Abstract
Aims
Ion channel reorganization is a critical step in the pro-arrhythmogenic remodelling process that occurs in heart disease. Ankyrin-B (AnkB) is required for targeting and stabilizing ion channels, exchangers, and pumps. Despite a wealth of knowledge implicating the importance of AnkB in human cardiovascular physiology, nothing is known regarding the role of AnkB in common forms of acquired human disease.
Methods and results
We present the first report of AnkB regulation following myocardial infarction (MI). AnkB protein levels were reduced in the infarct border zone 5 days following coronary artery occlusion in the canine. We also observed a dramatic increase in AnkB mRNA levels 5 days post-occlusion. Surprisingly, the expression of the upstream AnkB cytoskeletal component β2-spectrin was unchanged in post-infarct tissues. However, protein levels and/or membrane expression of downstream AnkB-associated ion channels and transporters Na+/K+ ATPase, Na+/Ca2+ exchanger, and IP3 receptor were altered 5 days post-occlusion. Interestingly, protein levels of the protein phosphatase 2A, an AnkB-associated signalling protein, were significantly affected 5 days post-occlusion. AnkB and PP2A protein levels recovered by 14 days post-occlusion, whereas Na+/K+ ATPase levels recovered by 2 months post-occlusion.
Conclusion
These findings reveal the first evidence of ankyrin remodelling following MI and suggest an unexpected divergence point for regulation between ankyrin and the underlying cytoskeletal network. These findings suggest a logical, but unexpected, molecular mechanism underlying ion channel and transporter remodelling following MI.
KEYWORDS: Arrhythmia (mechanisms), Infarction, Remodelling, Signal transduction, Cytoskeleton
1. Introduction
Heart disease is a leading cause of death in the US, with arrhythmia being the immediate cause of death in most cases.1 Myocardial infarction (MI) triggers dramatic structural and electrical remodelling of the heart, creating an arrhythmogenic substrate and promoting advancement of disease (e.g. heart failure).2 Electrical remodelling has been studied extensively in a thin rim of surviving tissue neighbouring the infarct (infarct border zone, BZ), because re-entrant arrhythmias are highly localized to the BZ region.2–4
Electrical remodelling is a complex, time-dependent process involving changes to a host of sarcolemmal ion channels and transporters.4,5 An elegant series of studies have shown subcellular redistribution of ion channels within days following coronary artery occlusion in areas corresponding to locations of re-entrant circuits,4–8 suggesting a pro-arrhythmic role for ion channel subcellular redistribution post-infarction. The molecular mechanisms responsible for electrical remodelling are not well understood.
Ankyrins are a family of adapter proteins with critical roles in vertebrate physiology. Over the past 5 years, ankyrins have been implicated in cardiovascular function. Specifically, both ankyrin-G and ankyrin-B (AnkB) have been associated with human ventricular tachycardia.9,10 Ankyrin-B (encoded by human ANK2) dysfunction is associated with a complex cardiac phenotype in humans [type 4 long QT syndrome (LQTS) or ‘ankyrin-B arrhythmia syndrome’].10 Human ANK2 variant carriers display a range of cardiac phenotypes, including sinus node dysfunction, conduction defects, and ventricular tachycardia.10–12 To date, nine ANK2 loss-of-function variants have been identified in the human population, each with unique cellular properties.10,12 Mice lacking AnkB phenocopy type 4 LQTS and AnkB-deficient cardiomyocytes display abnormal calcium homeostasis due to abnormal targeting of key membrane proteins, resulting in cellular afterdepolarization.10,13
The role of AnkB in common acquired forms of heart disease is unknown. Here, we present the first report of AnkB regulation in a large animal model of MI. Specifically, we demonstrate that abnormal AnkB mRNA and protein levels are present following MI in a well-validated canine model system. Furthermore, we identify parallel changes in the protein levels and/or membrane expression of AnkB-associated proteins Na+/K+ ATPase, Na+/Ca2+ exchanger, inositol 1,4,5-trisphosphate (IP3) receptor, and protein phosphatase 2A (PP2A). These findings identify a potential molecular mechanism underlying ion channel and transporter remodelling in hearts following MI and suggest a new class of functional channelopathies in common heart disease due to abnormal cellular localization.
2. Methods
2.1. Experimental model of myocardial infarction
A well-characterized and widely utilized protocol was employed to produce MI in healthy mongrel dogs.6,14–21 Briefly, MI was produced by total coronary artery occlusion, as described previously.19 A cardiectomy was performed 48 h, 5 days, 14 days, or 2 months after surgery. Thin tissue slices from visible epicardial BZ, where previous studies have shown re-entry to occur,6 and from a remote area away from the infarct (left ventricular base) were flash frozen for analysis or subjected to a cell dispersion protocol. The collection and extensive characterization of myocytes from this preparation has been performed and published by our group.8,15,18,19,22,23 Specifically, isolated myocytes were identical in appearance to cells used previously for electrophysiological studies,6,18,19,22 showing triangular action potentials and reduced Na+ currents in the BZ.18 This investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (Pub. No. 85-23, 1996).
Tissue lysates were prepared as described previously.10 RNA was isolated using the RNeasy Kit (Qiagen) and quantified by spectrophotometer. cDNA was amplified using SuperScript III Reverse Transcriptase (Invitrogen) and the antisense primers for each specific product. Semi-quantitative polymerase chain reaction (PCR) was performed in 20 µL reaction volumes. Sense and antisense primers were used at a concentration of 10 µM. The PCR was performed using Taq polymerase and 40 cycles of 95°C for 30 s, 63°C for 30 s, and 72°C for 30 s. Four dilutions (1:5) of each PCR reaction were run on a 2% agarose gel with no-template controls.
