Abstract
Retinal blood flow has been reported to decrease early in human diabetes as well as in diabetic animal models. The purpose of the present study is to investigate the role of thromboxane receptor binding in the decrease of flow. C57BL/6 mice were injected with streptozotocin (STZ) at 11–12 weeks of age and remained hyperglycemic for 4 weeks. The mice were treated with a selective thromboxane receptor antagonist, GR32191B (vapiprost), in drinking water for the final three weeks at a dose of 1 mg/kg/day. In separate experiments, vapiprost was administered only once, as an acute injection 25 minutes prior to the experimental measurements. The measurements included retinal arteriolar and venular diameters and red blood cell (RBC) velocities, from which retinal blood flow was calculated. STZ induced decreases in vascular diameters and RBC velocities, resulting in an approximate 30% decrease in overall retinal blood flow. However, these decreases were not seen in mice given the three-week administration of vapiprost. Acute administration to diabetic mice of 1 mg/kg vapiprost, but not 0.1 mg/kg, induced arteriolar vasodilation, with the dilation more substantial in smaller feed arterioles. In summary, STZ-induced decreases in retinal blood flow can be attenuated by the thromboxane receptor antagonist vapiprost.
Keywords: Vapiprost, Thromboxane Receptor Antagonist, Streptozotocin, Mouse, Diabetes, Retina, Blood Flow
Introduction
Microvascular complications in diabetes leads to sight-threatening diabetic retinopathy (Rossing, 2005), which is the most prevalent cause of blindness in western countries (Rossing, 2005). Diabetic retinopathy affects 1 in 300 Americans over the age of 18 (Roy et al., 2004). The mechanisms of retinopathy and microvascular complications in diabetes are still not well understood. Prior to the progression of clinical manifestations of diabetes, decreases in retinal arteriolar diameters and/or blood flow to the human retina have been reported (Clermont et al., 1997; Klein et al., 2003; Wong et al., 2002). Retinal ischemia may develop during diabetes and may lead to the development of new blood vessels on the surface of the retina, with a subsequent enhancement of blood flow (Clermont et al., 1997; Fong et al., 2003; Yam and Kwok, A. K., 2007).
Data from animal models of hyperglycemia also demonstrate decreases in blood flow and/or decreases in retinal arteriolar diameter early in the diabetic retina (Bursell et al., 1992; Clermont et al., 1994; Granstam and Granstam, S. O., 1999; Higashi et al., 1998; Small et al., 1987). The extent to which decreased blood flow might induce retinal hypoxia has not been established, although elevated tissue hypoxia has been reported for the streptozotocin mouse model (de Gooyer et al., 2006). It is hypothesized that tissue hypoxia is a major component which stimulates the development of new blood vessels via vascular endothelial growth factor (VEGF) in humans with proliferative diabetic retinopathy as well as in experimental models (Ayalasomayajula and Kompella, U. B., 2003; Pe’er et al., 1996).
Mechanisms for the early changes in blood flow have not been fully elucidated. However, one potential mediator is the vasoconstrictor thromboxane. Various models of hyperglycemia have reported increases in thromboxane levels (De La Cruz et al., 1998; De La Cruz et al., 2000; Lasserre et al., 2000; Moreno et al., 1995; Quilley and McGiff, J. C., 1985; Tesfamariam et al., 1989) that could lead to vasoconstriction.
Thromboxane is produced via the cyclooxygenase pathway. Upon synthesis of prostaglandin G2 (PGG2), thromboxane synthase converts PGG2 into thromboxane A2 (TxA2) which binds to the thromboxane/prostanoid (TP) receptor, leading to constriction of vascular smooth muscle (Bos et al., 2004). In previous experiments in several animal models (Lee and Harris, N. R., 2008; Lee et al., 2008; Wright and Harris, N. R., 2008), we have given acute administrations of the thromboxane synthase inhibitor ozagrel, and found that it can rapidly (within 25–30 minutes or less) dilate retinal arterioles that are constricted in STZ mice, non-obese diabetic (NOD) mice, and STZ rats, at 3–4 weeks of hyperglycemia. However whether the same beneficial dilatory effect can be seen with acute, or prolonged, administration of a thromboxane receptor antagonist has yet to be determined. However, consistent with this possibility is a study of pial arterioles of diabetic rats, where the investigators found that endothelium-dependent vasodilation can be improved by TP receptor antagonism (Mayhan et al., 1991).
Therefore, the aims of the present study were to 1) determine whether the diabetes-induced constriction of retinal arterioles is mediated by the thromboxane receptor, and 2) determine whether prolonged antagonism of the thromboxane receptor can attenuate the diabetes-induced decreases in retinal blood flow.
