Abstract
The metabolism of the amino acid l-arginine is emerging as a crucial mechanism for the regulation of immune responses. Here, we characterized the impact of l-arginine deprivation on T cell and macrophage (MΦ) effector functions: We show that whereas l-arginine is required unconditionally for T cell activation, MΦ can up-regulate activation markers and produce cytokines and chemokines in the absence of l-arginine. Furthermore, we show that l-arginine deprivation does not affect the capacity of activated MΦ to up-regulate l-arginine-metabolizing enzymes such as inducible NO synthase and arginase 1. Thus, our results show that to exert their effector functions, T cells and MΦ have different requirements for l-arginine.
Keywords: arginase, immune regulation, t cell hyporesponsiveness, iNOS, cytokine, chemokine
INTRODUCTION
l-arginine is classified as a semi-essential or conditionally essential amino acid, depending on the age and health of an individual. Whereas newborns are unable to produce arginine efficiently, adults can synthesize enough l-arginine so that it is not a nutritionally essential amino acid. However, in cases of physical stress such as surgery or trauma, l-arginine needs to be supplemented [1, 2]. Free arginine in the body is derived from the diet, endogenous synthesis, and protein turnover. Thus, in healthy adults, l-arginine homeostasis is mainly achieved by modulation of l-arginine intake and modulation of its catabolism [3]. There are four main enzymes that can metabolize l-arginine in macrophages (MΦ), and importantly, two of these enzymes, arginase and inducible NO synthase (iNOS), have been involved in the regulation of immune responses. Arginase 1, which is induced in myeloid cells, is up-regulated upon activation by Th2 cytokines [4], GM-CSF [5, 6], PG [7,8,9], and catecholamines [9]; arginase hydrolyzes l-arginine into ornithine, an amino acid that is the main intracellular source for the synthesis of polyamines. iNOS is induced in myeloid cells by proinflammatory cytokines such as TNF-α/IFN-γ and LPS [4, 10] and oxidizes l-arginine in a two-step process into NO [11]. iNOS can control arginase by the generation of the NO intermediate, hydroxy-l-arginine, which is a competitive inhibitor of arginase activity; in turn, arginase can regulate NO production via the depletion of l-arginine from the extracellular milieu [12, 13]. l-arginine is transported from the extracellular milieu into myeloid cells as a result of increased expression of the high-affinity cationic amino acid transporters [14, 15], which are coinduced with the arginine-metabolizing enzymes [16].
The metabolism of l-arginine by iNOS and arginase 1 in myeloid cells is emerging as an important mechanism of T cell regulation:
NO, which is generated by the oxidation of l-arginine by iNOS, is an important effector molecule in host defense mounted by the immune system, but it can also act as a cytotoxic agent in pathological processes and can therefore play a central role in the regulation of immune responses [17]. For example, high concentration of NO has direct, proapoptotic effects on T cells [18, 19]. NO is also known to down-regulate intracellular signaling proteins directly or indirectly [20, 21]. One of the main factors that can regulate the expression of iNOS is the availability of extracellular l-arginine [22,23,24].
Arginase 1 has been shown to impair T cell responses by modulating the bioavailability of l-arginine: High arginase activity expressed by myeloid cells coincides with the transport of extracellular l-arginine into the cells, thereby causing a reduction of l-arginine in the microenvironment. In turn, this decrease in l-arginine results in T cell hyporesponsiveness [25,26,27,28,29,30,31,32]. This T cell dysfunction is attributed directly to l-arginine starvation that can regulate the cell cycle and arrest the cells in the G0-G1 phase [28]. Arginase-mediated l-arginine deprivation has been shown to cause T cell hyporesponsiveness in a variety of pathological and physiological responses [8, 31, 33]. Moreover, high arginase expression has been reported in a variety of diseases such as chronic inflammation [32], asthma [34], psoriasis [35], and infectious diseases [36,37,38,39].
During immune responses in vivo, T lymphocytes and MΦ communicate and cooperate for the execution of many effector functions. Therefore, arginine deprivation in the microenvironment could affect T cells directly or indirectly via the impairment of MΦ effector functions. Therefore, we characterized further the effects of l-arginine deprivation on T cell activation and on T cell effector functions. As MΦ can be induced by T cell-derived cytokines as well as other signals to up-regulate l-arginine-metabolizing enzymes such as arginase 1 or iNOS [40], we determined how l-arginine starvation affects MΦ biological functions.
MATERIALS AND METHODS
Mice
Female BALB/c mice (6–8 weeks old) were purchased from Charles River (UK) and were kept in individually vented cages. The animal colonies were screened regularly for mouse pathogens and tested negative consistently. Animal experiments were performed in accordance with home office and institutional guidelines.
T cell activation
Primary activation
Spleens of BALB/c mice were homogenized, and 5 × 106/ml cells were activated with 1 μg plate-bound anti-CD3 mAb (eBioscience, San Diego, CA, USA) and 200 ng soluble anti-CD28 mAb (eBioscience) in DMEM (400 μM l-arginine) or l-arginine-free DMEM (0 μM l-arginine), supplemented with 5% FBS, 50 IU/ml penicillin, 50 μg/ml streptomycin, and 292 μg/ml L-glutamine (Gibco, Grand Island, NY, USA). Cells were harvested after 3 days for further analysis.
Restimulation
Spleen cells from BALB/c mice were stimulated as described above. Four days later, cells were washed and rested in complete DMEM containing 400 μM l-arginine, and 3 days later, they were restimulated for 24 h in DMEM (400 μM l-arginine) or l-arginine-free DMEM (0 μM l-arginine) with 1 μg plate-bound anti-CD3 mAb and 200 ng soluble anti-CD28 mAb. Cells were harvested after 1 day for further analysis.
T cell purification
Spleens of BALB/c mice were homogenized and T cells purified using the EasySep Mouse CD90.2 (Thy-1.2) positive selection kit (Stem Cell Technologies, Canada), according to the supplier’s protocol. The purity of the CD90.2+ cells was determined by flow cytometry to be >96%. T cells (1×106/ml) were stimulated as described above.
Flow cytometry analysis
FcR blocking reagent (1 μg; PharMingen, San Diego, CA, USA) was added to the cells to block unspecific binding before further treatment.
T cell proliferation
BrdU (0.3 μg; Sigma Chemical Co., St. Louis, MO, USA) was added to the cells, and 18 h later, the proliferative capacity of TcRβ+ cells was determined by flow cytometry by measuring BrdU incorporation as described in ref. [41].
Intracellular cytokine production
The frequency of TcRβ+ cells expressing IL-4, IL-10, or IFN-γ was determined by flow cytometry as described in ref. [42].
Activation markers
All antibodies were used according to the suppliers’ protocols [42]. T cell activation markers included anti-CD25 (PharMingen) and anti-CD62 ligand (anti-CD62L) and anti-CD28 (eBioscience); MΦ activation and differentiation markers included anti-F4/80, anti-CD69, anti-CD40, anti-MHCII, anti-CD86, anti-CD80, and anti-programmed death ligand-1 (PDL1; eBioscience) and anti-CD206 (Serotec, UK). All flow cytometry analyses were performed on a FACSCalibur (Becton Dickinson, San Jose, CA, USA), and data were analyzed using Summit 4.0 software.
