Abstract
Clinical electrical muscle stimulation has been shown to alleviate muscle atrophy resulting from functional disuse, yet little is known about its effect on the skeleton. The objective of this study is to evaluate the potential of dynamic muscle stimulation on disused trabecular bone, and to investigate the importance of optimized stimulation frequency in the loading regimen. Fifty-six skeletally mature Sprague-Dawley rats were divided into seven groups for the 4-week experiment: baseline control, age-matched control, hindlimb suspended (HLS), and HLS with muscle stimulation at 1 Hz, 20 Hz, 50 Hz, and 100 Hz. Muscle stimulation was carried out for 10 minutes per day for 5 days per week, total of 4 weeks. The metaphyseal and epiphyseal trabecular regions of the distal femurs were analyzed with microcomputed tomography and histomorphometry methods. HLS alone for 4-week resulted in a significant amount of trabecular bone loss and structural deterioration. Muscle contraction at 1 Hz was not sufficient to inhibit trabecular bone loss and resulted in similar amount of loss to that of HLS alone. Bone quantity and structure were significantly improved by applying muscle stimulation at mid-frequency (20 Hz & 50 Hz). Dynamic stimulation at 50 Hz demonstrated the greatest preventive effect on the skeleton against functional disused alone animals (up to +147% in bone volume fraction, +38% in trabecular number and -36% in trabecular separation). Histomorphometric analysis showed that the stimulation, regardless of its frequency, did not have an effect on the bone formation indices, such as mineral apposition rate and bone formation rate. Overall, the data demonstrated the potentials of frequency-dependent dynamic muscle contraction in regulating skeletal adaptive responses under disuse conditions. Dynamic muscle stimulation, with a specific regimen, may be beneficial to future orthopedic research in developing a countermeasure for disuse osteopenia and osteoporosis.
Keywords: electrical muscle stimulation, bone fluid flow, loading frequency, bone adaptation, functional disuse, osteopenia, skeletal adaptation
Introduction
Conditions associated with disuse osteoporosis are often identified as decreased bone mass and deterioration of the skeletal microarchitecture. Disuse osteoporosis is a common skeletal disorder in the elderly, in patients subjected to prolonged immobility or bed-rest, e.g., fracture and spinal injury, and in astronauts who participate in long-duration spaceflight. In addition to bone loss, functional disuse and microgravity can cause muscle atrophy. Taken together, these physiological changes generate additional health complications, including increased risk of falls and fracture, and poor long-term recovery. Thus, there is a great need to develop a clinically applicable intervention for the prevention of progressive muscle atrophy and osteopenia.
Analyses of spinal cord injury (SCI) patients showed significant reduction in bone mineral density (BMD) in the disused limbs[1-3] and higher incidence of fracture [4, 5]. More than 1 year after spinal cord injury, 30% to 40% of demineralization was observed in the femoral neck, distal femur and proximal tibia [2]. It has been reported that SCI induced osteopenia/osteoporosis reached the fracture threshold (BMD of 1g/cm2) 1 to 5 years after the injury, with a fracture frequency of 5% to 34% [4, 6-8]. Similarly, analyses from space missions of 4 to 12 months duration have demonstrated that weightlessness can induce 1% to 2% BMD loss per month in the spine, 1.6% BMD loss in the hip, and 1.2% in the lower extremities [9, 10]. In addition, the reduction in trabecular BMD in both hip and femur regions was greater than 2% per month, while there was only minimal decrease in the cortical bone [11].
Clinical muscle stimulation has been examined extensively in SCI patients to strengthen skeletal muscle and alleviate muscle atrophy with promising outcomes [12, 13]. A few physical training studies further investigated this electrical stimulation technique to determine the effect on osteopenia. These studies showed mixed results in bone density data [14-16]. Using dual energy X-ray absorptiometry (DXA), BeDell et al. found no change in BMD of the lumbar spine and femoral neck regions after functional electrical stimulation-induced cycling exercise, while Mohr et al. illustrated a 10% increase in BMD in the proximal tibia after 12months of similar training [14, 16]. In a 24-week study of SCI patients in whom 25 Hz electrical stimulation was applied to the quadriceps muscles daily, Belanger and colleagues reported a 28% recovery of BMD in the distal femur and proximal tibia, along with increased muscle strength [15].
In order to explore the mechanism of disuse osteoporosis and to develop new interventions, animal models have been used to study skeletal adaptation, mimicking results from human disuse. Exposure of rats to 14 days of microgravity demonstrated an 11% reduction of trabecular BMD in the distal femur region [17]. Data from COSMOS flights (14-40days) showed similar outcomes, with decreased mineralized tissue mass, trabecular number and thickness, and increased resorption activity [18-20]. Ground-based hindlimb suspension (HLS) rodent models can induce significant 10% to 30% BMD reduction in both femur and tibia [21-23]. Histomorphometric analyses revealed a 66% decrease in trabecular osteoblast surface, and up to a 70% decrease in bone formation rate (BFR), while there was minimal change seen in resorptive activity [22, 24].
Electrical muscle stimulation with disuse animal models also demonstrated effects on the musculoskeletal system. Several investigators have shown that muscle contraction can prevent muscle atrophy to varying degrees, depending on the experimental regimen [25, 26]. Other changes included transition between type I and II muscle fibers and increased resistance to fatigue [27-29]. When studying bone adaptive response to electrical stimulus, results were mixed. Zerath et. al. found that electrical stimulation increased osteoblast activity after 3-weeks disuse, yet Midura et. al. reported partial preventive effect on osteopenia [30, 31]. One explanation for these discordant outcomes observed in both clinical and in vivo studies might be the selection of the applicable signals.
Our group has demonstrated previously that various mechanical stimuli frequencies can generate different level of surface strain and intramedullary pressure in long bone [32, 33]. Here, we hypothesized that daily induced dynamic muscle contraction can inhibit bone loss and maintain the trabecular network during a 4-week study using a functional disuse model, and that the adaptive response is dependent on the stimulation frequency. To further explore the potential of electrical stimulation as a non-invasive approach to the skeletal system, our objective was to investigate the effect of dynamic electrical muscle contraction on disused trabecular bone. In particular, we examined the importance of the stimulation frequency in the inhibition of osteopenia in a hindlimb suspension (HLS) animal model.