Materials and methods
2.1. Animals
Eleven to twelve week old C57BL/6 male mice (Jackson Laboratories) were randomly assigned to intraperitoneal (i.p.) injection of streptozotocin (STZ; Sigma, St. Louis, MO; 180 mg/kg dissolved in pH 4.5 sodium citrate buffer) or sodium citrate buffer alone. STZ was injected into the animals within 15 minutes of preparation. Non-fasting blood glucose levels were checked via a tail vein puncture on day six following STZ injection and on the day of the experiment using a One Touch Ultra Glucometer (Milpitas, CA). Mice remained on the protocol for four weeks and STZ-injected mice were included in the study if glucose values on day six and on the day of the experiment exceeded 250 mg/dl. Beginning on day six following STZ injection, body weight was recorded three times per week and mice received 0–2 units insulin/kg of body weight, (Humulin R; Eli Lilly & Co., Indianapolis, IN) 0–3 times per week as needed to attenuate any measured loss in body weight. Insulin was stopped at least 48 hours prior to experimental measurements. Mice were housed one per cage and received water and standard chow. Animals were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
2.2. Retinal Blood Flow – Daily Vapiprost Treatment
Mice were administered GR32191B (vapiprost; Sigma, St. Louis, MO; (4Z)-7-[(1R,2R,3S,5S)-5-([1,1′-Biphenyl]-4-ylmethoxy)-3-hydroxy-2-(1-piperidinyl) cyclopentyl]-4-heptenoic acid hydrochloride), a selective thromboxane receptor antagonist, in drinking water beginning on day six following STZ injection. Mice were segregated into four groups: 1) control with no vapiprost, 2) control + 1 mg/kg/day vapiprost, 3) STZ-injected with no vapiprost, and 4) STZ-injected + 1 mg/kg/day vapiprost.
Red blood cells (RBCs) were labeled according to the methods previously described (Braun et al., 2002; Unthank et al., 1993; Wright and Harris, N. R., 2008). Briefly, age-matched donor mice were used for collection of RBCs, collected in a 1-ml syringe containing 100 μl of anticoagulant citrate dextrose (ACD; Sigma, St. Louis, MO). Whole blood passed through a 5-ml syringe (with 1 g of cotton) was washed with 10 ml of phosphate buffered saline (PBS; Sigma, St. Louis, MO; pH 7.4) then centrifuged, with the supernatant removed and the pellets resuspended in 1ml PBS. The pellets were centrifuged and washed an additional two times in PBS. RBCs were labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate (DiI; Invitrogen Molecular Probes, Eugene, OR) and washed five times in PBS. The RBCs (100 μl DiI-labeled) were then resuspended in 1.2 ml PBS and were used within 36 hours of labeling. RBCs not used on the day of labeling were kept at 4° C overnight; prior to infusion the RBCs were centrifuged and washed three times in PBS then resuspended.
RBCs were infused and visualized using methods previously reported with slight modification (Wright and Harris, N. R., 2008). Briefly, at the end of four weeks, mice were anaesthetized with an intramuscular (i.m.) injection of Nembutal sodium solution (pentobarbital; 50 mg/kg) followed 4–5 minutes later with an i.m. injection of ketamine (50 mg/kg). After the femoral vein was cannulated, the mice were placed on the left side of the body with 3″×3″ gauze placed under the head for support. One drop of Tropicamide Ophthalmic Solution USP 1% (Falcon Pharmaceuticals Ltd., Ft. Worth, TX) was used to dilate the right eye, followed 1–2 minutes later with one drop of Gonak™ Hypromellose Ophthalmic Demulcent Solution, 2.5% (Akron Inc., Buffalo Grove, IL). A 5-mm glass cover slide was placed on the Gonak™ solution over the eye. The optic disk was visualized using a CoolSNAP ES camera (Photometrics, Tucson, AZ) attached to a Nikon Eclipse E600FN microscope with a Gibraltar™ Burleigh stand. Once the optic disc was visualized via intravital microscopy, labeled red blood cells were infused into the left femoral vein. Labeled RBCs were infused at a rate of 20 μl/min for 2 min with an infusion pump (Harvard Apparatus, South Natick, MA). Images were obtained using a 10x objective with a Nikon rhodamine filter. NIS Elements Basic Research software version 2.3 (Nikon Instruments Inc., Melville, NY) was used for capturing and analyzing data. With a video camera exposure time of 10 ms, DiI-labeled RBCs appeared as fluorescent streaks in the vessels, with the length of the streak proportional to RBC velocity. Images were recorded for each vessel leading into or out of the optic disk for a minimum of 6 seconds per vessel. Velocities were measured within 600 μm of the optic disk, with ten consecutive RBC streaks averaged per vessel, and the mean reported in mm/sec.
Arteriolar and venular diameter measurements were captured using methods previously reported (Wright and Harris, N. R., 2008), with minor changes. Following infusion of labeled RBCs and video capture of RBC streaks, the optic disk was placed in the center of the field of view with the arterioles and venules in focus against the autofluorescent tissue background (through the FITC filter). While visualizing the optic nerve using a 4x objective, a 40–60 μl bolus of 4.5 mg/kg FITC-dextran (MW 2,000,000) was injected while images were being recorded, to determine the vessel type (arteriole vs venule). Vessels in which the FITC-dextran appeared first were considered arterioles. Two to four minutes following the bolus injection of FITC-dextran, baseline retinal diameters were recorded by sequentially focusing on each vessel in a clockwise fashion. Images were captured using a 10x objective through a Nikon FITC filter. NIS Elements Basic Research software version 2.3 (Nikon Instruments Inc., Melville, NY) was used for video capture and analysis. Diameter measurements were taken at three different locations along the vessel at least 30 μm apart and within 600 μm of the optic disk. The diameter measurements were reported as averages of the three measurements. Data were obtained from each of the 4–7 primary arterioles extending from the optic and from each of the 4–7 primary venules draining toward the optic disk.