2.2. Primers and sequences
Primers were designed to canine sequences for ANK2 (Genbank XM_846341.1, sense 5′-CCC TGA ATG GTT TTA CTC CAC TGC-3′ and antisense 5′-GGC CAG ACT CTG TTA TAG CTT GG-3′) and CASQ2 (Genbank XM_845004.1, sense 5′-GCA GCT GTG GCC AAG AAA CTA GG-3′ and antisense 5′-AAT GCC TGC AGC TCT CGT TC-3′).
2.3. Immunoblotting
Ventricular lysates were prepared for immunoblotting analysis, as described previously.10 Briefly, frozen tissue was ground into fine powder and resuspended in four volumes of buffer [1 mM NaHCO3, 5 mM EDTA, 1 mM EGTA, pH 8.0 containing 1 mM phenylmethyl sulfonyl fluoride, 1 mM 4-(2-aminoethyl) benzenesulphonylfluoride hydrochloride, 10 µg/mL aprotinin, 10 µg/mL bestatin, 10 µg/mL leupeptin, and 10 µg/mL pepstatin] and homogenized using a Dounce homogenizer. Equal quantities of protein lysate [protein concentrations determined using bicinchoninic acid (BCA) protein assay (Pierce)] were analysed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (3–8% Tris acetate gels) and immunoblotting. Protein samples were analysed on the same gel to allow appropriate quantitative comparison between samples. Antibodies include affinity-purified antibodies to AnkB,24 β-spectrin,25 PP2A catalytic subunit (Upstate), Na+/K+ ATPase alpha,10 NCX1 (Swant), and IP3 receptor.24 Equal protein loading was verified by analysing Coomassie and Ponceau stains of gels and blots. Additionally, any slight differences in protein loading were corrected using an internal control standard [rabbit polyclonal antibody to actin (Sigma Cruz)].
2.4. Immunostaining
Myocytes were isolated from BZ and remote regions of infarcted hearts 5-days post-occlusion, as described previously.18 A series of previously published studies by our group and others have documented this protocol as appropriate for both electrophysiological and immunofluorescence studies of isolated myocytes.6,15,17–19,23,26,27 Moreover, isolated myocytes were identical in appearance to cells used in identical studies to define electrical remodelling after MI.6,15,18,23 After isolation, myocytes were fixed in 4% paraformaldehyde, blocked/permeabilized in phosphate-buffered saline (PBS) containing 0.075% Triton X-100 and 3% fish oil gelatin (Sigma), and incubated in primary antibody overnight at 4°C. After PBS washes, myocytes were incubated in secondary antibody (Alexa 488, 568; Molecular Probes) for 2 h at room temperature. Following secondary antibody treatment, cells were extensively washed, covered with Vectashield Imaging Medium (Vector), and cover slips (no. 1) were applied. Images were collected on a Zeiss 510 Meta confocal microscope [63 power oil 1.40 NA (Zeiss), pinhole equals 1.0 Airy Disc], using Carl Zeiss Imaging software (Release version 4.0 SP1). Images were collected using similar confocal protocols at room temperature. Images were imported into Adobe Photoshop CS for cropping and linear contrast adjustment. Antibodies include those described for immunoblotting and a mouse monoclonal antibody to alpha-actinin (Sigma). Alpha-actinin does not associate with AnkB and was used as a control protein to confirm that the reduced AnkB observed in BZ cells was not simply an artefact of the cell isolation procedure. For immunostaining experiments, antibodies were validated on cardiomyocytes by peptide blocking experiments or pre-immune sera. For confocal experiments, more than 20 cardiomyocytes were visualized for each condition.
2.5. Statistics
When appropriate, differences between groups were analysed with analysis of variance and least-squares difference post hoc test. A value of P < 0.05 was considered statistically significant. Values are expressed as mean ± SD.
3. Results
3.1. Ankyrin-B mRNA levels are increased following myocardial infarction
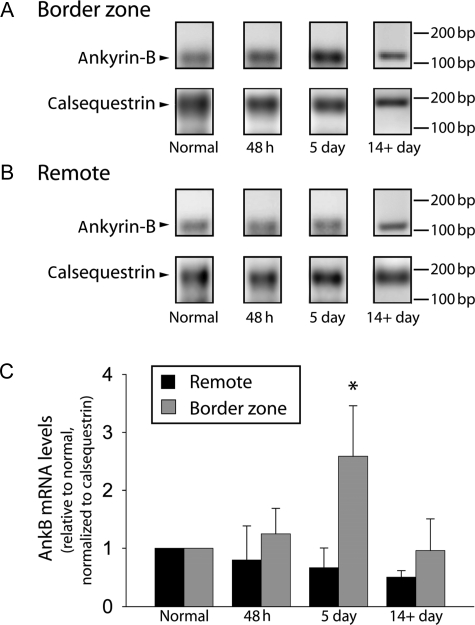
Despite clear roles for AnkB function in normal cardiac physiology, there are no reports detailing AnkB regulation in common cardiac electrical diseases (e.g. heart failure and ischaemia). We therefore analysed AnkB (ANK2) mRNA levels in a well-validated, large animal post-MI model of arrhythmias4 at 48 h, 5 days, and 14+ days post-occlusion (see section 2 for protocol details). Forty-eight hours post-occlusion, we observed no change in canine BZ AnkB mRNA levels (Figure 1; P = NS). However, ANK2 mRNA increased significantly in BZ 5-day post-occlusion samples (Figure 1; P < 0.01), whereas AnkB mRNA levels in remote regions of same hearts showed no change (Figure 1, 48 h, 5 days, or 14+ day; P = NS vs. normal). ANK2 mRNA levels in the BZ returned to normal by 14 days post-occlusion (Figure 1, P = NS vs. normal). Consistent with previous studies,28 we observed no change in calsequestrin 2 (CASQ2) mRNA levels post-infarction (data not shown).
