Abstract
Aims
CYP1A1 and CYP1B1, members of the cytochrome P450 protein family, are regulated by fluid shear stress. This study describes the effects of duration, magnitude and pattern of shear stress on CYP1A1 and CYP1B1 expressions in human endothelial cells, towards the goal of understanding the role(s) of these genes in pro-atherogenic or anti-atherogenic endothelial cell functions.
Methods and results
We investigated CYP1A1 and CYP1B1 expressions under different durations, levels, and patterns of shear stress. CYP1A1 and CYP1B1 mRNA, protein, and enzymatic activity were maximally up-regulated at ≥24 h of arterial levels of shear stress (15–25 dynes/cm2). Expression of both genes was significantly attenuated by reversing shear stress when compared with 15 dynes/cm2 steady shear stress. Small interfering RNA knockdown of CYP1A1 resulted in significantly reduced CYP1B1 and thrombospondin-1 expression, genes regulated by the aryl hydrocarbon receptor (AhR). Immunostaining of human coronary arteries showed constitutive CYP1A1 and CYP1B1 protein expressions in endothelial cells. Immunostaining of mouse aorta showed nuclear localization of AhR and increased expression of CYP1A1 in the descending thoracic aorta, whereas reduced nuclear localization of AhR and attenuated CYP1A1 expression were observed in the lesser curvature of the aortic arch.
Conclusion
CYP1A1 and CYP1B1 gene and protein expressions vary with time, magnitude, and pattern of shear stress. Increased CYP1A1 gene expression modulates AhR-regulated genes. Based on our in vitro reversing flow data and in vivo immunostained mouse aorta, we suggest that increased expression of both genes reflects an anti-atherogenic endothelial cell phenotype.
KEYWORDS: CYP1A1, CYP1B1, Shear stress, Vascular endothelial cell, Aryl hydrocarbon receptor
1. Introduction
Atherosclerosis is typically localized to the carotid artery sinus, the coronary arteries, the abdominal aorta, and the superficial femoral arteries.1 These are regions of complex blood flow patterns including recirculation (reversing flow) during each cardiac cycle, leading to the hypothesis that disturbed haemodynamic patterns are atherogenic. Such differences in haemodynamics alter the gene expression profile and ultimately the structure and function of endothelial cells, resulting in modulation of the interactions of endothelial cells with blood-borne factors and underlying smooth muscle cells, thus increasing the likelihood of atherogenesis.2,3
Cytochrome P450s are a class of membrane-bound haemoproteins which, when complexed with an NADPH-cytochrome P450 reductase and cytochrome b5, oxidize, peroxidize, or reduce endogenous fatty acids and steroids, as well as exogenous xenobiotics and pharmaceutical compounds.4 Cytochrome P450, family 1, subfamily A, polypeptide 1 (CYP1A1) and cytochrome P450, family 1, subfamily B, polypeptide 1 (CYP1B1) are classically recognized as enzymes that metabolize exogenous compounds for elimination.5,6 Halogenated aromatic hydrocarbons and polycyclic aromatic hydrocarbons have been shown to be strong exogenous inducers of CYP1A1 and CYP1B1 genes by binding to and activating the aryl hydrocarbon receptor (AhR), a ligand-activated basic helix–loop–helix transcription factor, which translocates from the cytoplasm to the nucleus upon activation.7 Recently, endogenous activators have been found for the AhR, which can also lead to increased CYP1A1 and CYP1B1 transcription.8,9 In addition, CYP1A1 and CYP1B1 enzymes can modulate cellular levels of a variety of lipid signalling molecules, including arachidonic acid and retinoic acid metabolites that can directly affect gene expression and vascular homeostasis.10 Recent work has shown that CYP1B1 is involved in the generation of retinoic acid during chick embryogenesis.11 Mutations of CYP1B1 in humans are associated with glaucoma,12 and CYP1B1 (−/−) mice have abnormalities in their ocular drainage structure that resembles those in human glaucoma patients.13,14 The ability of both genes to affect lipid signalling and the association of CYP1B1 mutations with a human pathology strongly suggest that CYP1A1 and CYP1B1 have important endogenous roles in the maintenance of homeostatic functions.
Microarray studies showed that gene expression of both CYP1A115–17 and CYP1B115–21 are dramatically up-regulated by arterial levels of shear stress in cultured human endothelial cells. Han et al.22 recently showed that the AhR is involved in CYP1A1 expression under laminar shear stress. The most highly up-regulated genes in our array studies under both steady shear stress16 and pulsatile non-reversing shear stress23 were CYP1A1 and CYP1B1. We postulated that their increases under shear stress resulted from unidirectional arterial shear stresses and that low steady shear stress or reversing pulsatile shear stress would not up-regulate either gene.
To better characterize the increased expression of CYP1A1 and CYP1B1 genes and proteins in human aortic endothelial cells (HAECs) and human umbilical vein endothelial cells (HUVECs) subjected to shear stress, we modelled the flow pattern in the common carotid sinus, a region prone to atherosclerosis, to examine the effects of this flow regimen on CYP1A1 and CYP1B1 gene and protein expression in vitro. We demonstrate that these genes are maximally up-regulated at arterial steady shear stresses of at least 15 dynes/cm2 and that reversing pulsatile shear stress attenuated expression of both genes. Furthermore, AhR nuclear localization and CYP1A1 protein expression correlate with the flow patterns in the mouse aortic arch. Our results strongly suggest that the AhR/CYP1 pathway promotes an anti-atherogenic phenotype in the endothelium.
2. Methods
An online supplement is available with expanded methods.
2.1. Cell culture and shear stress experiments
HUVECs or HAECs (Lonza, Walkersville, MD, USA) were grown as described previously.23 Steady shear stress experiments were performed with a parallel plate flow chamber, as described previously.24
A system was developed on the basis of computer simulated data25 to produce a reversing shear stress pattern that mimics the in vivo conditions at the carotid sinus wall (see Supplementary material online, Figure S1). The waveform has a time-averaged shear stress of −1 dynes/cm2, a maximum shear stress at +11 dynes/cm2, a minimum shear stress of −11 dynes/cm2, and a cycle frequency of 1 Hz.