Flow was estimated according to the calculation πVD2/4 where V = mean velocity of RBCs and D = the diameter of vessel. This relationship assumes a uniform cylindrical diameter for the vessels being measured. Total retinal blood flow was calculated by adding all arteriolar or venular flow measurements for each eye.
2.3. Arteriolar and Venular Diameter – Acute Vapiprost
In separate experiments, using the methods described above, the left femoral vein was cannulated and mice were prepared for intravital microscopy to determine the arteriolar diameter change following an acute intravenous infusion of vapiprost, in a protocol matched to our previous study of acute ozagrel infusion (Wright and Harris, N. R., 2008). Once the optic disk was in focus via intravital microscopy, a 10 μl bolus of 4.5 mg/kg FITC-dextran (MW 2,000,000) was injected while images were being recorded to determine the vessel type (arteriole vs venule), followed by a slow infusion of FITC-dextran for an additional 1.5 minutes at a rate of 15 μl/min. Baseline diameter images were recorded two minutes following the slow infusion of FITC-dextran.
Following capture of baseline images, vapiprost was infused over 2–4 minutes. Control and diabetic mice were infused with either 0.1 mg/kg (low-dose) or 1 mg/kg (high-dose) vapiprost. Retinal vessel images were recorded a second time, 25 min after the start of vapiprost infusion. At the conclusion of the experiment, mice were killed with a pentobarbital overdose. Arteriolar and venule diameters were analyzed as described above.
2.4. Statistics
Results are expressed as mean ± standard error of the mean. Statistical analysis was performed with GraphPad Instat version 3.05 software (San Diego, CA). A p-value < 0.05 was considered statistically significant. T-tests were performed unless multiple groups were being compared, for which ANOVA with Bonferroni correction was used.
Results
3.1. Animal Data
Table 1 provides data on body weight and glucose values for the experimental groups. Included in the study were 28 non-diabetic control mice (divided into groups of acute 0.1 mg/kg vapiprost, acute 1 mg/kg vapiprost, 3-week administration of 1 mg/kg vapiprost, and untreated; N=7 for each) and 28 STZ-diabetic mice divided into similar groups (N=6–8 each). STZ induced a 3–4 fold increase in glucose (compared with buffer-injected controls). By day 6 post-injection, 5/28 STZ mice had glucose values > 600 mg/dl (the upper limit of the glucometer), with an additional 11 mice exceeding that level by the end of the fourth week; therefore, in Table 1 we present median values. As expected, STZ induced a decrease in body weight.
Table 1.
Body weight and blood glucose values in the experimental groups: control (N=21), control + vapiprost (1 mg/kg/day; N=7), streptozotocin (STZ; N=20), and STZ + vapiprost (1 mg/kg/day; N=8). Glucose levels are reported as median values.
| Group | Day of injection | Day 6 after injection | Week 4 after injection | ||||
|---|---|---|---|---|---|---|---|
| Control | STZ | Control | STZ | Control | STZ | ||
| Weight (g) | 4 week | 25.0 ± 0.4 | 25.1 ± 0.3 | 26.0 ± 0.4 | 20.6 ± 0.6 *** | ||
| 4 week + vapiprost | 26.1 ± 0.4 | 26.4 ± 0.4 | 27.3 ± 0.3 | 22.2 ± 1.1 *** | |||
| Glucose (mg/dl) | 4 week | 182 | 509 | 212 | >600 | ||
| 4 week + vapiprost | 170 | 566 | 190 | >600 | |||
p<0.001 vs respective day of Control, and p<0.001 vs STZ day of injection.
3.2. Retinal Arteriolar and Venular Diameters
Retinal arteriolar and venular diameters were averaged and pooled per mouse (4–7 of each per mouse), with the mean values shown in Figure 1 (N = 21 control mice; 20 diabetics; which excludes the 7–8 mice per group given prolonged vapiprost treatment). STZ significantly decreased arteriolar diameters from 60.4 ± 0.7 μm to 55.9 ± 0.9 μm (p<0.001), and decreased venular diameters from 69.3 ± 1.3 μm to 59.7 ± 1.4 μm (p<0.001).
Figure 1.

Retinal arteriolar (A) and venular (B) diameters in control (N=21) and diabetic (N=20) mice. Data are pooled from 4–7 vessels per eye. ***p<0.001 vs Control.
Figure 2A shows a histogram of the diameters of all the arterioles (115 control vessels; 115 diabetic) from Figure 1A. As shown in the histogram, there were approximately twice as many arterioles with diameters > 60 μm in the control group as in the diabetic group, suggesting a constriction of the larger arterioles. However, the constriction may have been even more extensive (on a percentage basis) for the smaller arterioles, as presented in Fig 2B. The smallest one-fifth (quintile; 23 of the total 115 arterioles in both groups) were 11.6 % smaller in the diabetic group compared with controls (44.3 ± 1.2 μm vs 50.1 ± 1.3 μm, respectively). In the largest one-fifth of diameters (23 in both groups), diabetic arterioles were 3.8% smaller than controls. In successive order from smallest to largest diameter quintiles, the percentage decreases in the diabetic arteriolar diameters were 11.6, 9.6, 9.2, 6.5, and 3.8%.
