Abstract
Aims
Peripheral chemoreflex sensitivity is potentiated in both clinical and experimental chronic heart failure (CHF). NADPH oxidase-derived superoxide mediates angiotensin II (Ang II)-enhanced carotid body (CB) chemoreceptor sensitivity in CHF rabbits, and tempol, the superoxide dismutase (SOD) mimetic, inhibits this Ang II- and CHF-enhanced superoxide anion effect. Here we investigated the role of cytoplasmic SOD [CuZn superoxide dismutase (CuZnSOD)] in the CB on chemoreceptor activity and function in CHF rabbits.
Methods and results
CuZnSOD protein expression was decreased in CBs from CHF rabbits vs. sham (P < 0.05). Adenoviral CuZnSOD (Ad CuZnSOD) gene transfer to the CBs increased CuZnSOD protein expression and significantly reduced the baseline renal sympathetic nerve activity (RSNA) and the response of RSNA to hypoxia in the CHF rabbits (P < 0.05). Single-fibre discharge from CB chemoafferents during normoxia (baseline, at ∼100 mmHg PO2) and in response to hypoxia were enhanced in CHF vs. sham rabbits (P < 0.05). Ad CuZnSOD decreased the baseline discharge (7.6 ± 1.3 vs. 12.6 ± 1.7 imp/s at ∼100 mmHg PO2) and the response to hypoxia (22.4 ± 1.6 vs. 32.3 ± 1.2 imp/s at ∼40 mmHg PO2, P < 0.05) in CHF rabbits. Ad CuZnSOD also normalized the blunted outward K+ current (IK) in CB glomus cells from CHF rabbits (369 ± 14 vs. 565 ± 31 pA/pF at +70 mV, P < 0.05). In addition, Ad CuZnSOD reduced the elevation of superoxide level in CBs from CHF rabbits.
Conclusion
Downregulation of CuZnSOD in the CB contributes to the enhanced activity of CB chemoreceptors and chemoreflex function in CHF rabbits.
KEYWORDS: Superoxide dismutase, Adenoviral vector, Carotid body, Sympathetic nerve activity, Chemoreceptor, Glomus cell, Chronic heart failure
1. Introduction
In chronic heart failure (CHF), the progression of cardiac dysfunction is exacerbated by enhanced sympathetic nerve activity.1–8 Our previous studies have demonstrated that an enhanced sensitivity of the peripheral chemoreflex contributes to the sympathetic activation in pacing-induced CHF rabbits7,9–11 Augmented afferent input from the carotid body (CB) chemoreceptors is involved in the enhancement of peripheral chemoreflex function in the CHF state.1,7,11,12
The angiotensin (Ang) II -Ang II type 1 (AT1) receptor system plays an important role in the enhanced CB chemoreceptor sensitivity in CHF.7 The NADPH oxidase-derived superoxide signalling pathway mediates the Ang II-enhanced CB chemoreceptor sensitivity.12 This effect is consistent with the role of NADPH oxidase-derived superoxide anion to inhibit potassium channel activity in CB glomus cells from CHF rabbits.13,14
The causal relationship between oxidative stress and the enhanced CB chemoreceptor activity in CHF is evidenced by the efficacy of exogenous antioxidant tempol [an superoxide dismutase (SOD) mimetic] to attenuate the Ang II-enhanced CB chemoreceptor responses to hypoxia.12 However, it is not known whether SOD, the major anti-oxidant enzyme regulating superoxide anion metabolism, contributes to superoxide-mediated CB chemoreceptor hypersensitivity in CHF. In the present study, we investigated the effect of adenoviral CuZn superoxide dismutase (Ad CuZnSOD) gene transfer to the CB on the enhanced peripheral chemoreceptor activity in CHF rabbits.
2. Methods
2.1. Experimental animals and induction of chronic heart failure
All experiments were carried out on 72 male New Zealand White rabbits weighing 2.5–3.5 kg. The experimental protocols were approved by the University of Nebraska Medical Center Institutional Animal Care and Use Committee and conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). Rabbits were housed in individual cages under controlled temperature and humidity and a 12:12 h dark:light cycle and fed standard rabbit chow with water available ad libitum. They were randomly assigned to sham and CHF groups.
Rabbits were anaesthetized with a cocktail consisting of 1.2 mg acepromazine, 5.9 mg xylazine, 0.01 mg atropine, and 58.8 mg ketamine, given as an i.m. injection. Using sterile technique, a pin electrode was attached to the left ventricle for pacing via a left thoracotomy as previously described.1,7,9,11–13,15 After the rabbits recovered from the thoracotomy (about 2 weeks), the pacing was started at 320 bpm and gradually increased to 380 bpm. The progression of CHF was monitored by weekly echocardiograms (Acuson Sequoia 512C with a 4 MHz probe), with the pacemaker turned off for at least 30 min before the recordings were started. Sham-operated animals underwent a similar period of echocardiographic measurements. CHF was characterized by a >40% reduction in ejection fraction and fraction of shortening, and dilation of the left ventricle in both systole and diastole.
2.2. Gene transfer to the carotid body
The technique for gene transfer to CB was that originally described by Li et al.11 Briefly, the left and right sinus region was temporarily vascularly isolated (including the common carotid artery, internal carotid artery, and external carotid artery), and the tip of a PE-10 catheter was positioned at the level of the CB via the external maxillary artery using sterile surgical technique. After these arteries were occluded with snares, 200 µL of Ad Empty (as control adenoviruses) or Ad CuZnSOD [1 × 108 plaque-forming units (pfu) per mL, dissolved in 0.9% sodium chloride] was slowly injected into the CB via the catheter. The Ad CuZnSOD contained human CuZnSOD cDNA.16,17 The catheter and snares around the vessels were removed, incision closed, and animal allowed to recover. After 3–4 days, the chemoreflex and chemoreceptor sensitivity experiments (see below) were performed. The CB tissue was then collected.
