Abstract
Glial cell line-derived neurotrophic factor (GDNF), a member of the transforming growth factor beta (TGFβ) superfamily, is a potent neurotrophic protein promoting the survival and maintenance of dopaminergic (DA) neurons in the substantia nigra during development and adulthood. DA neurons that project to the striatum in the nigrostriatal pathway express GDNF receptors, GFRα1. The purpose of this study was to determine whether these neurons are especially sensitive to neurotoxic insults. Therefore, we examined effects of the dopaminergic toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) on locomotion and DA neurons in 26-month-old male GFRα1 heterozygous (GFRα1+/−) mice compared to aged-matched wild-type (WT) littermates. MPTP gave rise to increased locomotion, regardless of genotype, while GFRα1+/− mice treated with saline exhibited lower spontaneous locomotion, compared to WT mice. Moreover, GFRα1+/− saline mice had fewer TH-positive neurons, greater expression of inflammatory markers (CD45 immunostaining and phosphorylated p38 MAPK) in the nigra, and reduced striatal TH staining. MPTP exacerbated these effects, with the lowest density of striatal TH and highest density of nigral CD45 and phospho-p38 MAPK immunoreactivity observed in GFRα1+/− mice. The findings point to increased sensitivity of the DAergic system with age and neurotoxic exposure as a result of a genetic reduction of GFRα1.
Keywords: Growth factor receptors, Aging, Neurodegeneration, Neuroinflammation, Dopamine neurotoxins
1. INTRODUCTION
The neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) damages the nigrostriatal dopamine (DA) system and has been used to create parkinsonian-like models in non-human primates and rodents (Olanow and Tatton, 1999; Kopin and Markey, 1988). MPTP is a lipophilic molecule that crosses the blood brain barrier (BBB) and once converted to MPP+ it is taken up by DAergic terminals and cell bodies by the dopamine transporter (DAT; Gainetdinov et al., 1997; Smeyne and Jackson-Lewis, 2005). MPP+ accumulates in mitochondria resulting in oxidative stress and cell loss (Lander and Schork, 1994; Nickolas et al., 2007; Przedborski et al., 2004; Wu et al., 2002).
Increased cytokine expression and microglial activation occur with normal aging, especially in brain regions with large numbers of microglia, such as the substantia nigra (SN; see eg. Morgan et al, 2007). There is growing evidence suggesting that inflammation contributes to the pathogenesis of neurodegenerative disorders (see eg. Allan and Rothwell, 2001; Hartmann et al., 2003; Hirsch et al., 2003; for review see Granholm et al., 2008). Microglial activation has been found to occur in association with brain injury (Kato et al., 2000) and with exposure to neurotoxins in animal models of Parkinson’s and Alzheimer’s disease (Jorgensen et al., 1993; Scali et al., 1999; Fiedorowicz et al., 2001; Francis et al., 1995; Czlonkowska et al., 1996; Langston et al., 1999). It has been suggested that lesion-related neuroinflammation is triggered by protein aggregates, degenerating neurons, or dysregulation of inflammatory control mechanisms in the aged brain (Wyss-Coray and Mucke, 2002).
GDNF is a member of the transforming growth factor-β superfamily of neurotrophic factors (Krieglstein et al., 1995; Saarma, 2000). It is required for survival of cultured midbrain DAergic neurons (Lin et al., 1993) and promotes recovery in rodent and nonhuman primate models of PD (Bowenkamp et al., 1995; Gash et al., 1996; Mandel et al., 1997; Kordower et al., 2000; Cass et al., 1999; Granholm et al., 1997a,b; Dowd et al., 2005). GDNF levels are decreased in the SN of PD patients (Jenner and Olanow, 1998) and in normal aged rodents (Yurek et al., 2001) suggesting its involvement in motor dysfunction and DA neuron degeneration. Previous work in our laboratory has demonstrated that GDNF heterozygous mice (GDNF+/−) exhibit DA loss and motor dysfunction with aging, suggesting that a partial genetic depletion of GDNF results in greater aging-related degeneration of the DA system, at least in rodents (Boger et al., 2006). Cellular responses to GDNF are mediated via a multi-component receptor consisting of the RET receptor tyrosine kinase and the GPI-linked ligand-binding GDNF-receptor α1 (GFR α1; see eg. Harvey et al., 2005; Airaksinen and Saarma, 2002). Animal models have been constructed to assess long-term effects of decreasing RET and GFRa1 levels (Sarabi et al., 2003; Kramer et al., 2007; Cacalano et al., 1998; Enomoto et al., 1998). These previous studies demonstrated that RET and GFR α1 are essential for GDNF-induced neuroprotection of the nigrostriatal DA system since mice with a reduction in these receptor proteins demonstrated increased age-related decline in nigro-striatal DA expression and function (Zaman et al., 2008; Kramer et al., 2007). Furthermore, over-expression of RET leads to increased number of DA neurons and increased brain dopamine concentrations (Mijatovic et al., 2007), suggesting again that this receptor complex is involved with DA neuron survival and function. However, studies have not been directed toward determining whether loss of GDNF receptors increases the vulnerability of dopamine neurons to neurotoxins. Therefore, the goal of the present study was to determine the long-term impact of GFR α1 reduction on the neurotoxic and neuroinflammatory effects of MPTP.
2. RESULTS
Locomotor activity
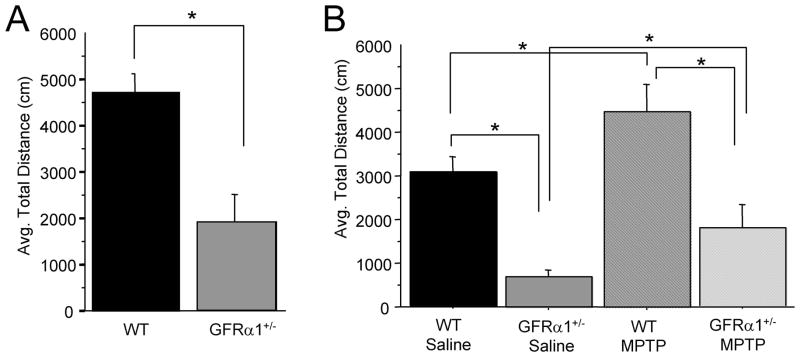
Basal motor activity of 26-month-old GFR α1+/− and WT mice over a one hour period in open-field locomotor activity chambers the day prior to MPTP treatment is summarized in Fig. 1A. Analysis showed that spontaneous locomotion was significantly lower in untreated GFR α1+/− mice than in untreated WT controls [t(25)=−3.746, p<0.001]. Fig. 1B summarizes motor activity over an hour for the four groups (WT and GFRα1+/− mice with or without MPTP injections) using a Genotype ×Treatment design two days after termination of the 4-day MPTP treatment paradigm. A 2(Genotype) × 2(Treatment: Saline or MPTP) ANOVA indicated significant Genotype [F(1,25)=22.526, p<0.0001] and Treatment [F(1,25)=5.587, p<0.05] effects but no interaction [F(1,25)=0.100, p=0.7547] of the two factors. As noted in the graph, GFRα1+/− mice were less active than WT mice whether treated with saline or MPTP similar to the findings of the baseline activity assessment prior to MPTP injections (Fig. 1A). In addition, MPTP elevated motor activity to a similar extent regardless of genotype, even though the GFRα1+/− mice had lower baseline activity (Fig. 1A and B).
Figure 1. Partial loss of GFR α1 resulted in lower locomotor activity compared to WT mice.
(A) Twenty-four hrs prior to MPTP administration, 26-month-old GFR α1+/− mice had less locomotor activity than age-matched WT mice. (B) Two days following the administration of sub-chronic MPTP, mice given MPTP had significantly higher locomotor activity than saline-treated mice, regardless of genotype, with the activity of GFR α1+/− mice treated with MPTP significantly lower than WT mice even though the incremental increase in motor activity was higher in MPTP-treated GFR α1+/− mice than MPTP-treated WT mice. N=8 per group. Error bars indicate SEM.