Materials and methods
Experimental Design
All experimental procedures were approved by the Laboratory Animal Use Committee at Stony Brook University. Fifty-six 6-months-old female Sprague-Dawley retired breeder rats (Taconic, NY) were used to investigate the effects of frequency-dependent dynamic muscle stimulation (MS) on skeletal adaptation under disuse environment. They were housed individually in 18”×18”×24” (L×W×H) stainless steel HLS cages in a temperature-controlled room with a 12:12 hours light:dark cycle, and were provided standard rodent chow and water ad libitum. Animals were transferred to these cages one week prior to the experiment start date in order to acclimate them to their environment. Animals were randomly assigned to seven groups with n=8 per group: (1) baseline control, (2) age-matched control, (3) HLS, (4) HLS+1 Hz MS, (5) HLS+20 Hz MS, (6) HLS+50 Hz MS, and (7) HLS+100 Hz MS. Functional disuse was induced by HLS, setup modified from Morey-Holton and Globus [34]. Briefly, animal’s tail was cleaned with 70% alcohol and lightly coated with tincture of benzoin. Once the tail was dried and sticky, a tail harness was attached to the tail with a piece of surgical tape. The tape was secured with two strips of elastic adhesive bandage; one over the end of the tape at the base of the tail and the other about half-way up to the end of the tail. The tail harness was then attached to a swivel apparatus suspended from the top of the cage. An approximately 30° head-down tilt was set to prevent contact of the animal’s hindlimbs with the cage bottom. The animal’s forelimbs were allowed full access to the entire cage bottom. The body weight of each animal was weighted three times per week throughout the study.
Electrical MS Protocol
For the four experimental groups, dynamic MS was applied in conjunction with HLS for 4 weeks. For the daily stimulation, animals were anesthetized and remained suspended on a counter-top with the experimental set-up. Muscle contraction was induced with two disposable needle-size electrodes (L-type guage #3, Seirin, Weymouth, MA); one electrode was placed at the right lateral proximal quadriceps, ~5 mm away from the greater trochanter, and the other was placed at the lateral distal quadriceps, above the condyles. The electrodes were then connected to a 100 MHz arbitrary waveform generator (Model 395, Wavetek) to transmit a 1 ms square pulse with various stimulation frequencies (1 Hz, 20 Hz, 50 Hz and 100 Hz) for 10 minutes per day, 5 days per week, for a total of 4 weeks. Age-matched and HLS animals were also subjected to anesthesia for the same amount of time per day as the experimental animals to account for any potential effect due to isoflurane inhalation. A rest-insertion period (2 seconds contraction followed by 8 seconds rest) was added in the MS regimen to avoid muscle fatigue.
Microcomputed Tomography (μCT)
After 4 weeks of study, animals were euthanized, and the right femurs were harvested and preserved in 70% ethanol. Using a high resolution μCT scanner (μCT-40, SCANCO Medical AG, Bassersdorf, Switzerland), the distal portion of the femur was scanned with a spatial resolution of 15μm. All images were evaluated using Gaussian filter, with specific sigma, support and threshold values of 0.5, 1, and 347, respectively. Three consecutive 750μm regions of trabecular bone (M1, M2 and M3) were analyzed in the distal metaphysis, immediately proximal to the growth plate (Figure 1). M1 is the section closest to the diaphysis, M2 is the middle section between M1 and M3, and M3 is the section closest to the growth plate. One 750μm region of trabecular bone was also analyzed in the distal epiphysis of each femur (Figure 1). Values for bone volume fraction (BV/TV, given as %), connectivity density (Conn.D, 1/mm3), structural model index (SMI), trabecular number (Tb.N, 1/mm), thickness (Tb.Th, mm) and separation (Tb.Sp, mm) were evaluated for each region [35].
Figure 1.
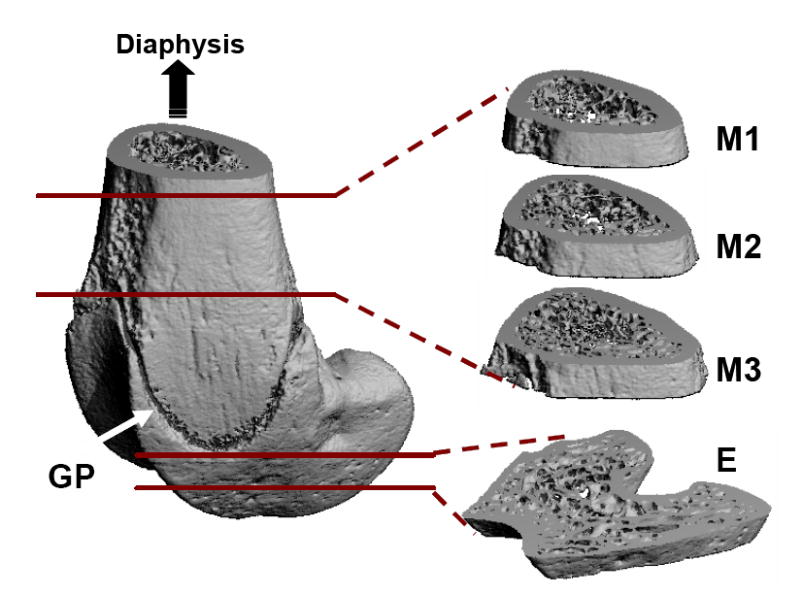
Trabecular bone at three metaphyseal sections and one epiphyseal region of the distal femurs was evaluated using microcomputed tomography. GP = growth plate (arrow); M = metaphysis; E = epiphysis.
Static and Dynamic Histomorphometry
Two intraperitoneal injections of calcein (10mg/kg) were administered to each animal two and 16 days prior to euthanasia. After scanning with μCT, the distal portions of the femurs (10 mm) were cut and dehydrated with isopropanol. The samples were then infiltrated and embedded with mixture of methyl methacrylate, n-butyl phthalate, and benzoyl peroxide. Longitudinal slices were sectioned to 8 μm using a Leica 2165 microtome (Leica, Wetzlar, Germany). Histomorphometric measurements were made by tracing calcein labels in the trabecular bone at both metaphyseal and epiphyseal regions (5mm2 and 3mm2 per section, respectively), using the Osteomeasure software (OsteoMetrics Inc, Decatur, GA). Histomorphometric bone volume fraction (BV/TV – Histo, %), mineralizing surface/bone surface (MS/BS, %), mineral apposition rate (MAR, μm/day), and bone formation rate (BFR/BS, μm3/μm2/yr) were calculated from the raw single- and double-label values [36, 37].
Statistical Analyses
Results are reported as mean ± SD for all of the analyses. For body weight measurements and all bone morphological indices, differences between groups were determined using the SigmaStat 2.03 (Systat Software Inc, San Jose, CA). Two-way analysis of variance (ANOVA) with Tukey’s pairwise multiple comparison tests was performed on the μCT data with normal equal variance. The factors in the two-way ANOVA were the different groups and the various metaphyseal regions within the distal femur. For the histomorphometric data, significant differences between groups were evaluated with one-way ANOVA with Tukey’s pairwise multiple comparison tests. The level of significance was established at p<0.05.