Figure 2.

A. Histogram of the number of arterioles segregated by baseline diameter. Data were obtained from 115 arterioles in both control and diabetic mice. B. Average diameters separated by equal percentiles of the total. N = 23 arterioles per quintile. **p<0.01 and ***p<0.001 vs respective Control.
3.3. Daily Vapiprost Administration
The thromboxane receptor antagonist vapiprost was administered over a period of three weeks to see if the drug could attenuate the STZ-induced retinal vasoconstriction. Arteriolar diameters, pooled per mouse from the four groups (control with and without vapiprost; diabetic with and without vapiprost), are shown in Figure 3A. There was no statistically significant decrease in arteriolar diameter induced by STZ with vapiprost treatment, as was present without vapiprost. Neither was there an STZ-induced decrease in venular diameter during vapiprost treatment, as shown in Figure 3B.
Figure 3.
Arteriolar diameter (A), venular diameter (B), arteriolar velocity (C), venular velocity (D), arteriolar blood flow rate (E), and venular blood flow rate (F), each with and without daily vapiprost administration. Values are reported as whole eye averages, N = 7–8 animals per group. *p<0.05, **p<0.01, and ***p<0.001 vs Control without vapiprost treatment.
Average RBC velocities in the arterioles and venules are shown in Figures 3C and 3D. In the absence of vapiprost treatment, arteriolar velocities were significantly lower in diabetics (22.8 ± 1.0 mm/s) compared to controls (28.3 ± 1.4 mm/s), but this was not the case with vapiprost treatment. Similarly, in the absence of vapiprost treatment, venular velocities were significantly lower in diabetics (22.3 ± 0.5 mm/s) compared to controls (26.3 ± 1.2 mm/s). Although this difference was not found with vapiprost treatment, it was noted that venular velocities were significantly lower in vapiprost-treated controls compared with untreated controls.
Blood flow rates were estimated from the diameters and velocities of the individual vessels, then summed for an overall retinal blood flow rate for each retina. As shown in Figures 3E and 3F, total retinal blood flow in response to STZ decreased by 28% (when calculated from the arteriolar flow rates which decreased from 439± 42 nl/s in controls to 315 ± 22 nl/s in diabetics) or by 39% (when calculated from the venular flow rates which decreased from 530 ± 40 nl/s in controls to 325 ± 17 nl/s in diabetics). These decreases were not present with vapiprost treatment; however, it should be noted that flow rates in control mice given vapiprost tended to be lower (although this was not statistically significant).
3.4. Acute Vapiprost Administration
In separate animals, vapiprost was given acutely to investigate its effect on retinal arteriolar diameters (measured prior to and 25 min following vapiprost infusion in the same animal). In 37–40 arterioles per group (N=7 mice per group), paired t-tests of the individual vessel responses to acute 1 mg/kg vapiprost indicated a statistically significant dilation for the arterioles in the diabetic mice (58.3 ± 1.1 μm vs baseline diameters of 55.9 ± 1.3 μm; highly significant at p<0.0001), but no significant changes were found in arterioles of control mice (59.1 ± 1.1 vs 59.2 ± 1.1μm baseline). Additionally, 1 mg/kg vapiprost did not significantly alter venular diameters in either controls (67.0 ± 2.6 vs 66.2 ± 2.5 μm baseline) or diabetics (59.6 ± 2.4 vs 61.2 ± 2.5μm baseline).
Arteriolar diameter changes following acute vapiprost administration in diabetic mice were broken into quintiles based on baseline diameters. In general, acute vapiprost administration (1 mg/kg) induced an approximate 5–10% increase in arteriolar diameters of STZ-injected mice (Fig 4A); however, the percentage vasodilation was more substantial in the smallest arterioles. In the smallest quintile of baseline diameters, vapiprost induced an 8.8 ± 3.2% vasodilation. Statistically significant vasodilation also was observed in the next two quintiles (6.1 ± 1.9% and 6.4 ± 1.2%, respectively), but not in the largest arterioles. At a lower dose of vapiprost (0.1 mg/kg), no vasodilation was observed in the STZ mice (Fig 4B).
Figure 4.
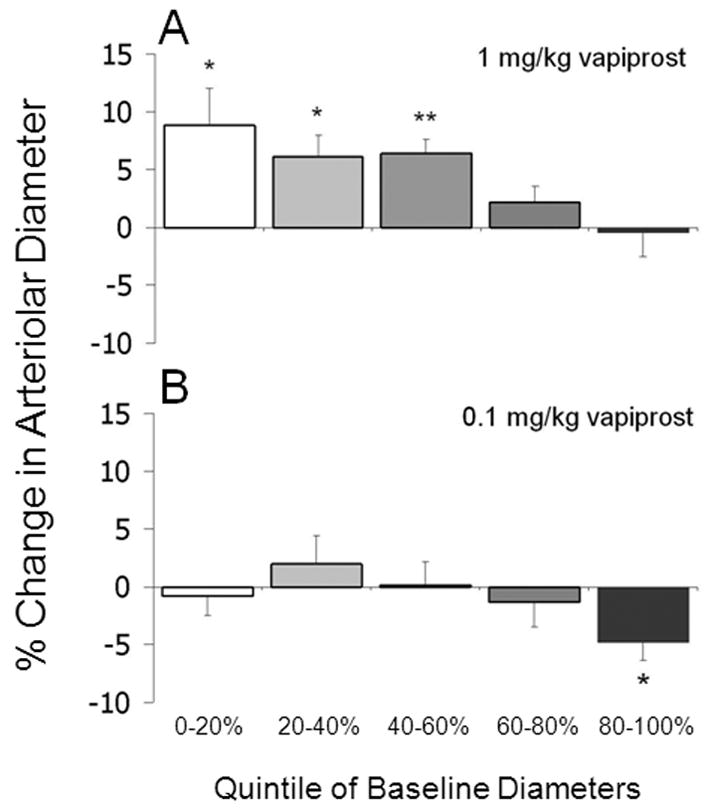
Percent change in arteriolar diameter in STZ-injected mice following acute administration of (A) 1.0 mg/kg vapiprost (N=7–8 arterioles per group) or (B) 0.1 mg/kg vapiprost (N=6–7 arterioles per group). Data are segregated by baseline diameter percentiles. *p<0.05, **p<0.01 vs a value of 0.