Nigral and striatal microglial immunoreactivity
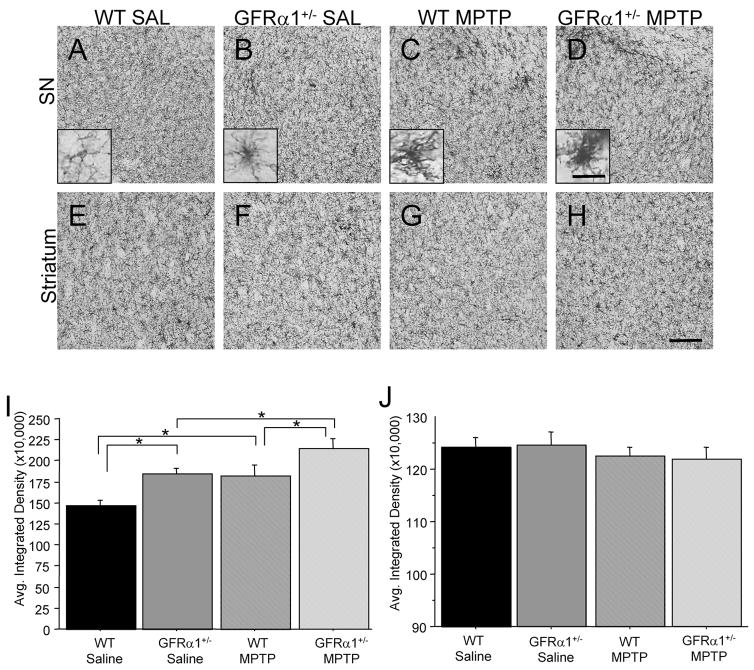
All mice were sacrificed two days following cessation of the MPTP injections, since our primary focus in this study was related to inflammatory responses following the neurotoxin treatment, rather than the slower neurodegenerative process which occurs within a week or so following cessation of repeated MPTP injections (see e.g. Du et al., 2001). An antibody directed against the cluster differentiation (CD) antigen, CD45, was employed to evaluate degree of microglial activation in the four treatment groups. Microglia were stained with a CD45 antibody that detects cluster differentiation marker 45. Resting state microglia are characterized by a smaller cell body with long, thin processes (see Figure 2A inset), whereas reactive microglia are characterized by increased cell body volume, short, thick processes, and increased intensity of staining for cell surface markers, such as CD45 (see Fig. 2B–D insets; LaVoie et al., 2004). Because CD45 is expressed by both resting and activated microglia, qualitative assessment of the morphological appearance of microglia supplemented density measurements. When assessing the CD45-ir in the SN, significant Genotype [F(1,25)=11.238, p<0.01] and Treatment [F(1,25)=10.059, p<0.01] effects were confirmed but not a Genotype × Treatment interaction [F(1,25)=0.055, p=0.8167] indicating that the increase in microglial activation resulting from the MPTP treatment did not differ between the two genotypes, although an additive effect of the GFRα1 receptor deletion and the MPTP treatment was noted (see Figure 2I). Thus, at 26 months of age, saline-treated GFRα1+/− mice demonstrated greater nigral CD45-ir when compared to saline-treated WT mice (Fig. 2A, B, I). WT mice treated with MPTP had a 24% increase in CD45-ir in the SN when compared to WT mice treated with saline (Fig. 2A, C, I), and GFRα1+/− mice treated with MPTP also demonstrated a significant increase (16%) in CD45-ir in the SN when compared to saline-treated GFRα1+/− mice (Fig. 2B, D, I). Similarly, a significant difference existed in SN CD45-ir between genotypes when treated with MPTP (Fig. 2B, D, I) with GFRα1+/− mice having 18% more CD45-ir. When assessing the CD45-ir in the striatum at the same time point following MPTP injections, no differences were observed between the four treatment groups (Fig. 2E-H, J).
Figure 2. MPTP treatment resulted in a greater inflammatory response in the SN of 26-month-old GFR α1+/− mice two days post-injection.
Photomicrographs of CD45-immunoreactivity in the (A-D) SN and (E-H) striatum of (A, E) saline-treated WT mice, (B, F) saline-treated GFR α1+/−, (C, G) MPTP-treated WT mice, (D, H) MPTP-treated GFR α1+/− mice. Quantification of the average integrated density of CD45-ir is depicted in the (I) SN and (J) striatum using one-way ANOVA analysis followed by Student-Nuemann-Kuels post-hoc test. Saline-treated GFR α1+/− mice had elevated CD45 immunoreactivity (ir) in the SN compared to saline-treated WT mice (*p<0.05). MPTP treatment caused a significantly greater increase in CD45-ir in the SN of GFR α1+/− mice than in either saline-treated GFR α1+/− mice or MPTP-treated WT mice (*p<0.05). No differences in CD45-ir existed in the striatum between the four treatment groups. N=8 per group. Standard photomicrograph magnification 20x with scale bar=0.5mm (inset magnification =60x). Error bars indicate SEM.
Substantia nigra p38 MAPK phosphorylation
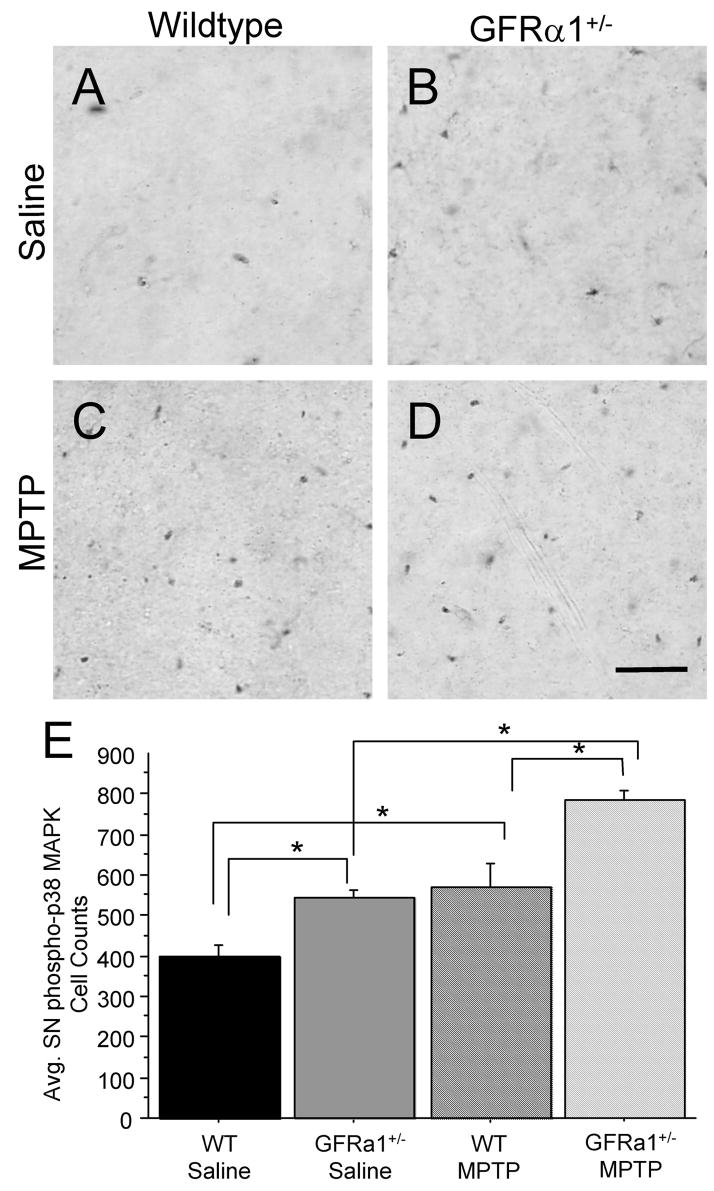
Growing evidence suggests that glial phosphorylation of p38 MAPK is essential for the synthesis and release of pro-inflammatory mediators (Hunter et al., 2004; Du et al., 2001; Wu et al., 2002). In the present study, we utilized a phospho-specific antibody to detect activation of phospho-p38 MAPK in the SN (Fig. 3). Using a 2(Genotype) x 2(Treatment) ANOVA, significant Genotype [F(1,25)=20.0478, p<0.01] and Treatment [F(1,25)=33.341, p<0.01] effects were confirmed but no Genotype × Treatment interaction [F(1,25)=0.963, p=0.0.3410] in terms of the number of SNpc phospho-p38 MAPK positive cells, again most likely due to an additive effect of the GFRα1 loss and the MPTP injections (Fig. 3E). Aged GFRα1+/− mice treated with saline displayed a greater number of phospho-p38 MAPK cells when compared to age-matched saline-treated WT mice (Fig. 3A, B, E). In addition, MPTP-treated resulted in a higher number of phospho-p38 MAPK positive cells when compared to saline-treated mice, regardless of genotype (Fig. 3). MPTP-treated GFRα1+/− mice displayed a slightly greater increase in phosho-p38 MAPK positive cells (42%) than MPTP-treated WT mice (37%; Fig. 3C, D, E), when compared to saline-treated controls. Thus, our findings provide support for the “dual-hit” hypothesis but suggest that additive rather than synergistic effects of the two detrimental events occur, adding insult to the otherwise normal aging process in these mice.