Results
Body weight
Throughout the entire experimental period, the animals’ body weight was monitored. The body weights were not significantly different between groups at the beginning of the study, with an average of 320g ± 47g. Age-matched control animals were able to maintain a steady body weight throughout the study, with only a -0.15% difference between the start and end date. Animals subjected to 4-week functional disuse lost a significant amount of body mass. These weight reductions were similar in HLS and HLS+MS groups, with -10% for HLS (p < 0.05), -8% for 1 Hz (p = 0.07), -9% for 20 Hz (p < 0.05), -11% for 50 Hz (p < 0.01) and -8% for (p = 0.09).
μCT - M1 region
M1 is the distal metaphyseal region 1.5mm above the growth plate (Figure 2). The lack of weight-bearing activity for 4 weeks significantly reduced trabecular bone quantity and quality, demonstrated by a 70% decreases in BV/TV, an 86% decrease in Conn.D, a 28% decrease in Tb.N, a 57% increases in SMI, and a 43% increase in Tb.Sp compared with baseline (p < 0.001). Similar results were observed when compared with age-matched control (p < 0.001); decreases in BV/TV (66%), Conn.D (86%) and Tb.N (26%), as well as increases in SMI (39%), and Tb.Sp (39%) were observed. Trabecular BV/TV in electrically stimulated animals, with the exception of 1 Hz, was significantly greater than that of disused bone. Animals with MS at 20 Hz and 50 Hz showed an increase in BV/TV by 143% (p < 0.05) and 147% (p < 0.01), respectively. Stimulation at 100 Hz showed an 86% increase in BV/TV, but this change was not statistically different from the HLS group. The other outcome measures of Conn.D, Tb.N and Tb.Sp were also significantly affected by MS at 20 Hz, 50 Hz and 100 Hz frequencies. There were up to 600% and 38% increases for Conn.D and Tb.N, and up to a 36% decrease for trabecular separation (20 Hz p < 0.01, 50 Hz p < 0.001 and 100 Hz p < 0.05). SMI and Tb.Th were not affected by the stimulus, regardless of its frequency. The animals subjected to 4 weeks of 1 Hz MS showed the same level of bone loss and structural deterioration as did the HLS animals without MS, and were significant differences compared to stimulation at higher frequencies.
Figure 2.
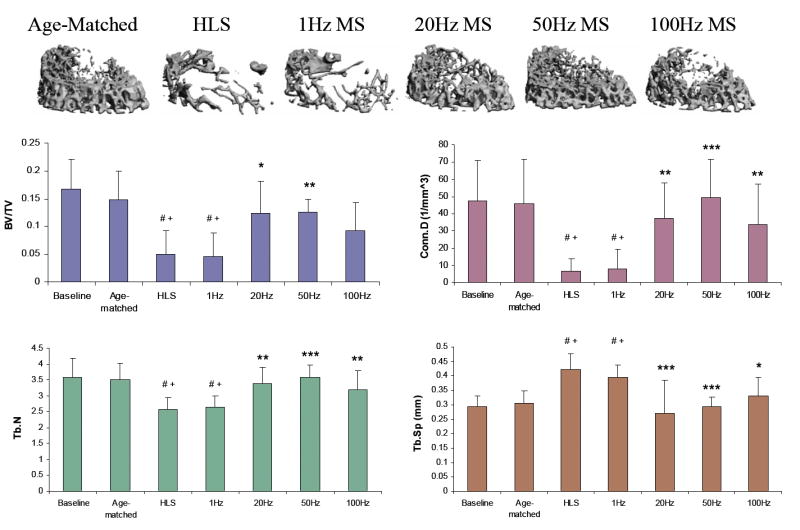
Representative 3D μCT images of trabecular bone in the M1 region (750 μm, closest to femoral diaphysis). Graphs show mean + SD values for bone volume fraction (BV/TV, %), connectivity density (Conn.D, 1/mm3), trabecular number (Tb.N, 1/mm), and separation (Tb.Sp, mm) at the M1 region. MS at 50 Hz produced a significant change in all indices, compared with values obtained in 4-week HLS. #p <0.001 vs. baseline; +p <0.001 vs. age-matched; *p <0.05 vs. HLS & 1 Hz MS; **p <0.01 vs. HLS & 1 Hz MS; ***p <0.001 vs. HLS & 1 Hz MS.
μCT - M2 region
M2, the distal metaphyseal region 750 μm above the growth plate, is a region with a moderate amount of trabecular bone under normal condition (Figure 3). Four weeks of unloading with no MS and 1 Hz MS demonstrated significant trabecular bone loss. Compared with controls, HLS animals experienced -54% for BV/TV, -77% for Conn.D, -29% for Tb.N and +45% for Tb.Sp (all p < 0.001). In this region, 50 Hz MS was the only stimulation that showed positive statistically significant effects in all measured indices against functional disuse, with increased BV/TV (66%; p < 0.05), Conn.D (371%; p < 0.001), and Tb.N (41%; p < 0.001), and reduced Tb.Sp (31%; p < 0.001). The stimulation at 50 Hz also had significantly difference compared to 1 Hz for all the parameters (p < 0.05). The changes in trabecular bone parameters observed with 20 Hz and 100 Hz stimulations had a trend similar to those of the 50 Hz MS, but with smaller percentage differences (up to +48% for BV/TV, +241% Conn.D, +29% Tb.N, and -23% Tb.Sp).
Figure 3.
