Abstract
Hypoadiponectinemia has been implicated in the development of obesity-related conditions including dyslipidemia and coronary heart disease (CHD). This study examined the association of adiponectin with CHD prevalence, incidence and mortality among 1513 community-dwelling men and women, aged 50 to 91, who were followed from 1984–87 through 2004. In cross-sectional analyses, adiponectin concentrations were positively related to female sex, age, and HDL cholesterol, and inversely related to waist girth, triglycerides, and fasting plasma glucose (all p<0.001). Adiponectin levels in the highest, compared to the lowest, sex-specific quintile were associated with 44% decreased odds (p=0.03) of prevalent CHD; adjustment for HDL cholesterol and/or triglycerides eliminated this association. In 20 year prospective analyses, higher adiponectin concentrations predicted reduced risk of non-fatal myocardial infarction in men only; adiponectin was not associated with fatal incident CHD events or 20 year CHD mortality (N=215 deaths) in either sex. Adiponectin levels in the highest sex-specific quintile, compared to lower levels, were associated with a 40% increased risk of cardiovascular disease death (N=441) and death from all causes (N=925), independent of age, sex, waist girth, lipids, and glucose (both p<0.001). These results suggest that use of adiponectin for CVD risk stratification is premature.
Keywords: adiponectin, coronary heart disease, mortality
INTRODUCTION
Adiponectin, the most abundant circulating adipocyte-derived protein identified thus far, appears to play an important role in glucose and lipid metabolism, vascular biology and energy homeostasis (1, 2). In animal studies, recombinant adiponectin improves insulin sensitivity, inhibits inflammatory responses and reverses diet-induced lipid abnormalities (3). Cross-sectional population-based studies in humans show that low levels of adiponectin are associated with an adverse cardiovascular disease risk profile, even among relatively healthy individuals (4–7). Hypoadiponectinemia is strongly linked to central adiposity, low HDL cholesterol, high triglycerides, insulin resistance, and high blood pressure, all characteristics of the metabolic syndrome (4–8). The identification of low adiponectin levels in men and women with prevalent coronary heart disease (CHD) (9–11), coupled with its known anti-atherogenic effects (3, 12), has generated enthusiasm for the idea that hypoadiponectinemia might be a pathogenic element in the development of CHD (3, 13).
There have been few prospective studies of adiponectin and CHD in healthy populations and results are mixed. The first, a nested case-control study of men in the Health Professionals Follow-up Study, supported the thesis that adiponectin is cardioprotective; a doubling of adiponectin was associated with a 30% decreased risk for incident myocardial infarction over 6 years (14). This study was followed by two negative nested case-control studies, one from the Strong Heart Study of American Indians (15), the other from the British Women’s Heart and Health Study (16); neither found an association of adiponectin with incident CHD events during 4 years of follow-up. More recently, high baseline levels of adiponectin were associated with a favorable cardiovascular disease (CVD) risk profile, but with increased risk of 15 year mortality in older Dutch men and women (17). Thus, the nature of the adiponectin link to the pathogenesis of CHD and its long term sequelae is not clear.
We report here the association of adiponectin with CHD prevalence, incidence and mortality among 1513 community-dwelling older men and women from the Rancho Bernardo Study who were followed for 20 years. Sex differences were evaluated and analyses are based on sex-specific adiponectin distributions. Adiponectin associations with CVD death and all cause mortality are also reported.
MATERIALS AND METHODS
Study population
The Rancho Bernardo Study is a population-based study of healthy aging in Caucasian residents of a Southern California community. Between 1984 and 1987, 82% (n=2480) of surviving community-dwelling older participants attended a research clinic visit. During this visit, information regarding medical history, date of last menstrual cycle for women, medication use, physical activity (exercise 3+ times per week, yes/no), alcohol consumption (1+drinks/day versus less or none), and current smoking (yes/no) was obtained using standard questionnaires. Current medication use was validated by examination of pills and prescriptions brought to the clinic for that purpose. The study protocol was approved by the Institutional Review Board of the University of California, San Diego; all participants gave written informed consent. Eligibility criteria for the present analysis included 1) age 50 or older when evaluated at the 1984–87 visit, 2) availability of stored serum, 3) postmenopausal status (for women), and 4) no estrogen or insulin use at the time of the clinic visit. Of the 2480 participants who attended the 1984–87 clinic visit, 332 women were excluded for current estrogen use; 1588 (891 men and 697 women) of the remaining participants had sufficient stored sera for measurement of adiponectin. Of these, 28 were excluded for age less than 50, 8 for pre-menopausal status, 11 because of insulin use, and 28 because they had sex hormone levels outside the normal physiologic range (18). The remaining 835 men and 678 postmenopausal, non-estrogen using women are the subject of this report. Height, weight, and waist girth were measured in 1984–87 and 99% also had height and weight recorded in 1972–74. Compared with those without adiponectin assays, participants included in this study were slightly older and more likely to be male, but did not differ in terms of weight, BMI, lifestyle characteristics, prevalent heart disease, or weight loss ≥ 10 pounds in the previous 10 years.
Measurements
Height, weight, and waist girth were measured in the clinic with participants wearing light clothing and no shoes. Body mass index (BMI) (kg/m2) and waist girth (cm) were used as estimates of overall and central adiposity. Weight change was determined by subtracting the participant’s weight at the 1972–74 visit from that at the 1984–87 visit. Weight loss was defined as a loss of 10 or more pounds based on a previous report from the same Rancho Bernardo cohort showing a significant association of this degree of weight loss with survival (19). Systolic blood pressure was measured twice in seated resting subjects using the Hypertension Detection and Follow–Up Program protocol (20); the mean of two readings was used in analyses. Pulse pressure was calculated as the difference between systolic and diastolic blood pressure.
Blood samples were obtained by venipuncture between 0730 h and 1100 h after a requested 12-h fast; serum and plasma were separated and frozen at −70° C. In 2004, adiponectin levels were measured by RIA on twice-thawed serum samples at Linco Diagnostics Laboratory, St. Louis, MO. The Linco adiponectin assay measures total adiponectin, i.e. all molecular forms. The sensitivity and the intra- and interassay coefficients of variation were 0.8 mg/L, 6%, and 7%, respectively. Linco reports reproducible results for the adiponectin assay after two freeze-thaw cycles and adiponectin levels did not vary by years of frozen sample storage or hour of sample collection.