2.3. Recording of renal sympathetic nerve activity
Renal sympathetic nerve activity (RSNA) recording electrodes were implanted as described previously.1,7,9,11,12 At the same time, catheters were inserted into the jugular vein for injections and femoral artery or right carotid artery for arterial pressure. The experiments were carried 3 days after surgery. Changes of RSNA in response to stimulation of peripheral chemoreceptors were recorded in sham and CHF rabbits in the conscious state as in our previous studies.1,7,9,11,12 RSNA was expressed as % maximum, and maximal RSNA was determined in each rabbit by an intravenous bolus injection of sodium nitroprusside (100 µg/kg).9,18 Peripheral chemoreceptors were stimulated preferentially by allowing the rabbits to breathe graded mixtures of hypoxic gas 3 min under isocapnic conditions. Because hypoxic stimulation of ventilation induces hyperventilatory hypocapnia, 2–4% CO2 was added to the hypoxic gases to maintain relatively constant PaCO2 during hyperventilation.1
2.4. Immunofluorescence detection of CuZn superoxide dismutase in the carotid body
The animals were transcardially perfused with saline containing 100 U of heparin for 5 min, then continued to perfuse with 4% paraformaldehyde for 30 min. Both CBs in each rabbit rapidly removed and post-fixed in 4% paraformaldehyde for 12 h at 4°C, followed by soaking the CBs in 30% sucrose for 12 h at 4°C for cryostat protection. The CB was cut into 10 µm-thick sections. The CB sections were mounted on pre-coated glass slides for immunofluorescence for CuZnSOD and tyrosine hydroxylase (TH) detection. CB sections on the glass slide were incubated with 10% normal donkey serum for 1 h, followed by incubation with primary anti-CuZnSOD and anti-TH antibodies overnight at 4°C. Then the sections were incubated with appropriate secondary antibody for 60 min at room temperature. Slides were observed under a Leica fluorescent microscope with appropriate excitation/emission filters. Pictures were captured by a digital camera system. No staining was observed when the procedure described above was repeated using PBS instead of the primary antibody.
2.5. Western blot analysis for CuZn superoxide dismutase in the carotid body
CBs were rapidly removed and immediately frozen in dry ice and stored at −80°C until analysed. In brief, the protein in CBs was extracted in lysing buffer (10 mM PBS, 1% Nonidet P-40, 0.5% sodium deoxycholate, 1% SDS) plus protease inhibitor cocktail (100 µL/mL). Following a centrifugation at 12 000g for 20 min at 4°C, protein concentration in the supernatant was measured using a BCA protein assay kit (Pierce Chemical, Rockford, IL, USA). Samples were adjusted to the same concentrations of protein with equal volumes of loading buffer containing β-mercaptoethanol and boiled for 5 min. For electrophoresis, 5 µg protein was loaded using a Bio-Rad minigel apparatus at 80 mA for 45 min. Proteins were fractionated in a 10% polyacrylamide gel along with molecular weight standards and were electrophoretically transferred onto the polyvinylidene difluoride membrane (Millipore). The membrane was probed with primary antibody (1:1000 dilutions of anti-CuZnSOD) and secondary antibody (1:5000 dilutions) IgG-horseradish peroxidase, respectively, and then treated with enhanced chemiluminescence substrate for 5 min at room temperature. The bands in the membrane were visualized and analysed using UVP BioImaging Systems. CuZnSOD protein intensity was normalized by β-tubulin.
2.6. Recording of afferent discharge of carotid body chemoreceptor
Single unit action potentials were recorded from CB chemoreceptor fibres in the carotid sinus nerve (CSN) as in previous studies.7,10–12 Briefly, the left or right carotid sinus region was vascularly isolated and perfused with Krebs–Henseleit solution (in mM: 120 NaCl, 4.8 KCl, 2.0 CaCl2, 2.5 MgSO4, 1.2 KH2PO4, 25 NaHCO3, and 5.5 glucose; at 10 mL/min, at 37°C). Perfusate was bubbled with O2/CO2/N2 gas mixture to maintain PO2 at 100–110 mmHg, PCO2 at 30–35 mmHg, and pH 7.4 as the normoxic condition. Flow through the isolated sinus was set at 10 mL/min at a perfusion pressure of 80 mmHg via a variable resistor on the effluent line. PO2 of the perfusate was altered by bubbling with gas mixtures of different O2 concentrations to achieve a PO2 of 55–65 and 35–45 mmHg, respectively. The gas mixture contained a constant fraction of CO2 and a balance of N2.
The CSN was exposed and transected near the petrosal ganglion to interrupt neural efferents to the CB. The CSN was covered with mineral oil, and fine slips of nerve filaments were placed on a silver electrode. Impulses were amplified with a bandwidth of 100 Hz–3 kHz (Grass P511, Grass Instrument, Quincy, MA, USA), displayed on an oscilloscope (2120 Oscilloscope, BK Precision, Taiwan), and fed into a rate meter (FHC, Brunswick, ME, USA) whose window discriminators were set to accept potentials of the particular amplitude. Bundles that had one, or at most two, easily distinguishable active fibres were used. Chemoreceptor afferents were identified by their sparse and irregular discharge at normoxia and by their response to hypoxia and NaCN.
