding SD, Diaz LA, Giudice GJ. A recombinant form of the human BP180 ectodomain forms a collagen-like homotrimeric complex. Biochemistry. 1997;36:8821–30. doi: 10.1021/bi970675n. [DOI] [PubMed] [Google Scholar]
- 17.Hirako Y, Usukura J, Nishizawa Y, Owaribe K. Demonstration of the molecular shape of BP180, a 180-kDa bullous pemphigoid antigen and its potential for trimer formation. J Biol Chem. 1996;271:13739–45. doi: 10.1074/jbc.271.23.13739. [DOI] [PubMed] [Google Scholar]
- 18.Giudice GJ, Emery DJ, Zelickson BD, Anhalt GJ, Liu Z, Diaz LA. Bullous pemphigoid and herpes gestationis autoantibodies recognize a common non-collagenous site on the BP180 ectodomain. J Immunol. 1993;151:5742–50. [PubMed] [Google Scholar]
- 19.Zillikens D, Rose PA, Balding SD, Liu Z, Olague-Marchan M, Diaz LA, Giudice GJ. Tight clustering of extracellular BP180 epitopes recognized by bullous pemphigoid autoantibodies. J Invest Dermatol. 1997;109:573–9. doi: 10.1111/1523-1747.ep12337492. [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Diaz LA, Troy JL, Taylor AF, Emery DJ, Fairley JA, Giudice GJ. A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J Clin Invest. 1993;92:2480–8. doi: 10.1172/JCI116856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwatsuki K, Tagami H, Yamada M. Induction of leukocyte adherence at the basement membrane zone with subsequent activation of their metabolic pathway by pemphigoid antibodies and complement. Acta Derm Venereol. 1983;63:290–5. [PubMed] [Google Scholar]
- 22.Nestor MS, Cochran AJ, Ahmed AR. Mononuclear cell infiltrates in bullous disease. J Invest Dermatol. 1987;88:172–5. doi: 10.1111/1523-1747.ep12525315. [DOI] [PubMed] [Google Scholar]
- 23.Nishioka K, Hashimoto K, Katayama I, Sarashi C, Kubo T, Sano S. Eosinophilic spongiosis in bullous pemphigoid. Arch Dermatol. 1984;120:1166–8. [PubMed] [Google Scholar]
- 24.Wintroub BU, Mihm MC, Jr, Goetzl EJ, Soter NA, Austen KF. Morphologic and functional evidence for release of mast-cell products in bullous pemphigoid. N Engl J Med. 1978;298:417–21. doi: 10.1056/NEJM197802232980803. [DOI] [PubMed] [Google Scholar]
- 25.Dvorak AM, Mihm MC, Jr, Osage JE, Kwan TH, Austen KF, Wintroub BU. Bullous pemphigoid, an ultrastructural study of the inflammatory response: eosinophil, basophil and mast cell granule changes in multiple biopsies from one patient. J Invest Dermatol. 1982;78:91–101. doi: 10.1111/1523-1747.ep12505711. [DOI] [PubMed] [Google Scholar]
- 26.Baba T, Sonozaki H, Seki K, Uchiyama M, Ikesawa Y, Toriisu M. An eosinophil chemotactic factor present in blister fluids of bullous pemphigoid patients. J Immunol. 1976;116:112–6. [PubMed] [Google Scholar]
- 27.Endo H, Iwamoto I, Fujita M, Okamoto S, Yoshida S. Increased immunoreactive interleukin-5 levels in blister fluids of bullous pemphigoid. Arch Dermatol Res. 1992;284:312–4. doi: 10.1007/BF00372588. [DOI] [PubMed] [Google Scholar]
- 28.Grando SA, Glukhenky BT, Drannik GN, Epshtein EV, Kostromin AP, Korostash TA. Mediators of inflammation in blister fluids from patients with pemphigus vulgaris and bullous pemphigoid. Arch Dermatol. 1989;125:925–30. [PubMed] [Google Scholar]
- 29.Katayama I, Doi T, Nishioka K. High histamine level in the blister fluid of bullous pemphigoid. Arch Dermatol Res. 1984;276:126–7. doi: 10.1007/BF00511070. [DOI] [PubMed] [Google Scholar]
- 30.Kawana S, Ueno A, Nishiyama S. Increased levels of immunoreactive leukotriene B4 in blister fluids of bullous pemphigoid patients and effects of a selective 5-lipoxygenase inhibitor on experimental skin lesions. Acta Derm Venereol. 1990;70:281–5. [PubMed] [Google Scholar]