Figure 3. GFR α1+/− mice displayed an increase in phosphorylated p38 MAPK in the SN after a subchronic MPTP regimen.
Phospho-p38 MAPK immunoreactivity (ir) in the SN of (A) saline-treated WT mice, (B) saline-treated GFR α1+/− mice, (C) MPTP-treated WT mice, and (D) MPTP-treated GFR α1+/− mice. (E) Quantification of the average integrated density of phospho-p38 MAPK-ir in the SNpc using one-way ANOVA analysis followed by Student-Nuemann-Kuels post-hoc test. Twenty-six month-old GFR α1+/− mice treated with saline had increased phospho-p38 MAPK-ir in the SN compared to saline-treated WT mice (*p<0.05). MPTP treatment caused a significantly greater increase in phospho-p38 MAPK-ir in the SN of GFR α1+/− mice than in saline-treated GFR α1+/− mice or MPTP-treated WT mice (*p<0.05). Photomicrograph magnification=40x. N=8 per group. Scale bar=0.5mm. Error bars indicate SEM.
Tyrosine hydroxylase immunoreactivity
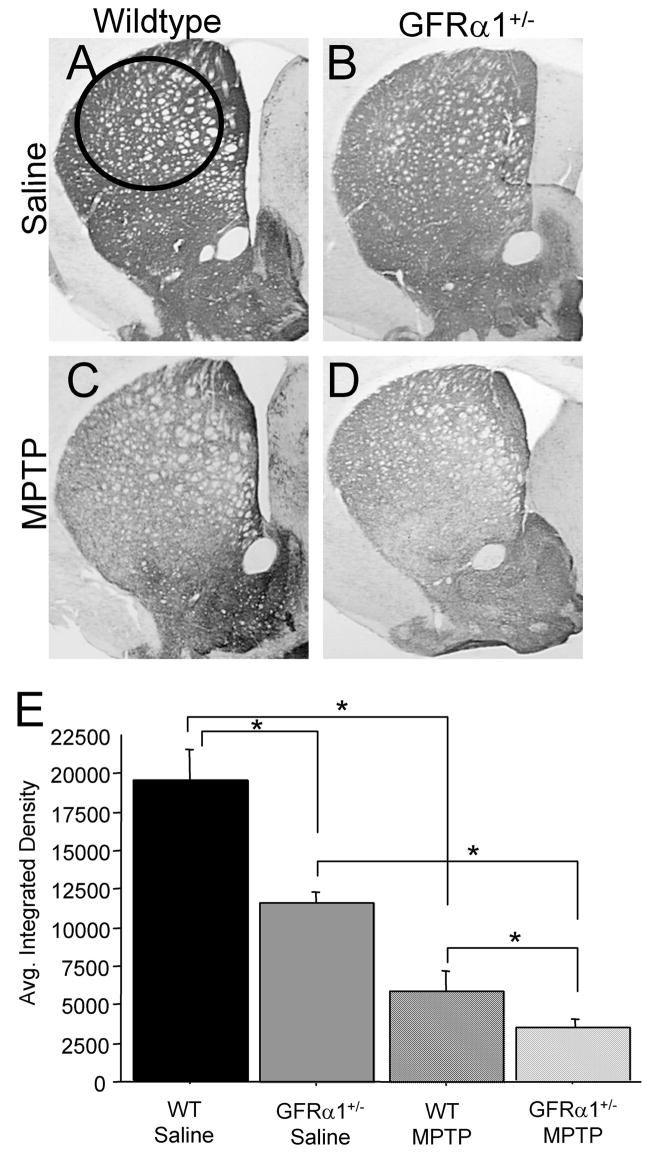
Although our primary focus in this study was to explore inflammatory effects of the MPTP injections, since inflammation is an immediate response to neurotoxic insults, we also examined TH-ir in SN and striatum of the four groups. Not surprisingly, two days following cessation of the MPTP injections, observable differences were found in the expression of TH-ir in the striatum (Fig. 4). A 2(Genotype) ×2(Treatment) ANOVA revealed significant Genotype [F(1,25)=10.820, p<0.01] and Treatment [F(1,25)=48.147, p<0.0001] effects but no Genotype × Treatment interaction [F(1,25)=2.995, p=0.1040]. The lack of an interactive effect could be due to the percent loss between MPTP-treated mice and saline-treated mice, regardless of genotype, was similar (GFRα1+/− mice=68% reduction, WT mice=70% reduction). As noted by Fig. 4, TH-ir in the striatum of saline-treated GFRα1+/− mice was significantly lower than saline-treated WT mice (Fig. 4A, B, E). Mice treated with MPTP, had lower striatal TH-ir expression, regardless of genotype, when compared to saline-treated mice, with MPTP-treated GFRα1+/− mice exhibiting the lowest expression of striatal TH-ir.
Figure 4. GFR α1+/− mice treated with MPTP demonstrated a greater loss of striatal tyrosine hydroxylase (TH) immunoreactivity (ir) than WT mice.
Photomicrographs of TH-ir in coronal hemisections of the striatum from (A) saline-treated WT mice, (B) saline-treated GFR α1+/−, (C) MPTP-treated WT mice, and (D) MPTP-treated GFR α1+/−. (E) Quantification of the average integrated density using one-way ANOVA analysis followed by Student-Nuemann-Kuels post-hoc test from the four treatment groups at 26 months of age confirmed that there were significant differences in the striatum between GFR α1+/− and WT mice treated with saline (*p<0.05). The administration of MPTP resulted in a significant reduction of striatal TH-ir compared to saline-treated mice, regardless of genotype (*p<0.05), with the greatest loss of TH-ir in the striatum of GFR α1+/− mice. Photomicrograph magnification=2x. N=8 per group. Error bars indicate SEM.
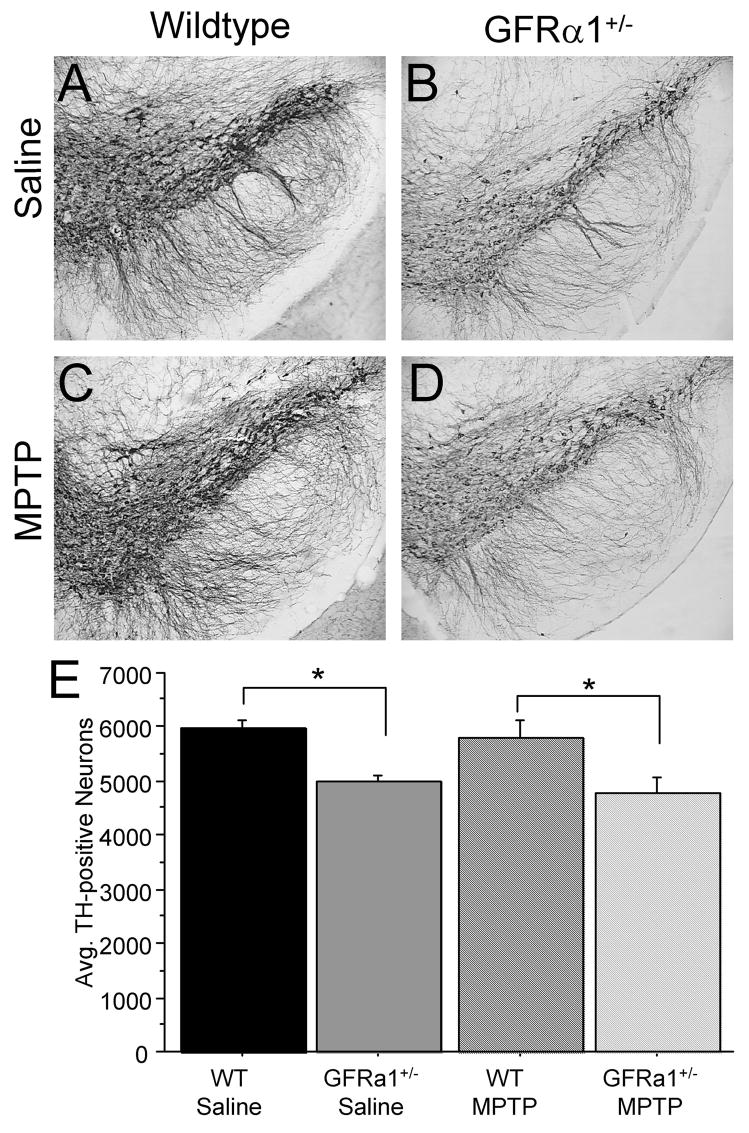
To assess direct effects of MPTP on DA neurons, stereological cell counts were carried out on TH-positive neuronal cell bodies in the SN. A significant genotype effect [F(1,24)=14.148, p<0.01] was confirmed by the 2(Genotype) ×2(Treatment) ANOVA, but not a treatment effect [F(1,24)=0.548, p=0.0.4663] or a Genotype × Treatment interaction [F(1,24)=0.020, p=0.8894]. Saline-treated GFRα-1+/− mice exhibited significantly fewer TH-positive neurons in the SN compared to saline-treated WT mice (Fig. 5A, B, E) at 26 months of age. We have recently found significantly fewer SN DA neurons in untreated GFRα1+/− mice than age-matched WT mice at 18 months, but not 8 months of age (Zaman et al., 2008; in press), suggesting that this is an age-related phenomenon. No statistical differences were found between MPTP-treated mice and saline-treated mice, regardless of genotype, possibly because two days was not sufficient time to allow for neuronal loss after a subchronic regimen of MPTP, as suggested by other investigators (eg. Vila et al., 2000). Interestingly, a negative correlation was found between the number of nigral TH-positive neurons and CD45-ir in GFRα1+/− mice, so that fewer TH-positive neurons was correlated with increased CD45-ir (r=−0.830, p<0.001), suggesting a link between the health of substantia nigra DA neurons and neuroinflammation. No correlation between these two markers was found in the saline-treated WT group (r=0.037, p=0.9411), MPTP-treated WT mice (r=−0.423, p=0.3127), or MPTP-treated GFRα1+/− mice (r=−0.489, p=0.2850). The lack of correlation after MPTP administration between nigral TH-positive neurons and CD45-ir is possibly due to the short time-point at which the mice were anesthetized after treatment, but clearly suggests that inflammation may play a role in mice with a chronic reduction in the GFRα1 receptor.