2.7. Measurement of superoxide level in the carotid body
CB samples were homogenized and centrifuged (3000 rpm) at 4°C for 5 min. The supernatant was used to measure the superoxide level. Total protein concentration was determined using a bicinchoninic acid protein assay kit (Pierce). The superoxide level was measured using the lucigenin chemiluminescence method.12,19,20 The supernatant was placed in 0.5 mL microfuge containing dark-adapted lucigenin (5 µM), then read in a TD-20/20 Luminometer (Turner Designs, Sunnyvale, CA, USA) with NADPH (100 µM). Light emission was recorded for 5 min and expressed as mean light units (MLU)/min/100 µg protein. In order to investigate the effects of some drugs on the superoxide level, the homogenates were pre-treated for 30 min with phenylarsine oxide (PAO, 2 µM, an NADPH oxidase inhibitor) or CuZnSOD (200 IU/mL) from bovine liver.
2.8. Recording of outward K+ currents (IK) in the carotid body glomus cells
The procedures for isolation, identification of glomus cells, and recording of its K+ currents were as described previously.13,15 CB glomus cells were isolated by a two-step enzymatic digestion protocol and cultured at 37°C in a humidified atmosphere of 95% air–5% CO2, and studied within 24 h of dissociation. At the beginning of each experiment, cells were superfused with the normal extracellular solution containing the following composition (mM) 140 NaCl, 5.4 KCl, 2.5 CaCl2, 0.5 MgCl2, 5.5 HEPES, 11 glucose, 10 sucrose, pH 7.4, to identify glomus cells which exhibited Na+ current. Once the presence of Na+ current was confirmed, the extracellular solution was changed to solution containing 0.5 µM TTX (Na+ channel blocker) to record IK. The Pipettes solution contained the following composition (mM), 105 potassium aspartate, 20 KCl, 1 CaCl2, 10 EGTA, 5 Mg-ATP, 10 HEPES, and 25 glucose, pH 7.2.
All experiments were done at 22°C. Patch pipettes had resistances of 4–6 MΩ when filled with intracellular solution. Currents were measured in the whole-cell configuration of the patch-clamp technique using a Warner PC-501A patch-clamp amplifier (Warner Instrument Corp., Hamden, CT, USA) and pClamp 8.1 program (Axon Instruments, CA, USA). Current traces were sampled at 10 kHz and filtered at 5 kHz. Holding potential was −80 mV. Current–voltage (I–V) relations were elicited by 400 ms test pulses from −80 to +80 mV applied in 10 mV increments (5 s between steps). Peak currents were measured for each test potential and plotted against the corresponding test potential.
2.9. Drugs and reagents
The goat anti-CuZnSOD used for western blots and immunofluorescent labelling is reactive to both rabbit and human CuZnSOD and was obtained from Santa Cruz Biotechnology Inc., CA, USA. The secondary antibodies for immunofluorescent labelling were purchased from Molecular Probe Company. Chemicals for protein extraction, anti-β-tubulin antibody, secondary antibodies, IgG-horseradish peroxidase, and chemiluminescence substrate for western blotting were obtained from Pierce Chemical. Mouse anti-TH and other chemicals used in this study were obtained from Sigma-Aldrich Chemical Co., St Louis, MO, USA.
2.10. Data analysis
All values are presented as mean ± SEM. RSNA was expressed as percentage of the maximal nerve activity as described in the Methods. Peripheral chemoreflex function curves were analysed by plotting data points averaged over 30 s for RSNA against the corresponding PaO2. Statistical significance was determined by a two-way ANOVA, with a Bonferroni procedure for post hoc analysis for multiple comparisons. Statistical significance was accepted when P < 0.05. A power analysis was conducted to assess whether the sample size was sufficient.
3. Results
3.1. Characteristics of the chronic heart failure state
CHF was induced by 3–4 weeks of rapid ventricular pacing, consistent with our previous studies.7,9,11,12 CHF was characterized by an enlarged heart and impaired contractile function as evaluated by the changes of heart weight, LV chamber diameter, and the shortening and ejection fractions (Table 1). There was no difference in heart weight, LV chamber diameter, and the shortening and ejection fractions among CHF rabbits with/without Ad Empty or Ad CuZnSOD.
Table 1.
Body weight, ventricular weight and cardiac function in sham and CHF rabbits
| n | BW (kg) | LVW/BW (g/kg) | LVEDD (mm) | LVESD (mm) | FS (%) | EF (%) | |
|---|---|---|---|---|---|---|---|
| Sham | 16 | 3.6 ± 0.1 | 1.4 ± 0.1 | 13.8 ± 0.1 | 9.3 ± 0.3 | 39.3 ± 0.4 | 75.3 ± 1.2 |
| CHF | 24 | 3.8 ± 0.1 | 1.9 ± 0.1* | 16.9 ± 0.5* | 12.0 ± 0.4* | 20.2 ± 2.4* | 44.8 ± 0.7* |
| CHF + Ad Empty | 16 | 3.7 ± 0.1 | 1.9 ± 0.1* | 17.1 ± 0.5* | 11.9 ± 0.4* | 21.5 ± 2.2* | 45.2 ± 0.6* |
| CHF + Ad CuZnSOD | 16 | 3.8 ± 0.1 | 1.9 ± 0.1* | 17.2 ± 0.5* | 12.1 ± 0.4* | 20.1 ± 2.2* | 44.0 ± 0.6* |
Data are mean ± SEM; BW, body weight; LVW, left ventricular weight; LVEDD, left ventricular end diastolic diameter; LVESD, left ventricular end systolic diameter; FS, fractional shortening; EF, ejection fraction. *P < 0.05 compared with sham.