Fasting plasma total, HDL, and LDL cholesterol and triglyceride levels were measured in a Center for Disease Control Certified Lipid Research Clinic Laboratory. Total cholesterol and triglyceride levels were measured by enzymatic techniques using an ABA-200 biochromatic analyzer (Abbott Laboratories, Irving, TX). HDL was measured after precipitation of the other lipoproteins with heparin and manganese chloride. LDL was estimated using the Friedewald formula (21). Plasma glucose levels were measured by the glucose oxidase method, serum creatinine by the Jaffe reaction method.
Outcomes assessment
Medical history and incident CHD information was obtained using standardized questionnaires at baseline, at clinic visits approximately every 4 years thereafter, and from periodic mailings. Follow-up continued through 2004, a 20-yr follow-up. Prevalent CHD was defined as doctor-diagnosed myocardial infarction (MI) or cardiac revascularization. Incident CHD was defined as the first occurrence of a non-fatal MI or fatal CHD. Vital status was known for 96 percent of participants. Death certificates, obtained for 91 percent of decedents, were classified for underlying cause of death by a certified nosologist using the International Classification of Diseases, Ninth Revision; CHD death included codes 410-414; CVD, codes 401-414, 426-438, and 440-448; and all causes, codes 0-999.
Diabetes was defined by physician diagnosis, fasting plasma glucose ≥7.0 mmol/L (126 mg/dl), 2 hr post-challenge glucose ≥ 11.1 mmol/L (200 mg/dl), or use of diabetes medications (22). The metabolic syndrome was defined according to the 2002 Adult Treatment Panel III criteria (23). Hypertension was defined as blood pressure ≥130/85 mm Hg or use of antihypertensive medication.
Statistical analysis
Adiponectin, HDL cholesterol, and triglyceride levels were not normally distributed and were log10-transformed for analyses; reported values are geometric means or medians and interquartile ranges. Baseline characteristics were compared by prevalent CHD status using general linear models (GLM) adjusted for age and sex for continuous variables and Chi-square analysis for categorical variables. Age and sex-adjusted associations between adiponectin levels and selected CHD risk factors were examined using partial correlations for continuous variables and GLM for categorical variables. The association between adiponectin and prevalent CHD was assessed using logistic regression analyses; goodness of fit was confirmed by the Hosmer and Lemeshow method (24). The association between baseline adiponectin levels and incident CHD and all cause, CHD, and CVD mortality was investigated using Cox proportional hazards regressions; goodness of fit was confirmed by the May and Hosmer method (25). All models presented met the proportional hazards assumption. None of the regression results were significantly influenced by outliers.
For regression analyses, adiponectin levels were examined as a continuous variable (log10 adiponectin), as one sex-specific SD increase in log10 adiponectin, and as sex-specific quintiles based on the entire population. Three separate models were evaluated: the first adjusted for age and sex, the second added adjustment for waist girth, and the third added further adjustment for potential adiponectin covariates including HDL cholesterol, triglycerides, and fasting plasma glucose. There was no significant multicollinearity between the independent variables. No significant interactions were found between adiponectin and age and sex or any other risk factor; nevertheless sex-specific analyses are presented for incident CHD to allow comparisons with the literature. Incident CHD events were further analyzed based on whether the first event was fatal or non-fatal. These event-specific analyses included all participants. When considering fatal CHD as the first event, participants with non-fatal incident CHD were censored at the time of the non-fatal event, and vice versa for the non-fatal analysis. The same approach was used for cause-specific mortality analyses, that is, participants whose deaths were attributed to causes other than the one of interest were censored at the time of death.
All p-values presented are 2-tailed; p ≤0.05 was considered statistically significant. Data were analyzed using SAS version 9.1, SPSS version 11.5, and STATA version 9.
RESULTS
Baseline characteristics
The mean (SD) age of the population at baseline was 74.0 (8.1) years for women and 71.0 (9.7) years for men (range 50–91); 55 percent were male. Median adiponectin levels were 50 percent higher in women (15.5 mg/L) than men (9.8 mg/L) (p<0.001). Age and sex adjusted comparisons of characteristics for those with and without prevalent CHD are shown in table 1. Adiponectin levels were lower in individuals with prevalent CHD compared to those without, although this difference was of borderline statistical significance (p=0.056).
Table 1.
Age, sex and age and sex-adjusted baseline characteristics for 1513 older men and postmenopausal women according to prevalent and incident CHD: the Rancho Bernardo Study. Incident CHD excludes prevalent CHD cases.
| No Prevalent CHD (N=1352) | Prevalent CHD (N=161) | No Incident CHD (N=1100) | Incident CHD (N=252) | |
|---|---|---|---|---|
| Demographic, anthropomorphic | ||||
| Sex (male, %) | 52.7 | 75.8*** | 52.5 | 53.6 |
| Age, yr | 71.9 (71.5 ,72.4) | 75.6 (74.2 ,77.0)*** | 71.2 (70.7 ,71.8) | 75.4 (74.2 ,76.5)*** |
| Body mass index, kg/m2 | 25.1 (24.9 ,25.3) | 24.9 (24.2 ,25.5) | 25.0 (24.8 ,25.2) | 25.6 (25.1 ,26.0)* |
| Waist girth (cm) | 87.3 (86.8 ,87.8) | 86.0 (84.6 ,87.5) | 86.6 (86.0 ,87.1) | 88.9 (97.7 ,90.0)*** |
| Metabolic parameters | ||||
| Systolic blood pressure, mm Hg | 141.6 (140.5 ,142.6) | 139.9 (136.8 ,143.0) | 140.8 (139.6 ,141.9) | 143.6 (141.1 ,146.0)* |
| Diastolic blood pressure, mm Hg | 77.3 (76.8 ,77.8) | 74.6 (73.2 ,76.1)*** | 77.2 (76.6 ,77.8) | 77.3 (76.1 ,78.4) |
| Pulse pressure, mm Hg | 64.3 (63.4 ,65.1) | 65.2 (62.7 ,67.7) | 63.5 (62.6 ,64.4) | 66.2 (64.3 ,68.2)* |
| Total cholesterol (mmol/L) | 5.69 (5.63 ,5.74) | 5.79 (5.62 ,5.95) | 5.65 (5.59 ,5.71) | 5.91 (5.79 ,6.04)*** |
| LDL cholesterol (mmol/L) | 3.55 (3.50 ,3.60) | 3.63 (3.48 ,3.78) | 3.51 (3.46 ,3.57) | 3.78 (3.66 ,3.89)*** |
| HDL cholesterol (mmol/L)† | 1.48 (1.46 ,1.50) | 1.35 (1.30 ,1.41)*** | 1.51 (1.48 ,1.53) | 1.40 (1.35 ,1.45)*** |
| Triglycerides (mmol/L) † | 1.15 (1.12 ,1.19) | 1.38 (1.27 ,1.51)*** | 1.12 (1.09 ,1.16) | 1.31 (1.23 ,1.41)*** |
| Fasting plasma glucose (mmol/L) | 5.65 (5.58 ,5.71) | 5.85 (5.66 ,6.04) | 5.60 (5.53 ,5.68) | 5.82 (5.67 ,5.97)*** |
| Creatinine (μmol/L) | 102.1 (99.9 ,103.4) | 109.6 (105.2 ,114.0)** | 100.8 (99.1 ,102.5) | 103.2 (99.6 ,106.8) |
| Adiponectin, mg/L† | 11.9 (11.6 ,12.3) | 11.0 (10.1 ,11.9) | 12.1 (11.8 ,12.5) | 11.2 (10.5 ,11.9)* |
| Lifestyle parameters (%) | ||||
| Alcohol, (1+ drinks/day) | 44.3 | 45.3 | 44.2 | 42.7 |
| Current smoker | 12.4 | 10.9 | 12.4 | 13.9 |
| Exercise (3+ times/week) | 81.3 | 77.2 | 82.3 | 75.6* |
| Prevalent conditions (%) | ||||
| Diabetes | 14.3 | 23.0** | 13.0 | 19.2* |
| Metabolic syndrome | 17.3 | 23.0** | 15.4 | 25.5*** |
| vHypertension | 77.5 | 70.5* | 44.0 | 48.4 |
| Weight loss (≥ 10 lbs) | 21.7 | 24.4 | 21.5 | 19.9 |
| Family history of myocardial infarction | 4.3 | 5.7 | 4.1 | 5.0 |
| Aspirin use | 20.7 | 45.1*** | 21.3 | 17.0 |
p ≤ 0.05,
p ≤ 0.01,
p ≤ 0.001 comparing prevalent versus no prevalent CHD or incident versus no incident CHD
Values are adjusted means (95% CI) for continuous variables, proportions for categorical variables.