2.2. siRNA transfection
Cells were transfected 48 h prior to shear stress with 100 nM CYP1A1 (sense: 5′-gguauccaaaaauguguaatt-3′) or CYP1B1 (sense: 5′-gggcccaaugaauuauuautt-3′) siRNA (Silencer Pre-designed siRNAs, Ambion, Austin, TX, USA) using Oligofectamine reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions. siRNA sequences chosen knocked down target gene expression by at least 75%. Control cells were transfected with 100 nM Cy3-labelled nonsense siRNA (Silencer negative control #1, Ambion). Transfection was verified using fluorescent microscopy.
2.3. Quantitative real-time polymerase chain reaction and western blotting
Immediately after exposure to shear stress, total RNA and protein were extracted using TRI-zol (Invitrogen). RNA was reverse-transcribed into cDNA; the quantitative real-time–PCR (qRT–PCR) and analysis were performed on a MyiQ (BioRad, Hercules, CA, USA). Western blots were probed either with rabbit-anti-human CYP1A1 (1:1000, Santa Cruz, Santa Cruz, CA, USA) or with rabbit-anti-human CYP1B1 (1:1000, previously characterized26). Membranes were then re-probed with mouse anti-human β-actin (1:5000, Clone A-15, Sigma, St Louis, MO, USA) to normalize protein loading. Band intensities were quantified by densitometry with the EDAS 1D Imaging System (Kodak, Rochester, NY, USA).
2.4. CYP1A1 and CYP1B1 activity assay
Combined CYP1A1 and CYP1B1 activity was measured using the CYP1A1/CYP1B1 P450 Glo Assay (Promega, Madison, WI, USA).27 Cells were exposed to 25 dynes/cm2 for 48 h. Following shear stress, the medium was replaced with that containing 100 µM luciferin-6′ chloroethyl ether (luciferin-CEE), and cells were cultured for an additional 3 h under static conditions. Medium was then incubated 1:1 with the manufacturer-supplied luciferase-developing reagent for 20 min and luminescence was measured (Victor 3 plate reader, Perkin-Elmer, Waltham, MA, USA). CYP1B1 activity was inhibited with 1 µM 2,3′,4,5′-tetramethoxystilbene (TMS) (Cayman Chemical, Ann Arbor, MI, USA) added to the media simultaneously with luciferin-CEE. Luminescence was normalized to total protein (DC Protein Assay, BioRad).
2.5. Mouse aorta immunohistochemistry
C57BL/6 mice (6–8-week-old males, The Jackson Laboratory, Bar Harbor, ME, USA) were euthanized by CO2 inhalation, and the aortas were perfusion-fixed with 10% formalin. The aortas were carefully cleaned in situ, and the arches and thoracic aortas were dissected and stained with anti-AhR IgG (1:100, a gift from R. Pollenz, University of South Florida)28 or anti-CYP1A1 IgG (1:100, a gift from F. Guengerich, Vanderbilt University)29 antibodies. The aortas were opened and separated into regions of lesser curvature, greater curvature, and thoracic artery. En face images were collected with an LSM 510 META confocal microscope (Zeiss, Jena, Germany).30 The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85-23, revised 1996). Approval for use of animal studies was provided by the Emory University Institutional Animal Care and Use Committee.
2.6. Human coronary artery immunohistochemistry
Frozen sections of human coronary artery explants from patients undergoing heart transplants were prepared31 and stained with either rabbit anti-human CYP1A1 (1:100, a gift from F. Guengerich, Vanderbilt University)29 or rabbit anti-human CYP1B1 (1:100, previously characterized26). The investigation conforms with the principles outlined in the Declaration of Helsinki. Approval for use of human tissue was granted by the Emory University School of Medicine Institutional Review Board.
2.7. Statistics
Results are expressed as mean ± standard error of the mean. All measures of significance were performed using either Student's t-test or one-way analysis of variance (ANOVA), with post hoc analysis using Tukey's multiple comparison test as indicated. Values of P < 0.05 are considered significant.
3. Results
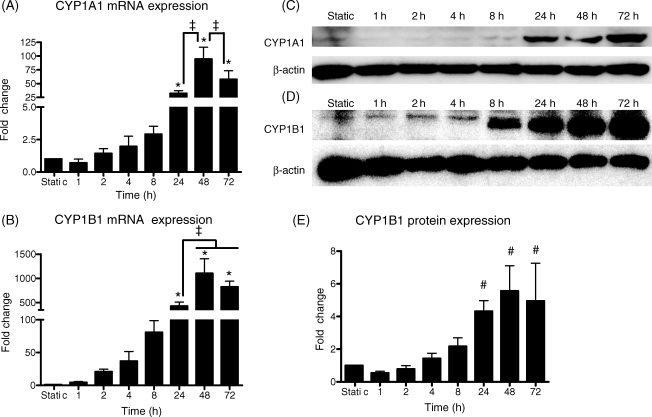
A time course of the response of CYP1A1 and CYP1B1 genes to 25 dynes/cm2 was performed at 1, 2, 4, 8, 24, 48, and 72 h and compared with cells cultured under static conditions (Figure 1). Shear stress up-regulated CYP1A1 and CYP1B1 mRNA in a time-dependent manner (Figures 1A and 1B). Following 24 h of 25 dynes/cm2, CYP1A1 mRNA was up-regulated 32.1 ± 5.0-fold and CYP1B1 mRNA was up-regulated 428.9 ± 82.6-fold. CYP1A1 protein was not quantifiable in static cultures and at 1, 2, 4, and 8 h, but was observed at 24–72 h under 25 dynes/cm2 (Figure 1C). CYP1B1 protein was elevated time-dependently, reaching a maximal induction of 5.6 ± 1.5-fold at 48 h (Figure 1D and E). Static controls isolated at each time point (0–72 h) showed no significant differences in CYP1A1 and CYP1B1 mRNA expressions (data not shown).
Figure 1.