3.2. Immunofluorescence location of CuZn superoxide dismutase protein expression in carotid bodies
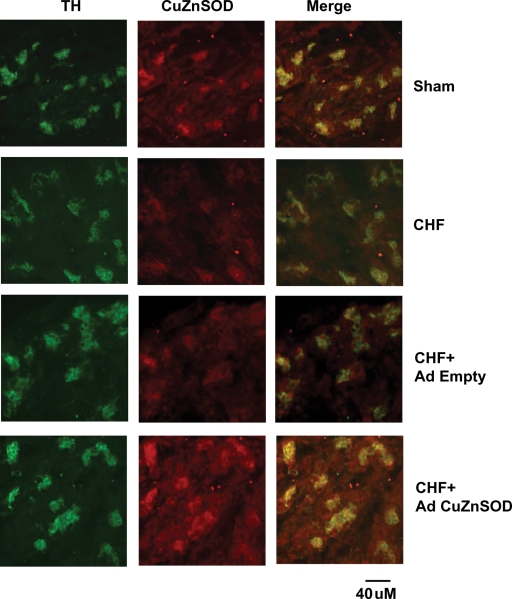
Using immunofluorescence analysis, we found significant overlap of immunolabelled CuZnSOD with identified glomus cell clusters. The immunofluorescence level of CuZnSOD was lower in the CB from CHF rabbits than that from sham rabbits (Figure 1). Three days after gene transfer of Ad CuZnSOD to the CB of CHF rabbits, the level of immunolabelled CuZnSOD was significantly increased as compared with that in the non-infected CB from CHF rabbits. Ad Empty did not affect the level of CuZnSOD in the CBs of CHF rabbits.
Figure 1.
Co-localization of tyrosine hydroxylase (TH) and copper/zinc superoxide dismutase (CuZnSOD) in carotid bodies from sham and chronic heart failure (CHF) rabbits. Green immunofluorescent image for TH, red immunofluorescent image for CuZnSOD, and merged image, yellow, for overlap of TH and CuZnSOD. TH is a marker for glomus cells. n = 3 each group.
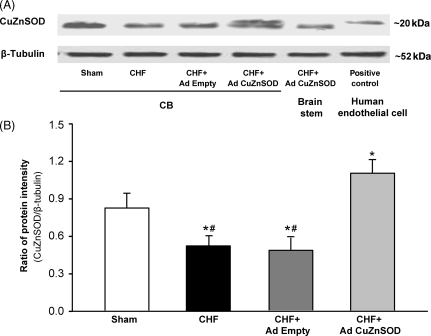
3.3. Western blot analysis of CuZn superoxide dismutase protein expression
Figure 2 illustrates a marked decrease in CuZnSOD protein in CBs from CHF rabbits compared with that from sham rabbits. Ad CuZnSOD increased CuZnSOD protein expression in CHF CBs as compared with CHF CBs infected by Ad Empty (P < 0.05, Figure 2). Ad Empty did not affect CuZnSOD levels in the CBs of CHF rabbits (Figure 2). The levels of Cu/Zn SOD protein expression in the CB measured by western blot were consistent with the degree of immunofluorescence staining of CuZn SOD observed for each group. The expression of β-tubulin protein was not affected by CHF or adenoviral transfections.
Figure 2.
Protein expression of copper/zinc superoxide dismutase (CuZnSOD) in the carotid bodies from sham and chronic heart failure (CHF) rabbits. (A) Representative bands from western blot analysis. Anti-CuZnSOD antibody was used to detect a ∼22 kDa protein corresponding to the molecular mass of CuZnSOD on immunoblots from human tissue extracts vs. a ∼19 kDa protein on blots from rabbit tissue extracts. (B) Total relative CuZnSOD protein expression (rabbit + human CuZnSOD). Data are mean ± SEM. n = 4 each group. *P < 0.05 compared with sham group, #P < 0.05 compared with CHF + Ad CuZnSOD group.
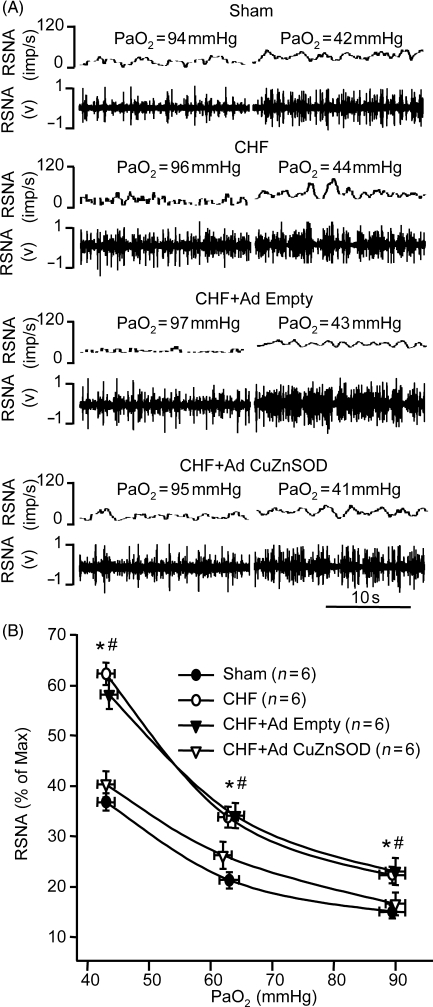
3.4. Effect of gene transfer of CuZn superoxide dismutase on chemoreflex function in chronic heart failure rabbits
CHF increased baseline RSNA (normoxia) and enhanced the response of RSNA to hypoxia compared with that in sham rabbits (P < 0.05, Figure 3A and B), which is consistent with those in our previous studies.1,7,9,11 Ad CuZn SOD gene transfer to both CBs in CHF rabbits markedly reduced the baseline RSNA and the response of RSNA to hypoxia (P < 0.05, Figure 3). Bilateral CB infection with Ad Empty did not alter the enhanced RSNA at normoxic and hypoxic states in CHF rabbits (Figure 3).