log10-transformed for analysis; values are geometric means.
Adiponectin and CHD risk factors
Age and sex-adjusted associations of adiponectin with CHD risk factors were similar for those with and without prevalent CHD (table 2). Adiponectin levels were negatively correlated with BMI, waist girth, triglycerides and fasting plasma glucose and positively correlated with age and HDL cholesterol. Adiponectin concentrations were higher in individuals who reported drinking one or more alcohol beverages daily compared to those who drank less or not at all. Current smoking and exercise were not related to adiponectin. Adiponectin levels were higher in those who had lost 10 or more pounds of weight in the preceding 10 years compared to those who had not, irrespective of CHD status, and independent of current weight (data not shown). The metabolic syndrome was associated with lower adiponectin levels, independent of CHD status, whereas diabetes was associated with significantly lower adiponectin in participants without prevalent CHD, but not in those with CHD (p for interaction=0.15).
Table 2.
Age and sex-adjusted associations of adiponectin with selected cardiovascular risk factors in those with and without prevalent CHD: the Rancho Bernardo Study.
| No CHD (N=1352) | CHD (N=161) | |
|---|---|---|
| Cardiovascular risk factors | R† | R† |
| Age | .32*** | .32*** |
| Body mass index | −.29*** | −.23** |
| Waist girth | −.30*** | −.26*** |
| Waist hip ratio | −.26*** | −.21** |
| Systolic blood pressure | −.02 | −.11 |
| Diastolic blood pressure | −.03 | −.09 |
| Pulse pressure | −.01 | −.08 |
| Total cholesterol | .01 | .02 |
| LDL cholesterol | −.05 | .02 |
| HDL cholesterol‡ | .43*** | .52*** |
| Triglycerides‡ | −.35*** | −.47*** |
| Fasting plasma glucose | −.17*** | −.18* |
| Creatinine | −.03 | −.06 |
| Mean† | Mean† | |
| Lifestyle variables (yes/no) | ||
| Current smoking | 12.2/11.9 | 12.1/10.4 |
| Alcohol, 1+ dks/dy | 12.7/11.4*** | 11.7/9.6* |
| Exercise, 3+ x/wk | 11.9/12.1 | 10.7/10.2 |
| Concomitant conditions (yes/no) | ||
| Diabetes | 9.6/12.4*** | 9.7/10.9 |
| Metabolic syndrome | 8.5/12.9*** | 7.1/11.9*** |
| Hypertension | 11.8/12.5 | 10.3/11.3 |
| Weight loss ≥ 10 lbs (yes/no) | 13.9/11.5*** | 13.8/9.6*** |
| Family history of MI§ (yes/no) | 12.0/11.9 | 11.8/10.5 |
| Aspirin use (yes/no) | 12.4/11.9 | 10.7/10.4 |
p ≤ 0.05,
p ≤ 0.01,
p ≤ 0.001 versus adiponectin
values are Pearson partial correlation coefficients for continuous variables and adjusted geometric means for categorical variables.
log10-transformed for analysis; values are geometric means.
MI, myocardial infarction
Adiponectin and prevalent CHD
The age-adjusted prevalence of CHD was 3-fold higher (p<0.001) in men (15.2 percent) than women (5.0 percent). Table 3 shows age and sex-adjusted odds ratios for prevalent CHD by sex-specific adiponectin quintiles (quintile cut points were 10.6, 13.9, 17.5, and 22.4 mg/L for women; and 6.2, 8.5, 11.4, and 15.8 mg/L for men). The odds of CHD were 44 percent lower in participants whose adiponectin values were in the highest quintile compared to those in the lowest quintile (ptrend = 0.03); adjustment for waist girth strengthened this association to a 55 percent reduction in odds. Adjusting for other CHD risk factors including lifestyle variables (exercise, current smoking, and alcohol intake), family history of myocardial infarction, aspirin use, serum creatinine, weight loss and prevalent diabetes and hypertension did not materially alter these results (data not shown). However, addition of HDL cholesterol, triglycerides, and fasting plasma glucose together (Model 3), HDL cholesterol alone, or triglycerides alone (data not shown), eliminated the association between adiponectin and prevalent CHD. Results were similar in separate analyses excluding 230 participants with diabetes; adiponectin was not significantly related to prevalent CHD in analyses excluding 271 individuals with the metabolic syndrome.
Table 3.