Time course of CYP1A1 and CYP1B1 mRNA and protein expression under shear stress. Human umbilical vein endothelial cells were exposed to 0 (static), 1, 2, 4, 8, 24, 48, or 72 h of 25 dynes/cm2 steady shear stress (n = 3–8). Both CYP1A1 (A) and CYP1B1 (B) mRNA were maximally induced by 24–72 h of shear stress. CYP1A1 protein (C) was usually detected only after 24 h of shear stress. CYP1B1 (D and E) protein was maximally induced after 24 h. Equal loading in (C) and (D) is indicated by reblotting the membranes with a β-actin antibody. Fold changes are relative to static values. Significance was assessed using analysis of variance followed by Tukey's post hoc test. *P < 0.05 vs. 0–8 h shear stress, ‡P < 0.05 vs. indicated durations, #P < 0.05 vs. 0–8 h shear stress.
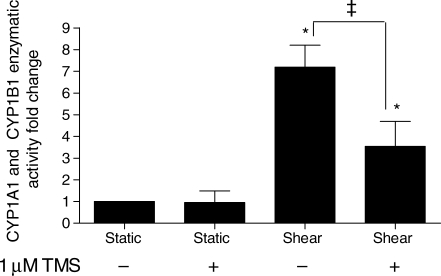
Total CYP1A1 and CYP1B1 activity, measured by dechloroethylation of luciferin-6′ CEE, was 7.2 ± 1.0-fold higher in cells shear stressed for 48 h at 25 dynes/cm2 compared with static controls (Figure 2). Shear-stressed cells simultaneously treated with the CYP1B1 inhibitor (1 µM TMS) had a 3.5 ± 1.1-fold increase in activity over static cells, a decrease of over 50% when compared with shear-stressed cells assayed without the inhibitor. The activity of static controls treated with TMS was not significantly different from untreated static cells.
Figure 2.
Effect of shear stress on CYP1A1 and CYP1B1 activity. Cells were exposed to 25 dynes/cm2 or maintained under static conditions for 48 h and subsequently incubated under static conditions with 100 µM luciferin-chloroethyl ether (CEE) for an additional 3 h (n = 8). The CYP1B1 inhibitor [1 µM 2,3′,4,5′-tetramethoxystilbene (TMS)] was added to the media simultaneously with luciferin-CEE in the indicated samples. Luminescence was normalized to total protein. Significance was assessed using analysis of variance, followed by Tukey's post hoc test; *P < 0.05 vs. static conditions and ‡P < 0.05 between shear-stressed cells with and without TMS.
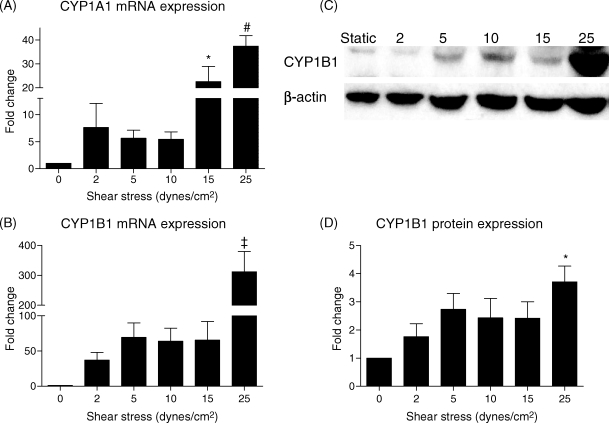
To determine whether the expression level of these genes depended on shear stress magnitude, cells were exposed to 0 (static), 2, 5, 10, 15, or 25 dynes/cm2 shear stress for 24 h (Figure 3). CYP1A1 and CYP1B1 mRNA and CYP1B1 protein expression varied with shear stress magnitude. CYP1A1 protein was not detectable in cells subjected to shear stresses <15 dynes/cm2 (data not shown). Both CYP1A1 and CYP1B1 mRNA and CYP1B1 protein were up-regulated by as little as 2 dynes/cm2 and maximally induced at 25 dynes/cm2.
Figure 3.
Effect of shear stress magnitude on CYP1A1 and CYP1B1 mRNA and protein expression. Human umbilical vein endothelial cells were subjected to 0 (static), 2, 5, 10, 15, or 25 dynes/cm2 shear stress for 24 h (n = 4–5). Both CYP1A1 (A) and CYP1B1 (B) mRNA expression and CYP1B1 protein (C and D) expression were maximal at 25 dynes/cm2 shear stress. Significance was assessed using analysis of variance, followed by Tukey's post hoc test. *P < 0.05 with respect to static conditions, #P < 0.05 vs. 0–10 dynes/cm2, ‡P < 0.05 vs. 0–15 dynes/cm2.
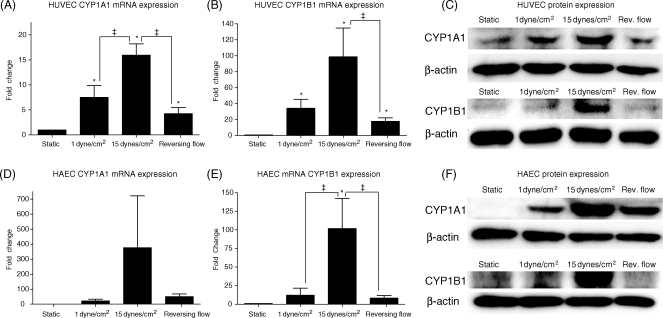
The expression of CYP1A1 and CYP1B1 genes was investigated in HUVECs under reversing shear stress when compared with steady shear stresses (15 or 1 dyne/cm2) (Figure 4). The increased expression of both CYP1A1 and CYP1B1 mRNA by 15 dynes/cm2 shear stress was significantly attenuated under reversing shear stress (Figure 4A and B), and under steady shear stress of 1 dyne/cm2 (the time average of the reversing shear stress profile). CYP1A1 protein levels were highest at 15 dynes/cm2 and were reduced under reversing flow, 1 dyne/cm2, and static conditions (Figure 4C). CYP1B1 protein levels were consistent with CYP1B1 mRNA levels (Figure 4C). Similar results were observed in HAECs, with attenuation of CYP1A1 and CYP1B1 mRNA and protein under reversing shear stress and 1 dyne/cm2 compared with 15 dynes/cm2 (Figure 4D–F).