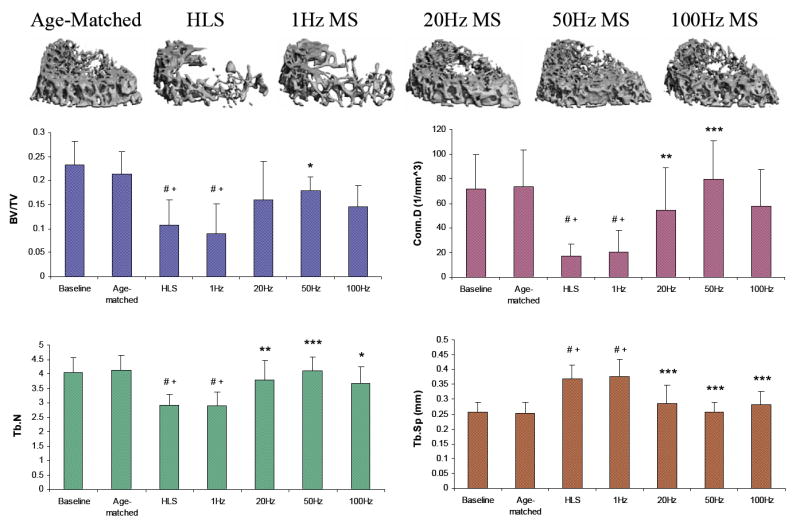
Representative 3D μCT images of trabecular bone in the M2 region (750 μm, in between M1 and M3, 750 μm above the growth plate). Graphs show mean + SD values for bone volume fraction (BV/TV, %), connectivity density (Conn.D, 1/mm3), trabecular number (Tb.N, 1/mm), and separation (Tb.Sp, mm) at the M2 region. Only 50 Hz MS showed significant effects for all indices against 4-week HLS. #p <0.001 vs. baseline; +p <0.001 vs. age-matched; *p <0.05 vs. HLS & 1 Hz MS; **p <0.01 vs. HLS & 1 Hz MS; ***p <0.001 vs. HLS & 1 Hz MS.
μCT - M3 region
M3, the distal metaphyseal portion directly above the growth plate, is a region with the most abundant trabecular network with 0.3±0.05 BV/TV and 4.72±0.64 Tb.N (Figure 4). Disuse induced a 38% bone loss, 75% decrease in Conn.D, 30% reduction in Tb.N and 43% more spacing within this region. Similar to the results reported for the M2 portion, 50 Hz MS resulted in the greatest preventive effects against disuse osteopenia, with increased BV/TV (40%; p < 0.05), Conn.D (305%; p < 0.001), and Tb.N (41%; p < 0.001), and reduced Tb.Sp (31%; p < 0.001). While BV/TV was not significantly altered by MS at 20 Hz (+26%) and 100 Hz (+20%), trabecular qualities, Conn.D, Tb.N and Tb.Sp, were improved (up to 226%, 28% and 24% respectively, p < 0.001). Like the other metaphyseal regions, SMI and Tb.Th were not affected by the stimulation.
Figure 4.
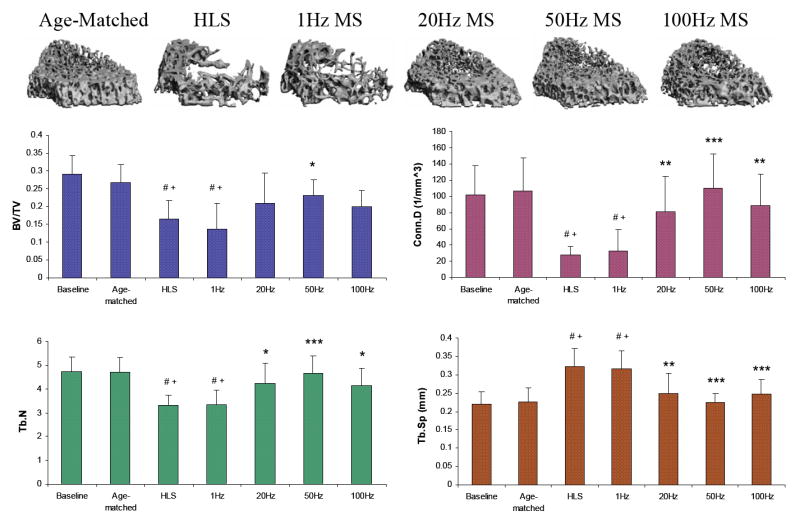
Representative 3D μCT images of trabecular bone in the M3 region (750 μm, immediately above the growth plate). Graphs show mean + SD values for bone volume fraction (BV/TV, %), connectivity density (Conn.D, 1/mm3), trabecular number (Tb.N, 1/mm), and separation (Tb.Sp, mm) at the M3 region. Only 50 Hz MS demonstrated significant preventive effects for all indices against 4-week HLS. #p <0.001 vs. baseline; +p <0.001 vs. age-matched; *p <0.05 vs. HLS & 1 Hz MS; **p <0.01 vs. HLS & 1 Hz MS; ***p <0.001 vs. HLS & 1 Hz MS.
μCT – Differences between metaphyseal regions
Figure 5 summarizes the levels of changes in BV/TV and Conn.D in electrically stimulated experimental animals compared with those of disused bone in unstimulated animals at the three metaphyseal regions. With the exception of 1 Hz, stimulation frequencies at 20 Hz, 50 Hz, and 100 Hz had greater effects on the tracbecular bone 2.25 cm away from the growth plate, closer to the diaphysis. With MS at 50 Hz, the percent changes at M1 were significantly different from those measured at M2 and M3 for BV/TV (both, p < 0.001) and from those measured at M3 for Conn.D only (p < 0.05). Also, BV/TV inhibition at M1 was significantly higher (p < 0.05) than that of M3 with 20 Hz MS. Although following a trend similar to that of the above indices, at 50 Hz and 20 Hz MS, the percent changes of the μCT measurements were not statistically significant between the three metaphyseal regions.
Figure 5.
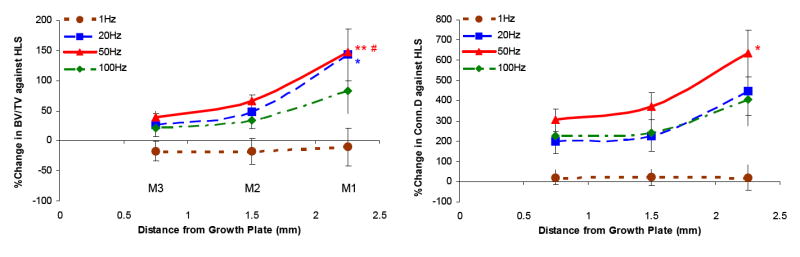
Graphs show the percentage differences between HLS and MS experimental groups ± SD values in all three metaphyseal regions for bone volume fraction (BV/TV) and connectivity density (Conn.D). For MS with mid to high stimulation frequencies, the levels of effectiveness on trabecular bone against functional disuse alone were always greatest at M1 and least at M3. **p <0.001 vs. M3; *p <0.05 vs. M3; #p <0.001 vs. M2.
μCT - E region
The epiphyseal trabecular bone was not significantly affected by the 4-week HLS. The percentage changes were minor versus the metaphyseal regions, with -5% BV/TV, -44% Conn.D, -7% Tb.N, and +4% Tb.Sp. In this region, MS did not induce any measurable effect on bone volume and trabecular integrity at any stimulation frequency. All stimulated values were comparable to age-matched and HLS animals, with up to 10% greater in BV/TV, 8% greater in Tb.N, and a 9% reduction in Tb.Sp. These changes were not statistically significant.