Integrated mean fluorescense intensity (iMFI)
The iMFI [43] was obtained by multiplying the percent of TcRβ+ cells by the value of the MFI for BrdU or by the value of the MFI for the relevant cytokine.
ELISA
For the determination of IFN-γ, IL-4, and IL-10 by ELISA, 5 × 106/ml spleen cells were activated with 1 μg plate-bound anti-CD3 mAb (eBioscience) and 200 ng soluble anti-CD28 mAb (eBioscience). Cells were harvested after 3 days, and the levels of cytokines were determined using an ELISA kit (ELISA development kit, PeproTech, Rocky Hill, NJ, USA) following the supplier’s protocol.
Bone marrow MΦ (BMMΦ)
BM was obtained by flushing the femurs of naïve BALB/c mice, and precursor cells were cultured in hydrophobic Teflon bags as described in ref. [38]. After 8 days in culture, mature BMMΦ were harvested, and classically activated MΦ (CAMΦ) were obtained by stimulating 5 × 105/ml mature MΦ with 100 U/ml IFN-γ (PeproTech) and 500 U/ml TNF-α (PeproTech); alternatively activated MΦ (AAMΦ) were obtained by stimulating 5 × 105/ml mature MΦ with 20 U/ml IL-4 (PeproTech). IL-10 (10 U/ml; PeproTech) and 1 μg/ml LPS (Sigma Chemical Co.) were also used. After 2 days in culture DMEM (400 μM l-arginine) or l-arginine-free DMEM (0 μM l-arginine) at 37°C/10% CO2, cells were harvested for further analysis.
MΦ phagocytosis assay
The assay was adapted using the Vybrant phagocytosis assay kit (Molecular Probes, Eugene, OR, USA). Briefly, activated MΦ were incubated for 2 h with fluorescein-labeled BioParticles at 37°C with 10% CO2; the fluorescence of any noninternalized particles was then quenched by the addition of Trypan blue. Detection of fluorescein was performed using a FACSCalibur (Becton Dickinson), and data were analyzed using Summit 4.3 software.
BMMΦ cytokine and chemokine analyses
BMMΦ were stimulated as described above, and 48 h later, supernatants were harvested, and IL-6, IL-10, IL-12p70, and MCP-1 were detected simultaneously in each sample by the Luminex-based Multiplexed assay (Luminex 100 system). Data were analyzed using STarstation V2.0.
Determination of arginase activity
Arginase activity was measured in MΦ lysates by the conversion of l-arginine to urea as described in refs. [4, 38]. One unit of enzyme activity was defined as the amount of enzyme that catalyzes the formation of 1 μmol urea per min.
Nitrite determination
NO2− accumulation was used as an indicator of NO production and measured using the Griess reagent [44].
Western blot analysis
An equal amount of protein [determined by bicinchoninic acid protein assay reagent (Pierce, Rockford, IL, USA)] was resolved by the addition of reducing buffer (Invitrogen, Carlsbad, CA, USA) and sample buffer (Invitrogen) following the suppliers’ recommendations before separation on SDS 4–12% gels (Invitrogen). Following electrophoresis, the protein was transferred to polyvinylidene difluoride membranes (Invitrogen) using a semi-dry transfer cell (Bio-Rad, Hercules, CA, USA) at 450 mA for 90 min. For immunodetection, membranes were blocked with 1% BSA in PBS (Sigma Chemical Co.) before the primary antibody [antiarginase 1 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-NOS 2 (Santa Cruz Biotechnology), anti-Ym1 (Stem Cell Technologies), and anti-found in inflammatory zone 1 (Fizz1; PeproTech)] was added following the suppliers’ recommendations, and goat anti-mouse HRP (Santa Cruz Biotechnology) was used as the secondary antibody. Detection of bound antibodies was visualized by chemiluminescence using the Western blotting Luminol reagent kit (Santa Cruz Biotechnology).
MΦ and T cell coculture
MΦ
Mature BMMΦ were obtained as described above in BMMΦ; 5 × 105 BMMΦ were stimulated with IL-4 (20 U/ml) and IL-10 (10 U/ml) for 2 days in complete DMEM (100 μM l-arginine).
T cells
Purified T cells (1×106) were activated with anti-CD3 mAb (1 μg/ml) and anti-CD28 mAb (200 ng/ml) in complete DMEM (400 μM l-arginine) for 24 h.
Cocultures
MΦ were washed after 2 days of activation and resuspended in complete DMEM (100 μM l-arginine). T cells were harvested, washed, and resuspended in DMEM without l-arginine and added to the MΦ cultures. l-arginine (400 μM) was added twice/day to some of the cultures. T cells were harvested for further analysis after 2 days of coculture.
Statistical analyses
Data were evaluated using a Student’s t-test with GraphPad PRISM, Version 2.0.
RESULTS
l-arginine deprivation impairs T cell effector functions
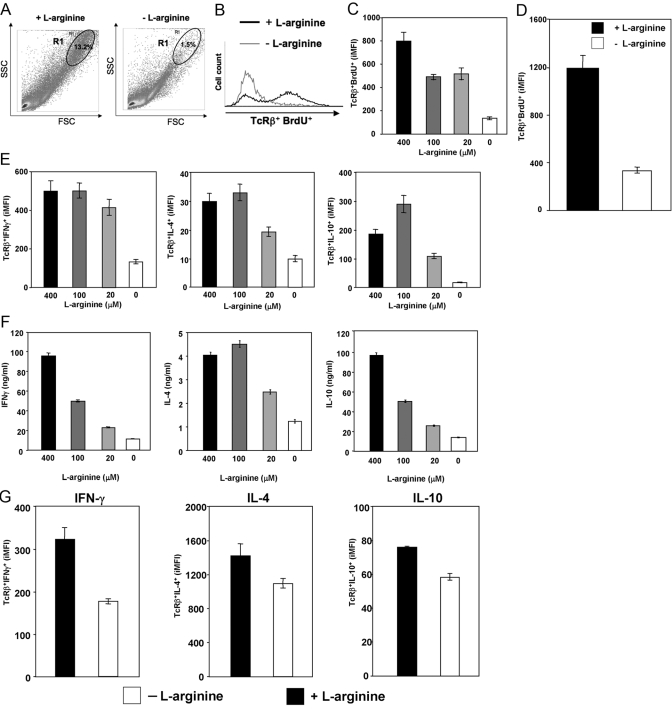
It has been shown previously that l-arginine deprivation induces T cell hyporesponsiveness, as defined by profound reduction of T cell proliferation and reduced CD3ζ chain expression [25, 26, 29, 31, 32, 45]. Here, we extended these results by further characterizing T cell activation and effector functions in the absence of l-arginine (l-arginine-free medium). We first assessed the capacity of T cells to undergo blast transformation and proliferate following anti-CD3 and anti-CD28 mAb stimulation. Our results show that after polyclonal stimulation of spleen cells in the presence (400 μM) of l-arginine, the large majority (>95%) of proliferating T cells (TcRβ+ BrdU+) is found in a distinct region of cells with a larger side- and forward-scatter (SSC and FSC, respectively; blasts), R1; this represents 13.2% of all gated cells (Fig. 1A). However, a sharp reduction in the percentage of cells in R1 was observed when cells were stimulated in l-arginine-free medium (Fig. 1A), as only 1.5% of all gated cells were found in R1 (88.6% reduction). In addition, l-arginine deprivation induced a clear decrease in the frequency of proliferating T cells [80.7% in the presence of l-arginine to 3.9% in the absence of l-arginine (Fig. 1B)] and in the MFI (14.0–9.1) of TcRβ+ BrdU+ (Fig. 1B). A recent study has identified a metric parameter, the iMFI, which reflects more accurately the total functional response of activated cells [43]. iMFI is calculated by multiplying the frequency (the magnitude of the response) by the MFI (the quality of the response). As shown in Figure 1C, BrdU iMFI of TcRβ+ decreases in the media containing 100 and 20 μM, as compared with 400 μM; however, the sharpest reduction was observed in the absence of l-arginine (0 μM). These results demonstrate that the magnitude and the quality of the proliferative response are impaired greatly with decreasing levels of l-arginine.