Figure 4.
Effect of shear stress pattern on CYP1A1 and CYP1B1 mRNA and protein expression. Human umbilical vein endothelial cells (HUVECs) (n = 5–9) or human aortic endothelial cells (HAECs) (n = 4) were subjected to one of three different flow regimes: low shear stress (1 dyne/cm2), steady arterial shear stress (15 dynes/cm2), or carotid sinus reversing shear stress for 24 h. CYP1A1 and CYP1B1 mRNA and protein expression in HUVECs (A–C) were highest under 15 dynes/cm2 and greatly reduced under reversing shear stress. CYP1A1 and CYP1B1 had similar trends of expression in HAECs (D–F). Significance was assessed using analysis of variance, followed by Tukey's post hoc test; *P < 0.05 vs. static conditions and ‡P < 0.05 between indicated conditions.
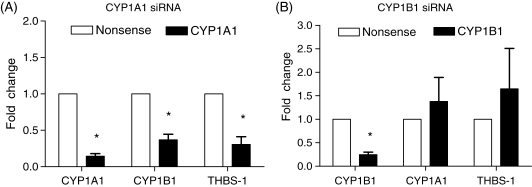
To examine whether CYP1A1 or CYP1B1 modulates downstream gene expression, siRNA against CYP1A1 or CYP1B1 was transfected into HUVECs, which were then subjected to 24 h of 25 dynes/cm2 (Figure 5). Gene expression in these conditions was compared with that occurring in cells transfected with non-silencing nonsense siRNA exposed to identical shear stress conditions. qRT–PCR was performed on target genes as well as genes previously shown to be regulated by AhR activation to determine whether these effects were mediated by either CYP1A1 or CYP1B1. Knockdown of CYP1A1 resulted in significant decreases in CYP1B1 and thrombospondin-1 mRNA expression under shear stress (Figure 5A). Knockdown of CYP1B1 did not result in significant gene expression changes of potential downstream genes examined (thrombospondin-1, CYP1A1) after shear stress (Figure 5B).
Figure 5.
Effect of siRNA knockdown of CYP1A1 and CYP1B1 gene expressions. Human umbilical vein endothelial cells were treated with either CYP1A1 (A) or CYP1B1 (B) siRNA 48 h prior to 24 h of 25 dynes/cm2 shear stress (n = 4). mRNA levels of CYP1A1, CYP1B1, and thrombospondin-1 were compared with cells treated with a non-silencing nonsense control and subjected to identical shear conditions. The knockdown of CYP1A1 caused significant decreases in CYP1B1 and thrombospondin-1. The knockdown of CYP1B1 caused no significant gene changes other than the target knockdown of CYP1B1. Significance was measured using paired Student's t-test; *P < 0.05 between targeted knockdown and nonsense control.
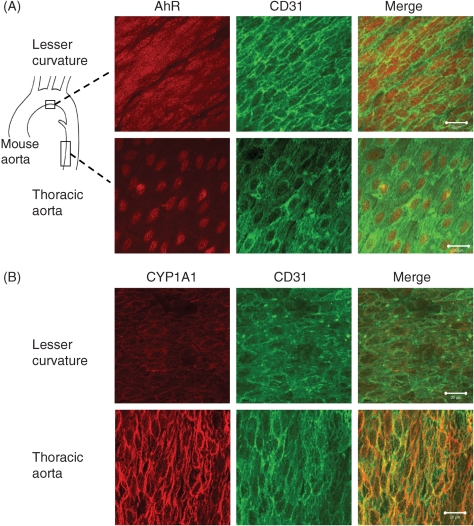
Immunohistochemical staining of AhR and CYP1A1 protein was performed on fixed mouse aortas, which were then isolated into regions of the greater curvature, lesser curvature, and thoracic aorta. AhR (Figure 6A) was localized to the endothelial nuclei in the thoracic aorta, but in the region of the lesser curvature, the AhR was found in both the nuclei and the cytoplasm of the endothelial cells. The expression of CYP1A1 (Figure 6B) was increased in the thoracic aorta, but attenuated in the region of lesser curvature. Staining of the greater curvature showed trends in AhR localization and CYP1A1 expression similar to that of the thoracic aorta (data not shown). Negative control samples (without primary antibody) showed no positive staining (data not shown). Immunohistochemistry of CYP1A1 and CYP1B1 proteins was performed on cross-sections of human coronary artery from two individuals. The sections stained positive for CYP1A1 exclusively in the endothelium, whereas CYP1B1 was present in the endothelium, media, and adventitia (see Supplementary material online, Figure S2).
Figure 6.
Expression of aryl hydrocarbon receptor (AhR) and CYP1A1 protein in mouse aorta. Whole mouse aortas were stained for AhR (n = 3) or CYP1A1 (n = 7), and the lesser curvature and thoracic aorta were mounted en face. Endothelial cell integrity was confirmed with CD31 counterstaining. AhR (A) was expressed in the cytoplasm and nucleus in the region of lesser curvature, but was principally localized in the nucleus in the thoracic aorta. CYP1A1 (B) staining was strongest in the thoracic aorta, with attenuated staining in the region of lesser curvature. Bar is 20 µm.