Figure 5. Aged GFR α1+/− mice had fewer nigral TH-positive neurons than age-matched WT mice.
TH-immunoreactivity in the midbrain of (A) saline-treated WT mice, (B) saline-treated GFR α1+/− mice, (C) MPTP-treated WT mice, and (D) MPTP-treated GFR α1+/− mice. (E) Quantification of the average number of SN TH-positive neurons using one-way ANOVA analysis followed by Student-Nuemann-Kuels post-hoc test reveals that 26 month old GFR α1+/− mice treated with saline have significantly fewer SN TH-positive neurons than age-matched WT mice (*p<0.05). Two days after the completion of the subchronic MPTP regimen, no differences existed in the number of SN TH-positive neurons between saline-treated mice and MPTP-treated mice, regardless of genotype. Photomicrograph magnification=10x. N=8 per group. Error bars indicate SEM.
3. DISCUSSION
In the present study on aged (26-month-old) WT and GFRα1+/− mice, the genetic partial deletion of the GFRα1 receptor resulted in lower spontaneous locomotor activity and reduced striatal TH-ir compared to age-matched WT mice. GFRα1+/− mice also had elevated CD45-ir and phosphorylated p38 MAPK in the SN, indicating chronic inflammatory activation in this brain region. The MPTP treatment produced increases in spontaneous locomotor activity and reductions in striatal TH-ir as well as elevated immunoreactivity of the inflammatory markers CD45 and phospho-p38 MAPK in the SN but not the striatum, regardless of genotype. Although genotype did not interact with the MPTP treatment on any of the measures, the largest difference on all measures relative to the basic WT saline control group was for the MPTP treated GFRα1+/− group suggesting possible additive effects of the gene deletion and MPTP toxicity in GFRα1+/− mice on these measures.
The nigrostriatal DA system plays an essential role in the control of voluntary movement (Chinta and Anderson, 2005; Ljungberg et al., 1992). The lower spontaneous activity exhibited by 26-month-old GFRα1+/− mice may be due to reductions in the DA system suggested by 40% less striatal TH-ir and 28% fewer TH-positive SN neurons in GFRα1+/− mice compared to controls. The findings in these studies on GFRα1+/− mice are in accordance with previous studies conducted on mice with regionally selective ablation of the tyrosine kinase receptor RET which demonstrated progressive and late loss of DA neurons in the SNpc, a loss of striatal DAergic terminals, and reduced levels of evoked DA levels (Kramer et al., 2007). In addition, RET knock-in animal models have demonstrated that constitutively active RET increased DA levels and TH expression in the SN and striatum of these animals (Mijatovic et al., 2007). Studies from our laboratory established that a partial loss of GDNF in GDNF+/− mice accelerates aging-related decline in motor function and the DA system (Boger et al., 2006; 2007). Similar to the GFRα1+/− mice in the present study, older GDNF+/− mice were less active, had reduced TH-ir in striatum, and reduced numbers of TH-positive neurons in the SN, beginning at 12 months of age (Boger et al., 2006). Since DA loss is a slow, progressive process in both these mouse models, the studies on GDNF+/− and GFRα1+/− mice may provide better models for age-related motor dysfunction in humans and can therefore be used to evaluate biological mechanisms as well as treatment paradigms for age-related parkinsonism leading to progressive DA loss (see eg. Smith et al., 2005). Taken together, these data point to the importance of the GDNF-GFRα1-RET complex in the survival and function of the DA system, especially as it relates to increased vulnerability of this system with aging, and also suggest that additive effects of genetic alterations and environmental toxins may be part of the pathology observed in humans with parkinsonian disease patterns.
In addition to alterations in the nigrostriatal dopamine system resulting in motor impairments associated with a partial loss of GFRα1, we cannot rule out the possible impact the GDNF-GFRα1-RET complex plays on spinal motoneurons. GDNF family ligands are potent neurotrophic factors that regulate the survival of spinal motoneurons during development (see eg. Henderson et al., 1994; Oppenheim et al., 1995). Studies have demonstrated that mice with a loss of GFRa1 or RET have a loss of a specific type of motoneurons, muscle spindle-innervating gamma-motoneurons (Gould et al., 2008; Garces et al., 2000). Furthermore, Gould and colleagues (2008) stated that the developmental period during which GDNF regulates the survival of gamma-motoneurons is transient, such that the postnatal elimination of RET had no effect on gamma-motoneurons whereas, the pan-RET knockout mouse as well as the adult motoneuron-specific RET knockout mouse demonstrated a failure of the motoneurons to innervate their targets. Another study from our laboratory indicates that the reduction in spontaneous locomotion and DA neuronal loss observed in GFRα1+/− mice are age dependent, since the GFRα1+/− mice exhibited their alterations at 18 but not 8 months of age (Zaman et al., 2008, in press with revisions). The study also established that GFRα1+/− mice were hypersensitive to the DA D1 receptor agonist SKF 82958 at 18 but not 8 months of age. These results suggest hypersensitivity of DA D1 receptors due to a partial loss of SN DA neurons in 18-month-old GFRα1+/− mice. The hypersensitivity to the DA D1 receptor agonist also provides evidence that the nigrostriatal system played a role in the observed motor dysfunction of aged GFRα1+/− mice, and that the motor activity reduction was not due to muscle or spinal cord abnormalities, since the mice were able to increase spontaneous locomotion significantly in the presence of the DA D1 receptor agonist (Zaman et al., 2008, in press), and thus did not exhibit inability to move following the partial gene deletion.