Static and Dynamic Histomorphometry
In the metaphyseal trabecular bone, BV/TV measured by the 2-D histomorphometric method, was 43% lower in HLS group than in age-matched controls (p < 0.001). Animals subjected to MS also experienced 22 – 29% bone loss (p < 0.01). Using the Pearson Product Moment Correlation (SigmaStat 2.03, CA), this result was correlated with the BV/TV values from the μCT analysis of the M2 region, giving an R2 value of 0.84 (p < 0.05).
In other bone formation indices, HLS animals also showed significant decline in MS/BS (76%, p < 0.001), MAR (80%, p < 0.001) and BFR/BS (92%, p < 0.001). As expected, 1 Hz MS showed no ameliorative effect. To our surprise, electrical stimulation at 20 Hz, 50 Hz and 100 Hz did not enhance bone formation indices during the 4-week disuse. These values were all significantly lower than the normal animals. MS at 50 Hz resulted in the greatest percentage changes in bone histomorphometry indices compared with those in unstimulated HLS: 86% for MS/BS, 78% for MAR and 181% for BFR/BS. However, these differences were not statistically significant between the HLS and experimental animals due to the high variability between animals (Table 1). Disuse had an insignificant effect on the trabecular BV/TV (-10%) at the epiphyseal region, similar to the results of the μCT analysis. Bone formation indices were reduced due to HLS (52% for MS/BS, 147% for MAR and 59% for BFR/BS), and daily MS failed to prevent such reduction of bone formation activity.
Table 1.
Distal femur metaphysis histomorphometry.
| Control | HLS | 1 Hz | 20 Hz | 50 Hz | 100 Hz | |
|---|---|---|---|---|---|---|
| BV/TV - Histo (%) | 41.2±7.7 | 23.3±4.3** | 24.0±8.1** | 29.6±3.9* | 32.1±4.3*# | 29.1±3.5* |
| MS/BS (%) | 9.15±5.5 | 2.11±1.0** | 1.38±1.3** | 2.37±1.7** | 3.94±2.8** | 2.21±1.7** |
| MAR (μm/day) | 1.77±0.5 | 0.36±0.3** | 0.38±0.5** | 0.48±0.3** | 0.65±0.4* | 0.57±0.4** |
| BFR/BS (μm3/μm2/yr) | 46.9±21 | 3.67±5.1** | 3.15±2.6** | 6.63±7.8** | 10.3±8.5* | 6.56±7.1* |
Values are mean ± SE. BV/TV, bone volume/tissue volume; MS/BS, mineralized surface/bone surface; MAR, mineral apposition rate; BFR/BS, bone formation rate/bone surface.
p <0.01 vs. age-matched;
p <0.001 vs. age-matched;
p =0.07 vs. HLS.
Discussion
The data strongly indicated that dynamic electrical stimulation was able to partially inhibit bone loss and trabecular architectural deterioration caused by a lack of daily weight-bearing activity. The importance of selecting an effective loading regimen was investigated in this study, in which a wide range of stimulation frequency was tested to determine its effects on skeletal adaptive responses. Throughout this study, we have referred to 1 Hz as low-frequency, 20 Hz and 50 Hz as mid-frequency, and 100 Hz as high-frequency.
From our results, we concluded that the effectiveness of dynamic MS was greatly dependent on the stimulation frequency. In addition, the degree of effectiveness varied in different regions of the distal femur. While low-frequency MS was unsuccessful in preventing osteopenia, mid-frequency MS applied to the quadriceps was able to maintain trabecular bone mass.
In this study, 4 weeks of functional disuse significantly reduced the metaphyseal bone volume fraction, mainly via a decrease in trabecular number, thereby reducing its connectivity and augmenting the marrow space. Dynamic MS at the mid-frequency range was capable in preserving bone mass, trabecular number, and connectivity. Although the changes in bone formation indices were not statistically significant, increases in MAR and BFR were quite promising. The partial enhancement of bone formation may not fully explain our results; bone volume and structures in the metaphyseal regions were maintained at a level similar to that of age-matched controls.
One limitation of our study is that we did not examine bone resorption, which may play a crucial component in regulating skeletal adaptation induced by MS. Our methodology in ex vivo processing of the femurs precluded analysis of tartrate-resistant acid phosphatase as a marker of bone resorptive process; hence, resorption analysis will be the focus for future experiments.
The nonlinear relationship between femoral strain measured at the mid-diaphysis and mechanical stimulation applied at various frequencies has been widely reported [30, 33, 37-40]. It has been hypothesized that strain information integrates over time [39], suggesting that low-level mechanical strain induced by mid-frequency electrical MS may dominate in the skeleton, and initiate osteogenic activities. Both osteoblasts and osteocytes have been shown to response to mechanical stimulus. Mechanotransduction induces biochemical signal cascades, including those that produce hormones and growth factors, which in turn affect the coupled processes of formation and resorption [41-44].
Previous experiments, using an 8 weeks disused turkey ulna model, demonstrated that a low strain magnitude (100 με) with high loading cycle number (108,000) was sufficient to maintain cortical bone mass [33]. Here, our 50 Hz and 100 Hz MS, which both induced extremely low-magnitude microstrain and displayed protective effects against disuse osteopenia, delivered only 6,000 and 12,000 daily pulses, respectively, to the skeletal muscle. MS of 50 Hz or100 Hz generates tetanic muscle contraction, in which summation of subsequent stimuli occurs and skeletal muscles are not allowed to relax during stimulation. During induced tetanic contraction of the quadriceps, the femurs may be subjected to maximal dynamic compressive loading creating the measurable low-magnitude bone strain. Other studies with low-magnitude stimulus, e.g., vibration, have also been shown to enhance bone morphology, promote bone formation, and increase bone strength [38, 40, 45]. An important conclusion from these experiments is that small strain, e.g., induced by muscle contraction, might be one of the key determinants in regulating skeletal adaptation.
Although there were no statistical differences between the 20 Hz, 50 Hz, and 100 Hz experimental groups, our data indicated that mid-frequency MS resulted in greater percentage changes in all bone quantity and quality indices. In addition to bone strain, another factor involved in mediating bone remodeling is bone fluid flow induced by intramedullary pressure (ImP) [46]. Similar to the strain profile, dynamic mechanical stimulation also yielded a nonlinear ImP distribution, and changes in ImP is highly depended on the stimulus frequency [32, 46, 47]. For MS with mid to high stimulation frequencies, the effect on trabecular bone was always greater at M1, closer to the mid-diaphysis. This suggests that there may be an ImP gradient, perhaps generated by the induced muscle contraction, influencing the magnitude of mechanotransductory signals on the trabecular bone at the different regions.