4. Discussion
This study shows that both CYP1A1 and CYP1B1 gene expressions are regulated by fluid shear stress in a time- and magnitude-dependent manner. Both CYP1A1 and CYP1B1 mRNA and protein expressions are strongly elevated in response to prolonged exposure to arterial levels of shear stress (15–25 dynes/cm2),32 demonstrating that the increase of levels in these two genes is not transiently induced by the initial application of shear stress (Figure 1). The continued elevation in expressions of CYP1A1 and CYP1B1 out to 72 h suggests that endothelial cells upregulate these two genes as an adaptation to chronic arterial levels of shear stress. It may be that high expression levels of CYP1A1 and CYP1B1 reflect a more physiological phenotype that is lost in statically cultured endothelial cells. This is consistent with our observation that these two genes are expressed in sections of human coronary arteries (see Supplementary material online, Figure S2). Also consistent with our results, Dekker et al.20 showed maximal induction of CYP1B1 mRNA of HUVECs exposed to 25 dynes/cm2 for 6 and 24 h, although our data showed an order of magnitude greater induction of this gene. Our finding that increased shear stress magnitude led to increased expression of CYP1A1 and CYP1B1 mRNA and protein (Figure 3) suggests that in vivo these genes would likely have higher expression levels in arterial than venous endothelial cells (human mean arterial shear stresses range from 10 to 30 dynes/cm2, whereas mean venous shear stresses range from 1 to 7 dynes/cm2).32
The reversing shear stress results (Figure 4) suggest that CYP1A1 and CYP1B1 gene expressions would be higher in the arterial regions of the vasculature, which have non-reversing flow than in those regions exposed to reversing flow, this hypothesis is confirmed by our mouse en face staining data (Figure 6), which showed decreases in AhR nuclear localization and CYP1A1 expression in the lesser curvature of the mouse aorta, a region that experiences fluid shear stress reversal.30 The development of lipid deposits at the lesser curvature in mice lacking low-density lipoprotein (LDL) receptors or apolipoprotein E30 correlates disturbed flow with atherogenesis in this part of the aorta. Our results are also supported by Dai et al.,17 who showed reduced CYP1A1 and CYP1B1 mRNA expressions under reversing, athero-prone shear stress when compared with athero-protective, pulsatile non-reversing shear stress. As reversing flow has been hypothesized to be atherogenic and non-reversing flow to be athero-protective, CYP1A1 and CYP1B1 gene expressions may be important in maintaining an athero-protective endothelial phenotype. There were no significant differences in CYP1A1 and CYP1B1 expressions under 1 dyne/cm2 steady shear stress compared with reversing shear stress, suggesting that this pathway is more sensitive to the time-averaged shear stress than to shear stress reversal.
Combined CYP1A1 and CYP1B1 enzymatic activity was increased significantly (7.2-fold) following shear stress (Figure 2). TMS, a specific CYP1B1 inhibitor,33 reduced the measured activity in shear stressed cells by over 50%, indicating that both CYP1A1 and CYP1B1 contribute significantly to the dramatic change in activity. We also used alpha-naphthoflavone in an effort to specifically block CYP1A1 activity, but found that it blocked all activity (data not shown). Although alpha-naphthoflavone has been used as a CYP1A1 inhibitor, it has been shown to equally inhibit CYP1B1.34 The change in activity we observed under shear stress is nearly two-fold greater than that reported by Han et al.22 and is likely due to the more sensitive luciferase activity assay. Our data also have the added advantage of distinguishing CYP1A1 from CYP1B1 through the use of a specific CYP1B1 inhibitor.
Recent studies have shown the existence of endogenous AhR-activating ligands.7–9,35 We previously reported that conditioned media from shear-stressed cells increase CYP1A1 and CYP1B1 in naive cells in static culture.36 McMillan and Bradfield37 showed that in the absence of cells, shear stress alone can directly modify LDL into an AhR ligand capable of up-regulating CYP1A1 and CYP1B1 when shear stress-conditioned media are applied to static cells. Our finding that CYP1A1 and CYP1B1 expressions depend on shear stress magnitude (Figure 3) supports the hypothesis that shear stress produces a circulating AhR ligand in a shear stress magnitude-dependent manner. Interestingly, the observation of regional differences in AhR nuclear localization in the mouse aorta (Figure 6) suggests a novel (and possibly synergistic) mechanism, in which the local haemodynamic environment directly affects the cellular response to circulating AhR ligands through a mechanotransduction-sensitive signalling pathway.
Expression of target genes and thrombospondin-1 as a marker of AhR activation8,38 were examined following siRNA treatment and shear stress exposure. In addition to knocking down CYP1A1, CYP1A1 siRNA treatment significantly decreased both CYP1B1 and thrombospondin-1 mRNA expressions compared with the nonsense control (Figure 5A). As the CYP1A1 siRNA sequence is not complementary to CYP1B1 mRNA, a possible explanation is that CYP1A1 is necessary for endogenous AhR activation. Chiaro et al.9 have shown the existence of an auto-regulatory feedback loop between AhR and CYP1A1. This study showed that AhR null mice had dramatically reduced CYP1A1 mRNA expression and lower levels of AhR ligands in heart tissue when compared with wild-type animals, suggesting that in the heart, CYP1A1 was necessary for the activation of endogenous AhR ligands. Thrombospondin-1 has been recently shown in endothelial cells to be up-regulated by activated AhR through an AhR-binding site in its promoter.8 Isenberg et al.39 showed that thrombospondin-1 is an antagonist of NO-induced proliferation in HUVECs. Given our observation that CYP1A1 knockdown decreased thrombospondin-1 expression and the observation by Han et al.22 that AhR activation is involved in shear stress cell cycle arrest, increased CYP1A1 expression may decrease endothelial cell proliferation. Other shear stress responsive genes (Kruppel-like factor 2, endothelial nitric oxide synthase, and endothelin-1) were measured following CYP1A1 or CYP1B1 siRNA treatment, but no significant differences in gene expression were found (data not shown). Taken together, these results suggest that CYP1A1 and CYP1B1 gene expressions affect the endothelial cell differently and that CYP1A1 mediates gene expression through its involvement in AhR activation.