The elevated motor activity for both GFRα1+/− and WT mice two days following a sub-chronic regimen of MPTP is consistent with previous reports on mice with no genetic manipulation (Rousselet et al., 2003). This elevated activity commonly observed in non-human primates as well as rodents treated with MPTP could be due to co-activation of striatal DA D1 and D2 receptors, or to a loss of functional segregation in cortico-basal ganglia circuits resulting from the partial loss of DA innervation of the striatum associated with an MPTP lesion (Kuno, 1997; Nicholas, 2007; Pessiglione et al., 2005; Rousselet et al., 2003). In addition to lower striatal TH-ir, GFRα1+/− mice also had 23% fewer TH-positive nigral neurons than controls even though MPTP administration did not further exacerbate this loss at the time point examined. Interestingly, the overall locomotor activity of MPTP-treated GFRα1+/− mice was increased by 155% compared to saline-treated GFRα1+/− mice, whereas MPTP-treated WT mice displayed an increase of only 47%. Thus, the partial loss of GFRα1 receptor expression gave rise to increased sensitivity of the DA system, manifested by increased hyperactivity following the toxic administration of MPTP, and similar to the increased sensitivity to the DA D1 receptor agonist observed in the other study (Zaman et al., 2008, in press). It is quite possible that the hyperactivity observed in GFRα1+/− mice after MPTP administration is due to an additive effect of nigral DA neuronal loss coupled with MPTP-induced terminal loss, due to a lack of significant interactions between genotype and treatment with any of the presented measures. Previous studies have been conducted assessing long-term effects of MPTP administration in RET ablated mice (Kowsky et al., 2007). In this study, RET-deficient mice did not exhibit a greater reduction in DAergic markers following MPTP. However, they did have reduced regeneration of DAergic terminals compared to control mice. The data from this study suggest that RET-dependent signaling does not modulate MPTP-induced degeneration of DAergic neurons and terminals (Kowsky et al., 2007). Therefore, it will be interesting, in future studies, to determine long-term consequences of the MPTP administration on behavior and DA morphology and biochemistry in GFRα1 deficient mice.
In addition to reductions in DA markers and locomotor activity, staining for inflammatory markers in the SN was elevated in GFRα1+/− compared to WT mice. This finding is in accordance with RET-deficient mice in which they displayed increased gliosis in the SN (Kramer et al., 2007), suggesting the importance of the GDNF-GFRα1-RET complex in the suppression of inflammation. The neurotoxic effects of MPTP were examined at 48 hrs after treatment in the present study to allow investigation of the inflammatory response utilizing the initial microglial response that subsides 48–72 hrs post-insult (see eg. Cardenas and Bolin, 2003; Pattarini et al., 2007). The aged GFRα1+/− saline-treated mice in our study had higher CD45-ir and phosphorylated p38 MAPK than age-matched WT mice in the SN. The greater CD45-ir and phospho-p38 MAPK cell counts in aged GFRα1+/− mice compared to WT mice was coupled with a reduction in TH-positive neurons in the SN suggesting that inflammation might contribute to deterioration of the DA system in these mice. The greater CD45-ir density and phosphorylated p38 MAPK in the SN of GFRα1+/− mice compared to age-matched WT mice subjected to the subchronic MPTP regimen in our study suggests that the impaired DA system of GFRα1+/− mice plus exposure to the DA neurotoxin results in a greater inflammatory response which in turn increases the deterioration of the aged DA system. It is also possible that the elevated phospho-p38 MAPK imunoreactivity may result from reactive gliosis of astrocytes in the SN region, since p38 MAPK phosphorylation can be observed in microglia, neurons, and astrocytes (Cuenda and Rousseau, 2007). Nonetheless, recent studies have demonstrated that GDNF can directly reduce p38 MAPK and c-Jun N-terminal kinase (JNK) phosphorylation while elevating both AKT and ERK signaling (Du et al., 2008) effectively in tissue culture, suggesting that the results obtained in the present study are in line with previous published roles for this growth factor in the SN also in vivo. Elevated phospho-p38 MAPK immunoreactivity seen in the SNpc of GFRα1+/− mice at baseline (without MPTP) would therefore predict that this particular signaling pathway is involved in the ensuing pathology reported. Future studies will therefore focus on the p38 pathway for therapeutic interventions of this additive neurodegenerative mouse model. The MPTP rodent model of PD has been well documented to induce inflammation and result in DA toxicity (Grunblatt et al., 2000; 2001; Mandel et al., 2003). This hypothesis is supported by previous studies with IL-6 knockout (−/−) mice (see Cardenas and Bolin, 2003). These investigators found that microgliosis in the substantia nigra after an MPTP lesion was severely compromised in IL-6 (−/−) mice, leading to transient reactive microgliosis and a complete absence of reactive microglia at day 7 post-lesion (Cardenas and Bolin, 2003). Cardenas and Bolin (2003) further suggested that the long-term microgliosis missing in IL-6 (−/−) mice may be beneficial for survival purposes in the substantia nigra, based on their findings that IL-6 (−/−) mice exhibited increased sensitivity to the DA toxin. These are interesting conclusions, which warrant further studies. However, in the present study, the increased CD45 and phospho-p38 expression appears to be damaging instead, as evidenced by the negative correlation with TH-ir. Other studies have indicated a double edged sword for microglia activation in degeneration and aging. Gemma and colleagues (2007) demonstrated that injection with the anti-sense oligodeoxyribonucleotide to a pro-inflammatory cytokine, TNFα, can lead to increased response to the DA neurotoxin, 6-OHDA, in rats, suggesting that the initial microglial response to a neurotoxic paradigm may in fact exert a beneficial effect in terms of recouperation from the injury. Further studies utilizing better markers for beneficial versus detrimental microglia will reveal the complicated influence of microglia upon the integrity of the SN DA neurons.
In conclusion, the present study suggested that mice with a partial loss of the GDNF receptor, GFRα1, are more susceptible to aging-related effects on the DA system possibly due to an increased inflammatory response. Furthermore, an additive effect was found in terms of DA system damage and inflammatory markers when these mice were given the DA neurotoxin MPTP, thus demonstrating a particular role for GFRα1, and their ligand, for maintenance of function of the DA system during aging.
4. EXPERIMENTAL PROCEDURES
Animals
Twenty-six month old male GFRα1+/− mice were compared to WT littermates in all experiments. The nonfunctional allele for the GFRα1 gene was generated by replacing part of the first coding exon that encodes the GFRα1 protein with a phosphoglycerate kinase (pgk) neo-resistance expression cassette, as described in detail previously (Tomac et al., 2000). After introducing this construct into embryonic stem cells, six clones were identified with the predicted mutant allele. CD1 or C57BL/6 recipient strains were used to obtain germline transmission of the targeted allele. The mice were backcrossed to a C57/B6 background. Heterozygous offspring are viable and fertile, whereas mice homozygous for the mutant GFRα1 allele (GFRα1−/−) die within 24 hours of birth. The mice were housed in groups of 3–4 to a cage and had free access to food and water. GFRα1+/− breeding pairs were obtained from the National Institutes on Drug Abuse (NIDA), and were maintained and bred at the Medical University of South Carolina according to Institutional Animal Care and Use Committee (IACUC) approved protocols. They were maintained on a 12-h light: 12-h dark cycle at an ambient temperature of 20–22°C.
Experimental design
Twenty-six month old GFRα1+/− and WT mice were injected with a sub-clinical does of MPTP (20 mg/kg, i.p., daily for 4 days; Sigma-Aldrich., Missouri, USA) or 0.9% physiological saline (0.2 ml); N=8 per group, total of 32 mice used in experiment. Motor activity was recorded in photocell chambers for 60 min, one day prior to injections and then two days after cessation of the injections as described below. After the motor activity was recorded two days post-MPTP injections, the mice were perfused with 4% paraformaldehyde for assessment of various morphological markers.
Locomotor testing
Locomotor activity (total distance traveled) was assessed in a Digiscan Animal Activity Monitor system [Omnitech Electronics Model RXYZCM(8) TAO, Columbus, OH]. The details of the apparatus have been described previously (Halberda et al., 1997). On the day of testing, the mice were transferred from the animal colony into the laboratory in groups of six and tested in a darkened environment. Data were collected in five minute intervals for 60 minutes at the same time of day (8am to 12pm) for each test period.
Immunohistochemistry
After mice were assessed for locomotor activity two days following MPTP treatment, the mice were anesthetized with halothane and perfused transcardially with saline followed by 4% paraformaldehyde in phosphate buffer (0.1 M, pH 7.4) for immunohistochemical analysis. The brains were removed, postfixed in 4% paraformaldehyde for 24 hrs, and then transferred to 30% sucrose in 0.1 M PBS for at least 24 hrs before sectioning for histochemical analysis, according to our standard protocol (see eg. Granholm et al., 1997a, b; Boger et al., 2006). The striatum and midbrain were sectioned on a cryostat (Microm, Zeiss, Thornwood, NY, USA) at 45 μm. Serial sections through the striatum (every 6th section) and SN (every 3rd section) were processed for free-floating immunohistochemistry using a rabbit polyclonal antibody against tyrosine hydroxylase (TH; 1:1000; Pel-Freeze Biologicals, Inc., Roger, AZ, USA), a rat anti-mouse antibody against the inflammatory MHC Class II induced cluster differentiation marker, CD45 (MCA1388–1:1000; Serotec North America, Raleigh, NC, USA) or a rabbit polyclonal antibody against phospho-p38 MAPK (1:500; Chemicon, Temecula, CA, USA). Immunodetection was performed using the avidin-biotin-immunoperoxidase method (Granholm et al., 1997a, b; Boger et al., 2006). Briefly, after a 5 min. pretreatment with 2% Triton-X to allow penetration into the tissue, a subset of sections from each mouse was incubated in the primary antisera against TH, CD45, or phospho-p38 MAPK for 24 hrs at 4°C. The sections were then rinsed and incubated for 1hr in biotin-conjugated goat anti-rabbit (TH and phospho-p38 MAPK) or goat anti-rat (CD45) IgG, rinsed, and incubated for 1h with avidin-biotin-peroxidase reagents (Elite Vectastain kit, Vector Labs, Burlingame, CA). Diaminobenzidine (DAB, Sigma) was used as a chromagen to develop the reaction using 0.05% of 3% H202 and nickel ammonium sulfate (2.5%, Sigma) was used to enhance the reaction. Each of the above steps was separated by 3 × 10 min. washes in PBS. Sections were mounted on glass slides and coverslipped with DPX.