Previous ImP measurement in mice showed a 23% drop in ImP upon HLS, indicate a decrease in interstitial fluid flow via the cortex [47]. On the contrary, increasing the pressure gradient in HLS animals via venous ligation was able to compensate such drop in ImP [47]. In vivo fluid pressure measurements have investigated the close relationship between loading frequency and fluid flow regulation in bone, where 20-50 Hz stimulation could initiate anabolic activities [46, 48]. In the absence of matrix deformation, an increase in ImP induced by direct manipulation of marrow cavity was able to inhibit resorption related to disuse by osteotomy [32]. These observations clearly pointed out that fluid flow can be altered by both ImP and bone strain.
In addition, changes in the fluid environments i.e., pressure gradient and shear stress, have the potential to modulate bone remodeling via autocrine and paracrine signaling cascades, e.g., nitric oxide and prostaglandins [47, 49, 50]. With 50 Hz MS, bone cells on the trabecular surface may sense the 4-fold increase of ImP, accompanying with the low-magnitude strain (<20 με), thereby activating mechanotransduction and increasing the transport of nutrients and other signaling molecules within the tissues.
Both strain and ImP have the potentials to induce bone fluid flow, thus mediating adaptive responses in the trabecular bone. Another limitation of this presented study was that by using MS, we were unable to isolate the two main fluid flow inducing factors using MS. In order to investigate the influence of MS-induced ImP, a more invasive experimental design is required to eliminate the matrix deformation, in which muscle bundles might be disrupted or damaged in process. In our system to examine the effects of dynamic electrical contraction in a functional disuse model, MS was delivered to the quadriceps muscles in an extremely minimal invasive manner, as the key concept in translational research. The stimulation frequencies were chosen so that different combinations of strain and ImP would be achieved via electrically induced muscle contraction.
The interactive relationships between muscle and bone adaptations are not fully understood. It is clear that MS promotes blood flow, yet the mechanism remains highly controversial. One theory (muscle pump), suggests that at the onset of muscle contraction, veins within skeletal muscle are compressed thus increasing the arteriovenous pressure gradient and promoting blood flow to the vascular bed [51-53]. The pressure gradient in the muscle vasculature may be relayed to the nutrient vessels in bone and further increasing ImP and inducing fluid flow in bone [54]. Alternatively, vasodilation, with an onset of 5-20 seconds, might also play a role in the rapid release of metabolites, contributing to the initial blood flow response to tetanic MS [55, 56]. Aside from avoiding muscle fatigue, the pattern of 2 seconds stimulation with 8s seconds rest inserted into our loading regimen was designed to address both theories, attempting to maximize the fluid perfusion and nutrient delivery to the musculoskeletal tissues. Further investigations will concentrate on characterizing the effects of frequency-dependent MS on muscular morphology and its vasculature, and exploring other mechanical parameters to optimize the dynamic MS regimen.
It is important to note that microgravity and functional disuse affect both muscle and bone. The potential of MS in regulating musculoskeletal adaptation would be an ideal clinical intervention. The main objective of this study is to demonstrate that dynamic MS can prevent osteopenia. Furthermore, the skeletal adaptive responses induced by MS are highly depended on its stimulation frequency. Although we evaluated only indices of bone formation, it is likely that inhibition of bone resorption activity is also contributory to the observed effects. The findings from this study may assist in development of future clinical interventions and beneficial to future countermeasure for disuse osteoporosis.
Acknowledgments
This research is kindly supported by the National Institute of Health (R01 AR52379 and R01 AR49286, Qin), the US Army Medical Research and Materiel Command, and the National Space Biomedical Research Institute through NASA Cooperative Agreement NCC 9-58. The authors are grateful to Dr. C. Rubin and Dr. S. Judex for friendly discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Biering-Sorensen F, Bohr HH, Schaadt OP. Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur J Clin Invest. 1990;20:330–5. doi: 10.1111/j.1365-2362.1990.tb01865.x. [DOI] [PubMed] [Google Scholar]
- 2.Dauty M, Perrouin Verbe B, Maugars Y, Dubois C, Mathe JF. Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone. 2000;27:305–9. doi: 10.1016/s8756-3282(00)00326-4. [DOI] [PubMed] [Google Scholar]
- 3.Garland DE, Adkins RH, Stewart CA, Ashford R, Vigil D. Regional osteoporosis in women who have a complete spinal cord injury. J Bone Joint Surg Am. 2001;83-A:1195–200. doi: 10.2106/00004623-200108000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Lazo MG, Shirazi P, Sam M, Giobbie-Hurder A, Blacconiere MJ, Muppidi M. Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord. 2001;39:208–14. doi: 10.1038/sj.sc.3101139. [DOI] [PubMed] [Google Scholar]
- 5.Vestergaard P, Krogh K, Rejnmark L, Mosekilde L. Fracture rates and risk factors for fractures in patients with spinal cord injury. Spinal Cord. 1998;36:790–6. doi: 10.1038/sj.sc.3100648. [DOI] [PubMed] [Google Scholar]
- 6.Heaney RP. Pathophysiology of osteoporosis. Endocrinol Metab Clin North Am. 1998;27:255–65. doi: 10.1016/s0889-8529(05)70004-9. [DOI] [PubMed] [Google Scholar]
- 7.Ingram RR, Suman RK, Freeman PA. Lower limb fractures in the chronic spinal cord injured patient. Paraplegia. 1989;27:133–9. doi: 10.1038/sc.1989.20. [DOI] [PubMed] [Google Scholar]