Figure 3.
Effect of normoxic and hypoxic states on renal sympathetic nerve activity (RSNA) in sham, chronic heart failure (CHF), and CHF treated with either adenoviral copper/zinc superoxide dismutase (Ad CuZnSOD) or Ad Empty to bilateral carotid bodies. (A) Representative recording of RSNA. (B). RSNA response to hypoxia. Data are mean ± SEM. n = 6 each group. *P < 0.05 compared with sham group, #P < 0.05 compared with CHF + Ad CuZnSOD group.
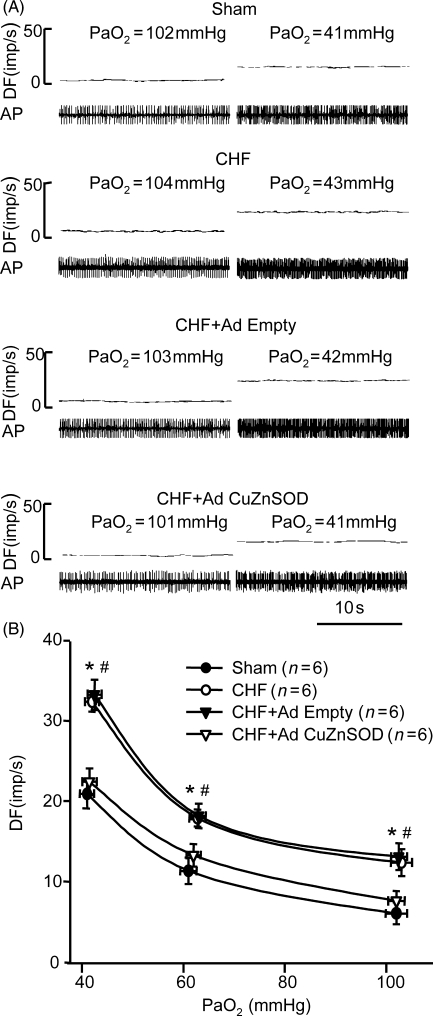
3.5. Effect of gene transfer of CuZn superoxide dismutase on carotid body chemoreceptor activity in chronic heart failure rabbits
The basal discharge of CB chemoreceptors during normoxia and the response to isocapnic hypoxia were elevated in CHF rabbits compared with that in sham rabbits (P < 0.05, Figure 4A and B), which is similar to our previous results.10–12 Ad CuZnSOD transfection to the CB in CHF rabbits significantly blunted CB chemoreceptor activity during normoxia and hypoxia as compared with that from the non-infected CB in the same CHF animals, and the activity was similar to that in sham rabbits (Figure 4). Ad Empty infection showed no effect on CB chemoreceptor activity (Figure 4).
Figure 4.
Effect of normoxic and hypoxic PO2 on carotid body (CB) chemoafferent discharge in CB from sham, chronic heart failure (CHF), and CHF treated with either adenoviral copper/zinc superoxide dismutase (Ad CuZnSOD) or Ad Empty. (A) Representative recording of CB chemoreceptor afferent discharge. (B) CB chemoafferent response to hypoxia. DF, discharge frequency; AP, action potential. Data are mean ± SEM. n = 6 each group. *P < 0.05 compared with sham group, #P < 0.05 compared with CHF + Ad CuZnSOD group.
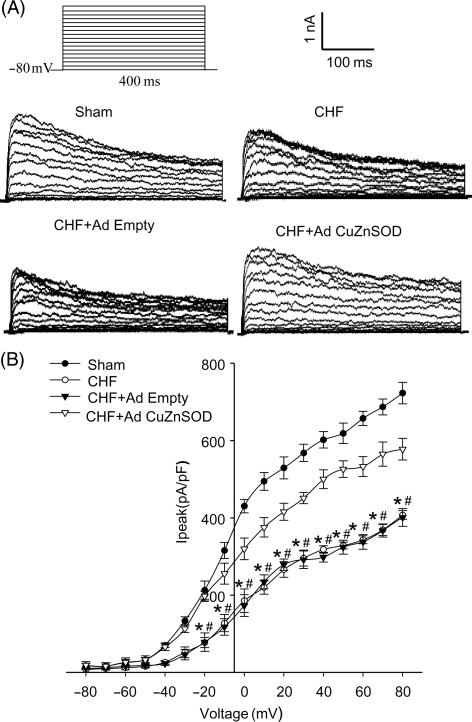
3.6. Effect of gene transfer of CuZn superoxide dismutase on IK of carotid body glomus cells in chronic heart failure rabbits
The cell membrane capacitances were 2.80 ± 0.16, 3.12 ± 0.20, 3.23 ± 0.36, and 3.13 ± 0.34 pF in sham, CHF, CHF + Ad Empty, and CHF + Ad CuZnSOD groups, respectively. There was no significant difference among groups. Figure 5 illustrates typical IK recordings and I–V curves obtained from glomus cells, with voltage steps from a holding potential of −80 mv under conventional whole-cell patch technique. IK was attenuated in CB glomus cells from CHF rabbits when compared with that in shams (P < 0.05, Figure 5). Ad CuZnSOD transfection of the CB in CHF rabbits significantly increased glomus cell IK as compared with that in glomus cells from Ad Empty-infected CHF CBs (P < 0.05, Figure 5).