Figure 1.
Ankyrin-B RNA levels increase in the border zone 5 days post-occlusion. Representative gels showing AnkB and calsequestrin RNA levels in (A) border zone and (B) remote regions of non-infarcted (normal) and at 48 h, 5 days, and 14+ days post-occlusion. (C) Densitometric measurements of AnkB RNA levels in remote and BZ regions of non-infarcted (normal) hearts and at 48 h, 5 days, and 14+ days post-occlusion. Error bars designate standard deviation (*P < 0.01 compared with normal BZ or remote, n = 4). AnkB values are presented in reference to a well-characterized internal control (calsequestrin28) to ensure consistent sample preparation and gel loading.
3.2. Ankyrin-B protein levels are reduced following myocardial infarction
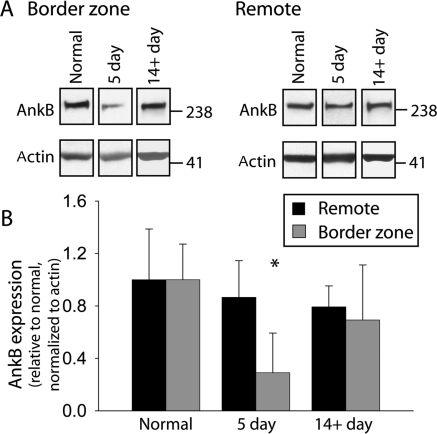
We next performed immunoblot analysis to determine whether changes in ANK2 mRNA following MI would be reflected in AnkB polypeptide expression (Figure 2). Surprisingly, AnkB protein levels were dramatically reduced in the 5-day BZ (P < 0.01), despite increased mRNA levels (Figure 1). AnkB protein expression in the BZ returned to normal levels by 14 days post-occlusion (Figure 2, P = NS vs. normal), consistent with the time course of recovery in BZ ANK2 mRNA levels. We observed no statistical difference in AnkB levels in remote regions from normal, 5 day, or 14+ day post-occlusion hearts (Figure 2; P = NS). Actin levels (loading control) were unchanged in either BZ or remote regions (Figure 2).
Figure 2.
Ankyrin-B protein levels decrease in the border zone 5 days post-occlusion. (A) Representative immunoblots and (B) densitometric measurements (normalized to actin and expressed relative to normal levels) of AnkB from remote and BZ regions of normal and infarcted hearts. Error bars designate standard deviation (*P < 0.01 compared with normal BZ or remote, n = 4 for normal, n = 5 for 5-day, and n = 3 for 14+ day infarct). Densitometric measurements were made from all samples analysed on same gel and normalized to the corresponding actin levels from the same blot. Equal protein loading was ensured by BCA assay and verified by analysis of Coomassie and Ponceau stains of gel and blots.
3.3. Ankyrin-B localization is decreased following myocardial infarction
We next investigated whether AnkB redistribution occurred in single myocytes isolated from the BZ 5 days post-occlusion using immunofluorescence. Previous studies demonstrate that AnkB is distributed both on the ventricular cardiomyocyte transverse-tubule network and on M-lines.10,25 Confocal analysis of 5-day post-occlusion BZ myocytes revealed reduced AnkB expression at myocyte membrane domains compared with remote myocytes (Figure 3; note punctate intracellular staining in the right panel), consistent with changes in AnkB protein expression (Figure 2). Importantly, alpha-actinin cellular expression was nearly identical between remote and BZ myocytes (Figure 3), an indicator that AnkB reduction in BZ myocytes is not simply an artefact of the cell isolation procedure.
Figure 3.
Cellular expression and distribution of ankyrin-B are affected 5 days post-occlusion. Isolated myocytes from remote and BZ regions of 5-day infarcted hearts were immunostained using antibodies against AnkB (middle) and alpha-actinin (left). Although AnkB membrane expression is reduced 5 days post-occlusion, alpha-actinin expression and localization are unaffected. Scale bars, 10 µm. Right panels show higher magnification images of AnkB localization.
3.4. Ankyrin-upstream cytoskeletal elements are unaffected in post-myocardial infarction heart
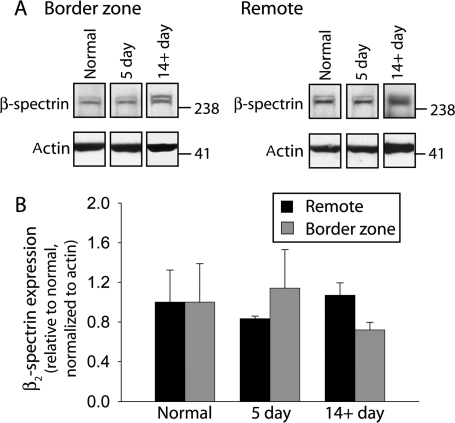
In excitable cells including cardiomyocytes and neurons, ankyrins are tightly linked with the underlying cytoskeleton by the large actin-associated molecule β2-spectrin.25 Mice lacking spectrin isoforms display abnormal targeting of ankyrin polypeptides and down-stream ankyrin–protein partners (e.g. ion channels/transporters).29 We therefore tested whether loss of β2-spectrin (primary cardiac spectrin isoform25) occurred in parallel with loss of AnkB in 5-day post-occlusion BZ tissues. Unexpectedly, immunoblot analysis revealed no difference in β2-spectrin levels in BZ or remote regions at 5 days or 14+ days post-occlusion (Figure 4). Furthermore, we observed no difference in BZ or remote regions at 5 days in protein levels of a structurally similar member of the ankyrin family, ankyrin-R (see Supplementary material online, Figure S1). These surprising data demonstrate that BZ remodelling occurs for AnkB, but not for functionally coupled upstream cytoskeletal elements.