- 31.Schmidt E, Ambach A, Bastian B, Brocker EB, Zillikens D. Elevated levels of interleukin-8 in blister fluid of bullous pemphigoid compared with suction blisters of healthy control subjects. J Am Acad Dermatol. 1996;34:310–2. doi: 10.1016/s0190-9622(96)80146-0. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt E, Bastian B, Dummer R, Tony HP, Brocker EB, Zillikens D. Detection of elevated levels of IL-4, IL-6, and IL-10 in blister fluid of bullous pemphigoid. Arch Dermatol Res. 1996;288:353–7. doi: 10.1007/BF02507102. [DOI] [PubMed] [Google Scholar]
- 33.Takiguchi Y, Kamiyama O, Saito E, Nagao S, Kaneko F, Minagawa T. Cell-mediated immune reaction in the mechanism of blister formation in bullous pemphigoid. Dermatologica. 1989;179(Suppl 1):137. doi: 10.1159/000248478. [DOI] [PubMed] [Google Scholar]
- 34.Gissler HM, Simon MM, Kramer MD. Enhanced association of plasminogen/plasmin with lesional epidermis of bullous pemphigoid. Br J Dermatol. 1992;127:272–7. doi: 10.1111/j.1365-2133.1992.tb00127.x. [DOI] [PubMed] [Google Scholar]
- 35.Kramer MD, Reinartz J. The autoimmune blistering skin disease bullous pemphigoid. The presence of plasmin/alpha 2-antiplasmin complexes in skin blister fluid indicates plasmin generation in lesional skin. J Clin Invest. 1993;92:978–83. doi: 10.1172/JCI116674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oikarinen AI, Zone JJ, Ahmed AR, Kiistala U, Uitto J. Demonstration of collagenase and elastase activities in the blister fluids from bullous skin diseases. Comparison between dermatitis herpetiformis and bullous pemphigoid. J Invest Dermatol. 1983;81:261–6. doi: 10.1111/1523-1747.ep12518285. [DOI] [PubMed] [Google Scholar]
- 37.Stahle-Backdahl M, Inoue M, Guidice GJ, Parks WC. 92-kD gelatinase is produced by eosinophils at the site of blister formation in bullous pemphigoid and cleaves the extracellular domain of recombinant 180-kD bullous pemphigoid autoantigen. J Clin Invest. 1994;93:2022–30. doi: 10.1172/JCI117196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishie W, Sawamura D, Goto M, Ito K, Shibaki A, McMillan JR, Sakai K, Nakamura H, Olasz E, Yancey KB, et al. Humanization of autoantigen. Nat Med. 2007;13:378–83. doi: 10.1038/nm1496. [DOI] [PubMed] [Google Scholar]
- 39.Dopp R, Schmidt E, Chimanovitch I, Leverkus M, Brocker EB, Zillikens D. IgG4 and IgE are the major immunoglobulins targeting the NC16A domain of BP180 in Bullous pemphigoid: serum levels of these immunoglobulins reflect disease activity. J Am Acad Dermatol. 2000;42:577–83. [PubMed] [Google Scholar]
- 40.Haase C, Budinger L, Borradori L, Yee C, Merk HF, Yancey K, Hertl M. Detection of IgG autoantibodies in the sera of patients with bullous and gestational pemphigoid: ELISA studies utilizing a baculovirus-encoded form of bullous pemphigoid antigen 2. J Invest Dermatol. 1998;110:282–6. doi: 10.1038/sj.jid.5602955. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt E, Obe K, Brocker EB, Zillikens D. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol. 2000;136:174–8. doi: 10.1001/archderm.136.2.174. [DOI] [PubMed] [Google Scholar]
- 42.Liu Z, Giudice GJ, Swartz SJ, Fairley JA, Till GO, Troy JL, Diaz LA. The role of complement in experimental bullous pemphigoid. J Clin Invest. 1995;95:1539–44. doi: 10.1172/JCI117826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Broide DH, Campbell K, Gifford T, Sriramarao P. Inhibition of eosinophilic inflammation in allergen-challenged, IL-1 receptor type 1-deficient mice is associated with reduced eosinophil rolling and adhesion on vascular endothelium. Blood. 2000;95:263–9. [PubMed] [Google Scholar]