Discussion
Previous studies from our lab have indicated that retinal blood flow decreases within the first few weeks of hyperglycemia induced by STZ injection (Lee and Harris, N. R., 2008; Lee et al., 2008; Wright and Harris, N. R., 2008). Our data have come from a variety of models, that is, STZ injection of mice, STZ injection of rats, and in the non-obese diabetic (NOD) mouse model. In each case, the decrease in flow could be acutely reversed upon administration of a thromboxane synthase inhibitor, ozagrel. Data from the current study give an important confirmation of a role for thromboxane in the retina, inasmuch as the thromboxane receptor antagonist vapiprost was able to acutely reverse the arteriolar constriction due to STZ-induced diabetes. Moreover, the current study demonstrated that extended (three-week) administration of vapiprost could attenuate the STZ-induced vasoconstriction.
Characterizing the mediators of diabetes-induced vasoconstriction could have clinical relevance given the decreases in human retinal blood flow reported in the early stages of diabetes (Bursell et al., 1996; Clermont et al., 1997). One potential consequence of a decrease in flow is an insufficient supply of oxygen, and retinal hypoxia has been measured in diabetic animal models (de Gooyer et al., 2006; Linsenmeier et al., 1998). It has been hypothesized that increases in hypoxia lead to the development of new blood vessels on the surface of the retina. The growth of new blood vessels is mediated by vascular endothelial growth factor (VEGF), which has been shown to increase in diabetic patients in the inner retinal layers (Pe’er et al., 1996). In an experimental model of diabetes, VEGF has been reported to increase in the retina within seven days of STZ injection in the rat, with the increase inhibited by a selective cyclooxygenase-2 (COX-2) inhibitor (Ayalasomayajula and Kompella, U. B., 2003). In the mouse, retinal VEGF has been reported to increase six weeks after STZ injection (Nagai et al., 2007).
Thromboxane is produced from arachidonic acid through the cyclooxygenase pathway via the enzyme thromboxane synthase, with its receptors found on vascular smooth muscle as well as platelets (Shen and Tai, H. H., 1998). Thromboxane works in tissues in an autocrine and paracrine manner and has a short half-life, as it is converted to the stable inactive thromboxane B2 (Shen and Tai, H. H., 1998). Potential sources for increased thromboxane production are platelets (De La Cruz et al., 2002) and macrophages (Sun et al., 2001), which have both been reported to increase in the diabetic retina (Esser et al., 1993; Yamashiro et al., 2003). Although controversial, another potential source of thromboxane is the endothelial cell. Human aortic endothelial cells exposed to high levels of glucose have been found to increase the production of thromboxane, which was associated with increased COX-2 levels (Cosentino et al., 2003). Bovine retinal endothelial cells incubated with high glucose increased production of the stable metabolite thromboxane B2, levels of which were attenuated by a thromboxane synthase inhibitor (Sone et al., 1996). However, others have reported that platelets within primary cultures of human umbilical vein endothelial cells contribute to the thromboxane measurements (Pfister et al., 2002).
The STZ-induced decrease in retinal blood flow is likely to be caused by the observed constriction of arterioles. Consistent with this possibility is the relationship between changes in flow predicted by the observed arteriolar constriction. For Poiseuille flow with a given pressure gradient, changes in vascular resistance are inversely proportional the fourth power of diameter, and therefore, flow will be predicted to decrease by 34% in a vessel when the diameter constricts by 10% (0.94 = 0.66). This prediction is within our observations of a 25–40% decrease in flow associated with 5–15% vascular constrictions in the diabetic mice.
It should be noted that a decrease in flow also could be attributed to changes in ocular perfusion pressure (OPP), which is defined as the mean arteriole pressure (MAP) minus the intraocular pressure (IOP). However, we have previously measured and reported no change in MAP or IOP in the same protocol, that is, after 4 weeks of hyperglycemia in a STZ-injected C57BL/6 mouse model of diabetes (Wright and Harris, N. R., 2008). Our findings were consistent with other studies that have found no change in MAP or IOP at early time points in various experimental models of hyperglycemia (Horio et al., 2004; Kanamori et al., 2004; Lee and Harris, N. R., 2008). If our treatments altered MAP, this could influence retinal flow rates; however, evidence in the literature using acute (receptor antagonist) and chronic (knockout) models do not indicate any alterations in MAP due to inhibition of the TP receptor pathway (Guth and Muller, T. H., 1997; Thomas et al., 1998; Xu et al., 2006).