Figure 5.
Outwards K+ currents (IK) in glomus cells from carotid body from sham, chronic heart failure (CHF), and CHF treated with either adenoviral copper/zinc superoxide dismutase (Ad CuZnSOD) or Ad Empty. (A) Representative IK recording evoked by 400 ms depolarizing test pulses from a holding potential of −80 to +80 mv in 10 mV increments. (B) Peak current–voltage relationship curves. Data are mean ± SEM, n = 10 cells from five rabbits each group. *P < 0.05 compared with sham group, #P < 0.05 compared with CHF + Ad CuZnSOD group.
3.7. Effect of gene transfer of CuZn superoxide dismutase on superoxide anion levels in carotid bodies of chronic heart failure rabbits
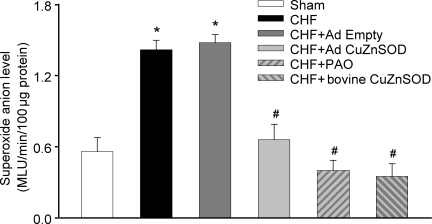
Using the lucigenin chemiluminescence method, we found that superoxide anion levels were higher in the CB homogenate from CHF rabbits than that from sham rabbits (P < 0.05, Figure 6). Ad CuZnSOD infection of the CB decreased the superoxide anion levels as compared with non-infected CB in CHF rabbits, and the decrease reached a similar level found in CBs from sham rabbits (P < 0.05, Figure 6). Ad Empty infection of CBs in CHF rabbits did not affect superoxide anion levels. We further observed that both PAO (an NADPH oxidase inhibitor) and external SOD from bovine liver reduced superoxide anion levels in CBs from CHF animals (P < 0.05, Figure 6).
Figure 6.
NADPH oxidase-derived superoxide anion levels in carotid bodies from sham and CHF rabbits using lucigenin chemiluminescence method. The NADPH oxidase activity was assessed by 100 µM of NADPH-stimulated superoxide and dark-adapted lucigenin (5 µM) in carotid body homogenates. In some experiments, homogenates were pre-incubated with NADPH oxidase inhibitor phenylarsine oxide (PAO) 2 µm and copper/zinc superoxide dismutase (CuZnSOD) (200 U/mL) from bovine liver before measurement of lucigenin chemiluminescence. Light emission was recorded for 5 min and expressed as mean light units (MLU). Data are mean ± SEM. n = 4 pairs of CBs in each group. *P < 0.05 compared with sham group; #P < 0.05 compared with CHF + Ad Empty.
Discussion
The present study has revealed that the expression of CuZnSOD is suppressed in the CBs from CHF rabbits, which correlates with the enhanced CB chemoreceptor activity and reflex function. Ad CuZnSOD localized to the CB enhanced the expression of CuZnSOD in the CB and reversed the enhanced CB chemoreflex sensitivity and chemoafferent activity in CHF rabbits. Ad CuZnSOD gene transfer to the CB also increased the IK of glomus cells and effectively reduced the elevated superoxide anion levels in the CB homogenates from CHF rabbits. These results indicate that downregulation of CuZnSOD with reduced scavenging of superoxide anion in the CB contributes to the enhanced CB chemoafferent and reflex function in CHF.
Our previous studies have shown that Ang II-NADPH oxidase-derived superoxide anion signalling pathway contributes to the enhanced CB chemoreceptor sensitivity to hypoxia in CHF rabbits.7,12 However, the relative contribution of SOD, an important antioxidant to scavenge superoxide anion production, to these effects was not known. In agreement with our previous report,12 the present study found superoxide anion levels in the CB are increased in CHF rabbits. We further demonstrate that CuZnSOD protein expression is markedly lower in the CB from CHF rabbits and that gene transfer of CuZnSOD to the CB in CHF augmented protein expression and decreased superoxide anion levels in CBs (Figure 6). Therefore, decreased CuZnSOD as well as the increased Ang II-NADPH oxidase contributes to the elevated superoxide anion levels in the CB in CHF.
Gao et al.21–23 has demonstrated both CuZnSOD downregulation and increased NADPH oxidase-derived radical stress also are involved in the exaggerated sympathoexcitatory responses from autonomic areas of the brain in CHF rabbits. Collectively, those results from the RVLM with our present results from the CB suggest that decreased CuZnSOD expression together with the increased Ang II-NADPH oxidase-derived superoxide anion signalling is a common feature of heightened autonomic neuronal activity at peripheral and central loci in CHF.
We found IK is reduced in CB glomus cells from HF rabbits compared with that in sham rabbits, as in previous studies.13–15 The suppression of outward K+ channels in gloms cells is likely to contribute to a heightened excitability of these cells to drive the elevated afferent activity from the CB in CHF rabbits.15,24,25 Ad CuZnSOD enhanced IK of glomus cell in CHF rabbits (Figure 5), which is consistent with the effect of Ad CuZnSOD to reduce CB chemoafferent and chemoreflex hypersensitivity induced by CHF (Figures 3 and 4). Our previous experiments have shown that Ang II-NADPH oxidase-derived superoxide anion inhibits K+ channel activity in CB glomus cells from CHF rabbits.13,14 Taken together, we suggest that CuZnSOD acts to enhance IK of glomus cells by reducing superoxide anion. This regulatory component of glomus cell function is downregulated in CHF.