Fig. 1.
l-arginine deprivation impairs T cells proliferation and cytokine production. Spleen cells or purified T cells were stimulated with anti-CD3 and anti-CD28 mAb in grading concentration of l-arginine (+l-arginine=400 μM; -l-arginine=0 μM). The capacity of T cells from total spleen (A–C) or purified T cells (D) to proliferate and the capacity of splenic T cells (E) and purified T cells (G) to produce cytokines were determined by flow cytometry. (F) The level of cytokine production was determined by ELISA. The error bars represent sd, and data show the results of one representative experiment out of four independent experiments.
To determine whether the absence of l-arginine impacts directly on T cell activation, T cells were purified from spleens of BALB/c mice and stimulated with anti-CD3 and anti-CD28 mAb in the presence or absence of l-arginine. As shown in Figure 1D, the absence of l-arginine clearly results in impaired T cell proliferation in response to polyclonal T cell stimulation.
To characterize further the impact of l-arginine deprivation on T cell effector functions, we assessed the ability of splenic T cells to express IFN-γ, IL-4, and IL-10 in response to anti-CD3 and anti-CD28 mAb. The strongest reduction in the iMFI of TcRβ+ IFN-γ+, IL-4+, and IL-10+ was observed in the absence of l-arginine (Fig. 1E). Similar results were obtained when the levels of cytokine were determined by ELISA in the supernatant of stimulated cells (Fig. 1F). Cytokine production was also reduced when T cells, purified from spleen cell suspensions, were stimulated with anti-CD3 and anti-CD28 in the absence of l-arginine (Fig. 1G).
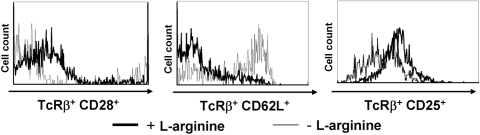
In agreement with the reduced capacity of T cells to proliferate and express cytokines in the absence of l-arginine, the expression of T cell activation markers such as CD25, CD28, and CD62L was also impaired (Fig. 2). Importantly, the total number of cells per well was only decreased minimally after 72 h in culture (27.1±4.6×106 in the presence of l-arginine vs. 23.8±4.1×106 in the absence of l-arginine; data not illustrated), and there was no increase in the frequency of apoptotic T cells in the absence of l-arginine, as the percentage of TcRβ+ caspase+ cells was similar in the presence (400 μM) and absence of l-arginine (1.2±0.3 vs. 2.2±0.1; data not illustrated); these results confirm previously published results that show that cell death is not the cause for reduced T cell activation and function [45].
Fig. 2.
l-arginine deprivation impairs expression of activation markers by T cells. Spleen cells were stimulated with anti-CD3 and anti-CD28 mAb in the presence (400 μM, black line) or absence (0 μM, gray line) of l-arginine, and the expression of CD28, CD62L, and CD25 on TcRβ+ cells was determined by flow cytometry. Data show the results of one representative experiment out of three independent experiments.
Thus, the results presented in Figures 1 and 2 clearly show that l-arginine deprivation during priming of T cells impairs their effector functions.
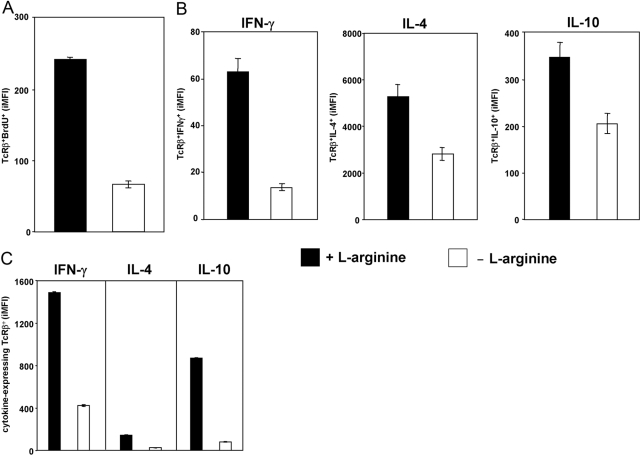
Naïve and effector T cells have different activation thresholds. Therefore, we assessed next whether restimulation of resting T cells in the absence of l-arginine also affects their effector functions. As presented in Figure 3A, l-arginine deprivation clearly impaired the capacity of restimulated T cells to proliferate, as shown by the reduced iMFI of TcRβ+ BrdU+. Furthermore, there was a systematic decrease in T cell cytokine iMFI when total spleen cells (Fig. 3B) or purified T cells (Fig. 3C) were polyclonally stimulated in the absence of l-arginine.
Fig. 3.
l-arginine is essential for efficient effector functions following restimulation of activated T cells. Spleen cells were stimulated with anti-CD3 and anti-CD28 mAb in the presence (400 μM) of l-arginine for 4 days, rested for 3 day, and restimulated for 24 h in the presence (400 μM) or absence (0 μM) of l-arginine. The capacity of T cells to proliferate (A) and produce cytokines (B) was determined by flow cytometry. The capacity of purified T cells from splenocytes to produce cytokines (C) was also determined. The error bars represent sd, and data show the results of one representative experiment out of three independent experiments.
The results presented in Figures 123 clearly demonstrate that activated T cells cannot exert their effector functions efficiently in the absence of l-arginine.