In the present study, we found that the role of TP receptor-induced vasoconstriction may be more dominant in the smaller subset of arterioles. As shown in Figure 2A, feed arterioles extending out of the optic disk range in diameter from 30–80 μm. Although vasoconstriction may occur throughout the size range (see Fig 2B), acute administration of the thromboxane receptor antagonist caused a more potent vasodilation in the smaller arterioles (Fig 4A). In our previous study using the thromboxane synthase inhibitor ozagrel (Wright and Harris, N. R., 2008), we also found heterogeneity in the arteriolar response, and we interpreted the variability as being influenced by the proximity of the arterioles to the neighboring venules. In that study, we noted that the dilation produced by ozagrel occurred to the greatest extent when the arterioles were more closely paired with countercurrent venules, which could be consistent with a mechanism by which venules contribute to the delivery of tissue-derived thromboxane to the arterioles. However, in the present study we noted that more closely venule-paired arterioles were typically smaller, and therefore, an alternative or additional explanation for the variability in the vapiprost response could relate to differences in phenotype depending on vascular size.
In summary, we have shown that STZ-induced retinal vasoconstriction can be attenuated by both acute and extended administration of the thromboxane receptor antagonist vapiprost. Future studies may address the mechanisms by which retinal thromboxane levels may increase, and the extent to which either thromboxane or its receptor increase in the diabetic retina.
Acknowledgments
This study was funded by the National Institutes of Health (EY017599; NRH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Ayalasomayajula SP, Kompella UB. Celecoxib, a selective cyclooxygenase-2 inhibitor, inhibits retinal vascular endothelial growth factor expression and vascular leakage in a streptozotocin-induced diabetic rat model. Eur J Pharmacol. 2003;458:283–289. doi: 10.1016/s0014-2999(02)02793-0. [DOI] [PubMed] [Google Scholar]
- Bos CL, Richel DJ, Ritsema T, Peppelenbosch MP, Versteeg HH. Prostanoids and prostanoid receptors in signal transduction. Int J Biochem Cell Biol. 2004;36:1187–1205. doi: 10.1016/j.biocel.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Braun RD, Abbas A, Bukhari SO, Wilson W., III Hemodynamic parameters in blood vessels in choroidal melanoma xenografts and rat choroid. Invest Ophthalmol Vis Sci. 2002;43:3045–3052. [PMC free article] [PubMed] [Google Scholar]
- Bursell SE, Clermont AC, Kinsley BT, Simonson DC, Aiello LM, Wolpert HA. Retinal blood flow changes in patients with insulin-dependent diabetes mellitus and no diabetic retinopathy. Invest Ophthalmol Vis Sci. 1996;37:886–897. [PubMed] [Google Scholar]
- Bursell SE, Clermont AC, Shiba T, King GL. Evaluating retinal circulation using video fluorescein angiography in control and diabetic rats. Curr Eye Res. 1992;11:287–295. doi: 10.3109/02713689209001782. [DOI] [PubMed] [Google Scholar]
- Clermont AC, Aiello LP, Mori F, Aiello LM, Bursell SE. Vascular endothelial growth factor and severity of nonproliferative diabetic retinopathy mediate retinal hemodynamics in vivo: a potential role for vascular endothelial growth factor in the progression of nonproliferative diabetic retinopathy. Am J Ophthalmol. 1997;124:433–446. doi: 10.1016/s0002-9394(14)70860-8. [DOI] [PubMed] [Google Scholar]
- Clermont AC, Brittis M, Shiba T, McGovern T, King GL, Bursell SE. Normalization of retinal blood flow in diabetic rats with primary intervention using insulin pumps. Invest Ophthalmol Vis Sci. 1994;35:981–990. [PubMed] [Google Scholar]
- Cosentino F, Eto M, De Paolis P, van der LB, Bachschmid M, Ullrich V, Kouroedov A, Delli GC, Joch H, Volpe M, Luscher TF. High glucose causes upregulation of cyclooxygenase-2 and alters prostanoid profile in human endothelial cells: role of protein kinase C and reactive oxygen species. Circulation. 2003;107:1017–1023. doi: 10.1161/01.cir.0000051367.92927.07. [DOI] [PubMed] [Google Scholar]
- de Gooyer TE, Stevenson KA, Humphries P, Simpson DA, Gardiner TA, Stitt AW. Retinopathy Is Reduced during Experimental Diabetes in a Mouse Model of Outer Retinal Degeneration. Invest Ophthalmol Vis Sci. 2006;47:5561–5568. doi: 10.1167/iovs.06-0647. [DOI] [PubMed] [Google Scholar]