Adenovirus-mediated gene transfer has been extensively used in many studies.11,26,27 Our lab has successfully used adenoviral gene transfer localized to the CBs to explore the mechanisms of altered CB function in CHF rabbits.11 The enhanced expression of CuZnSOD in the CBs of CHF rabbits by gene transfer was confirmed by immunofluorescence and western blot detection. The Ad CuZnSOD cDNA expressed human CuZnSOD, which could be distinguished from rabbit CuZnSOD on immunoblots (Figure 2A). These results are consistent with findings of Zanetti et al.,26 in which the transgenic human CuZn SOD was detected at ∼22 kDa and endogenous CuZnSOD was found at ∼19 kDa with Ad CuZnSOD gene transfer to rabbit aortas. We did not find human CuZnSOD expressed in the brain stem or other organs after local Ad CuZnSOD gene transfer to CBs in our experiments (Figure 2A). These data confirm that the gene transfer was successfully localized to CB tissue in our present study.
The present study addresses the role of the CuZnSOD isoform on CB chemoafferent hyperactivity in CHF rabbits. Three known isoforms of SOD function in mammals. The intracellular CuZnSOD is located predominantly in the cytoplasm and nucleus of cells. MnSOD is found predominantly in the mitochondria. The third isoenzyme of SOD is extracellular SOD.26,28 The relative contribution of the other isoforms of SOD to the superoxide signalling effects in the CB is not known and deserves further study.
Sympathetic hyperactivity in CHF contributes to late-stage deterioration of cardiac function.8,29,30 Previous studies have shown that an enhanced peripheral chemoreflex sensitivity contributes significantly to the generalized sympathetic overactivation in CHF patients and experimental animals.1–7,9 We could not assess the impact of SOD transfection in the CB on cardiac function in the present study due to the short duration of efficacy of the adenoviral vector. Upregulation of CB SOD in CHF rabbits over the course of several days did not alter cardiac function. But, it is not clear that one might expect a significant improvement in cardiac function over that period, considering that significant remodelling of the ventricle had already occurred. Additional studies using strategies to alter gene expression over the long term are needed to address this issue.
In summary, we found that Ad CuZnSOD gene transfer to the CB normalizes the enhanced CB chemoreflex sympathoexcitation, CB chemoafferent hypersensitivity, and the suppressed IK of glomus cells in the CB in the CHF state. Our results clearly implicate that the CuZnSOD deficiency of the CB plays an important role in the elevated superoxide anion levels and signalling in the CB in CHF.
Funding
This study was supported by a Program Project Grant from the Heart, Lung, and Blood Institute of National Institutes of Health (PO1-HL62222) and a postdoctoral fellowship to Y.D. from the American Heart Association, Heartland Affiliate (no. 0725749Z).
Acknowledgements
The authors wish to thank Kurtis Cornish and Kaye Talbitzer for their surgical assistance and management of the heart failure animal core at UNMC and Mary Ann Zink for her technical support.
Conflict of interest: none declared.
References
- 1.Sun SY, Wang W, Zucker IH, Schultz HD. Enhanced peripheral chemoreflex function in conscious rabbits with pacing-induced heart failure. J Appl Physiol. 1999;86:1264–1272. doi: 10.1152/jappl.1999.86.4.1264. [DOI] [PubMed] [Google Scholar]
- 2.Schultz HD, Sun SY. Chemoreflex function in heart failure. Heart Fail Rev. 2000;5:45–56. doi: 10.1023/A:1009846123893. [DOI] [PubMed] [Google Scholar]
- 3.Chua TP, Ponikowski P, Webb-Peploe K, Harrington D, Anker SD, Piepoli M, et al. Clinical characteristics of chronic heart failure patients with an augmented peripheral chemoreflex. Eur Heart J. 1997;18:480–486. doi: 10.1093/oxfordjournals.eurheartj.a015269. [DOI] [PubMed] [Google Scholar]
- 4.Ponikowski P, Chua TP, Piepoli M, Ondusova D, Webb-Peploe K, Harrington D, et al. Augmented peripheral chemosensitivity as a potential input to baroreflex impairment and autonomic imbalance in chronic heart failure. Circulation. 1997;96:2586–2594. doi: 10.1161/01.cir.96.8.2586. [DOI] [PubMed] [Google Scholar]
- 5.Ponikowski P, Chua TP, Piepoli M, Banasiak W, Anker SD, Szelemej R, et al. Ventilatory response to exercise correlates with impaired heart rate variability in patients with chronic congestive heart failure. Am J Cardiol. 1998;82:338–344. doi: 10.1016/s0002-9149(98)00303-8. [DOI] [PubMed] [Google Scholar]
- 6.Chugh SS, Chua TP, Coats AJS. Peripheral chemoreflex in chronic heart failure: friend and foe. Am Heart J. 1996;132:900–904. doi: 10.1016/s0002-8703(96)90333-6. [DOI] [PubMed] [Google Scholar]
- 7.Li YL, Xia XH, Zheng H, Gao L, Li YF, Liu D, et al. Angiotensin II enhances carotid body chemoreflex control of sympathetic outflow in chronic heart failure rabbits. Cardiovasc Res. 2006;71:129–138. doi: 10.1016/j.cardiores.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Schultz HD, Li YL, Ding Y. Arterial chemoreceptors and sympathetic nerve activity: implications for hypertension and heart failure. Hypertension. 2007;50:6–13. doi: 10.1161/HYPERTENSIONAHA.106.076083. [DOI] [PubMed] [Google Scholar]