Arginase-induced l-arginine depletion impairs T cells functions
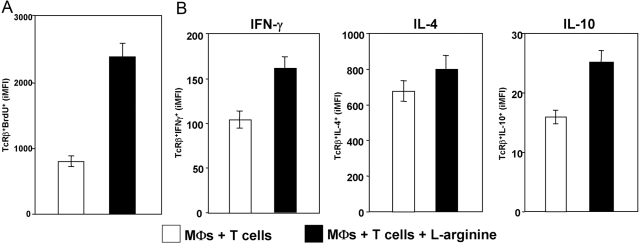
To assess whether l-arginine depletion by arginase-expressing MΦ can modulate T cell responses, a well-defined in vitro system was used: Arginase expression was induced in BMMΦ by stimulation with a combination of IL-4 and IL-10 [4], and purified T cells were added to the cultures. l-arginine was added daily to control cultures, and the impact of the presence of l-arginine-metabolizing MΦ on T cell effector functions was determined. As shown in Figure 4, addition of l-arginine clearly increases the capacity of T cells to proliferate (Fig. 4A) and to produce IFN-γ, IL-4, and IL-10 (Fig. 4B). These results demonstrate that arginase-induced l-arginine deletion by MΦ impaired T cell responses.
Fig. 4.
l-arginine depletion by arginase-expressing MΦ impairs T cells functions. Mature BMMΦs were stimulated with IL-4/IL-10 in the presence of 100 μM l-arginine. Two days later, MΦ were washed, and purified T cells (preactivated with anti-CD3 and anti-CD28 for 24 h) were added to the MΦ. l-arginine (400 μM) was added twice/day to some of the wells (+l-arginine). The capacity of T cells to proliferate (A) and produce cytokines (B) was determined by flow cytometry. The error bars represent sd, and data show the results of one representative experiment out of three independent experiments.
Effects of l-arginine deprivation on MΦ activation and effector functions
Whereas l-arginine deprivation is emerging as an important immunoregulatory mechanism for T lymphocytes, its role on other cells of the immune system is not well characterized. Up-regulation of l-arginine-metabolizing enzymes in MΦ induces uptake of l-arginine from the microenvironment into the cells [14, 38, 46], thereby controlling the availability of this amino acid in the extracellular milieu. Therefore, in the next step, we determined whether l-arginine deprivation also affects MΦ activation and effector functions. We tested several biological functions of physiologically distinct MΦ subsets: CAMΦ and AAMΦ in the presence of l-arginine (400 μM) or in l-arginine-free DMEM (0 μM). To obtain CAMΦ, we used IFN-γ and TNF-α, which induces iNOS rather than IFN-γ and LPS, as the latter have been shown to induce iNOS and arginase 1 (refs. [4, 47]; B-S. Choi and P. Kropf, unpublished results), and we used IL-4 for differentiation of AAMΦ. We first quantified the effects of l-arginine deprivation on phagocytic functions by determining the capacity of activated MΦ to phagocytose fluorescent particles. All groups of MΦ were capable of internalizing similar levels of fluorescent particles, even in the absence of l-arginine (data not shown).
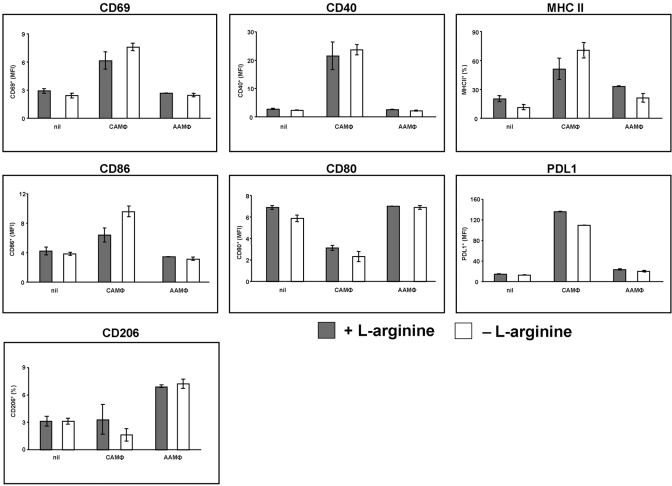
To characterize the impact of l-arginine deprivation on MΦ activation, we analyzed the expression levels of activation markers on both MΦ subsets. As shown in Figure 5, the absence of l-arginine during the differentiation of mature MΦ into CAMΦ or AAMΦ did not significantly affect the expression of activation markers (P>0.05). Of note, CD69, CD40, MHCII, CD86, and PDL1 were up-regulated in CAMΦ, whereas CD80 was down-regulated in CAMΦ, suggesting that these markers might be useful in physiologically distinguishing different types of activated MΦ; as shown previously [48], CD206 was up-regulated in AAMΦ exclusively.
Fig. 5.
l-arginine is not required for the expression of MΦ activation markers. Mature BMMΦ were differentiated in CAMΦ or AAMΦ or left unstimulated (nil) in the presence (400 μM, shaded bars) or absence (0 μM, open bars) of l-arginine, and the capacity to express cell-surface markers was analyzed by flow cytometry. The error bars represent sd, and data show the results of one representative experiment out of three independent experiments.
In the next step, we assessed whether CAMΦ and AAMΦ require l-arginine for the production of cytokines (IL-6, IL-10, and IL-12p70) and chemokine (MCP-1) and found that in the presence or absence of l-arginine, CAMΦ produce similar levels of IL-6. Similarly, the production of MCP-1 by CAMΦ and AAMΦ was not impaired in the absence of l-arginine (data not shown). No IL-10 or IL-12p70 was detectable under those conditions (data not shown).
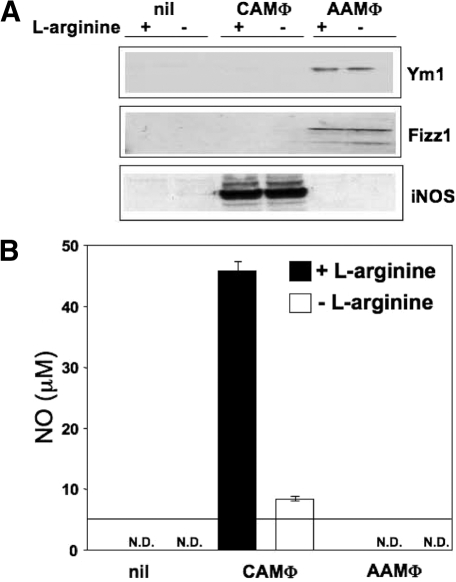
l-arginine deprivation also did not influence the expression of two markers of AAMΦ: YM1 (an eosinophilic chemotactic factor), and FIZZ1 (a resistin-like molecule; Fig. 6A).
Fig. 6.
l-arginine is not required for the expression of Ym1 and Fizz1, two hallmarks of AAMΦ and does not impair iNOS expression by CAMΦ. (A) Mature BMMΦ were differentiated in CAMΦ or AAMΦ or left unstimulated in the presence (400 μM) or absence (0 μM) of l-arginine, and the expression of Ym1, Fizz1, and iNOS was determined by Western blot. The same concentration of protein (14 μg) was loaded for each group. Data show the results of one representative experiment out of three independent experiments. (B) Mature BMMΦ were differentiated in CAMΦ or AAMΦ or left unstimulated in the presence (400 μM) or absence (0 μM) of l-arginine, and the production of NO was measured by the Griess reaction (B). The horizontal line represents the detection limit (5 μM). The error bars represent sd, and data show the results of one representative experiment out of five independent experiments. N.D., Not detected.