Figure 4.
β2-spectrin protein levels are unchanged in the border zone post-occlusion. (A) Representative immunoblots and (B) densitometric measurements (normalized to actin and expressed relative to normal levels) of β2-spectrin from remote and BZ regions of normal and infarcted hearts. Error bars designate standard deviation (n = 4 for each group, except 14+ day where n = 3). Densitometric measurements were made from samples ran on same gel and normalized to the corresponding actin levels. Equal protein loading was ensured by BCA assay and Coomassie and Ponceau stains of gel and blots.
3.5. Remodelling of ankyrin-B-associated proteins following myocardial infarction
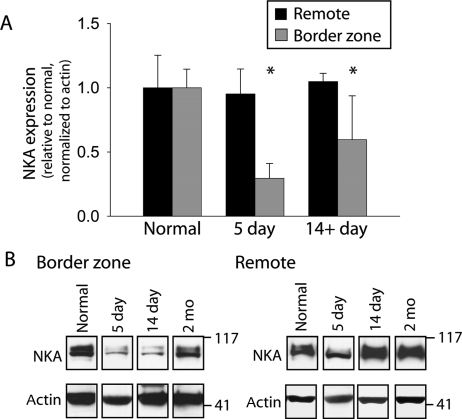
Dramatic electrical remodelling occurs in myocytes isolated from the BZ 5 days post-occlusion, characterized by changes in ion channel properties and action potential morphology.4 For example, loss of the transient outward K+ current, Ito, and dramatic reduction of Na+ current, INa, contribute to the reduced amplitude and diminished phase 1 early repolarization ‘notch’ of the BZ action potential 5 days post-occlusion.18,19 Based on our data showing reduced AnkB expression in the BZ 5 days post-occlusion, we next determined whether parallel remodelling occurs for the known AnkB-associated ion channels and transporters Na+/K+ ATPase, Na+-Ca2+ exchanger, and IP3 receptor.30 We found no significant difference in expression levels of the IP3R and NCX1 in BZ at 5 days or 14+ days post-occlusion when compared with normal (1.00 ± 0.29, n = 4; 1.05 ± 0.54, n = 4; and 0.66 ± 0.37, n = 3 in normal, 5 days, and 14+ days, respectively, for IP3R, P = NS and 1.00 ± 0.43, n = 4; 1.00 ± 0.18, n = 4; and 0.79 ± 0.54, n = 3 in normal, 5 days, and 14+ days, respectively, for NCX1, P = NS). However, NKA protein expression was dramatically reduced in the BZ 5 days post-occlusion when compared with normal (Figure 5), consistent with the reduction in AnkB expression (Figure 2). Interestingly, NKA expression remained significantly reduced even at 14+ days, revealing time course of recovery that follows recovery of AnkB. Immunoblots in Figure 5B indicate that, more specifically, NKA expression remains depressed at 14 days post-occlusion, but recovers to normal by 2 months.
Figure 5.
NKA protein levels decrease in the infarct border zone. (A) Densitometric measurements and (B) representative immunoblots of NKA from remote and BZ regions of normal, 5 day, and 14+ day infarcted hearts. Error bars designate standard deviation (n = 4 except n = 3 for 14+ day).
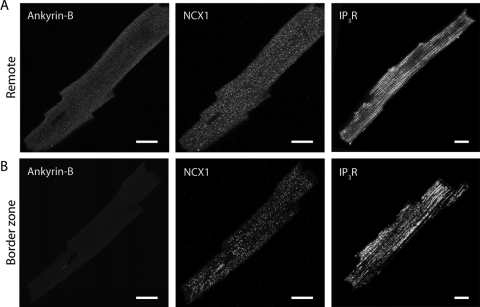
Although we measured decreased expression of NKA by immunoblot in the BZ, we detected no difference in protein levels of NCX or IP3R. On the basis of the role of ankyrin in targeting ion channels to specific subcellular domains, we next determined whether subcellular redistribution of NCX and IP3R occurred in 5-day post-occlusion BZ myocytes. Immunofluorescence studies revealed cellular redistribution of both IP3R and NCX in BZ myocytes 5 days post-occlusion (Figure 6). Specifically, prominent t-tubular localization of NCX and IP3R is apparent in remote but not BZ myocytes. Thus, cellular distribution but not total protein levels of NCX and IP3R changes in the BZ 5-day post-occlusion.
Figure 6.
Cellular redistribution of NCX and IP3R in border zone myocytes. Isolated myocytes from remote and BZ regions of 5-day infarcted hearts were immunostained using antibodies against AnkB (left), NCX1 (middle), and IP3R (right). Localization of NCX1 and IP3R in BZ myocytes is less pronounced at t-tubules in BZ myocytes, where AnkB expression is reduced. Scale bars, 10 µm.