The presence of CYP1A1 and CYP1B1 expression in the endothelium of human coronary arteries (see Supplementary material online, Figure S2) and AhR nuclear localization and CYP1A1 expression in mouse aortas (Figure 6) are consistent with the observation that fluid shear stress activates AhR resulting in the increased expression of CYP1A1 and CYP1B1 genes in vitro. To the authors' knowledge, these are the first reports of constitutive CYP1A1 and CYP1B1 protein expressions in the endothelium of human arteries and of endogenous AhR nuclear localization in the mouse aorta. CYP1A1 has been shown to be inducible in vivo in vascular endothelium by many AhR ligands in many different species, including human; however, in vivo constitutive expression of CYP1A1 has not been reported.40 Previous work by Dekker et al.20 did not detect CYP1B1 mRNA in human aorta sections using in situ hybridization. Farin et al.41 showed constitutive expression of CYP1A1 in cultured HUVECs; however, Zhao et al.42 did not find constitutive expression of CYP1A1 or CYP1B1 in HUVECs. Constitutive CYP1A1 expression was also observed in CD31-positive rat aortic endothelial cells.43 Previous reports that CYP1A1 or CYP1B1 mRNA or protein was not constitutively expressed may be attributed to the use of the less-sensitive northern blotting technique when compared with qRT–PCR and to different sources of antibodies. Recent work has shown constitutive CYP1B1 expression in mouse corneal44 and retinal endothelial cells45 and human adult and foetal eyes.46 In this study, we also observed positive staining for CYP1B1 in the media and adventitia of human coronary arteries. Previous studies have shown that CYP1B1 is constitutively expressed in cultured human42,47 and mouse48 vascular smooth muscle cells.
CYP1A1 and CYP1B1 genes and proteins represent potentially novel mechanosensitive pathways that merit additional detailed study. In the present study, we also observed differences in CYP1A1 and CYP1B1 genes and proteins at different levels of shear stress (Figure 3) and between arterial steady shear stress and reversing shear stress (Figure 4), suggesting that the magnitude and time-average of shear stress may be important in the regulation of these two genes. The gene signalling capacities of CYP1A1 and CYP1B1 mRNA need additional investigation, particularly by focusing on the identity of substrates for these two genes, and a more detailed investigation of mechanisms of putative AhR/CYP feedback loops with cell regulatory functions. Interestingly, both CYP1A1 and CYP1B1 can produce vasodilating compounds that could act as endothelial-derived hyperpolarizing factors.10,49,50
The ability of cytochrome P450s to produce signalling and vasoreactive molecules from fatty acids and steroids, such as epoxyeicosatrienoic acids, has long been recognized in cardiovascular research. These acids are a group of P450 metabolites that are increased following shear stress.51,52 CYP1A1 and CYP1B1 represent two of the three reported cytochrome P450 genes (out of a total of 57 human P450 isoforms),53 directly affected by shear stress (CYP27A1 has been shown recently to also be shear-sensitive54). Although it is possible that some of the previously observed changes in P450 metabolites by shear stress could be due to post-transcriptional modifications of other P450 enzymes or changes in substrate availability,55 CYP1A1 and CYP1B1 are important contributors to gene expression and metabolite profile changes in endothelial cells subjected to long-term shear stress. The possibility of lipid metabolism changes driven by CYP1A1 and CYP1B1, along with our in vivo data showing regional differences in AhR localization and CYP1A1 expression (Figure 6) that correlate with known sites of lipid deposition, strongly suggests that changes in CYP1A1 and CYP1B1 could affect atherosclerosis development and progression.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by National Institutes of Health (HL018672 and HL070537) and by National Science Foundation (Graduate Research Fellowship to D.E.C.).
Supplementary Material
References
- 1.Wootton DM, Ku DN. Fluid mechanics of vascular systems, diseases, and thrombosis. Annu Rev Biomed Eng. 1999;1:299–329. doi: 10.1146/annurev.bioeng.1.1.299. [DOI] [PubMed] [Google Scholar]
- 2.Chien S. Effects of disturbed flow on endothelial cells. Ann Biomed Eng. 2008;36:554–562. doi: 10.1007/s10439-007-9426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davies PF. Endothelial transcriptome profiles in vivo in complex arterial flow fields. Ann Biomed Eng. 2008;36:563–570. doi: 10.1007/s10439-007-9400-0. [DOI] [PubMed] [Google Scholar]
- 4.Michaelis UR, Fleming I. From endothelium-derived hyperpolarizing factor (EDHF) to angiogenesis: epoxyeicosatrienoic acids (EETs) and cell signaling. Pharmacol Ther. 2006;111:584–595. doi: 10.1016/j.pharmthera.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Barouki R, Morel Y. Repression of cytochrome P450 1A1 gene expression by oxidative stress: mechanisms and biological implications. Biochem Pharmacol. 2001;61:511–516. doi: 10.1016/s0006-2952(00)00543-8. [DOI] [PubMed] [Google Scholar]
- 6.Murray GI, Melvin WT, Greenlee WF, Burke MD. Regulation, function, and tissue-specific expression of cytochrome P450 CYP1B1. Annu Rev Pharmacol Toxicol. 2001;41:297–316. doi: 10.1146/annurev.pharmtox.41.1.297. [DOI] [PubMed] [Google Scholar]