Finally, we determined the expression of the two main enzymes that use l-arginine as substrate and are associated with the depletion of l-arginine from the extracellular milieu: arginase 1 and iNOS. Importantly, we show that activation of MΦ in the absence of l-arginine did not abrogate protein expression of iNOS: As shown in Figure 6A, CAMΦ did not have an impaired capacity to express iNOS when differentiated in the absence of l-arginine. As expected, in the absence of l-arginine as a substrate, the production of NO was reduced strongly (Fig. 6B). NO production by MΦ stimulated by IFN-γ alone in the presence of l-arginine was just above the detection limit (7.0±1.7 μMol), and no NO was detectable when MΦ were stimulated with TNF-α alone (data not illustrated).
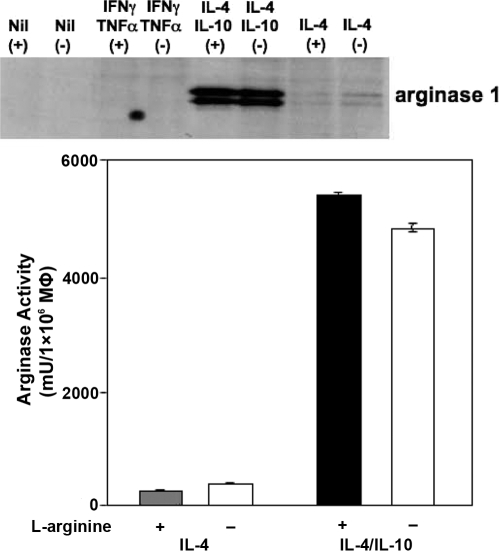
l-arginine deprivation did not affect the capacity of AAMΦ to up-regulate arginase, as arginase protein expression and arginase activity were not impaired in the absence of l-arginine (Fig. 7). IL-10 synergizes with IL-4 and strongly enhances the expression levels of arginase [48]; therefore, we tested whether a combination of these two cytokines could still induce arginase expression in the absence of l-arginine. As shown in Figure 7, IL-4 and IL-10 induced similar expression of arginase by AAMΦ in the presence and absence of l-arginine. To evaluate a possible contribution of l-arginine that is present in the FBS used in the culture medium, we did similar experiments as those presented in Figures 6B and 7 with dialyzed FBS [30] and show that even in the complete absence of l-arginine in the medium, small levels of NO were produced by CAMΦ; arginase activity expressed by AAMΦ remains constant under those conditions (Table 1).
Fig. 7.
l-arginine is not required for the expression and activity of arginase. Mature BMMΦ were activated with IFN-γ/TNF-α (CAMΦ), IL-4 (AAMΦ), or IL-4/IL-10 or left unstimulated in the presence (400 μM) or absence (0 μM) of l-arginine, and the expression of arginase was determined by Western blot (upper panel), and the activity of arginase was determined by enzymatic assay (lower panel). The same concentration of protein (14 μg) was loaded for each group. The error bars represent sd, and data show the results of one representative experiment out of five independent experiments.
TABLE 1.
Production of NO and Expression of Arginase by Activated BMMΦs in Dialyzed FBS
| A. NO (μM)
| ||
|---|---|---|
| CAMΦ (+ l-arginine) | CAMΦ (−l-arginine) | |
| Dialyzed FBS | 59.6 ± 1.1 | 4.5 ± 2.9 |
| FBS | 63.5 ± 2.4 | 2.9 ± 0.3 |
| B. Arginase (mU/1 × 106 MΦ)
| ||
|---|---|---|
| AAMΦ (+l-arginine) | AAMΦ (−l-arginine) | |
| Dialyzed FBS | 1657.3 ± 347.5 | 1345.4 ± 58.1 |
| FBS | 995.2 ± 16.2 | 1034.3 ± 13.1 |
BMMΦ were stimulated as described in Materials and Methods for 48 h. (A) Supernatants were tested for their content in NO by the Griess reagent; the values have been corrected for the levels of NO in the medium without macrophages. (B) The activity of arginase was determined by enzymatic assay as described in Materials and Methods. Values are ± sd, and data show the results of one representative experiment out of three independent experiments.
The results presented here show that to exert their effector functions, T cells and MΦ have different requirements for l-arginine: T cells primed or restimulated in the absence of l-arginine cannot be activated, proliferate, and produce cytokines efficiently, whereas MΦ activation and biological functions tested here are not altered by the lack of l-arginine.
DISCUSSION
The metabolism of l-arginine is emerging as a crucial mechanism for local immune cell regulation [25,26,27,28,29,30,31,32]. Arginase 1-induced l-arginine depletion has been shown to impair T cell responses by modulating the bioavailability of l-arginine. Indeed, human and mouse T cells display an impaired capacity to proliferate and express the CD3ζ chain in the absence of l-arginine in vitro; this can be reversed by addition of exogenous l-arginine. Suppression of T cell responses by l-arginine deprivation has also been demonstrated in physiological [31] and pathological [8, 27, 39] conditions. Here, we characterized further the impact of l-arginine deprivation on T cell activation and effector functions: We measured proliferation and cytokine production by T cells as functional responses. Our results show that the expression of activation markers such as CD25, CD62L, and CD28, which play crucial roles in the activation of T cells, is altered in the absence of l-arginine. Furthermore, we show that in the absence of l-arginine during priming and restimulation of T cells, the quality and the magnitude of these functional responses are impaired drastically. To determine whether l-arginine deprivation impacts directly on T cells, we also assessed the capacity of purified T cells to proliferate and produce cytokines in the absence of l-arginine. In agreement with our previous observations [32] and those of others [27], we show here that purified T cells cannot proliferate or produce cytokine efficiently in the absence of l-arginine. Of note, the decrease in proliferation and cytokine production of T cells stimulated in the absence of l-arginine was consistently lower in the cultures of total spleen cells as compared with purified T cells. This is likely to be a result of the absence of costimulation and/or bystander activation of other cells. These results demonstrate unequivocally that T cells cannot be activated efficiently and become competent effector cells in the absence of l-arginine.
Several immune dysfunctions have been associated with a change in the metabolism of l-arginine. For example, in different forms of cancer, high arginase activity in myeloid suppressor cells (MSCs) has been shown to down-regulate T cell responses; inhibition of arginase 1 alone [26, 33] or of iNOS and arginase 1 [49] has been shown to improve T cell responsiveness. These results demonstrate clearly that the metabolism of l-arginine regulates T cell responses.