Age and sex adjusted odds ratios (95% confidence intervals) for prevalent CHD (N=161) according to levels of adiponectin in 1513 older men and postmenopausal women: the Rancho Bernardo Study.
| Model 1† | Model 2‡ | Model 3§ | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| Per quintile | (# cases) | ||||||
| 1 | (38) | 1 | 1 | 1 | |||
| 2 | (29) | 0.64 | 0.38, 1.08 | 0.58 | 0.34, 1.00 | 0.72 | 0.42, 1.26 |
| 3 | (33) | 0.66 | 0.40, 1.11 | 0.64 | 0.38, 1.07 | 0.87 | 0.50, 1.49 |
| 4 | (27) | 0.53 | 0.31, 0.90 | 0.47 | 0.27, 0.82 | 0.71 | 0.40, 1.28 |
| 5 | (34) | 0.56 | 0.33, 0.94 | 0.45 | 0.26, 0.79 | 0.83 | 0.45, 1.55 |
| p-for trend | 0.025 | 0.006 | .56 | ||||
| Per unit increase in log | 0.49* | 0.24, 0.98 | 0.39* | 0.19, 0.81 | 0.96 | 0.41, 2.25 | |
| Per SD in log increase | 0.84* | 0.71, 0.99 | 0.80* | 0.67, 0.95 | 0.98 | 0.80, 1.21 | |
| -excluding diabetes # | 0.81* | 0.67, 0.99 | 0.78* | 0.64, 0.95 | 0.92 | 0.73, 1.15 | |
| -excluding MS†† | 0.90 | 0.73, 1.10 | 0.86 | 0.69, 1.06 | 1.00 | 0.78, 1.28 | |
p ≤ 0.05
Model 1: adjusted for age and sex
Model 2: adjusted for age, sex, and waist girth
Model 3: adjusted for Model 2 variables plus HDL cholesterol, triglycerides, and fasting plasma glucose
Excluding 230 participants with diabetes, 38 with CHD
Excluding 271 participants with MS, the metabolic syndrome; 37 with CHD
Adiponectin and incident CHD
After excluding the 161 participants with prevalent CHD at baseline, 252 (18.6 percent) of the remaining 1352 participants had a first CHD event during the 20 year follow-up, of which 144 (57.1 percent) were fatal. The age-adjusted event rate did not significantly differ (p=0.14) for men (20.1 percent) and women (17.0 percent); the median time to event was 7.5 years. In comparisons adjusted for age and sex, those who experienced an incident CHD event differed significantly from those who did not in almost every attribute (table 1). They were older, had greater BMI and waist girth, more adverse blood pressure and lipid profiles, and higher fasting plasma glucose levels. They also tended to exercise less and were more likely to have diabetes and the metabolic syndrome. Baseline age and sex-adjusted adiponectin levels were significantly lower among those with incident CHD compared to those without (mean: 11.99 mg/L vs 12.22 mg/L, respectively, p=0.03); this difference was significant for non-fatal (mean: 10.54 mg/L vs 11.99 mg/L, respectively, p=0.01), but not fatal, (mean: 11.72 vs 12.16 mg/L, respectively, p=0.41) CHD.
In Cox regression analyses (table 4), the association between adiponectin levels and incident CHD was of borderline significance for the population as a whole, with lower incidences across groups of increasing adiponectin (p=0.056). Sex-specific analyses were also conducted, first because sex differences have been reported in the literature (14, 16) and second because the p-value for the sex by log10 adiponectin interaction term was 0.10, which in our opinion justifies further exploration. Age-adjusted adiponectin levels were lower for men with an incident CHD event compared to those without (mean: 8.66 versus 9.93 mg/L, respectively, p=0.01), and higher adiponectin predicted reduced risk of incident CHD in age-adjusted analysis. This association was not independent of waist girth. Age-adjusted adiponectin levels did not differ at baseline for women with or without incident CHD (mean: 15.03 vs 15.20 mg/L, respectively, p=0.84), and adiponectin was not associated with future CHD risk in women.
Table 4.
Age-adjusted hazards ratios (95% confidence intervals) for incident fatal and non-fatal CHD according to levels of adiponectin in 1352 older men and postmenopausal women: the Rancho Bernardo Study.
| Total population | Men | Women | |||||
|---|---|---|---|---|---|---|---|
| Total N/No. of events | 1352/252 | 713/135 | 639/117 | ||||
| HR | 95% CI | HR | 95% CI | HR | 95% CI | ||
| Per quintile† | (# cases) | ||||||
| 1 | (63) | 1 | 1 | 1 | |||
| 2 | (70) | 0.88 | 0.59, 1.27 | 0.64 | 0.38, 1.09 | 1.25 | 0.70, 2.23 |
| 3 | (61) | 0.70 | 0.47, 1.04 | 0.59 | 0.35, 0.99 | 0.84 | 0.45, 1.56 |
| 4 | (58) | 0.70 | 0.48, 1.04 | 0.53 | 0.32, 0.90 | 0.99 | 0.55, 1.79 |
| 5 | (56) | 0.69 | 0.48, 1.01 | 0.55 | 0.32, 0.94 | 1.00 | 0.56, 1.81 |
| p for trend | .056 | .02 | .74 | ||||
| Per log increase† | 0.62 | 0.34, 0.94 | 0.46* | 0.23, 0.93 | 1.02 | 0.41, 2.54 | |
| Per SD log increase | |||||||
| Model 1† | 0.90 | 0.79, 0.99 | 0.83* | 0.69, 0.98 | 0.99 | 0.86, 1.21 | |
| Model 2‡ | 0.96 | 0.82, 1.05 | 0.87 | 0.72, 1.05 | 1.09 | 0.88, 1.33 | |
| Model 3§ | 1.10 | 0.89, 1.17 | 0.97 | 0.79, 1.18 | 1.30* | 1.03, 1.65 | |
| Model 1 | |||||||
| -excluding diabetes# | 0.95 | 0.82, 1.10 | 0.90 | 0.73, 1.09 | 1.02 | 0.82, 1.28 | |
| -excluding MS†† | 1.06 | 0.90, 1.25 | 0.95 | 0.76, 1.19 | 1.20 | 0.94, 1.53 | |
p ≤ 0.05
Adjusted for age and sex
Adjusted for age, sex, and waist girth
Adjusted for Model 2 variables plus HDL cholesterol, triglycerides, and fasting plasma glucose
Excluding 192 participants with diabetes, 54 with incident CHD
Excluding 234 participants with the metabolic syndrome (MS), 75 with incident CHD
We investigated whether the association of adiponectin levels with incident CHD differed for fatal and non-fatal events. For men, adiponectin was protective for future non-fatal MI, but not fatal CHD, independent of waist girth (table 5). This association became borderline significant (p=0.07) after additional adjustment for HDL cholesterol, triglycerides and fasting plasma glucose. For women, adiponectin was not associated with incident CHD in analyses of either a first non-fatal MI or fatal CHD as first presentation (data not shown). Age-adjusted adiponectin levels did not differ for those with a first fatal CHD event compared to those with a non-fatal CHD event in either sex (data not shown).