- 7.Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Ann Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- 8.Dabir P, Marinic TE, Krukovets I, Stenina OI. Aryl hydrocarbon receptor is activated by glucose and regulates the thrombospondin-1 gene promoter in endothelial cells. Circ Res. 2008;102:1558–1565. doi: 10.1161/CIRCRESAHA.108.176990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiaro CR, Patel RD, Marcus CB, Perdew GH. Evidence for an aryl hydrocarbon receptor-mediated cytochrome p450 autoregulatory pathway. Mol Pharmacol. 2007;72:1369–1379. doi: 10.1124/mol.107.038968. [DOI] [PubMed] [Google Scholar]
- 10.Choudhary D, Jansson I, Stoilov I, Sarfarazi M, Schenkman JB. Metabolism of retinoids and arachidonic acid by human and mouse cytochrome P450 1b1. Drug Metab Dispos. 2004;32:840–847. doi: 10.1124/dmd.32.8.840. [DOI] [PubMed] [Google Scholar]
- 11.Chambers D, Wilson L, Maden M, Lumsden A. RALDH-independent generation of retinoic acid during vertebrate embryogenesis by CYP1B1. Development. 2007;134:1369–1383. doi: 10.1242/dev.02815. [DOI] [PubMed] [Google Scholar]
- 12.Vincent AL, Billingsley G, Buys Y, Levin AV, Priston M, Trope G, et al. Digenic inheritance of early-onset glaucoma: CYP1B1, a potential modifier gene. Am J Hum Genet. 2002;70:448–460. doi: 10.1086/338709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasiliou V, Gonzalez FJ. Role of CYP1B1 in glaucoma. Annu Rev Pharmacol Toxicol. 2007;48:333–358. doi: 10.1146/annurev.pharmtox.48.061807.154729. [DOI] [PubMed] [Google Scholar]
- 14.Libby RT, Smith RS, Savinova OV, Zabaleta A, Martin JE, Gonzalez FJ, et al. Modification of ocular defects in mouse developmental glaucoma models by tyrosinase. Science. 2003;299:1578–1581. doi: 10.1126/science.1080095. [DOI] [PubMed] [Google Scholar]
- 15.Fledderus JO, van Thienen JV, Boon RA, Dekker RJ, Rohlena J, Volger OL, et al. Prolonged shear stress and KLF2 suppress constitutive pro-inflammatory transcription through inhibition of ATF2. Blood. 2007;109:4249–4257. doi: 10.1182/blood-2006-07-036020. [DOI] [PubMed] [Google Scholar]
- 16.McCormick SM, Eskin SG, McIntire LV, Teng CL, Lu CM, Russell CG, et al. DNA microarray reveals changes in gene expression of shear stressed human umbilical vein endothelial cells. Proc Natl Acad Sci USA. 2001;98:8955–8960. doi: 10.1073/pnas.171259298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, et al. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci USA. 2004;101:14871–14876. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohura N, Yamamoto K, Ichioka S, Sokabe T, Nakatsuka H, Baba A, et al. Global analysis of shear stress-responsive genes in vascular endothelial cells. J Atheroscler Thromb. 2003;10:304–313. doi: 10.5551/jat.10.304. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Cardena G, Comander J, Anderson KR, Blackman BR, Gimbrone MA., Jr Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc Natl Acad Sci USA. 2001;98:4478–4485. doi: 10.1073/pnas.071052598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, et al. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2) Blood. 2002;100:1689–1698. doi: 10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- 21.Parmar KM, Larman HB, Dai G, Zhang Y, Wang ET, Moorthy SN, et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han Z, Miwa Y, Obikane H, Mitsumata M, Takahashi-Yanaga F, Morimoto S, et al. Aryl hydrocarbon receptor mediates laminar fluid shear stress-induced CYP1A1 activation and cell cycle arrest in vascular endothelial cells. Cardiovasc Res. 2008;77:809–818. doi: 10.1093/cvr/cvm095. [DOI] [PubMed] [Google Scholar]
- 23.Yee A, Bosworth KA, Conway DE, Eskin SG, McIntire LV. Gene expression of endothelial cells under pulsatile non-reversing vs. steady shear stress; comparison of nitric oxide production. Ann Biomed Eng. 2008;36:571–579. doi: 10.1007/s10439-008-9452-9. [DOI] [PubMed] [Google Scholar]
- 24.Frangos JA, McIntire LV, Eskin SG. Shear stress induced stimulation of mammalian cell metabolism. Biotech Bioeng. 1987;32:1053–1060. doi: 10.1002/bit.260320812. [DOI] [PubMed] [Google Scholar]
- 25.Perktold K, Rappitsch G. Computer simulation of local blood flow and vessel mechanics in a compliant carotid artery bifurcation model. J Biomech. 1995;28:845–856. doi: 10.1016/0021-9290(95)95273-8. [DOI] [PubMed] [Google Scholar]
- 26.Vidal JD, Vandevoort CA, Marcus CB, Lazarewicz NR, Conley AJ. 2,3,7,8-tetrachlorodibenzo-p-dioxin induces CYP1B1 expression in human luteinized granulosa cells. Arch Biochem Biophys. 2005;439:53–60. doi: 10.1016/j.abb.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 27.Cali JJ, Ma D, Sobol M, Simpson DJ, Frackman S, Good TD, et al. Luminogenic cytochrome P450 assays. Expert Opin Drug Metab Toxicol. 2006;2:629–645. doi: 10.1517/17425255.2.4.629. [DOI] [PubMed] [Google Scholar]
- 28.Pollenz RS, Sattler CA, Poland A. The aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator protein show distinct subcellular localizations in Hepa 1c1c7 cells by immunofluorescence microscopy. Mol Pharmacol. 1994;45:428–438. [PubMed] [Google Scholar]
- 29.Soucek P, Martin MV, Ueng YF, Guengerich FP. Identification of a common cytochrome P450 epitope near the conserved heme-binding peptide with antibodies raised against recombinant cytochrome P450 family 2 proteins. Biochemistry. 1995;34:16013–16021. doi: 10.1021/bi00049a015. [DOI] [PubMed] [Google Scholar]
- 30.Suo J, Ferrara DE, Sorescu D, Guldberg RE, Taylor WR, Giddens DP. Hemodynamic shear stresses in mouse aortas: implications for atherogenesis. Arterioscler Thromb Vasc Biol. 2007;27:346–351. doi: 10.1161/01.ATV.0000253492.45717.46. [DOI] [PubMed] [Google Scholar]
- 31.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, et al. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 32.Kao S-H, McIntire LV, Rheology . In: Thrombosis and Hemorrhage. 3rd ed. Loscalzo J, Schafer AI, editors. Philadelphia: Lippincott Williams & Wilkins; 2003. pp. 294–314. [Google Scholar]