Another line of evidence suggests an important immunoregulatory role for the metabolism of l-arginine: Plasma l-arginine levels in patients with pulmonary tuberculosis were significantly lower than those of negative controls; this correlated with higher levels of arginase activity and impaired T cell activation, as shown by a decreased expression of CD3ζ [39]. These studies as well as those from others [1, 30,31,32, 50], in combination with the results presented here, show clearly that l-arginine deprivation plays a central role in the regulation of T cell activation.
The study of the impact of l-arginine deprivation has been mainly restricted to T cells; however, its role on other cells of the immune system is not well characterized. MΦ not only interact with T cells, but they also can express two l-arginine-metabolizing enzymes—iNOS and arginase 1—and therefore control l-arginine availability and play an important immunoregulatory role. Indeed, the levels of l-arginine not only affect T cell activation, but they also regulate the stability of iNOS expression and NO production [22,23,24]. In addition, increased levels of polyamines, the metabolic products of the degradation of l-arginine by arginase, have also been shown to down-regulate MΦ activation [51]. Thus, in the next step, we tested the impact of l-arginine deprivation on activated MΦ. Interestingly, we show that the phagocytic capacity of CAMΦ and AAMΦ as well as the expression of activation markers and the production of IL-6 and MCP-1 production were not altered in the absence of l-arginine. Importantly, we also show that deprivation of l-arginine did not impair the up-regulation of iNOS and arginase; similar results were obtained with peritoneal exudate MΦ (data not illustrated). It has been shown recently that in the absence of l-arginine, activated T cells are unable to up-regulate cyclin D3 and cyclin-dependent kinase 4 and are therefore arrested in the G0-G1 phase of the cell cycle [28]. As activated MΦ do not proliferate [52], our results suggest that activated MΦ do not require l-arginine to the same extent as T cells for their activation and up-regulation of iNOS, arginase, and phagocytosis.
The key finding that l-arginine starvation does not abrogate biological functions of MΦ might explain how MΦ maintain their ability to express iNOS and arginase and to phagocytose, therefore regulating immune functions in critical conditions such as l-arginine deprivation. For example, in cancer, it has been demonstrated that MSCs, expressing high levels of arginase, are efficient in depleting l-arginine from their microenvironment [29, 50] and therefore, down-regulate T cell activation. Similarly, our results might also explain how myeloid cells can maintain their expression of arginase in other diseases that are associated with lower levels of l-arginine and immune dysfunctions, such as tuberculosis [39] or asthma [53]. Furthermore, this mechanism of differential l-arginine requirement might also explain the observed T cell hyporesponsiveness in diseases such as leishmaniasis and schistosomiasis, in which increased MΦ arginase activity has been observed [36, 38]. Indeed, as high arginase expression has been associated with these pathological conditions, the subsequent depletion of l-arginine might explain impaired T cell responses, while leaving the functionality of arginase-expressing MΦ unaltered.
The results showing that l-arginine deprivation does not affect iNOS expression are in apparent contradiction to those presented by El-Gayar et al. [23] and Lee et al [54], who showed that arginine availability regulates the stability of iNOS protein. Whereas both studies show that iNOS protein is clearly expressed in the absence of l-arginine, they show that the stability of the protein is altered. Although we did not test protein stability in our system, it is worth mentioning that different sources of MΦ have been used, as well as different stimuli. In the study by El-Gayar et al. [23], IFN-γ and LPS were used to induce iNOS, whereas we used IFN-γ and TNF-α to induce CAMΦ. As LPS also induces arginase 1 [4], it cannot be excluded that induction of arginase activity might have played a role in the down-regulation of iNOS expression. It has to be noted that whereas we used l-arginine-free DMEM, the FBS contained in the culture medium does contain l-arginine; however, even when we used dialyzed FBS, the residual levels of NO detected following stimulation without l-arginine were not significantly lower. Similarly, the expression of arginase was also not reduced when MΦ were stimulated in the absence of l-arginine in medium containing dialyzed FBS, as compared with nondialyzed FBS. The use of intracellular arginine pools represents another possibility to circumvent the consequences of l-arginine starvation in the extracellular environment. At least two pools of intracellular l-arginine have been described [55]; however, the authors suggest that these pools are not freely exchangeable with the intracellular milieu [55] and are therefore not available to be catabolized. Importantly, a complete urea cycle has been described in MΦ [56]. Therefore, it is feasible that MΦ use their own synthesized l-arginine for the induction of arginase and iNOS protein as well as the production of cytokines and chemokines and expression of activation markers.
It is important to note that during the course of diseases that are associated with lower levels of l-arginine in the plasma, the latter is still relatively high as compared with controls: 45 μM versus 94 μM (asthma; ref. [53]), 42 μM versus 77 μM (severe cerebral malaria; ref. [57]), and 88 μM versus 153 μM (tuberculosis; ref. [39]). These results, as well as those presented in our study, suggest that T cells react differently than MΦ to limiting levels of l-arginine in the extracellular milieu.
The findings presented here further our understanding about the mechanism by which l-arginine depletion regulates immune responses. Targeting the metabolism of l-arginine and the myeloid cells expressing l-arginine-metabolizing enzymes is likely to represent an important therapeutic and prophylactic strategy to treat diseases such as cancer, psoriasis, allergic asthma, and infectious diseases such as tuberculosis, leishmaniasis, and schistosomiasis.
Acknowledgments
This work was supported by grants from The Wellcome Trust (076078/Z/04/Z and 07664/Z/05/Z to P. K.).