- De La Cruz JP, Moreno A, Ruiz-Ruiz MI, Garcia CJ, Sanchez dlC. Effect of camonagrel, a selective thromboxane synthase inhibitor, on retinal vascularization in experimental diabetes. Eur J Pharmacol. 1998;350:81–85. doi: 10.1016/s0014-2999(98)00233-7. [DOI] [PubMed] [Google Scholar]
- De La Cruz JP, Moreno A, Ruiz-Ruiz MI, Sanchez dlC. Effect of DT-TX 30, a combined thromboxane synthase inhibitor and thromboxane receptor antagonist, on retinal vascularity in experimental diabetes mellitus. Thromb Res. 2000;97:125–131. doi: 10.1016/s0049-3848(99)00173-5. [DOI] [PubMed] [Google Scholar]
- De La Cruz P, Guerrero A, Paniego J, Arranz I, Moreno A, Sanchez dlC. Effect of aspirin on prostanoids and nitric oxide production in streptozotocin-diabetic rats with ischemic retinopathy. Naunyn Schmiedebergs Arch Pharmacol. 2002;365:96–101. doi: 10.1007/s00210-001-0507-9. [DOI] [PubMed] [Google Scholar]
- Esser P, Heimann K, Wiedemann P. Macrophages in proliferative vitreoretinopathy and proliferative diabetic retinopathy: differentiation of subpopulations. Br J Ophthalmol. 1993;77:731–733. doi: 10.1136/bjo.77.11.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong DS, Aiello L, Gardner TW, King GL, Blankenship G, Cavallerano JD, Ferris FL, III, Klein R. Diabetic retinopathy. Diabetes Care. 2003;26:226–229. doi: 10.2337/diacare.26.1.226. [DOI] [PubMed] [Google Scholar]
- Granstam E, Granstam SO. Regulation of uveal and retinal blood flow in STZ-diabetic and non-diabetic rats; involvement of nitric oxide. Curr Eye Res. 1999;19:330–337. doi: 10.1076/ceyr.19.4.330.5300. [DOI] [PubMed] [Google Scholar]
- Guth BD, Muller TH. DTTX30, a combined thromboxane receptor antagonist and thromboxane synthetase inhibitor, prevents coronary thrombosis in anesthetized dogs. Basic Res Cardiol. 1997;92:181–190. doi: 10.1007/BF00788635. [DOI] [PubMed] [Google Scholar]
- Higashi S, Clermont AC, Dhir V, Bursell SE. Reversibility of retinal flow abnormalities is disease-duration dependent in diabetic rats. Diabetes. 1998;47:653–659. doi: 10.2337/diabetes.47.4.653. [DOI] [PubMed] [Google Scholar]
- Horio N, Clermont AC, Abiko A, Abiko T, Shoelson BD, Bursell SE, Feener EP. Angiotensin AT(1) receptor antagonism normalizes retinal blood flow and acetylcholine-induced vasodilatation in normotensive diabetic rats. Diabetologia. 2004;47:113–123. doi: 10.1007/s00125-003-1262-x. [DOI] [PubMed] [Google Scholar]
- Kanamori A, Nakamura M, Mukuno H, Maeda H, Negi A. Diabetes has an additive effect on neural apoptosis in rat retina with chronically elevated intraocular pressure. Curr Eye Res. 2004;28:47–54. doi: 10.1076/ceyr.28.1.47.23487. [DOI] [PubMed] [Google Scholar]
- Klein R, Klein BE, Moss SE, Wong TY, Hubbard L, Cruickshanks KJ, Palta M. Retinal vascular abnormalities in persons with type 1 diabetes: the Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVIII. Ophthalmology. 2003;110:2118–2125. doi: 10.1016/S0161-6420(03)00863-7. [DOI] [PubMed] [Google Scholar]
- Lasserre B, Navarro-Delmasure C, Pham Huu CA, Catala J, Hollande E. Modifications in the TXA(2) and PGI(2) plasma levels and some other biochemical parameters during the initiation and development of non-insulin-dependent diabetes mellitus (NIDDM) syndrome in the rabbit. Prostaglandins Leukot Essent Fatty Acids. 2000;62:285–291. doi: 10.1054/plef.2000.0156. [DOI] [PubMed] [Google Scholar]
- Lee S, Harris NR. Losartan and ozagrel reverse retinal arteriolar constriction in non-obese diabetic mice. Microcirculation. 2008;15:379–387. doi: 10.1080/10739680701829802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Morgan GA, Harris NR. Ozagrel reverses streptozotocin-induced constriction of arterioles in rat retina. Microvascular Research. 2008 doi: 10.1016/j.mvr.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmeier RA, Braun RD, McRipley MA, Padnick LB, Ahmed J, Hatchell DL, McLeod DS, Lutty GA. Retinal hypoxia in long-term diabetic cats. Invest Ophthalmol Vis Sci. 1998;39:1647–1657. [PubMed] [Google Scholar]
- Mayhan WG, Simmons LK, Sharpe GM. Mechanism of impaired responses of cerebral arterioles during diabetes mellitus. Am J Physiol. 1991;260:H319–H326. doi: 10.1152/ajpheart.1991.260.2.H319. [DOI] [PubMed] [Google Scholar]
- Moreno A, De La Cruz JP, Garcia CJ, Sanchez dlC. Prostacyclin-thromboxane balance and retinal vascular pattern in rats with experimentally induced diabetes. Can J Ophthalmol. 1995;30:117–123. [PubMed] [Google Scholar]