Table 5.
Hazards ratios (95% confidence intervals) for incident fatal CHD and non-fatal myocardial infarction (MI) according to levels of adiponectin in 713 older men: the Rancho Bernardo Study.
| Fatal CHD | Non-fatal MI | |||
|---|---|---|---|---|
| No. of events | 74 | 61 | ||
| HR | 95% CI | HR | 95% CI | |
| Per SD log increase | ||||
| Model 1† | 0.95 | 0.74, 1.20 | 0.67** | 0.52, 0.86 |
| Model 2‡ | 1.04 | 0.80, 1.35 | 0.69** | 0.53, 0.90 |
| Model 3§ | 1.18 | 0.88, 1.58 | 0.76 | 0.57, 1.08 |
p ≤ 0.05,
p ≤ 0.01
Model 1: adjusted for age
Model 2: adjusted for age and waist girth
Model 3: adjusted for Model 2 variables plus HDL cholesterol, triglycerides, and fasting plasma glucose
Adiponectin and mortality
Among the 1361 participants with complete follow-up and death certificate data, the age-adjusted proportion of deaths was 70.9 percent for men and 64.0 percent for women during the 20 year follow-up; the age-adjusted mortality rates per 1000 person-years were 61.9 and 46.4, respectively. Of 925 total deaths, 441 (48 percent) were attributed to CVD, 215 (49 percent) of these were CHD.
The age-adjusted proportion of CHD deaths was higher among men than women (17.5 versus 13.5 percent; p=0.04); age-adjusted CHD mortality rates per 1000 person-years were 16.5 and 9.9, respectively. Age and sex-adjusted baseline adiponectin levels did not differ for those with compared to those without CHD death (mean: 11.32 vs 11.83 mg/L, p=0.27), adiponectin concentrations were not significantly related to CHD mortality before or after adjusting for covariates (table 6), and there was no evidence for a trend across adiponectin quintiles (figure 1A).
Table 6.
Age and sex-adjusted hazards ratios (95% confidence interval) for CHD, CVD, and all cause mortality during 20 years of follow-up according to baseline serum adiponectin levels among 1361 older men and postmenopausal women.
| Number of events | CHD Death N=215 | CVD Death N=441 | All cause death N=925 | |||
|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | |
| Per quintile† | ||||||
| Quintile 1 (ref) | 1 | 1 | 1 | |||
| 2 | 0.74 | 0.47, 1.18 | 0.87 | 0.63, 1.22 | 0.89 | 0.71, 1.11 |
| 3 | 0.94 | 0.62, 1.43 | 1.01 | 0.74, 1.39 | 1.08 | 0.87, 1.33 |
| 4 | 0.72 | 0.46, 1.11 | 0.92 | 0.67, 1.25 | 0.96 | 0.77, 1.18 |
| 5 | 0.79 | 0.51, 1.20 | 1.15 | 0.84, 1.53 | 1.22 | 0.99, 1.51 |
| P for linear trend | 0.28 | .34 | 0.036 | |||
| Per log increase† | 0.88 | 0.49, 1.58 | 1.36 | 0.89, 2.09 | 1.38* | 1.03, 1.85 |
| Quintile 5 vs lower | ||||||
| Model 1† | 0.92 | 0.67, 1.27 | 1.19 | 0.96, 1.48 | 1.24** | 1.07, 1.45 |
| Model 2‡ | 0.98 | 0.70, 1.37 | 1.21 | 0.96, 1.50 | 1.26** | 1.08, 1.48 |
| Model 3§ | 1.18 | 0.83, 1.69 | 1.38** | 1.09, 1.76 | 1.36*** | 1.15, 1.60 |
| + weight loss # | 1.17 | 0.81, 1.69 | 1.38** | 1.08, 1.76 | 1.35*** | 1.14, 1.60 |
| -excluding diabetes †† | 1.13 | 0.79, 1.62 | 1.30* | 1.01, 1.67 | 1.30** | 1.09, 1.54 |
| -excluding MS ‡‡ | 1.11 | 0.75, 1.62 | 1.30* | 1.01, 1.66 | 1.35*** | 1.13, 1.60 |
| -excluding CHD §§ | 1.14 | 0.76, 1.72 | 1.38** | 1.07, 1.79 | 1.36*** | 1.14, 1.63 |
p ≤ 0.05,
p ≤ 0.01,
p ≤ 0.001
Number of cases/quintile 1–5 – CHD: 40, 34, 50, 42, 49; CVD: 70, 70, 92, 92, 117; All cause: 151, 153, 192, 194, 235
Adjusted for age and sex
Adjusted for age, sex, and waist girth
Adjusted for Model 2 variables plus HDL cholesterol, triglycerides, and fasting plasma glucose
Weight loss ≥ 10 pounds in the 10 years prior to baseline
No prevalent diabetes: total n=1155; n=172 CHD, 360 CVD, 765 all cause deaths
No prevalent MS: total n=1122; n=170 CHD, 357 CVD, 742 all cause deaths
No prevalent CHD: total n=1210; n=166 CHD, 363 CVD, 793 all cause deaths
Figure 1.