- 8.Szollar SM, Martin EM, Sartoris DJ, Parthemore JG, Deftos LJ. Bone mineral density and indexes of bone metabolism in spinal cord injury. Am J Phys Med Rehabil. 1998;77:28–35. doi: 10.1097/00002060-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 9.LeBlanc A. Summary of research issues in human studies. Bone. 1998;22:117S–118S. [PubMed] [Google Scholar]
- 10.McCarthy I, Goodship A, Herzog R, Oganov V, Stussi E, Vahlensieck M. Investigation of bone changes in microgravity during long and short duration space flight: comparison of techniques. Eur J Clin Invest. 2000;30:1044–54. doi: 10.1046/j.1365-2362.2000.00719.x. [DOI] [PubMed] [Google Scholar]
- 11.Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res. 2004;19:1006–12. doi: 10.1359/JBMR.040307. [DOI] [PubMed] [Google Scholar]
- 12.Perez M, Lucia A, Rivero JL, Serrano AL, Calbet JA, Delgado MA, Chicharro JL. Effects of transcutaneous short-term electrical stimulation on M. vastus lateralis characteristics of healthy young men. Pflugers Arch. 2002;443:866–74. doi: 10.1007/s00424-001-0769-6. [DOI] [PubMed] [Google Scholar]
- 13.Rodgers MM, Glaser RM, Figoni SF, Hooker SP, Ezenwa BN, Collins SR, Mathews T, Suryaprasad AG, Gupta SC. Musculoskeletal responses of spinal cord injured individuals to functional neuromuscular stimulation-induced knee extension exercise training. J Rehabil Res Dev. 1991;28:19–26. doi: 10.1682/jrrd.1991.10.0019. [DOI] [PubMed] [Google Scholar]
- 14.BeDell KK, Scremin AM, Perell KL, Kunkel CF. Effects of functional electrical stimulation-induced lower extremity cycling on bone density of spinal cord-injured patients. Am J Phys Med Rehabil. 1996;75:29–34. doi: 10.1097/00002060-199601000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Belanger M, Stein RB, Wheeler GD, Gordon T, Leduc B. Electrical stimulation: can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Arch Phys Med Rehabil. 2000;81:1090–8. doi: 10.1053/apmr.2000.7170. [DOI] [PubMed] [Google Scholar]
- 16.Mohr T, Podenphant J, Biering-Sorensen F, Galbo H, Thamsborg G, Kjaer M. Increased bone mineral density after prolonged electrically induced cycle training of paralyzed limbs in spinal cord injured man. Calcif Tissue Int. 1997;61:22–5. doi: 10.1007/s002239900286. [DOI] [PubMed] [Google Scholar]
- 17.Lafage-Proust MH, Collet P, Dubost JM, Laroche N, Alexandre C, Vico L. Space-related bone mineral redistribution and lack of bone mass recovery after reambulation in young rats. Am J Physiol. 1998;274:R324–34. doi: 10.1152/ajpregu.1998.274.2.R324. [DOI] [PubMed] [Google Scholar]
- 18.Cann CE, Adachi RR. Bone resorption and mineral excretion in rats during spaceflight. Am J Physiol. 1983;244:R327–31. doi: 10.1152/ajpregu.1983.244.3.R327. [DOI] [PubMed] [Google Scholar]
- 19.Jee WS, Wronski TJ, Morey ER, Kimmel DB. Effects of spaceflight on trabecular bone in rats. Am J Physiol. 1983;244:R310–4. doi: 10.1152/ajpregu.1983.244.3.R310. [DOI] [PubMed] [Google Scholar]
- 20.Vico L, Bourrin S, Genty C, Palle S, Alexandre C. Histomorphometric analyses of cancellous bone from COSMOS 2044 rats. J Appl Physiol. 1993;75:2203–8. doi: 10.1152/jappl.1993.75.5.2203. [DOI] [PubMed] [Google Scholar]
- 21.Bloomfield SA, Allen MR, Hogan HA, Delp MD. Site- and compartment-specific changes in bone with hindlimb unloading in mature adult rats. Bone. 2002;31:149–57. doi: 10.1016/s8756-3282(02)00785-8. [DOI] [PubMed] [Google Scholar]
- 22.Bourrin S, Palle S, Genty C, Alexandre C. Physical exercise during remobilization restores a normal bone trabecular network after tail suspension-induced osteopenia in young rats. J Bone Miner Res. 1995;10:820–8. doi: 10.1002/jbmr.5650100520. [DOI] [PubMed] [Google Scholar]
- 23.Inman CL, Warren GL, Hogan HA, Bloomfield SA. Mechanical loading attenuates bone loss due to immobilization and calcium deficiency. J Appl Physiol. 1999;87:189–95. doi: 10.1152/jappl.1999.87.1.189. [DOI] [PubMed] [Google Scholar]
- 24.Dehority W, Halloran BP, Bikle DD, Curren T, Kostenuik PJ, Wronski TJ, Shen Y, Rabkin B, Bouraoui A, Morey-Holton E. Bone and hormonal changes induced by skeletal unloading in the mature male rat. Am J Physiol. 1999;276:E62–9. doi: 10.1152/ajpendo.1999.276.1.e62. [DOI] [PubMed] [Google Scholar]
- 25.Dupont Salter AC, Richmond FJ, Loeb GE. Prevention of muscle disuse atrophy by low-frequency electrical stimulation in rats. IEEE Trans Neural Syst Rehabil Eng. 2003;11:218–26. doi: 10.1109/TNSRE.2003.817674. [DOI] [PubMed] [Google Scholar]
- 26.Nemirovskaya TL, Shenkman BS. Effect of support stimulation on unloaded soleus in rat. Eur J Appl Physiol. 2002;87:120–6. doi: 10.1007/s00421-002-0603-7. [DOI] [PubMed] [Google Scholar]
- 27.Gordon T, Tyreman N, Rafuse VF, Munson JB. Fast-to-slow conversion following chronic low-frequency activation of medial gastrocnemius muscle in cats. I. Muscle and motor unit properties. J Neurophysiol. 1997;77:2585–604. doi: 10.1152/jn.1997.77.5.2585. [DOI] [PubMed] [Google Scholar]
- 28.Jarvis JC, Sutherland H, Mayne CN, Gilroy SJ, Salmons S. Induction of a fast-oxidative phenotype by chronic muscle stimulation: mechanical and biochemical studies. Am J Physiol. 1996;270:C306–12. doi: 10.1152/ajpcell.1996.270.1.C306. [DOI] [PubMed] [Google Scholar]
- 29.Mabuchi K, Szvetko D, Pinter K, Sreter FA. Type IIB to IIA fiber transformation in intermittently stimulated rabbit muscles. Am J Physiol. 1982;242:C373–81. doi: 10.1152/ajpcell.1982.242.5.C373. [DOI] [PubMed] [Google Scholar]