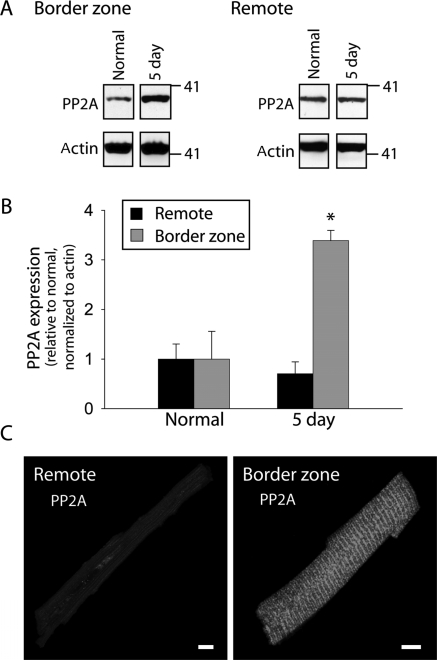
Recent studies demonstrate that AnkB directly associates with the regulatory subunit of PP2A (B56α subunit).31,32 Furthermore, transgenic expression of a mutant PP2A that cannot bind B56α subunit (or any other B subunit) results in the increased expression of PP2A (catalytic subunit) and dilated cardiomyopathy.33,34 Based on these findings, we hypothesized that reduced AnkB levels would phenocopy this genetic intervention and also result in increased PP2A levels following MI. We therefore determined whether expression and/or distribution of PP2A was altered in the BZ. Interestingly, by 5-days post-occlusion, the expression of the PP2A catalytic subunit (constituent of the core enzyme) increased to more than three times its normal level (Figure 7). These changes in the protein expression were paralleled by increased membrane expression of PP2A in BZ myocytes compared with remote 5 days post-occlusion (Figure 7C). By 14+ days post-occlusion, PP2A protein levels in the BZ returned to normal levels (1.00 ± 0.56, n = 4 and 0.54 ± 0.18, n = 3 in normal and BZ, respectively, P = NS, data not shown), following a similar time course as changes in AnkB expression (Figure 2). These surprising data indicate that remodelling of AnkB post-infarction may trigger downstream remodelling, not only of ion channels and transporters (Figures 5 and 6) but also of signalling molecules critical for normal cardiac function and linked to cardiovascular disease.35,36
Figure 7.
Altered PP2A expression in the border zone 5 days post occlusion. (A) Representative immunoblots and (B) densitometric measurements (normalized to actin and expressed relative to normal levels) of PP2A catalytic subunit from remote and BZ regions of normal and 5-day infarcted hearts. Error bars designate standard deviation (n = 4 for each group). (C) Isolated myocytes from remote and BZ regions of 5-day infarcted hearts were immunostained using antibody against the PP2A catalytic subunit. Scale bars, 10 µm.
4. Discussion
We present the first report of AnkB regulation in an animal model of MI. Specifically, AnkB mRNA levels increase, whereas protein levels decrease in the 5-day BZ post-infarction. At the single myocyte level, expression and distribution of AnkB are dramatically affected post-infarction. We report that β2-spectrin levels are surprisingly unaffected by MI. Finally, we identify significant remodelling of downstream AnkB-associated proteins PP2A, NKA, IP3R, and NCX1. Together, these findings provide the first data on AnkB remodelling post-infarction and reveal that while functionally coupled ankyrin-upstream cytoskeletal elements are unaffected, downstream ankyrin-associated proteins important for cell signalling, excitability, and homeostasis are dramatically altered.
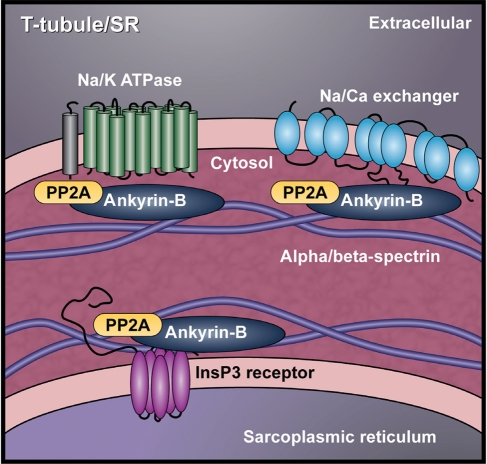
Abnormal action potentials in human heart disease were first reported almost 50 years ago.5 Since then, important studies from several groups have revealed specific ion channel changes responsible for action potential remodelling after MI.4–7,15,18,19,23,37–41 Although significant attention has been paid to remodelling of ion channel/transporter expression, distribution, and/or kinetics in heart disease, relatively little is known about roles or mechanism of abnormal channel localization in the remodelling process. AnkB targets multiple ion channels, transporters, and signalling molecules in heart13 (Figure 8). Our findings that AnkB levels are reduced in BZ myocytes suggest a potential mechanism for ion channel and transporter remodelling in disease. Namely, these new data raise the prospect of dysfunctional channel targeting as a mechanism for arrhythmogenesis post-MI. In fact, we identified remodelling changes in the expression and/or cellular distribution of the AnkB-associated proteins PP2A, NKA, IP3R, and NCX1.
Figure 8.
Cardiac ankyrin-B macromolecular complex. AnkB establishes a complex at the cardiomyocyte cell membrane consisting of the Na+/Ca2+ exchanger, Na+/K+ ATPase, the IP3 receptor, and PP2A. Reduction of AnkB in heart disease may disrupt the subcellular localization of these critical proteins.