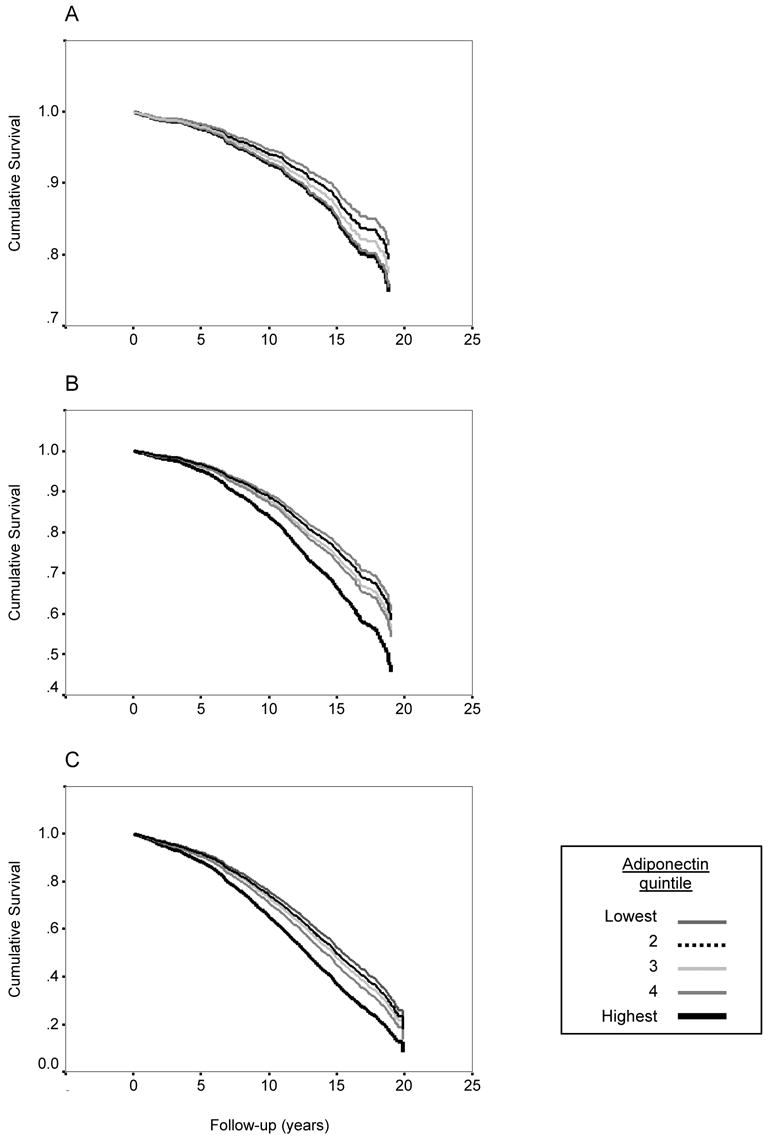
Survival curves according to quintiles of adiponectin for (A) CHD mortality, (B) CVD mortality, and (C) all cause mortality, adjusting for age, sex, waist girth, HDL cholesterol, triglycerides, and fasting plasma glucose. Hazards ratios for the highest versus the lowest quintile were: CHD 1.13, 95% CI 0.69, 1.85; CVD: 1.46, 95% CI 1.04, 2.07; all cause: 1.43, 95% CI 1.13, 1.82. Probability values for trend across the adiponectin quintiles were p=0.51, p=0.015, and p=0.002, respectively. Note differences in y-axis scales.
We also examined the association of adiponectin with CVD and all cause mortality. Age-and sex-adjusted baseline adiponectin levels did not differ on the basis of future CVD death or death from all causes (data not shown). In age and sex-adjusted analyses, there was a trend for increased risk of mortality from all causes across adiponectin quintiles (p=.036), which increased in strength all cause mortality and became significant for CVD mortality after additional adjustment for waist girth and other adiponectin covariates (HDL cholesterol, triglycerides, and fasting plasma glucose). As shown in Figure 1 (panels B and C), the risk of death due to CVD or all causes was similar for the first 4 quintiles of adiponectin and markedly elevated for the top quintile. When compared with those with lower levels, participants with adiponectin levels above the 80th percentile had 40 percent increased risk of fatal CVD and all cause mortality after adjusting for major adiponectin covariates (table 6).
Separate analyses adjusting for additional risk factors including lifestyle variables (exercise, current smoking, and alcohol intake), family history of myocardial infarction, aspirin use, serum creatinine and history of diabetes, hypertension, or the metabolic syndrome yielded similar results (data not shown). Although weight loss in the 10 years prior to baseline was a strong independent predictor of mortality (HR=1.54, 95% CI 1.32, 1.80, Model 3, table 6), adjustment for weight loss (table 6) or stratification by weight loss (data not shown) did not alter associations. Sequential exclusion of those with diabetes, the metabolic syndrome or CHD at baseline had negligible effect on mortality results (table 6).
DISCUSSION
This is the first long term prospective study to investigate the association of adiponectin with multiple CHD outcomes in men and women from the same population. We confirm that high serum adiponectin is associated with a favorable CHD risk profile and reduced odds of prevalent CHD in both sexes. Higher adiponectin concentrations also showed a protective association with risk of non-fatal MI over the following 20 years in men without diagnosed cardiovascular disease. However, adiponectin concentrations did not predict future CHD events in women and were not associated with 20 year fatal CHD in either sex. These observations, together with the unfavorable association of high adiponectin with mortality, suggest that use of serum adiponectin for cardiovascular risk stratification is premature.
The inverse and dose-dependent association between serum adiponectin levels and prevalent CHD was independent of adiposity, diabetes, hypertension and several other CHD risk factors, but was not independent of HDL cholesterol and triglyceride levels. These results suggest that a significant proportion of any protective effect of high concentrations of adiponectin may be mediated by lipid metabolism, specifically promotion of HDL cholesterol and reduction of circulating triglycerides. Although the exact mechanism by which adiponectin modulates lipid metabolism is unknown, adiponectin treatment reverses dyslipidemia in adiponectin-deficient mice (26, 27) and hypoadiponectinemia in humans is associated with increased hepatic lipase activity (28), a major determinant of HDL and triglyceride concentrations; both suggest a causal association.
Only longitudinal studies can distinguish cause from effect. In our prospective analysis, baseline levels of adiponectin were lower in men, but not women, who had a first ever CHD event during the 20 year follow-up, and higher adiponectin was associated with reduced risk of future CHD for men only. These results agree with two single sex studies reporting protective associations of adiponectin with incident CHD in men (14), but not women (16). Further analyses showed that the inverse association of adiponectin with incident CHD in men was specific to first ever non-fatal MI; adiponectin was not associated with incident CHD events that resulted in death. Although the number of events in our subgroup analysis was small, the Health Professionals Follow-up Study reported the same result in a larger dataset stratified by type of event (14).
Why would adiponectin protect against the development of non-fatal, but not fatal myocardial infarction, and why only in men? The lack of association of adiponectin with fatal CHD potentially reflects misclassification of CHD as cause of death in the elderly, whereas biological interactions between sex hormones, adipocytokines and CHD may account for the sex differences. Alternatively, adiponectin has been shown to influence thrombus formation and platelet aggregation in mouse models (29), thus adiponectin may influence MI through thrombotic as well as atherosclerotic processes. Larger prospective studies comparing the sexes and fatal versus non-fatal disease are needed to resolve these issues.
Participants in the present study were older and follow-up was longer than any of the published longitudinal studies of incident CHD, which varied between 4 and 6 years. However, restriction of the analysis to the first five years of follow-up did not alter results for incident disease. The presence of unrecognized CHD in the group identified as CHD-free at baseline could lead to overestimation of the association between adiponectin and incident events as these individuals may be more likely to have low levels of adiponectin as well as increased event risk. However, differences in baseline characteristics between those who did and did not experience CHD events were strong and consistent with established CHD risk profiles, and this bias is unlikely to have preferentially influenced results for women.