- Nagai N, Izumi-Nagai K, Oike Y, Koto T, Satofuka S, Ozawa Y, Yamashiro K, Inoue M, Tsubota K, Umezawa K, Ishida S. Suppression of diabetes-induced retinal inflammation by blocking the angiotensin II type 1 receptor or its downstream nuclear factor-kappaB pathway. Invest Ophthalmol Vis Sci. 2007;48:4342–4350. doi: 10.1167/iovs.06-1473. [DOI] [PubMed] [Google Scholar]
- Pe’er J, Folberg R, Itin A, Gnessin H, Hemo I, Keshet E. Upregulated expression of vascular endothelial growth factor in proliferative diabetic retinopathy. Br J Ophthalmol. 1996;80:241–245. doi: 10.1136/bjo.80.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister SL, Hughes MJ, Rosolowsky M, Campbell WB. Role of contaminating platelets in thromboxane synthesis in primary cultures of human umbilical vein endothelial cells. Prostaglandins Other Lipid Mediat. 2002;70:39–49. doi: 10.1016/s0090-6980(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Quilley J, McGiff JC. Arachidonic acid metabolism and urinary excretion of prostaglandins and thromboxane in rats with experimental diabetes mellitus. J Pharmacol Exp Ther. 1985;234:211–216. [PubMed] [Google Scholar]
- Rossing P. The changing epidemiology of diabetic microangiopathy in type 1 diabetes. Diabetologia. 2005;48:1439–1444. doi: 10.1007/s00125-005-1836-x. [DOI] [PubMed] [Google Scholar]
- Roy MS, Klein R, O’Colmain BJ, Klein BE, Moss SE, Kempen JH. The prevalence of diabetic retinopathy among adult type 1 diabetic persons in the United States. Arch Ophthalmol. 2004;122:546–551. doi: 10.1001/archopht.122.4.546. [DOI] [PubMed] [Google Scholar]
- Shen RF, Tai HH. Thromboxanes: synthase and receptors. J Biomed Sci. 1998;5:153–172. doi: 10.1007/BF02253465. [DOI] [PubMed] [Google Scholar]
- Small KW, Stefansson E, Hatchell DL. Retinal blood flow in normal and diabetic dogs. Invest Ophthalmol Vis Sci. 1987;28:672–675. [PubMed] [Google Scholar]
- Sone H, Okuda Y, Kawakami Y, Yamashita K. Effects of high glucose concentration and a thromboxane synthase inhibitor on the production of thromboxane A2 and prostaglandin I2 and E2 by retinal endothelial cells. Life Sci. 1996;58:239–243. doi: 10.1016/0024-3205(95)02281-3. [DOI] [PubMed] [Google Scholar]
- Sun LK, Wahl P, Bilic G, Wuthrich RP. CD44-mediated cyclooxygenase-2 expression and thromboxane A2 production in RAW 264.7 macrophages. Inflamm Res. 2001;50:496–499. doi: 10.1007/PL00000224. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B, Jakubowski JA, Cohen RA. Contraction of diabetic rabbit aorta caused by endothelium-derived PGH2-TxA2. Am J Physiol. 1989;257:H1327–H1333. doi: 10.1152/ajpheart.1989.257.5.H1327. [DOI] [PubMed] [Google Scholar]
- Thomas DW, Mannon RB, Mannon PJ, Latour A, Oliver JA, Hoffman M, Smithies O, Koller BH, Coffman TM. Coagulation defects and altered hemodynamic responses in mice lacking receptors for thromboxane A2. J Clin Invest. 1998;102:1994–2001. doi: 10.1172/JCI5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unthank JL, Lash JM, Nixon JC, Sidner RA, Bohlen HG. Evaluation of carbocyanine-labeled erythrocytes for microvascular measurements. Microvasc Res. 1993;45:193–210. doi: 10.1006/mvre.1993.1018. [DOI] [PubMed] [Google Scholar]
- Wong TY, Klein R, Sharrett AR, Schmidt MI, Pankow JS, Couper DJ, Klein BE, Hubbard LD, Duncan BB. Retinal arteriolar narrowing and risk of diabetes mellitus in middle-aged persons. JAMA. 2002;287:2528–2533. doi: 10.1001/jama.287.19.2528. [DOI] [PubMed] [Google Scholar]
- Wright WS, Harris NR. Ozagrel attenuates early streptozotocin-induced constriction of arterioles in the mouse retina. Exp Eye Res. 2008;86:528–536. doi: 10.1016/j.exer.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Jiang B, Maitland KA, Bayat H, Gu J, Nadler JL, Corda S, Lavielle G, Verbeuren TJ, Zuccollo A, Cohen RA. The thromboxane receptor antagonist s18886 attenuates renal oxidant stress and proteinuria in diabetic apolipoprotein e-deficient mice. Diabetes. 2006;55:110–119. [PubMed] [Google Scholar]
- Yam JC, Kwok AK. Update on the treatment of diabetic retinopathy. Hong Kong Med J. 2007;13:46–60. [PubMed] [Google Scholar]
- Yamashiro K, Tsujikawa A, Ishida S, Usui T, Kaji Y, Honda Y, Ogura Y, Adamis AP. Platelets accumulate in the diabetic retinal vasculature following endothelial death and suppress blood-retinal barrier breakdown. Am J Pathol. 2003;163:253–259. doi: 10.1016/S0002-9440(10)63648-6. [DOI] [PMC free article] [PubMed] [Google Scholar]