Previous studies have reported changes to AnkB-binding partners in heart disease.17,27,42–45 Although NKA activity has not been characterized in the infarct BZ, impaired NKA function is believed to play a role in abnormal intracellular Na+ homeostasis and NCX function in heart failure.43–45 Our data indicate that similar downregulation of NKA in BZ myocytes may contribute to abnormal ion homeostasis post-infarction.17,27 Furthermore, our findings raise the important question of whether AnkB downregulation underlies NKA dysfunction in cardiac hypertrophy and heart failure.
Beyond changes in ankyrin-associated ion channels and transporters, we report altered PP2A expression in BZ myocytes 5 days post-occlusion. This unexpected finding has important implications for the role of AnkB in heart disease. PP2A upregulation has been associated with connexin43 dephosphorylation and slowed conduction in non-ischaemic heart failure.35 Overexpression of the PP2A catalytic subunit has also been shown to impair cardiac function.36 Previous studies from our group have shown that AnkB targets PP2A regulatory subunit (B56α) in cardiomyocytes.31 Interestingly, the transgenic expression of mutant PP2A with impaired binding of the beta-subunit increases levels of core enzyme and produces dilated cardiomyopathy in mice.34 It is intriguing to consider the possibility that AnkB downregulation in the infarct BZ promotes structural remodelling by impairing PP2A binding of the beta subunit and/or increasing the amount of the catalytic PP2A subunit, similar to the mechanism observed in transgenic mice.34 It will be important for future studies to determine whether expression and/or localization of these and other AnkB-binding partners are altered in the BZ as a direct result of AnkB remodelling.
It is interesting to note that remodelling of AnkB and associated proteins follows a time course similar to electrical remodelling documented previously in the canine infarct BZ.21,23 Five days post-occlusion, action potentials recorded from isolated BZ myocytes show a very different morphology from those recorded in normal myocytes due to electrical remodelling.21 These electrical differences largely disappear by 2 months (animals do not develop heart failure). Previous studies have shown that the time course of ion channel remodelling in the BZ closely follows the time course of AP changes.23 For example, the transient outward current, Ito, is completely absent from 5-day BZ myocytes, but re-emerges at 14 days post-occlusion and returns completely to normal by 2 months.23 Following a similar pattern, AnkB and NKA expression levels decrease by 5 days post-occlusion but recover by 14+ days. Our studies indicate that although AnkB expression is normal in the 14-day post-occlusion heart, NKA levels remained depressed, suggesting that changes in NKA follow changes in AnkB. In contrast, Ca2+ current shows a much slower time course of recovery with density and kinetics that remain different from normal even at 2 months post-occlusion.23 Thus, it is very likely that multiple non-redundant pathways govern the remodelling process after MI. Future studies are required to dissect these different pathways and to define their unique roles in creating an arrhythmogenic substrate after MI. Furthermore, it will be important to establish causality between changes in AnkB and associated proteins.
The molecular mechanism responsible for decreased AnkB expression post-infarction remains to be determined. However, the AnkB protein expression decreases despite increased mRNA levels in the BZ. Furthermore, AnkB downregulation occurs without any change in β2-spectrin or alpha-actinin. These findings suggest that AnkB downregulation in the BZ most likely occurs post-translationally and without changes to important upstream cytoskeletal binding partners. It will be interesting for future studies to address whether regulation of AnkB occurs in other disease states (e.g. atrial fibrillation and acute ischaemia).
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by the National Institutes of Health (HL084583 and HL083422 to P.J.M.; HL66140 to P.A.B.) and the Pew Scholars Trust (P.J.M.).
Supplementary Material
References
- 1.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Wit AL, Janse MJ. The Ventricular Arrhythmias of Ischemia and Infarction: Electrophysiological Mechanisms. Mount Kisco, NY: Futura Publishing Company, Inc.; 1992. [Google Scholar]
- 3.Dillon SM, Allessie MA, Ursell PC, Wit AL. Influences of anisotropic tissue structure on reentrant circuits in the epicardial border zone of subacute canine infarcts. Circ Res. 1988;63:182–206. doi: 10.1161/01.res.63.1.182. [DOI] [PubMed] [Google Scholar]
- 4.Pinto JM, Boyden PA. Electrical remodeling in ischemia and infarction. Cardiovasc Res. 1999;42:284–297. doi: 10.1016/s0008-6363(99)00013-9. [DOI] [PubMed] [Google Scholar]