References
- Popovic P J, Zeh H J, III, Ochoa J B. Arginine and immunity. J Nutr. 2007;137:1681S–1686S. doi: 10.1093/jn/137.6.1681S. [DOI] [PubMed] [Google Scholar]
- Morris S M., Jr Arginine metabolism: boundaries of our knowledge. J Nutr. 2007;137:1602S–1609S. doi: 10.1093/jn/137.6.1602S. [DOI] [PubMed] [Google Scholar]
- Castillo L, Chapman T E, Sanchez M, Yu Y M, Burke J F, Ajami A M, Vogt J, Young V R. Plasma arginine and citrulline kinetics in adults given adequate and arginine-free diets. Proc Natl Acad Sci USA. 1993;90:7749–7753. doi: 10.1073/pnas.90.16.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munder M, Eichmann K, Moran J M, Centeno F, Soler G, Modolell M. Th1/Th2-regulated expression of arginase isoforms in murine macrophages and dendritic cells. J Immunol. 1999;163:3771–3777. [PubMed] [Google Scholar]
- Jost MM, Ninci E, Meder B, Kempf C, van Royen N, Hua J, Berger B, Hoefer I, Modolell M, Buschmann I. Divergent effects of GM-CSF and TGF-(1 on bone marrow-derived macrophage arginase-1 activity, MCP-1 expresssion and matrix-metalloproteinase-12: a potential role during arteriogenesis. FASEB J. 2003;17:2281–2283. doi: 10.1096/fj.03-0071fje. [DOI] [PubMed] [Google Scholar]
- Martin L, Comalada M, Marti L, Closs E I, MacLeod C L, Martin del Rio R, Zorzano A, Modolell M, Celada A, Palacin M, Bertran J. Granulocyte-macrophage colony-stimulating factor increases L-arginine transport through the induction of CAT2 in bone marrow-derived macrophages. Am J Physiol Cell Physiol. 2006;290:C1364–C1372. doi: 10.1152/ajpcell.00520.2005. [DOI] [PubMed] [Google Scholar]
- Corraliza I M, Soler G, Eichmann K, Modolell M. Arginase induction by suppressors of nitric oxide synthesis (IL-4, IL-10 and PGE2) in murine bone-marrow-derived macrophages. Biochem Biophys Res Commun. 1995;206:667–673. doi: 10.1006/bbrc.1995.1094. [DOI] [PubMed] [Google Scholar]
- Rodriguez P C, Hernandez C P, Quiceno D, Dubinett S M, Zabaleta J, Ochoa J B, Gilbert J, Ochoa A C. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202:931–939. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard A C, Fitzpatrick E A, Maley M E, Gellin G L, Tsuei B J, Arden W A, Boulanger B R, Kearney P A, Ochoa J B. β Adrenoceptor regulation of macrophage arginase activity. Surgery. 2000;127:412–418. doi: 10.1067/msy.2000.104115. [DOI] [PubMed] [Google Scholar]
- Nathan C. Natural resistance and nitric oxide. Cell. 1995;82:873–876. doi: 10.1016/0092-8674(95)90019-5. [DOI] [PubMed] [Google Scholar]
- Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- Modolell M, Corraliza I M, Link F, Soler G, Eichmann K. Reciprocal regulation of the nitric oxide synthase/arginase balance in mouse bone marrow-derived macrophages by TH1 and TH2 cytokines. Eur J Immunol. 1995;25:1101–1104. doi: 10.1002/eji.1830250436. [DOI] [PubMed] [Google Scholar]
- Morris S M., Jr Enzymes of arginine metabolism. J Nutr. 2004;134:2743S–2747S. doi: 10.1093/jn/134.10.2743S. [DOI] [PubMed] [Google Scholar]
- Yeramian A, Martin L, Serrat N, Arpa L, Soler C, Bertran J, McLeod C, Palacin M, Modolell M, Lloberas J, Celada A. Arginine transport via cationic amino acid transporter 2 plays a critical regulatory role in classical or alternative activation of macrophages. J Immunol. 2006;176:5918–5924. doi: 10.4049/jimmunol.176.10.5918. [DOI] [PubMed] [Google Scholar]
- Kakuda D K, Sweet M J, Mac Leod C L, Hume D A, Markovich D. CAT2-mediated L-arginine transport and nitric oxide production in activated macrophages. Biochem J. 1999;340:549–553. [PMC free article] [PubMed] [Google Scholar]
- Verrey F, Closs E I, Wagner C A, Palacin M, Endou H, Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch. 2004;447:532–542. doi: 10.1007/s00424-003-1086-z. [DOI] [PubMed] [Google Scholar]
- Aktan F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004;75:639–653. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- Stefani M M A, Müller I, Louis J A. Leishmania major-specific CD8+ T cells are inducers and targets of nitric oxide produced by parasitized macrophages. Eur J Immunol. 1994;24:746–752. doi: 10.1002/eji.1830240338. [DOI] [PubMed] [Google Scholar]
- Macphail S E, Gibney C A, Brooks B M, Booth C G, Flanagan B F, Coleman J W. Nitric oxide regulation of human peripheral blood mononuclear cells: critical time dependence and selectivity for cytokine versus chemokine expression. J Immunol. 2003;171:4809–4815. doi: 10.4049/jimmunol.171.9.4809. [DOI] [PubMed] [Google Scholar]
- Duhe R J, Evans G A, Erwin R A, Kirken R A, Cox G W, Farrar W L. Nitric oxide and thiol redox regulation of Janus kinase activity. Proc Natl Acad Sci USA. 1998;95:126–131. doi: 10.1073/pnas.95.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingisser R M, Tilbrook P A, Holt P G, Kees U R. Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J Immunol. 1998;160:5729–5734. [PubMed] [Google Scholar]
- Lee J, Ryu H, Ferrante R J, Morris S M, Ratan R R. Translational control of inducible nitric oxide synthase expression by arginine can explain the arginine paradox. Proc Natl Acad Sci USA. 2003;100:4843–4848. doi: 10.1073/pnas.0735876100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gayar S, Thüring-Nahler H, Pfeilschifter J, Röllinghoff M, Bogdan C. Translational control of inducible nitric oxide synthase by IL-13 and arginine availability in inflammatory macrophages. J Immunol. 2003;171:4561–4568. doi: 10.4049/jimmunol.171.9.4561. [DOI] [PubMed] [Google Scholar]
- Chaturvedi R, Asim M, Lewis N D, Algood H M, Cover T L, Kim P Y, Wilson K T. L-arginine availability regulates inducible nitric oxide synthase-dependent host defense against Helicobacter pylori. Infect Immun. 2007;75:4305–4315. doi: 10.1128/IAI.00578-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez P C, Zea A H, Culotta K S, Zabaleta J, Ochoa J B, Ochoa A C. Regulation of T cell receptor CD3ζ chain expression by L-arginine. J Biol Chem. 2002;277:21123–21129. doi: 10.1074/jbc.M110675200. [DOI] [PubMed] [Google Scholar]
- Rodriguez P C, Zea A H, DeSalvo J, Culotta K S, Zabaleta J, Quiceno D G, Ochoa J B, Ochoa A C. L-arginine consumption by macrophages modulates the expression of CD3ζ chain in T lymphocytes. J Immunol. 2003;171:1232–1239. doi: 10.4049/jimmunol.171.3.1232. [DOI] [PubMed] [Google Scholar]
- Rodriguez P C, Quiceno D G, Zabaleta J, Ortiz B, Zea A H, Piazuelo M B, Delgado A, Correa P, Brayer J, Sotomayor E M, Antonia S, Ochoa J B, Ochoa A C. Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res. 2004;64:5839–5849. doi: 10.1158/0008-5472.CAN-04-0465. [DOI] [PubMed] [Google Scholar]