Despite the contradictory results in prospective studies, a large body of experimental evidence and several cross-sectional studies in humans, support the thesis that adiponectin is a protective factor for the cardiovascular system. The association of hypoadiponectinemia with increased cancer risk (30–33) suggests that this protein could also have beneficial effects on other physiologic systems. Thus, the absence of an inverse association of adiponectin with CHD mortality seems counterintuitive, and the association of high adiponectin levels with increased risk of death from any cause over 20 years is surprising. However, the present study is not the first to make this observation. Higher adiponectin levels were associated with increased risk of death in a 15 year population-based study of more than 2000 50–75 year old Dutch men and women (17), in a 4 year study of 195 patients with congestive heart failure (34), and in a 9 year follow-up of 1025 Danish patients with stable CHD (35). Not all mortality studies agree. Adiponectin did not predict overall mortality in a study of 227 patients with end stage renal failure over a mean follow-up of 2.5 years (36) and low, not high, levels were related to increased risk of 5-year mortality after first-ever ischemic stroke (37).
The physiology underlying increased mortality risk at higher adiponectin concentrations is likely to be complex, particularly in the elderly who have multiple causes of death. Adiponectin receptors have been identified in the brain, and both peripheral and central adiponectin administration decreases weight by increasing energy expenditure and fatty acid oxidation (38, 39). As proposed by Kistorp and colleagues (34), unusually high adiponectin levels may be a marker for catabolic processes leading to wasting, which presage death. In the present study, weight loss of 10 or more pounds in the 10 years prior to baseline was associated with higher adiponectin levels, independent of current weight, and, as previously reported for this cohort (19), weight loss was associated with a marked increase in mortality risk. However, weight loss did not account for the association of high adiponectin levels with mortality in multivariate analyses or in analyses stratified by weight loss status. The positive association of adiponectin with mortality may be due to confounding by some other unidentified factor, and not to direct detrimental effects of adiponectin.
Some limitations should be noted. Our results are based on a predominantly white and middle to upper-middle class community and may not apply to other ethnic and socioeconomic groups. Non-fatal CHD events were based on self-report which could have resulted in misclassification and biased results toward the null. However, at an earlier Rancho Bernardo visit, 30% of the cohort had medical records searched to validate self-reported CHD; 85% were confirmed. The number of events in several of the stratified analyses in this study is small and may have resulted in missed associations. Serum adiponectin values were measured on a single sample which may have limited associations, however adiponectin levels show minimal diurnal variation, are stable during the morning hours when these samples were collected, and single measurements have been shown to accurately reflect levels over a one year period (40–43). It is not known whether long-term storage affects adiponectin levels; in this cohort adiponectin concentrations were not significantly related to storage time, and levels were similar to published values for individuals of comparable age and adiposity (4, 15, 16).
CONCLUSIONS
We found divergent associations of circulating adiponectin levels with prevalent and incident CHD and with mortality. Although higher adiponectin concentrations were associated with decreased odds of prevalent CHD for both sexes, adiponectin had only a weak association with future non-fatal CHD events, and only in men. These results, together with those of others, bring into question whether serum adiponectin is an independent risk factor for CHD, or simply a risk marker. Proposals for pharmacologic and lifestyle interventions aimed at enhancing adiponectin levels may be premature.
Acknowledgments
G.A. Laughlin was supported by American Heart Association Award 0315024Y. The Rancho Bernardo Study was funded by research grant AG07181 from the National Institute on Aging and grant DK31801 from the National Institute of Diabetes and Digestive and Kidney Diseases. C. Langenberg was supported by a UK Medical Research Council Research Training Fellowship.
Abbreviations
- BMI
body mass index
- CHD
coronary heart disease
- CVD
cardiovascular disease
Footnotes
Competing interests: None
References
- 1.Havel PJ. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes. 2004;53 (Suppl 1):S143–51. doi: 10.2337/diabetes.53.2007.s143. [DOI] [PubMed] [Google Scholar]
- 2.Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: more than just another fat cell hormone? Diabetes Care. 2003;26:2442–50. doi: 10.2337/diacare.26.8.2442. [DOI] [PubMed] [Google Scholar]
- 3.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26:439–51. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 4.Cnop M, Havel PJ, Utzschneider KM, et al. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46:459–69. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- 5.Kazumi T, Kawaguchi A, Sakai K, Hirano T, Yoshino G. Young men with high-normal blood pressure have lower serum adiponectin, smaller LDL size, and higher elevated heart rate than those with optimal blood pressure. Diabetes Care. 2002;25:971–6. doi: 10.2337/diacare.25.6.971. [DOI] [PubMed] [Google Scholar]
- 6.Ryo M, Nakamura T, Kihara S, et al. Adiponectin as a biomarker of the metabolic syndrome. Circ J. 2004;68:975–81. doi: 10.1253/circj.68.975. [DOI] [PubMed] [Google Scholar]
- 7.Matsubara M, Maruoka S, Katayose S. Decreased plasma adiponectin concentrations in women with dyslipidemia. J Clin Endocrinol Metab. 2002;87:2764–9. doi: 10.1210/jcem.87.6.8550. [DOI] [PubMed] [Google Scholar]
- 8.Trujillo ME, Scherer PE. Adiponectin--journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med. 2005;257:167–75. doi: 10.1111/j.1365-2796.2004.01426.x. [DOI] [PubMed] [Google Scholar]
- 9.Hotta K, Funahashi T, Arita Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–9. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 10.Kumada M, Kihara S, Sumitsuji S, et al. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–9. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 11.Rothenbacher D, Brenner H, Marz W, Koenig W. Adiponectin, risk of coronary heart disease and correlations with cardiovascular risk markers. Eur Heart J. 2005 doi: 10.1093/eurheartj/ehi340. [DOI] [PubMed] [Google Scholar]