Semi-quantitation of immunostaining
We performed densitometry measurements on every 6th rostral-caudal section for TH-immunoreactivity (TH-ir) in the dorsal striatum and CD45-ir in the dorsal striatum and SNpc at a magnification of 10× using the NIH Image program as described previously (Boger et al., 2007). In the dorsal striatum a circle with area 500um2 was used to measure the integrated density of TH-ir and CD45-ir (as indicated in Figure 4A). The circle is placed in the dorso-lateral striatum with the upper edge of the circle being placed on the edge between the striatum and the corpus collosum. Measurements were taken from every 6th section starting with striatal section +1.18mm relative to Bregma (Paxinos and Franklin, 2001). For measurements of CD45-ir in the SNpc, an oval with area 1000um × 200um was utilized starting on a random nigral section approximately −2.92mm relative to Bregma (Paxinos and Franklin, 2001). Measurements were thereafter performed on every 6th section through the SNpc. The hippocampus and mammillary nucleus were used as landmarks in the rostro-caudal plane, and the lateral boundary of the VTA (A10) was used as the medial landmark. Further, the oval excluded the SNpr ventrally, so that the cell body region of the SN was exclusively investigated for CD45 immunoreactivity. The span of the sectioned tissues exceeded the rostral and caudal extents of the SN, as determined by TH immunostaining on adjacent section series, allowing a systematic random design for the study with a random start within the first sections.
Following placement of the density template, background was subtracted and the LUT scale was adjusted using density slicing. This approach captures all signal intensity above a threshold density and interactively discriminates them from density values below the threshold. The mean density and number of pixels per area were measured in the selected striatal and SNpc areas independently from 5 sections per mouse. The measurements were expressed as integrated density (number of pixels per area × mean density).
Stereological cell counts
The unbiased sampling techniques of stereology have been developed to avoid the inaccuracies of using ‘representative’ sections for morphometric studies. The region of interest must be available for sampling in its entirety, and boundaries must also be distinctly identifiable (see e.g. Granholm et al., 2003; Hunter et al., 2004). A systematic-random series of sections is retrieved from the multi-well plate, stained in series with the anti-tyrosine hydroxylase antibody (TH). Landmarks were as described for Cd45 staining above. The region of interest is identified and the labeled neurons are counted using the fractionator. Unilateral quantitative estimates of the total number of TH-positive neurons in the SNpc were achieved using an unbiased, stereological cell counting method used as previously described (Granholm et al., 2002; Gunderson et al., 1987). Briefly, the optical fractionator system consists of a computer assisted image analysis system including a Nikon Eclipse E-600 microscope hard-coupled to a Prior H128 computer controlled x-y-z motorized stage, an Olympus-750 video camera system, a Micron Pentium III 450 computer, and stereological software (Stereoinvestigator®, MicroBrightField Inc.; Colchester, VT). The SNpc was outlined under low magnification (10X) on every 3rd (for TH-positive neurons) and every 6th (for phospho-p38 MAPK-ir) section through the rostro-caudal extent of the midbrain starting at −2.92mm relative to Bregma (Paxinos and Franklin, 2001), the outlined region measured with a systematic random design of dissector-counting frames. The counting frame area was 2500 μm2 and the sampling grid area was 100 X 100 μm. Actual mounted section thickness was found to be on average 37 μm and a 2 μm guard zone was set for the top and bottom of each section. A 40X objective lens with a 1.4 numerical aperture was used to count cells within the counting frames as previously described (see Boger et al., 2006). Neurons exhibiting a clear nucleus, at least one axonal/dendritic process and stained for TH were counted. Outline contours for SN cell counts included areas predominantly containing TH labeled DA neurons (i.e. SN pars compacta and lateralis), and excluded the SN pars reticulata area, which contains mostly TH fiber network. Thickness was evaluated for each counting frame and staining penetration was evaluated by stepwise capturing of images at 5 μm increments through each section. In our previous experience, 40 μm sections are well penetrated using our free-floating immunostaining technique. The average coefficient error for counting was <0.05, according to recommendation by Gundersen et al., (1987). Furthermore, a counting grid and counting frame settings were adjusted to render a minimum of 200 neurons were counted per brain.
Statistical Analysis
Locomotor activity, stereological cell counts, and immunohistochemical densitometry data were analyzed by ANOVA followed by a Student-Neumann-Keuls post-hoc test when main effects were significant. A 2(Genotype) × 2(Treatment) ANOVA design was utilized to determine if there were Genotype × Treatment interactions.
Acknowledgments
Supported by DAMD 17-99-1-9480, AG023630, and AG15239. The authors are thankful to Ms. Claudia Umphlet, Mr. Joe Vallone, and Mr. Alfred Moore for expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Airaksinen MS, Saarma M. The GDNF family: signalling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3:383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci. 2001;2:734–744. doi: 10.1038/35094583. [DOI] [PubMed] [Google Scholar]
- Boger HA, Middaugh LD, Patrick KS, Ramamoorthy S, Denehy ED, Zhu H, Pacchioni AM, Granholm A-Ch, McGinty JF. Long-term consequences of methamphetamine exposure in young adults are exacerbated in glial cell line-derived neurotrophic factor heterozygous mice. J Neurosci. 2007;27:8816–8825. doi: 10.1523/JNEUROSCI.1067-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boger HA, Middaugh LD, Huang P, Smith AC, Zaman V, Hoffer BJ, Tomac AC, Granholm A-Ch. A partial GDNF depletion exacerbates age-related motor dysfunction and degeneration of nigral dopamine neurons. Exp Neurol. 2006;202:336–347. doi: 10.1016/j.expneurol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Bowenkamp KE, Hoffman AF, Gerhardt GA, Henry MA, Biddle PT, Hoffer BJ, Granholm A-Ch. Glial cell line-derived neurotrophic factor supports survival of injured midbrain dopaminergic neurons. J Comp Neurol. 1995;355:479–489. doi: 10.1002/cne.903550402. [DOI] [PubMed] [Google Scholar]
- Cacalano G, Farinas I, Wang LC, Hagler K, Forgie A, Moore M, Armanini M, Phillips H, Ryan AM, Reichardt LF, Hynes M, Davies A, Rosenthal A. GFRalpha1 is an essential receptor component for GDNF in the developing nervous system and kidney. Neuron. 1998;21:53–62. doi: 10.1016/s0896-6273(00)80514-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas H, Bolin LM. Compromised reactive microgliosis in MPTP-lesioned IL-6 KO mice. Brain Res. 2003;985:89–97. doi: 10.1016/s0006-8993(03)03172-x. [DOI] [PubMed] [Google Scholar]
- Cass WA, Manning MW. GDNF protection against 6-OHDA-induced reductions in potassium-evoked overflow of striatal dopamine. J Neurosci. 1999;19:1416–1423. doi: 10.1523/JNEUROSCI.19-04-01416.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinta SJ, Andersen JK. Dopaminergic neurons. Int J Biochem Cell Biol. 2005;37:942–946. doi: 10.1016/j.biocel.2004.09.009. [DOI] [PubMed] [Google Scholar]
- Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta. 2007;1773:1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Czlonkowska A, Kohutnicka M, Kurkowska-Jastrzebska I, Czlonkowski A. Microglial reaction in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) induced Parkinson’s disease mice model. Neurodegen. 1996;5:137–143. doi: 10.1006/neur.1996.0020. [DOI] [PubMed] [Google Scholar]
- Dowd E, Monville C, Torres EM, Wong LF, Azzouz M, Mazarakis Dunnett SB. Lentivector-mediated delivery of GDNF protects complex motor functions relevant to human Parkinsonism in a rat lesion model. Eur J Neurosci. 2005;22:2587–2595. doi: 10.1111/j.1460-9568.2005.04414.x. [DOI] [PubMed] [Google Scholar]
- Du Y, Li X, Yang D, Zhang X, Chen S, Huang K, Le W. Multiple molecular pathways are involved in the neuroprotection of GDNF against proteasome inhibitor induced dopamine neuron degeneration in vivo. Exp Biol Med (Maywood) 2008;233:881–90. doi: 10.3181/0712-RM-329. [DOI] [PubMed] [Google Scholar]
- Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, Chernet E, Perry KW, Nelson DLG, Luecke S, Phebus LA, Bymaster FP, Paul S. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2001;98:14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto H, Araki T, Jackman A, Heucheroth RO, Snider WD, Johnson EM, Midbrandt J. GFR alpha1-deficient mice have deficits in the enteric nervous system and kidneys. Neuron. 1998;21:317–324. doi: 10.1016/s0896-6273(00)80541-3. [DOI] [PubMed] [Google Scholar]
- Fiedorowicz A, Figiel I, Kaminska B, Zaremba M, Wilk S, Oderfeld-Nowak B. Dentate granule neuron apoptosis and glia activation in murine hippocampus induced by trimethyltin exposure. Brain Res. 2001;912:116–127. doi: 10.1016/s0006-8993(01)02675-0. [DOI] [PubMed] [Google Scholar]
- Francis JW, Von Visger J, Markelonis GJ, Oh TH. Neuroglial responses to the dopaminergic neurotoxicant 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mouse striatum. Neurotoxicol Teratol. 1995;17:7–12. doi: 10.1016/0892-0362(94)00048-i. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Fumagalli F, Jones SR, Caron MG. Dopamine transporter is required for in vivo MPTP neurotoxicity: evidence from mice lacking the transporter. J Neurochem. 1997;69:1322–1325. doi: 10.1046/j.1471-4159.1997.69031322.x. [DOI] [PubMed] [Google Scholar]
- Garces A, Haase G, Airaksinen MS, Livet J, Filippi P, de Lapeyriere O. GFRalpha 1 is required for development of distinct subpopulations of motoneuron. J Neurosci. 2000;20:4992–5000. doi: 10.1523/JNEUROSCI.20-13-04992.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L, Russell D, Martin D, Lapchak PA, Collins F, Hoffer BJ, Gerhardt GA. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380:252–255. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- Gemma C, Catlow B, Cole M, Hudson C, Samec A, Shah N, Vila J, Bachstetter A, Bickford PC. Early inhibition of TNFalpha increases 6-hydroxydopamine-induced striatal degeneration. Brain Res. 2007;1147:240–247. doi: 10.1016/j.brainres.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Gould TW, Yonemura S, Oppenheim RW, Ohmori S, Enomoto H. The neurotrophic effects of glial cell line-derived neurotrophic factor on spinal motoneurons are restricted to fusimotor subtypes. J Neurosci. 2008;28:2131–2146. doi: 10.1523/JNEUROSCI.5185-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm A-Ch, Boger HA, Emborg ME. Mood, memory, and movement: An age-related neurodegenerative complex? Current Aging Science. 2008;1:133–139. doi: 10.2174/1874609810801020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm A-Ch, Ford KA, Hyde LA, Bimonte HA, Hunter CL, Nelson M, Albeck D, Sanders LA, Mufson EJ, Crnic LS. Estrogen restores cognition and cholinergic phenotype in an animal model of Down syndrome. Physiol Behav. 2002;77:371–385. doi: 10.1016/s0031-9384(02)00884-3. [DOI] [PubMed] [Google Scholar]
- Granholm A-Ch, Mott JL, Bowenkamp K, Eken S, Henry S, Hoffer BJ, Lapchak PA, Palmer MR, van Horne C, Gerhardt GA. Glial cell line-derived neurotrophic factor improves survival of ventral mesencephalic grafts to the 6-hydroxydopamine lesioned striatum. Exp Brain Res. 1997a;116:29–38. doi: 10.1007/pl00005741. [DOI] [PubMed] [Google Scholar]
- Granholm A-Ch, Srivastava N, Mott JL, Henry S, Henry M, Westphal H, Pichel JG, Shen L, Hoffer BJ. Morphological alterations in the peripheral and central nervous systems of mice lacking glial cell line-derived neurotrophic factor (GDNF): immunohistochemical studies. J Neurosci. 1997b;17:1168–1178. doi: 10.1523/JNEUROSCI.17-03-01168.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grünblatt E, Mandel S, Maor G, Youdim MB. Gene expression analysis in N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mice model of Parkinson’s disease using cDNA microarray: effect of R-apomorphine. J Neurochem. 2001;78:1–12. doi: 10.1046/j.1471-4159.2001.00397.x. [DOI] [PubMed] [Google Scholar]
- Grünblatt E, Mandel S, Youdim MB. Neuroprotective strategies in Parkinson’s disease using the models of 6-hydroxydopamine and MPTP. Ann N Y Acad Sci. 2000;899:262–273. doi: 10.1111/j.1749-6632.2000.tb06192.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Hartmann A, Hunot S, Hirsch ES. Inflammation and dopaminergic neuronal loss in Parkinson’s disease: a complex matter. Exp Neurol. 2003;184:561–564. doi: 10.1016/j.expneurol.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Harvey BK, Hoffer BJ, Wang Y. Stroke and TGF-beta proteins: glial cell line-derived neurotrophic factor and bone morphogenetic protein. Pharmacol Ther. 2005;105:113–125. doi: 10.1016/j.pharmthera.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Halberda JP, Middaugh LD, Gard BE, Jackson BP. DAD1- and DAD2-like agonist effects on motor activity of C57 mice: differences compared to rats. Synapse. 1997;26:81–92. doi: 10.1002/(SICI)1098-2396(199705)26:1<81::AID-SYN9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simmons L, Moffet B, Vandlen RA, Simpson L. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994;266(5187):1062–1064. doi: 10.1126/science.7973664. Erratum in: Science 1995 Feb 10;267(5199):777. [DOI] [PubMed] [Google Scholar]
- Hirsch EC, Breidert T, Rousselet E, Hunot S, Hartmann A, Michel PP. The role of glial reaction and inflammation in Parkinson’s disease. Ann N Y Acad Sci. 2003;991:214–228. doi: 10.1111/j.1749-6632.2003.tb07478.x. [DOI] [PubMed] [Google Scholar]
- Hunter CL, Bachman D, Granholm A-Ch. Minocycline prevents cholinergic loss in a mouse model of Down’s syndrome. Ann Neurol. 2004;56:675–688. doi: 10.1002/ana.20250. [DOI] [PubMed] [Google Scholar]
- Jenner P, Olanow CW. Understanding cell death in Parkinson’s disease. Ann Neurol. 1998;44:S72–S84. doi: 10.1002/ana.410440712. [DOI] [PubMed] [Google Scholar]
- Jorgensen MB, Finsen BR, Jensen MB, Castellano B, Diemer NH, Zimmer J. Microglial and astroglial reactions to ischemic and kainic acid-induced lesions of the adult rat hippocampus. Exp Neurol. 1993;120:70–88. doi: 10.1006/exnr.1993.1041. [DOI] [PubMed] [Google Scholar]
- Kato H, Tanaka S, Oikawa T, Koike T, Takahashi A, Itovama Y. Expression of microglial response factor-1 in microglia and macrophages following cerebral ischemia in the rat. Brain Res. 2000;882:206–211. doi: 10.1016/s0006-8993(00)02811-0. [DOI] [PubMed] [Google Scholar]
- Kopin IJ, Markey SP. MPTP toxicity: implications for research in Parkinson’s disease. Annu Rev Neurosci. 1998;11:81–96. doi: 10.1146/annurev.ne.11.030188.000501. [DOI] [PubMed] [Google Scholar]