- 9.Ding Y, Li YL, Schultz HD. Downregulation of carbon monoxide as well as nitric oxide contributes to peripheral chemoreflex hypersensitivity in heart failure rabbits. J Appl Physiol. 2008;105:14–23. doi: 10.1152/japplphysiol.01345.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun SY, Wang W, Zucker IH, Schultz HD. Enhanced activity of carotid body chemoreceptors in rabbits with heart failure: role of nitric oxide. J Appl Physiol. 1999;86:1273–1282. doi: 10.1152/jappl.1999.86.4.1273. [DOI] [PubMed] [Google Scholar]
- 11.Li YL, Li YF, Liu D, Cornish KG, Patel KP, Zucker IH, et al. Gene transfer of neuronal nitric oxide synthase to carotid body reverses enhanced chemoreceptor function in heart failure rabbits. Circ Res. 2005;97:260–267. doi: 10.1161/01.RES.0000175722.21555.55. [DOI] [PubMed] [Google Scholar]
- 12.Li YL, Gao L, Zucker IH, Schultz HD. NADPH oxidase-derived superoxide anion mediates angiotensin II-enhanced carotid body chemoreceptor sensitivity in heart failure rabbits. Cardiovasc Res. 2007;75:546–554. doi: 10.1016/j.cardiores.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li YL, Schultz HD. Enhanced sensitivity of Kv channels to hypoxia in the rabbit carotid body in heart failure: role of angiotensin II. J Physiol (Lond) 2006;575:215–227. doi: 10.1113/jphysiol.2006.110700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li YL, Schultz HD. Role of NADPH oxidase-derived superoxide anion on angiotensin II-enhanced sensitivity of potassium channels to hypoxia in carotid body of congestive heart failure rabbits. (Abstract) FASEB J. 2007;21:910.12. [Google Scholar]
- 15.Li YL, Sun SY, Overholt JL, Prabhakar NR, Rozanski GJ, Zucker IH, et al. Attenuated outward potassium currents in carotid body glomus cells of heart failure rabbit: involvement of nitric oxide. J Physiol (Lond) 2004;555:219–229. doi: 10.1113/jphysiol.2003.057422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, et al. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res. 2002;91:1038–1045. doi: 10.1161/01.res.0000043501.47934.fa. [DOI] [PubMed] [Google Scholar]
- 17.Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–216. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]
- 18.Liu JL, Irvine S, Reid IA, Patel KP, Zucker IH. Chronic exercise reduces sympathetic nerve activity in rabbits with pacing-induced heart failure: a role for angiotensin II. Circulation. 2000;102:1854–1862. doi: 10.1161/01.cir.102.15.1854. [DOI] [PubMed] [Google Scholar]
- 19.Li JM, Shah AM. Differential NADPH- versus NADH-dependent superoxide production by phagocyte-type endothelial cell NADPH oxidase. Cardiovasc Res. 2001;52:477–486. doi: 10.1016/s0008-6363(01)00407-2. [DOI] [PubMed] [Google Scholar]
- 20.Li JM, Gall NP, Grieve DJ, Chen M, Shah AM. Activation of NADPH oxidase during progression of cardiac hypertrophy to failure. Hypertension. 2002;40:477–484. doi: 10.1161/01.hyp.0000032031.30374.32. [DOI] [PubMed] [Google Scholar]
- 21.Gao L, Wang W, Liu D, Zucker IH. Exercise training normalizes sympathetic outflow by central antioxidant mechanisms in rabbits with pacing-induced chronic heart failure. Circulation. 2007;115:3095–3102. doi: 10.1161/CIRCULATIONAHA.106.677989. [DOI] [PubMed] [Google Scholar]
- 22.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, et al. Sympathoexcitation by central ANG II: roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am J Physiol Heart Circ Physiol. 2005;288:H2271–H2279. doi: 10.1152/ajpheart.00949.2004. [DOI] [PubMed] [Google Scholar]
- 23.Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, et al. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res. 2004;95:937–944. doi: 10.1161/01.RES.0000146676.04359.64. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–898. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- 25.Prabhakar NR. Neurotransmitters in the carotid body. Adv Exp Med Biol. 1994;360:57–69. doi: 10.1007/978-1-4615-2572-1_6. [DOI] [PubMed] [Google Scholar]
- 26.Zanetti M, Sato J, Katusic ZS, O'Brien T. Gene transfer of superoxide dismutase isoforms reverses endothelial dysfunction in diabetic rabbit aorta. Am J Physiol Heart Circ Physiol. 2001;280:H2516–H2523. doi: 10.1152/ajpheart.2001.280.6.H2516. [DOI] [PubMed] [Google Scholar]
- 27.Baum BJ, Goldsmith CM, Kok MR, Lodde BM, van Mello NM, Voutetakis A, et al. Advances in vector-mediated gene transfer. Immunol Lett. 2003;90:145–149. doi: 10.1016/j.imlet.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Zelko IN, Mariani TJ, Folz RJ. Superoxide dismutase multigene family: a comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic Biol Med. 2002;33:337–349. doi: 10.1016/s0891-5849(02)00905-x. [DOI] [PubMed] [Google Scholar]
- 29.Zucker IH, Wang W, Brandle M, Schultz HD, Patel KP. Neural regulation of sympathetic nerve activity in heart failure. Prog Cardiovasc Dis. 1995;37:397–414. doi: 10.1016/s0033-0620(05)80020-9. [DOI] [PubMed] [Google Scholar]
- 30.Mark AL. Sympathetic dysregulation in heart failure: mechanisms and therapy. Clin Cardiol. 1995;18:I3–I8. doi: 10.1002/clc.4960181303. [DOI] [PubMed] [Google Scholar]