- 12.Yamauchi T, Hara K, Kubota N, et al. Dual roles of adiponectin/Acrp30 in vivo as an anti-diabetic and anti-atherogenic adipokine. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3:243–54. doi: 10.2174/1568008033340090. [DOI] [PubMed] [Google Scholar]
- 13.Koerner A, Kratzsch J, Kiess W. Adipocytokines: leptin--the classical, resistin--the controversical, adiponectin--the promising, and more to come. Best Pract Res Clin Endocrinol Metab. 2005;19:525–46. doi: 10.1016/j.beem.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–7. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 15.Lindsay RS, Resnick HE, Zhu J, et al. Adiponectin and coronary heart disease: the Strong Heart Study. Arterioscler Thromb Vasc Biol. 2005;25:e15–6. doi: 10.1161/01.ATV.0000153090.21990.8c. [DOI] [PubMed] [Google Scholar]
- 16.Lawlor DA, Davey Smith G, Ebrahim S, Thompson C, Sattar N. Plasma adiponectin levels are associated with insulin resistance, but do not predict future risk of coronary heart disease in women. J Clin Endocrinol Metab. 2005;90:5677–83. doi: 10.1210/jc.2005-0825. [DOI] [PubMed] [Google Scholar]
- 17.Dekker JM, Funahashi T, Nijpels G, et al. Adiponectin is associated with CVD risk profile but does not predict CVD mortality: The Hoorn Study. Abstract 1437-P.Annual Meeting of the American Diabetes Association; San Diego, CA. June 2005; [Google Scholar]
- 18.Laughlin GA, Barrett-Connor E, May S. Sex-specific determinants of adiponectin in older adults: the role of serum sex hormones. 2nd International Symposium on Triglycerides and HDL: Role in Cardiovascular Disease and the Metabolic Syndrome; New York, NY. July 2005. [Google Scholar]
- 19.Wedick NM, Barrett-Connor E, Knoke JD, Wingard DL. The relationship between weight loss and all-cause mortality in older men and women with and without diabetes mellitus: the Rancho Bernardo study. J Am Geriatr Soc. 2002;50:1810–5. doi: 10.1046/j.1532-5415.2002.50509.x. [DOI] [PubMed] [Google Scholar]
- 20.The hypertension detection and follow-up program: Hypertension detection and follow-up program cooperative group. Prev Med. 1976;5:207–15. doi: 10.1016/0091-7435(76)90039-6. [DOI] [PubMed] [Google Scholar]
- 21.Friedwald W, Levy R, Frederickson D. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of preparative ultracentrifuge. Clin Chem. 1972:459–502. [PubMed] [Google Scholar]
- 22.Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva: World Health Organization; 1999. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications: Report of a WHO Consultation. [Google Scholar]
- 23.National Cholesterol Education Program (NCEP) Expert Panel on Detection E, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–421. [PubMed] [Google Scholar]
- 24.Hosmer DW, Lemeshow S. Applied Logistic Regression. New York, NY: John Wiley & Sons Inc; 2000. [Google Scholar]
- 25.May S, Hosmer DW. A simplified method of calculating an overall goodness-of-fit test for the Cox proportional hazards model. Lifetime Data Anal. 1998;4:109–20. doi: 10.1023/a:1009612305785. [DOI] [PubMed] [Google Scholar]
- 26.Kubota N, Terauchi Y, Yamauchi T, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–6. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 27.Maeda N, Shimomura I, Kishida K, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–7. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 28.Schneider JG, von Eynatten M, Schiekofer S, Nawroth PP, Dugi KA. Low plasma adiponectin levels are associated with increased hepatic lipase activity in vivo. Diabetes Care. 2005;28:2181–6. doi: 10.2337/diacare.28.9.2181. [DOI] [PubMed] [Google Scholar]
- 29.Kato H, Kashiwagi H, Shiraga M, et al. Adiponectin acts as an endogenous antithrombotic factor. Arterioscler Thromb Vasc Biol. 2006;26:224–30. doi: 10.1161/01.ATV.0000194076.84568.81. [DOI] [PubMed] [Google Scholar]
- 30.Wei EK, Giovannucci E, Fuchs CS, Willett WC, Mantzoros CS. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J Natl Cancer Inst. 2005;97:1688–94. doi: 10.1093/jnci/dji376. [DOI] [PubMed] [Google Scholar]
- 31.Ishikawa M, Kitayama J, Kazama S, Hiramatsu T, Hatano K, Nagawa H. Plasma adiponectin and gastric cancer. Clin Cancer Res. 2005;11:466–72. [PubMed] [Google Scholar]
- 32.Dal Maso L, Augustin LS, Karalis A, et al. Circulating adiponectin and endometrial cancer risk. J Clin Endocrinol Metab. 2004;89:1160–3. doi: 10.1210/jc.2003-031716. [DOI] [PubMed] [Google Scholar]
- 33.Mantzoros C, Petridou E, Dessypris N, et al. Adiponectin and breast cancer risk. J Clin Endocrinol Metab. 2004;89:1102–7. doi: 10.1210/jc.2003-031804. [DOI] [PubMed] [Google Scholar]
- 34.Kistorp C, Faber J, Galatius S, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–62. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 35.Kragelund C, Kober L, Flyvbjerg A, Frystyk J, Steffensen R, Hildebrandt P. Adiponectin predicts mortality in stable coronary heart disease. Abstract 2763.Scientific sessions of the American Heart Association; Dallas, TX. November 2005; [Google Scholar]
- 36.Zoccali C, Mallamaci F, Tripepi G, et al. Adiponectin, metabolic risk factors, and cardiovascular events among patients with end-stage renal disease. J Am Soc Nephrol. 2002;13:134–41. doi: 10.1681/ASN.V131134. [DOI] [PubMed] [Google Scholar]
- 37.Efstathiou SP, Tsioulos DI, Tsiakou AG, Gratsias YE, Pefanis AV, Mountokalakis TD. Plasma adiponectin levels and five-year survival after first-ever ischemic stroke. Stroke. 2005;36:1915–9. doi: 10.1161/01.STR.0000177874.29849.f0. [DOI] [PubMed] [Google Scholar]
- 38.Ahima RS. Central actions of adipocyte hormones. Trends Endocrinol Metab. 2005;16:307–13. doi: 10.1016/j.tem.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Qi Y, Takahashi N, Hileman SM, et al. Adiponectin acts in the brain to decrease body weight. Nat Med. 2004;10:524–9. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 40.Gavrila A, Peng CK, Chan JL, Mietus JE, Goldberger AL, Mantzoros CS. Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J Clin Endocrinol Metab. 2003;88:2838–43. doi: 10.1210/jc.2002-021721. [DOI] [PubMed] [Google Scholar]
- 41.Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS. Independent Circadian And Sleep/Wake Regulation Of Adipokines And Glucose In Humans. J Clin Endocrinol Metab. 2005 doi: 10.1210/jc.2004-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yildiz BO, Suchard MA, Wong ML, McCann SM, Licinio J. Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc Natl Acad Sci U S A. 2004;101:10434–9. doi: 10.1073/pnas.0403465101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pischon T, Hotamisligil G, Rimm E. Adiponectin: stability in plasma over 36 hours and within-person variation over 1 year. Clin Chem. 2003;49:650–2. doi: 10.1373/49.4.650. [DOI] [PubMed] [Google Scholar]