- Kowsky S, Poppelmeyer C, Kramer ER, Falkenburger BH, Kruse A, Klein R, Schulz JB. RET signaling does not modulate MPTP toxicity but is required for regeneration of dopaminergic axon terminals. Proc Natl Acad Sci USA. 2007;104:20049–20054. doi: 10.1073/pnas.0706177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno S. Differential therapeutic effects of dopamine D1 and D2 agonists in MPTP-induced parkinsonian monkeys: clinical implications. Eur Neurol. 1997;38(Suppl 1):18–22. doi: 10.1159/000113452. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, McBride J, Chen EY, Palfi S, Roitberg BZ, Brown WD, Holden JE, Pyzalski R, Taylor MD, Carvey P, Ling Z, Trono D, Hantraye P, Deglon N, Aebischer P. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- Kramer ER, Aron L, Ramakers GM, Seitz S, Zhuang X, Beyer K, Smidt MP, Klein R. Absence of Ret signaling in mice causes progressive and late degeneration of the nigrostriatal system. PLoS Biol. 2007;5:e39. doi: 10.1371/journal.pbio.0050039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieglstein K, Suter-Crazzolara C, Unsicker K. Development of mesencephalic dopaminergic neurons and the transforming growth factor-beta superfamily. J Neural Transm Suppl. 1995;46:209–216. [PubMed] [Google Scholar]
- Lander ES, Schork NJ. Genetic dissection of complex traits. Science. 1994;265:2037–2048. doi: 10.1126/science.8091226. [DOI] [PubMed] [Google Scholar]
- Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol. 1999;46:598–605. doi: 10.1002/1531-8249(199910)46:4<598::aid-ana7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Lavoie MJ, Card JP, Hastings TG. Microglial activation preceded dopamine terminal pathology in methamphetamine-induced neurotoxicity. Exp Neurol. 2004;187:47–57. doi: 10.1016/j.expneurol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Ljungberg T, Apicella P, Schultz W. Responses of monkey dopamine neurons during learning of behavioral reactions. J Neurophysiol. 1992;67:145–163. doi: 10.1152/jn.1992.67.1.145. [DOI] [PubMed] [Google Scholar]
- Mandel S, Grünblatt E, Riederer P, Gerlach M, Levites Y, Youdim MB. Neuroprotective strategies in Parkinson’s disease: an update on progress. CNS Drugs. 2003;17:729–762. doi: 10.2165/00023210-200317100-00004. [DOI] [PubMed] [Google Scholar]
- Mandel RJ, Spratt SK, Snyder RO, Leff SE. Midbrain injection of recombinant adeno-associated virus encoding rat glial cell line-derived neurotrophic factor protects nigral neurons in a progressive 6-hydroxydopamine-induced degeneration model of Parkinson’s disease in rats. Proc Natl Acad Sci U S A. 1997;94:14083–14088. doi: 10.1073/pnas.94.25.14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijatovic J, Airavaara M, Planken A, Auvinen P, Raasmaja A, Piepponen TP, Costantini F, Ahtee L, Saarma M. Constitutive Ret activity in knock-in multiple endocrine neoplasia type B mice induces profound elevation of brain dopamine concentration via enhanced synthesis and increases the number of TH-positive cells in the substantia nigra. J Neurosci. 2007;27:4799–4809. doi: 10.1523/JNEUROSCI.5647-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan TE, Wong AM, Finch CE. Anti-inflammatory mechanisms of dietary restriction in slowing aging processes. Interdiscip Top Gerontol. 2007;35:83–97. doi: 10.1159/000096557. [DOI] [PubMed] [Google Scholar]
- Nicholas AP. Levodopa-induced hyperactivity in mice treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Movement Disorders. 2007;22:99–104. doi: 10.1002/mds.21235. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson’s disease. Annu Rev Neurosci. 1999;22:123–44. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW, Houenou LJ, Johnson JE, Lin LF, Li L, Lo AC, Newsome AL, Prevette DM, Wang S. Developing motor neurons rescued from programmed and axotomy-induced cell death by GDNF. Nature. 1995;373:344–346. doi: 10.1038/373344a0. [DOI] [PubMed] [Google Scholar]
- Pattarini R, Smeyne RJ, Morgan JI. Temporal mRNA profiles of inflammatory mediators in the murine 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrimidine model of Parkinson’s disease. Neuroscience. 2007;145:654–668. doi: 10.1016/j.neuroscience.2006.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates: Deluxe edition of the atlas. 2. Academic Press; San Diego, CA, USA: 2001. [Google Scholar]
- Pessiglione M, Guehl D, Rolland AS, Francois C, Hirsch EC, Feger J, Tremblay L. Thalamic neuronal activity in dopamine-depleted primates: evidence for a loss of functional segregation within basal ganglia circuits. J Neurosci. 2005;25:1523–1531. doi: 10.1523/JNEUROSCI.4056-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S, Tieu K, Perier C, Vila M. MPTP as a mitochondrial neurotoxic model of Parkinson’s disease. J Bioenergetics and Biomembranes. 2004;36:375–379. doi: 10.1023/B:JOBB.0000041771.66775.d5. [DOI] [PubMed] [Google Scholar]
- Rousselet E, Joubert C, Callebert J, Parain K, Tremblay L, Orieux G, Launay JM, Cohen-Salmon C, Hirsch EC. Behavioral changes are not directly related to striatal monoamine levels, number of nigral neurons, or dose of parkinsonian toxin MPTP in mice. Neurobiol Dis. 2003;14:218–228. doi: 10.1016/s0969-9961(03)00108-6. [DOI] [PubMed] [Google Scholar]
- Tomac AC, Grinberg A, Huang SP, Nosrat C, Wang Y, Borlongan C, Lin SZ, Chiang YH, Olson L, Westphal H, Hoffer BJ. Glial cell line-derived neurotrophic factor receptor alpha1 availability regulates glial cell line-derived neurotrophic factor signaling: evidence from mice carrying one or two mutated alleles. Neuroscience. 2000;95:1011–1023. doi: 10.1016/s0306-4522(99)00503-5. [DOI] [PubMed] [Google Scholar]
- Saarma M. GDNF - a stranger in the TGF-beta superfamily? Eur J Biochem. 2000;267:6968–6971. doi: 10.1046/j.1432-1327.2000.01826.x. [DOI] [PubMed] [Google Scholar]
- Sarabi A, Chang CF, Wang Y, Tomac AC, Hoffer BJ, Morales M. Differential expression of the cell line-derived neurotrophic factor (GDNF) receptor GFRalpha1 in heterozygous Gfralpha1 null-mutant mice after stroke. Neurosci Lett. 2003;341:241–245. doi: 10.1016/s0304-3940(03)00195-2. [DOI] [PubMed] [Google Scholar]
- Scali C, Prosperi C, Giovannelli L, Bianchi L, Pepeu G, Casamenti F. Beta(1–40) amyloid peptide injection into the nucleus basalis of rats induces microglia reaction and enhances cortical gamma-aminobutyric acid release in vivo. Brain Res. 1999;831:319–321. doi: 10.1016/s0006-8993(99)01492-4. [DOI] [PubMed] [Google Scholar]
- Smeyne RJ, Jackson-Lewis V. The MPTP model of Parkinson’s disease. Brain Res Mol Brain Res. 2005;134:57–66. doi: 10.1016/j.molbrainres.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Smith RG, Betancourt L, Sun Y. Molecular Endocrinology and Physiology of the Aging Central Nervous System. Endocrine Reviews. 2005;26:203–250. doi: 10.1210/er.2002-0017. [DOI] [PubMed] [Google Scholar]
- Vila M, Vukosavic C, Jackson-Lewis V, Neystat M, Jakowec M, Przedborski S. Alpha-synuclein up-regulation in substantia nigra dopaminergic neurons following administration of the parkinsonian toxin MPTP. J Neurochem. 2000;74:721–729. doi: 10.1046/j.1471-4159.2000.740721.x. [DOI] [PubMed] [Google Scholar]
- Yurek DM, Fletcher-Turner A. Differential expression of GDNF, BDNF, and NT-3 in the aging nigrostriatal system following a neurotoxic lesion. Brain Res. 2001;891:228–235. doi: 10.1016/s0006-8993(00)03217-0. [DOI] [PubMed] [Google Scholar]
- Wu DC, Jackson-Lewis V, Vila M, Tieu K, Teismann P, Vadseth C, Choi D-K, Ischiropoulos H, Przedborski S. Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s Disease. J Neurosci. 2002;22:1763–1771. doi: 10.1523/JNEUROSCI.22-05-01763.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease--a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Zaman V, Boger HA, Granholm A-Ch, Rohrer B, Moore AB, Gerhardt GA, Hoffer BJ, Middaugh LD. Nigrostriatal dopamine system of aging GFRα1 heterozygous mice: neurochemistry, morphology and behavior. Eur J Neurosci. 2008 doi: 10.1111/j.1460-9568.2008.06456.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]