- 30.Midura RJ, Dillman CJ, Grabiner MD. Low amplitude, high frequency strains imposed by electrically stimulated skeletal muscle retards the development of osteopenia in the tibiae of hindlimb suspended rats. Med Eng Phys. 2005;27:285–93. doi: 10.1016/j.medengphy.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 31.Zerath E, Canon F, Guezennec CY, Holy X, Renault S, Andre C. Electrical stimulation of leg muscles increases tibial trabecular bone formation in unloaded rats. J Appl Physiol. 1995;79:1889–94. doi: 10.1152/jappl.1995.79.6.1889. [DOI] [PubMed] [Google Scholar]
- 32.Qin YX, Kaplan T, Saldanha A, Rubin C. Fluid pressure gradients, arising from oscillations in intramedullary pressure, is correlated with the formation of bone and inhibition of intracortical porosity. J Biomech. 2003;36:1427–37. doi: 10.1016/s0021-9290(03)00127-1. [DOI] [PubMed] [Google Scholar]
- 33.Qin YX, Rubin CT, McLeod KJ. Nonlinear dependence of loading intensity and cycle number in the maintenance of bone mass and morphology. J Orthop Res. 1998;16:482–9. doi: 10.1002/jor.1100160414. [DOI] [PubMed] [Google Scholar]
- 34.Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol. 2002;92:1367–77. doi: 10.1152/japplphysiol.00969.2001. [DOI] [PubMed] [Google Scholar]
- 35.Laib A, Kumer JL, Majumdar S, Lane NE. The temporal changes of trabecular architecture in ovariectomized rats assessed by MicroCT. Osteoporos Int. 2001;12:936–41. doi: 10.1007/s001980170022. [DOI] [PubMed] [Google Scholar]
- 36.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 37.LaMothe JM, Zernicke RF. Rest insertion combined with high-frequency loading enhances osteogenesis. J Appl Physiol. 2004;96:1788–93. doi: 10.1152/japplphysiol.01145.2003. [DOI] [PubMed] [Google Scholar]
- 38.Judex S, Lei X, Han D, Rubin C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech. 2007;40:1333–9. doi: 10.1016/j.jbiomech.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 39.Rubin C, Turner AS, Bain S, Mallinckrodt C, McLeod K. Anabolism. Low mechanical signals strengthen long bones. Nature. 2001;412:603–4. doi: 10.1038/35088122. [DOI] [PubMed] [Google Scholar]
- 40.Xie L, Jacobson JM, Choi ES, Busa B, Donahue LR, Miller LM, Rubin CT, Judex S. Low-level mechanical vibrations can influence bone resorption and bone formation in the growing skeleton. Bone. 2006;39:1059–66. doi: 10.1016/j.bone.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 41.Bloomfield SA. Cellular and molecular mechanisms for the bone response to mechanical loading. Int J Sport Nutr Exerc Metab. 2001;11(Suppl):S128–36. doi: 10.1123/ijsnem.11.s1.s128. [DOI] [PubMed] [Google Scholar]
- 42.Chow JW. Role of nitric oxide and prostaglandins in the bone formation response to mechanical loading. Exerc Sport Sci Rev. 2000;28:185–8. [PubMed] [Google Scholar]
- 43.Robling AG, Castillo AB, Turner CH. Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng. 2006;8:455–98. doi: 10.1146/annurev.bioeng.8.061505.095721. [DOI] [PubMed] [Google Scholar]
- 44.Rubin J, Rubin C, Jacobs CR. Molecular pathways mediating mechanical signaling in bone. Gene. 2006;367:1–16. doi: 10.1016/j.gene.2005.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dickerson DA, Sander EA, Nauman EA. Modeling the mechanical consequences of vibratory loading in the vertebral body: microscale effects. Biomech Model Mechanobiol. 2007 doi: 10.1007/s10237-007-0085-y. [DOI] [PubMed] [Google Scholar]
- 46.Qin YX, Lin W, Rubin C. The pathway of bone fluid flow as defined by in vivo intramedullary pressure and streaming potential measurements. Ann Biomed Eng. 2002;30:693–702. doi: 10.1114/1.1483863. [DOI] [PubMed] [Google Scholar]
- 47.Stevens HY, Meays DR, Frangos JA. Pressure gradients and transport in the murine femur upon hindlimb suspension. Bone. 2006;39:565–72. doi: 10.1016/j.bone.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 48.Rubin C, Turner AS, Muller R, Mittra E, McLeod K, Lin W, Qin YX. Quantity and quality of trabecular bone in the femur are enhanced by a strongly anabolic, noninvasive mechanical intervention. J Bone Miner Res. 2002;17:349–57. doi: 10.1359/jbmr.2002.17.2.349. [DOI] [PubMed] [Google Scholar]
- 49.Johnson DL, McAllister TN, Frangos JA. Fluid flow stimulates rapid and continuous release of nitric oxide in osteoblasts. Am J Physiol. 1996;271:E205–8. doi: 10.1152/ajpendo.1996.271.1.E205. [DOI] [PubMed] [Google Scholar]
- 50.Kasten TP, Collin-Osdoby P, Patel N, Osdoby P, Krukowski M, Misko TP, Settle SL, Currie MG, Nickols GA. Potentiation of osteoclast bone-resorption activity by inhibition of nitric oxide synthase. Proc Natl Acad Sci U S A. 1994;91:3569–73. doi: 10.1073/pnas.91.9.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laughlin MH, Schrage WG. Effects of muscle contraction on skeletal muscle blood flow: when is there a muscle pump? Med Sci Sports Exerc. 1999;31:1027–35. doi: 10.1097/00005768-199907000-00016. [DOI] [PubMed] [Google Scholar]
- 52.Pollack AA, Wood EH. Venous pressure in the saphenous vein at the ankle in man during exercise and changes in posture. J Appl Physiol. 1949;1:649–62. doi: 10.1152/jappl.1949.1.9.649. [DOI] [PubMed] [Google Scholar]
- 53.Sheriff DD, Van Bibber R. Flow-generating capability of the isolated skeletal muscle pump. Am J Physiol. 1998;274:H1502–8. doi: 10.1152/ajpheart.1998.274.5.H1502. [DOI] [PubMed] [Google Scholar]
- 54.Winet H. A bone fluid flow hypothesis for muscle pump-driven capillary filtration: II. Proposed role for exercise in erodible scaffold implant incorporation. Eur Cell Mater. 2003;6:1–10. doi: 10.22203/ecm.v006a01. discussion 10-1. [DOI] [PubMed] [Google Scholar]
- 55.Naik JS, Valic Z, Buckwalter JB, Clifford PS. Rapid vasodilation in response to a brief tetanic muscle contraction. J Appl Physiol. 1999;87:1741–6. doi: 10.1152/jappl.1999.87.5.1741. [DOI] [PubMed] [Google Scholar]
- 56.Valic Z, Buckwalter JB, Clifford PS. Muscle blood flow response to contraction: influence of venous pressure. J Appl Physiol. 2005;98:72–6. doi: 10.1152/japplphysiol.00151.2004. [DOI] [PubMed] [Google Scholar]