- 33.Kim S, Ko H, Park JE, Jung S, Lee SK, Chun YJ. Design, synthesis, and discovery of novel trans-stilbene analogues as potent and selective human cytochrome P450 1B1 inhibitors. J Med Chem. 2002;45:160–164. doi: 10.1021/jm010298j. [DOI] [PubMed] [Google Scholar]
- 34.Shimada T, Yamazaki H, Foroozesh M, Hopkins NE, Alworth WL, Guengerich FP. Selectivity of polycyclic inhibitors for human cytochrome P450s 1A1, 1A2, and 1B1. Chem Res Toxicol. 1998;11:1048–1056. doi: 10.1021/tx980090+. [DOI] [PubMed] [Google Scholar]
- 35.Song J, Clagett-Dame M, Peterson RE, Hahn ME, Westler WM, Sicinski RR, et al. A ligand for the aryl hydrocarbon receptor isolated from lung. Proc Natl Acad Sci USA. 2002;99:14694–14699. doi: 10.1073/pnas.232562899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eskin SG, Turner NA, McIntire LV. Endothelial cell cytochrome P450 1A1 and 1B1: up-regulation by shear stress. Endothelium. 2004;11:1–10. doi: 10.1080/10623320490432434. [DOI] [PubMed] [Google Scholar]
- 37.McMillan BJ, Bradfield CA. The aryl hydrocarbon receptor is activated by modified low-density lipoprotein. Proc Natl Acad Sci USA. 2007;104:1412–1417. doi: 10.1073/pnas.0607296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo J, Sartor M, Karyala S, Medvedovic M, Kann S, Puga A, et al. Expression of genes in the TGF-beta signaling pathway is significantly deregulated in smooth muscle cells from aorta of aryl hydrocarbon receptor knockout mice. Toxicol Appl Pharmacol. 2004;194:79–89. doi: 10.1016/j.taap.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci USA. 2005;102:13141–13146. doi: 10.1073/pnas.0502977102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rifkind AB. CYP1A in TCDD toxicity and in physiology—with particular reference to CYP dependent arachidonic acid metabolism and other endogenous substrates. Drug Metab Rev. 2006;38:291–335. doi: 10.1080/03602530600570107. [DOI] [PubMed] [Google Scholar]
- 41.Farin FM, Pohlman TH, Omiecinski CJ. Expression of cytochrome P450s and microsomal epoxide hydrolase in primary cultures of human umbilical vein endothelial cells. Toxicol Appl Pharmacol. 1994;124:1–9. doi: 10.1006/taap.1994.1001. [DOI] [PubMed] [Google Scholar]
- 42.Zhao W, Parrish AR, Ramos KS. Constitutive and inducible expression of cytochrome P450IA1 and P450IB1 in human vascular endothelial and smooth muscle cells. In Vitro Cell Dev Biol Anim. 1998;34:671–673. doi: 10.1007/s11626-998-0060-7. [DOI] [PubMed] [Google Scholar]
- 43.Thum T, Haverich A, Borlak J. Cellular dedifferentiation of endothelium is linked to activation and silencing of certain nuclear transcription factors: implications for endothelial dysfunction and vascular biology. FASEB J. 2000;14:740–751. doi: 10.1096/fasebj.14.5.740. [DOI] [PubMed] [Google Scholar]
- 44.Choudhary D, Jansson I, Rezaul K, Han DK, Sarfarazi M, Schenkman JB. Cyp1b1 protein in the mouse eye during development: an immunohistochemical study. Drug Metab Dispos. 2007;35:987–994. doi: 10.1124/dmd.106.014282. [DOI] [PubMed] [Google Scholar]
- 45.Tang Y, Scheef EA, Wang S, Sorenson CM, Marcus CB, Jefcoate CR, Sheibani N. CYP1B1 expression promotes the proangiogenic phenotype of endothelium through decreased intracellular oxidative stress and thrombospondin-2 expression. Blood. doi: 10.1182/blood-2008-03-145219. Epub ahead of print November 12, 2008; doi:10.1182/blood-2008-03-145219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doshi M, Marcus C, Bejjani BA, Edward DP. Immunolocalization of CYP1B1 in normal, human, fetal and adult eyes. Exp Eye Res. 2006;82:24–32. doi: 10.1016/j.exer.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 47.Dubey RK, Gillespie DG, Zacharia LC, Barchiesi F, Imthurn B, Jackson EK. CYP450- and COMT-derived estradiol metabolites inhibit activity of human coronary artery SMCs. Hypertension. 2003;41:807–813. doi: 10.1161/01.HYP.0000048862.28501.72. [DOI] [PubMed] [Google Scholar]
- 48.Kerzee JK, Ramos KS. Constitutive and inducible expression of Cyp1a1 and Cyp1b1 in vascular smooth muscle cells: role of the Ahr bHLH/PAS transcription factor. Circ Res. 2001;89:573–582. doi: 10.1161/hh1901.097083. [DOI] [PubMed] [Google Scholar]
- 49.Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 50.Schwarz D, Kisselev P, Ericksen SS, Szklarz GD, Chernogolov A, Honeck H, et al. Arachidonic and eicosapentaenoic acid metabolism by human CYP1A1: highly stereoselective formation of 17(R),18(S)-epoxyeicosatetraenoic acid. Biochem Pharmacol. 2004;67:1445–1457. doi: 10.1016/j.bcp.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 51.Huang A, Sun D, Jacobson A, Carroll MA, Falck JR, Kaley G. Epoxyeicosatrienoic acids are released to mediate shear stress-dependent hyperpolarization of arteriolar smooth muscle. Circ Res. 2005;96:376–383. doi: 10.1161/01.RES.0000155332.17783.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y, Zhang Y, Schmelzer K, Lee TS, Fang X, Zhu Y, et al. The antiinflammatory effect of laminar flow: the role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc Natl Acad Sci USA. 2005;102:16747–16752. doi: 10.1073/pnas.0508081102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guengerich FP. Cytochrome p450 and chemical toxicology. Chem Res Toxicol. 2008;21:70–83. doi: 10.1021/tx700079z. [DOI] [PubMed] [Google Scholar]
- 54.Zhu M, Fu Y, Hou Y, Wang N, Guan Y, Tang C, et al. Laminar shear stress regulates liver X receptor in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:527–533. doi: 10.1161/ATVBAHA.107.143487. [DOI] [PubMed] [Google Scholar]
- 55.Aguiar M, Masse R, Gibbs BF. Regulation of cytochrome P450 by posttranslational modification. Drug Metab Rev. 2005;37:379–404. doi: 10.1081/dmr-46136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.