- Rodriguez P C, Quiceno D G, Ochoa A C. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109:1568–1573. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- Munder M, Mollinedo F, Calafat J, Canchado J, Gil-Lamaignere C, Fuentes J M, Luckner C, Doschko G, Soler G, Eichmann K, Müller F M, Ho A D, Goerner M, Modolell M. Arginase I is constitutively expressed in human granulocytes and participates in fungicidal activity. Blood. 2005;105:2549–2556. doi: 10.1182/blood-2004-07-2521. [DOI] [PubMed] [Google Scholar]
- Kropf P, Baud D, Marshall S E, Munder M, Mosley A, Fuentes J M, Bangham C R, Taylor G P, Herath S, Choi B S, Soler G, Teoh T, Modolell M, Müller I. Arginase activity mediates reversible T cell hyporesponsiveness in human pregnancy. Eur J Immunol. 2007;37:935–945. doi: 10.1002/eji.200636542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munder M, Schneider H, Luckner C, Giese T, Langhans C D, Fuentes J, Kropf P, Müller I, Kolb A, Modolell M, Ho A D. Suppression of T cell functions by human granulocyte arginase. Blood. 2006;108:1627–1634. doi: 10.1182/blood-2006-11-010389. [DOI] [PubMed] [Google Scholar]
- Zea A H, Rodriguez P C, Atkins M B, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O'Neill A, Mier J, Ochoa A C. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- Vercelli D. Arginase: marker, effector, or candidate gene for asthma? J Clin Invest. 2003;111:1815–1817. doi: 10.1172/JCI18908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruch-Gerharz D, Schnorr O, Suschek C, Beck K-F, Pfeilschnifter J, Ruzicka T, Kolb-Bachofen V. Arginase 1 overexpression in psoriasis. Am J Pathol. 2003;162:203–211. doi: 10.1016/S0002-9440(10)63811-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse M, Modolell M, La Flamme A C, Schito M, Fuentes J M, Cheever A W, Pearce E J, Wynn T A. Differential regulation of nitric oxide synthase-2 and arginase-1 by type1/type2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J Immunol. 2001;167:6533–6544. doi: 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- Vincendeau P, Gobert A P, Daulouede S, Moynet D, Mossalayi M D. Arginases in parasitic diseases. Trends Parasitol. 2003;19:9–12. doi: 10.1016/s1471-4922(02)00010-7. [DOI] [PubMed] [Google Scholar]
- Kropf P, Fuentes J M, Fahnrich E, Arpa L, Herath S, Weber V, Soler G, Celada A, Modolell M, Muller I. Arginase and polyamine synthesis are key factors in the regulation of experimental leishmaniasis in vivo. FASEB J. 2005;19:1000–1002. doi: 10.1096/fj.04-3416fje. [DOI] [PubMed] [Google Scholar]
- Zea A H, Culotta K S, Ali J, Mason C, Park H J, Zabaleta J, Garcia L F, Ochoa A C. Decreased expression of CD3ζ and nuclear transcription factor κ B in patients with pulmonary tuberculosis: potential mechanisms and reversibility with treatment. J Infect Dis. 2006;194:1385–1393. doi: 10.1086/508200. [DOI] [PubMed] [Google Scholar]
- Munder M, Eichmann K, Modolell M. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J Immunol. 1998;160:5347–5354. [PubMed] [Google Scholar]
- Herath S, Kropf P, Müller I. Cross-talk between CD8+ and CD4+ T cells in experimental cutaneous leishmaniasis: CD8+ T cells are required for optimal IFN-γ production by CD4+ T cells. Parasite Immunol. 2003;25:559–567. doi: 10.1111/j.0141-9838.2004.00668.x. [DOI] [PubMed] [Google Scholar]
- Kropf P, Herath S, Tewari R, Syed N, Klemenz R, Müller I. Identification of two distinct subpopulations of Leishmania major specific T helper 2 cells. Infect Immun. 2002;70:5512–5520. doi: 10.1128/IAI.70.10.5512-5520.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrah P A, Patel D T, De Luca P M, Lindsay R W, Davey D F, Flynn B J, Hoff S T, Andersen P, Reed S G, Morris S L, Roederer M, Seder R A. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- Kropf P, Brunson K, Etges R, Müller I. The leishmaniasis model. San Diego, CA, USA: Academic; 1998:419–458. [Google Scholar]
- Zea A H, Rodriguez P C, Culotta K S, Hernandez C P, DeSalvo J, Ochoa J B, Park H J, Zabaleta J, Ochoa A C. L-Arginine modulates CD3ζ expression and T cell function in activated human T lymphocytes. Cell Immunol. 2004;232:21–31. doi: 10.1016/j.cellimm.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Nicholson B, Manners C K, Kleeman J, MacLeod C L. Sustained nitric oxide production in macrophages requires the arginine transporter CAT2. J Biol Chem. 2001;276:15881–15885. doi: 10.1074/jbc.M010030200. [DOI] [PubMed] [Google Scholar]
- Currie G A. Activated macrophages kill tumor cells by releasing arginase. Nature. 1978;273:758–759. doi: 10.1038/273758a0. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Bronte V, Kasic T, Gri G, Gallana K, Borsellino G, Marigo I, Battistini L, Iafrate M, Prayer-Galetti T, Pagano F, Viola A. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201:1257–1268. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa A C, Zea A H, Hernandez C, Rodriguez P C. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res. 2007;13:721s–726s. doi: 10.1158/1078-0432.CCR-06-2197. [DOI] [PubMed] [Google Scholar]
- Zhang M, Caragine T, Wang H, Cohen P S, Botchkina G, Soda K, Bianchi M, Ulrich P, Cerami A, Sherry B, Tracey K J. Spermine inhibits proinflammatory cytokine synthesis in human mononuclear cells: a counterregulatory mechanism that restrains the immune response. J Exp Med. 1997;185:1759–1768. doi: 10.1084/jem.185.10.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xaus J, Cardo M, Valledor A F, Soler C, Lloberas J, Celada A. Interferon γ induces the expression of p21waf-1 and arrests macrophage cell cycle, preventing induction of apoptosis. Immunity. 1999;11:103–113. doi: 10.1016/s1074-7613(00)80085-0. [DOI] [PubMed] [Google Scholar]
- Morris C R, Poljakovic M, Lavrisha L, Machado L, Kuypers F A, Morris S M. Decreased arginine bioavailability and increased serum arginase activity in asthma. Am J Respir Crit Care Med. 2004;170:148–153. doi: 10.1164/rccm.200309-1304OC. [DOI] [PubMed] [Google Scholar]
- Lee J, Ryu H, Ferrante R J, Morris S M, Jr, Ratan R R. Translational control of inducible nitric oxide synthase expression by arginine can explain the arginine paradox. Proc Natl Acad Sci USA. 2003;100:4843–4848. doi: 10.1073/pnas.0735876100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Closs E I, Scheld J S, Sharafi M, Forstermann U. Substrate supply for nitric-oxide synthase in macrophages and endothelial cells: role of cationic amino acid transporters. Mol Pharmacol. 2000;57:68–74. [PubMed] [Google Scholar]
- Hofmann F, Kreusch J, Maier K-P, Munder P G, Decker K. The urea cycle in different types of macrophages. Biochem Soc Trans. 1978;6:990–993. doi: 10.1042/bst0060990. [DOI] [PubMed] [Google Scholar]
- Yeo T W, Lampah D A, Gitawati R, Tjitra E, Kenangalem E, McNeil Y R, Darcy C J, Granger D L, Weinberg J B, Lopansri B K, Price R N, Duffull S B, Celermajer D S, Anstey N M. Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med. 2007;204:2693–2704. doi: 10.1084/jem.20070819. [DOI] [PMC free article] [PubMed] [Google Scholar]