- 5.Nattel S, Maguy A, Le Bouter S, Yeh YH. Arrhythmogenic ion-channel remodeling in the heart: heart failure, myocardial infarction, and atrial fibrillation. Physiol Rev. 2007;87:425–456. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 6.Baba S, Dun W, Cabo C, Boyden PA. Remodeling in cells from different regions of the reentrant circuit during ventricular tachycardia. Circulation. 2005;112:2386–2396. doi: 10.1161/CIRCULATIONAHA.105.534784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peters NS, Coromilas J, Severs NJ, Wit AL. Disturbed connexin43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation. 1997;95:988–996. doi: 10.1161/01.cir.95.4.988. [DOI] [PubMed] [Google Scholar]
- 8.Mays D, Boyden PA, Tamkun M. Redistribution of the Kv1.5 K+ channel protein on the surface of myocytes from the epicardial border zone of the infarcted canine heart. CV Pathobiol. 1997;2:79–87. [Google Scholar]
- 9.Mohler PJ, Rivolta I, Napolitano C, LeMaillet G, Lambert S, Priori SG, et al. Nav1.5 E1053K mutation causing Brugada syndrome blocks binding to ankyrin-G and expression of Nav1.5 on the surface of cardiomyocytes. Proc Natl Acad Sci USA. 2004;101:17533–17538. doi: 10.1073/pnas.0403711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421:634–639. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 11.Le Scouarnec S, Bhasin N, Vieyres C, Hund TJ, Cunha SR, Koval O, et al. Dysfunction in ankyrin-B-dependent ion channel and transporter targeting causes human sinus node disease. Proc Natl Acad Sci USA. 2008;105:15617–15622. doi: 10.1073/pnas.0805500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohler PJ, Le Scouarnec S, Denjoy I, Lowe JS, Guicheney P, Caron L, et al. Defining the cellular phenotype of ‘ankyrin-B syndrome’ variants: human ANK2 variants associated with clinical phenotypes display a spectrum of activities in cardiomyocytes. Circulation. 2007;115:432–441. doi: 10.1161/CIRCULATIONAHA.106.656512. [DOI] [PubMed] [Google Scholar]
- 13.Cunha SR, Mohler PJ. Cardiac ankyrins: essential components for development and maintenance of excitable membrane domains in heart. Cardiovasc Res. 2006;71:22–29. doi: 10.1016/j.cardiores.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 14.Billman GE, Schwartz PJ, Stone HL. Baroreceptor reflex control of heart rate: a predictor of sudden cardiac death. Circulation. 1982;66:874–880. doi: 10.1161/01.cir.66.4.874. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal R, Boyden PA. Diminished Ca2+ and Ba2+ currents in myocytes surviving in the epicardial border zone of the 5-day infarcted canine heart. Circ Res. 1995;77:1180–1191. doi: 10.1161/01.res.77.6.1180. [DOI] [PubMed] [Google Scholar]
- 16.Harris AS. Delayed development of ventricular ectopic rhythms following experimental coronary occlusion. Circulation. 1950;1:1318–1328. doi: 10.1161/01.cir.1.6.1318. [DOI] [PubMed] [Google Scholar]
- 17.Licata A, Aggarwal R, Robinson RB, Boyden P. Frequency dependent effects on Cai transients and cell shortening in myocytes that survive in the infarcted heart. Cardiovasc Res. 1997;33:341–350. doi: 10.1016/s0008-6363(96)00246-5. [DOI] [PubMed] [Google Scholar]
- 18.Lue WM, Boyden PA. Abnormal electrical properties of myocytes from chronically infarcted canine heart. Alterations in Vmax and the transient outward current. Circulation. 1992;85:1175–1188. doi: 10.1161/01.cir.85.3.1175. [DOI] [PubMed] [Google Scholar]
- 19.Pu J, Boyden PA. Alterations of Na+ currents in myocytes from epicardial border zone of the infarcted heart. A possible ionic mechanism for reduced excitability and postrepolarization refractoriness. Circ Res. 1997;81:110–119. doi: 10.1161/01.res.81.1.110. [DOI] [PubMed] [Google Scholar]
- 20.Hund TJ, Decker KF, Kanter E, Mohler PJ, Boyden PA, Schuessler RB, et al. Role of activated CaMKII in abnormal calcium homeostasis and INa remodeling after myocardial infarction: insights from mathematical modeling. J Mol Cell Cardiol. 2008;45:420–428. doi: 10.1016/j.yjmcc.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ursell PC, Gardner PI, Albala A, Fenoglio JJ, Jr, Wit AL. Structural and electrophysiological changes in the epicardial border zone of canine myocardial infarcts during infarct healing. Circ Res. 1985;56:436–451. doi: 10.1161/01.res.56.3.436. [DOI] [PubMed] [Google Scholar]
- 22.Pu J, Balser JR, Boyden PA. Lidocaine action on Na+ currents in ventricular myocytes from the epicardial border zone of the infarcted heart. Circ Res. 1998;83:431–440. doi: 10.1161/01.res.83.4.431. [DOI] [PubMed] [Google Scholar]
- 23.Dun W, Baba S, Yagi T, Boyden PA. Dynamic remodeling of K+ and Ca2+ currents in cells that survived in the epicardial border zone of canine healed infarcted heart. Am J Physiol Heart Circ Physiol. 2004;287:H1046–H1054. doi: 10.1152/ajpheart.00082.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohler PJ, Davis JQ, Davis LH, Hoffman JA, Michaely P, Bennett V. Inositol 1,4,5-trisphosphate receptor localization and stability in neonatal cardiomyocytes requires interaction with ankyrin-B. J Biol Chem. 2004;279:12980–12987. doi: 10.1074/jbc.M313979200. [DOI] [PubMed] [Google Scholar]
- 25.Mohler PJ, Yoon W, Bennett V. Ankyrin-B targets beta2-spectrin to an intracellular compartment in neonatal cardiomyocytes. J Biol Chem. 2004;279:40185–40193. doi: 10.1074/jbc.M406018200. [DOI] [PubMed] [Google Scholar]