- 44.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–9. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 45.Liu Z, Giudice GJ, Zhou X, Swartz SJ, Troy JL, Fairley JA, Till GO, Diaz LA. A major role for neutrophils in experimental bullous pemphigoid. J Clin Invest. 1997;100:1256–63. doi: 10.1172/JCI119639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen R, Ning G, Zhao ML, Fleming MG, Diaz LA, Werb Z, Liu Z. Mast cells play a key role in neutrophil recruitment in experimental bullous pemphigoid. J Clin Invest. 2001;108:1151–8. doi: 10.1172/JCI11494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wershil BK, Wang ZS, Gordon JR, Galli SJ. Recruitment of neutrophils during IgE-dependent cutaneous late phase reactions in the mouse is mast cell-dependent. Partial inhibition of the reaction with antiserum against tumor necrosis factor-alpha. J Clin Invest. 1991;87:446–53. doi: 10.1172/JCI115016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Litjens SH, de Pereda JM, Sonnenberg A. Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol. 2006;16:376–83. doi: 10.1016/j.tcb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 49.Liu Z. Bullous pemphigoid: using animal models to study the immunopathology. J Investig Dermatol Symp Proc. 2004;9:41–6. doi: 10.1111/j.1087-0024.2004.00841.x. [DOI] [PubMed] [Google Scholar]
- 50.Gammon WR, Merritt CC, Lewis DM, Sams WM, Jr, Carlo JR, Wheeler CE., Jr An in vitro model of immune complex-mediated basement membrane zone separation caused by pemphigoid antibodies, leukocytes, and complement. J Invest Dermatol. 1982;78:285–90. doi: 10.1111/1523-1747.ep12507222. [DOI] [PubMed] [Google Scholar]
- 51.Sitaru C, Schmidt E, Petermann S, Munteanu LS, Brocker EB, Zillikens D. Autoantibodies to bullous pemphigoid antigen 180 induce dermal-epidermal separation in cryosections of human skin. J Invest Dermatol. 2002;118:664–71. doi: 10.1046/j.1523-1747.2002.01720.x. [DOI] [PubMed] [Google Scholar]
- 52.Shimanovich I, Mihai S, Oostingh GJ, Ilenchuk TT, Brocker EB, Opdenakker G, Zillikens D, Sitaru C. Granulocyte-derived elastase and gelatinase B are required for dermal-epidermal separation induced by autoantibodies from patients with epidermolysis bullosa acquisita and bullous pemphigoid. J Pathol. 2004;204:519–27. doi: 10.1002/path.1674. [DOI] [PubMed] [Google Scholar]
- 53.Verraes S, Hornebeck W, Polette M, Borradori L, Bernard P. Respective contribution of neutrophil elastase and matrix metalloproteinase 9 in the degradation of BP180 (type XVII collagen) in human bullous pemphigoid. J Invest Dermatol. 2001;117:1091–6. doi: 10.1046/j.0022-202x.2001.01521.x. [DOI] [PubMed] [Google Scholar]
- 54.Bernard P, Aucouturier P, Denis F, Bonnetblanc JM. Immunoblot analysis of IgG subclasses of circulating antibodies in bullous pemphigoid. Clin Immunol Immunopathol. 1990;54:484–94. doi: 10.1016/0090-1229(90)90060-4. [DOI] [PubMed] [Google Scholar]
- 55.Laffitte E, Skaria M, Jaunin F, Tamm K, Saurat JH, Favre B, Borradori L. Autoantibodies to the extracellular and intracellular domain of bullous pemphigoid 180, the putative key autoantigen in bullous pemphigoid, belong predominantly to the IgG1 and IgG4 subclasses. Br J Dermatol. 2001;144:760–8. doi: 10.1046/j.1365-2133.2001.04130.x. [DOI] [PubMed] [Google Scholar]
- 56.Bernard P, Prost C, Durepaire N, Basset-Seguin N, Didierjean L, Saurat JH. The major cicatricial pemphigoid antigen is a 180-kD protein that shows immunologic cross-reactivities with the bullous pemphigoid antigen. J Invest Dermatol. 1992;99:174–9. doi: 10.1111/1523-1747.ep12616797. [DOI] [PubMed] [Google Scholar]
- 57.Tamada Y, Yokochi K, Nitta Y, Ikeya T, Hara K, Owaribe K. Lichen planus pemphigoides: identification of 180 kd hemidesmosome antigen. J Am Acad Dermatol. 1995;32:883–7. doi: 10.1016/0190-9622(95)91554-0. [DOI] [PubMed] [Google Scholar]
- 58.Zone JJ, Taylor TB, Meyer LJ, Petersen MJ. The 97 kDa linear IgA bullous disease antigen is identical to a portion of the extracellular domain of the 180 kDa bullous pemphigoid antigen, BPAg2. J Invest Dermatol. 1998;110:207–10. doi: 10.1046/j.1523-1747.1998.00129.x. [DOI] [PubMed] [Google Scholar]