- 26.Aggarwal R, Pu J, Boyden PA. Ca2+-dependent outward currents in myocytes from epicardial border zone of 5-day infarcted canine heart. Am J Physiol Heart Circ Physiol. 1997;273:H1386–H1394. doi: 10.1152/ajpheart.1997.273.3.H1386. [DOI] [PubMed] [Google Scholar]
- 27.Pu J, Robinson RB, Boyden PA. Abnormalities in Cai handling in myocytes that survive in the infarcted heart are not just due to alterations in repolarization. J Mol Cell Cardiol. 2000;32:1509–1523. doi: 10.1006/jmcc.2000.1184. [DOI] [PubMed] [Google Scholar]
- 28.Hasenfuss G. Alterations of calcium-regulatory proteins in heart failure. Cardiovasc Res. 1998;37:279–289. doi: 10.1016/s0008-6363(97)00277-0. [DOI] [PubMed] [Google Scholar]
- 29.Komada M, Soriano P. BetaIV-spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. J Cell Biol. 2002;156:337–348. doi: 10.1083/jcb.200110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohler PJ, Davis JQ, Bennett V. Ankyrin-B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T-tubule/SR microdomain. PLoS Biol. 2005;3:e423. doi: 10.1371/journal.pbio.0030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhasin N, Cunha SR, Mduannayake M, Gigena MS, Rogers TB, Mohler PJ. Molecular basis for PP2A regulatory subunit B56 alpha targeting in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2007;293:H109–H119. doi: 10.1152/ajpheart.00059.2007. [DOI] [PubMed] [Google Scholar]
- 32.Cunha SR, Mohler PJ. Obscurin targets ankyrin-B and protein phosphatase 2A to the cardiac M-line. J Biol Chem. 2008;283:31968–31980. doi: 10.1074/jbc.M806050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruediger R, Brewis N, Ohst K, Walter G. Increasing the ratio of PP2A core enzyme to holoenzyme inhibits Tat-stimulated HIV-1 transcription and virus production. Virology. 1997;238:432–443. doi: 10.1006/viro.1997.8873. [DOI] [PubMed] [Google Scholar]
- 34.Brewis N, Ohst K, Fields K, Rapacciuolo A, Chou D, Bloor C, et al. Dilated cardiomyopathy in transgenic mice expressing a mutant A subunit of protein phosphatase 2A. Am J Physiol Heart Circ Physiol. 2000;279:H1307–H1318. doi: 10.1152/ajpheart.2000.279.3.H1307. [DOI] [PubMed] [Google Scholar]
- 35.Ai X, Pogwizd SM. Connexin 43 downregulation and dephosphorylation in nonischemic heart failure is associated with enhanced colocalized protein phosphatase type 2A. Circ Res. 2005;96:54–63. doi: 10.1161/01.RES.0000152325.07495.5a. [DOI] [PubMed] [Google Scholar]
- 36.Gergs U, Boknik P, Buchwalow I, Fabritz L, Matus M, Justus I, et al. Overexpression of the catalytic subunit of protein phosphatase 2A impairs cardiac function. J Biol Chem. 2004;279:40827–40834. doi: 10.1074/jbc.M405770200. [DOI] [PubMed] [Google Scholar]
- 37.Yuan F, Pinto JM, Li Q, Wasserlauf BJ, Yang X, Bassett AL, et al. Characteristics of IK and its response to quinidine in experimental healed myocardial infarction. J Cardiovasc Electrophysiol. 1999;10:844–854. doi: 10.1111/j.1540-8167.1999.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 38.Kim YK, Kim SJ, Kramer CM, Yatani A, Takagi G, Mankad S, et al. Altered excitation–contraction coupling in myocytes from remodeled myocardium after chronic myocardial infarction. J Mol Cell Cardiol. 2002;34:63–73. doi: 10.1006/jmcc.2001.1490. [DOI] [PubMed] [Google Scholar]
- 39.Litwin SE, Zhang D, Bridge JH. Dyssynchronous Ca2+ sparks in myocytes from infarcted hearts. Circ Res. 2000;87:1040–1047. doi: 10.1161/01.res.87.11.1040. [DOI] [PubMed] [Google Scholar]
- 40.Pinto JM, Yuan F, Wasserlauf BJ, Bassett AL, Myerburg RJ. Regional gradation of L-type calcium currents in the feline heart with a healed myocardial infarct. J Cardiovasc Electrophysiol. 1997;8:548–560. doi: 10.1111/j.1540-8167.1997.tb00823.x. [DOI] [PubMed] [Google Scholar]
- 41.Matsushita T, Oyamada M, Fujimoto K, Yasuda Y, Masuda S, Wada Y, et al. Remodeling of cell–cell and cell–extracellular matrix interactions at the border zone of rat myocardial infarcts. Circ Res. 1999;85:1046–1055. doi: 10.1161/01.res.85.11.1046. [DOI] [PubMed] [Google Scholar]
- 42.Go LO, Moschella MC, Watras J, Handa KK, Fyfe BS, Marks AR. Differential regulation of two types of intracellular calcium release channels during end-stage heart failure. J Clin Invest. 1995;95:888–894. doi: 10.1172/JCI117739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Semb SO, Lunde PK, Holt E, Tonnessen T, Christensen G, Sejersted OM. Reduced myocardial Na+/K+-pump capacity in congestive heart failure following myocardial infarction in rats. J Mol Cell Cardiol. 1998;30:1311–1328. doi: 10.1006/jmcc.1998.0696. [DOI] [PubMed] [Google Scholar]
- 44.Swift F, Birkeland JA, Tovsrud N, Enger UH, Aronsen JM, Louch WE, et al. Altered Na+/Ca2+-exchanger activity due to downregulation of Na+/K+-ATPase alpha2-isoform in heart failure. Cardiovasc Res. 2008;78:71–78. doi: 10.1093/cvr/cvn013. [DOI] [PubMed] [Google Scholar]
- 45.Verdonck F, Volders PG, Vos MA, Sipido KR. Intracellular Na+ and altered Na+ transport mechanisms in cardiac hypertrophy and failure. J Mol Cell Cardiol. 2003;35:5–25. doi: 10.1016/s0022-2828(02)00280-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.