Abstract
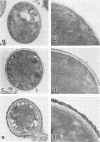
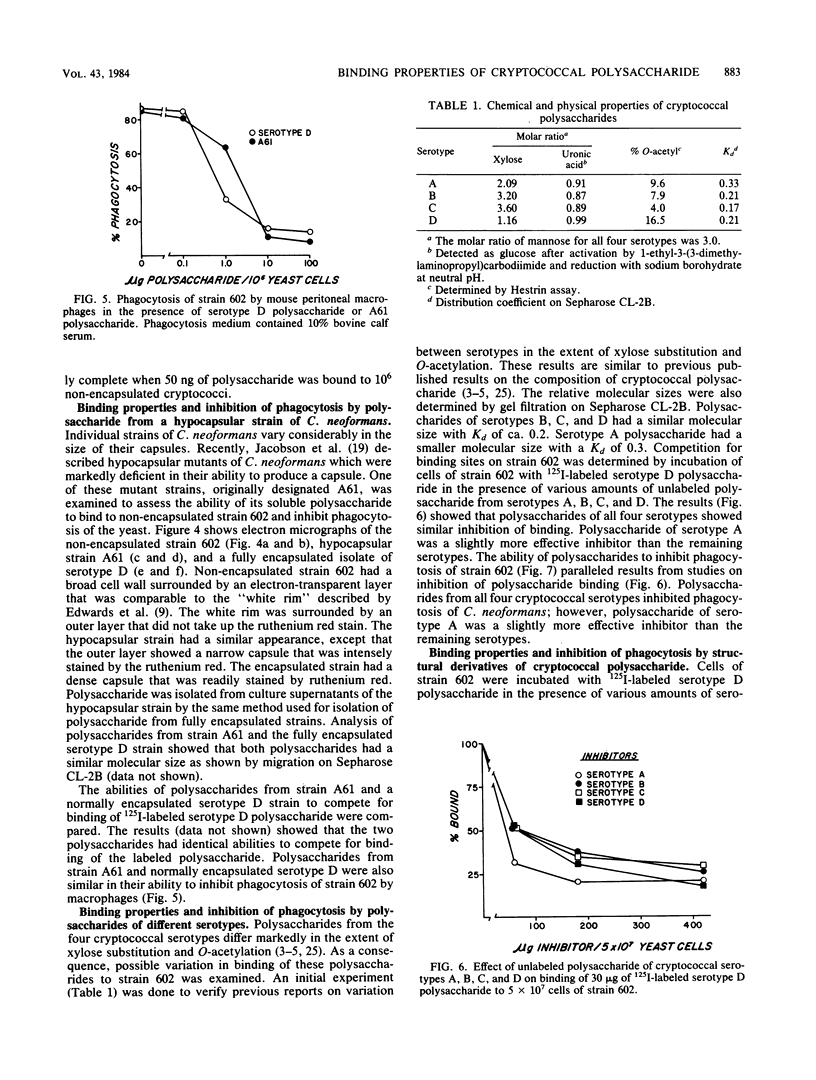
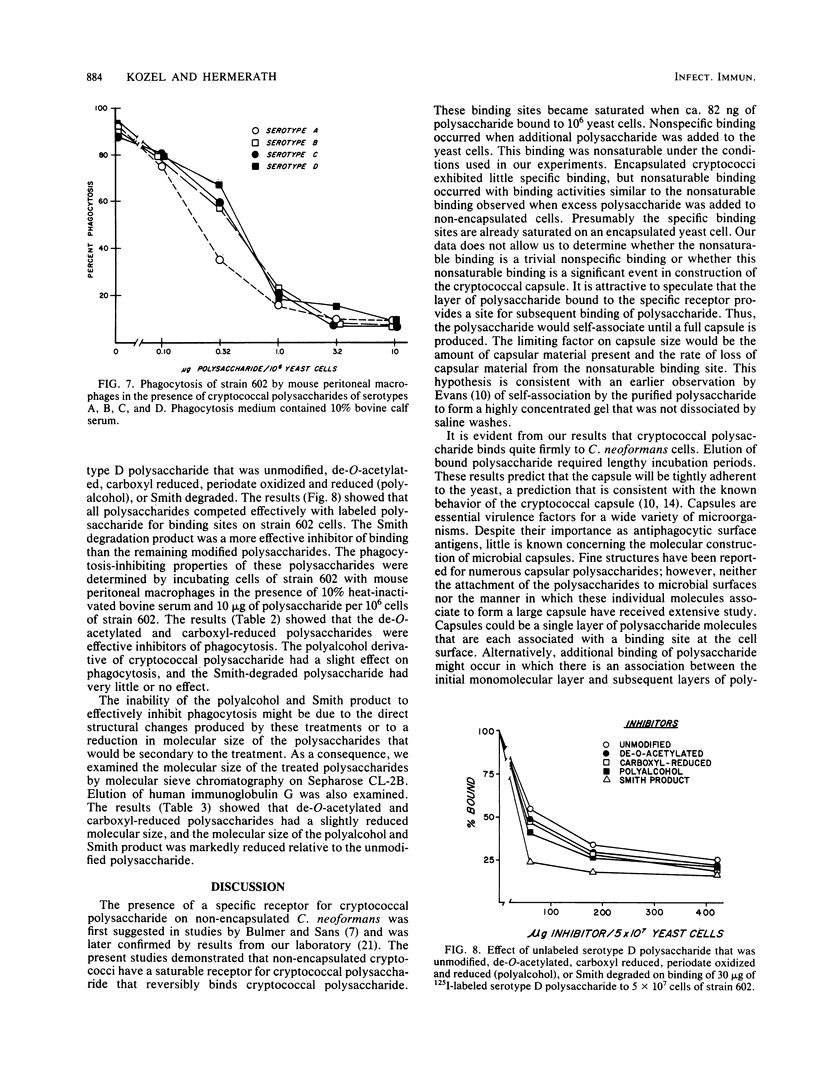
Radioiodinated cryptococcal polysaccharide was used to study binding of the soluble polysaccharide to encapsulated and non-encapsulated cryptoccoci. Binding of polysaccharide to non-encapsulated cryptococci occurred rapidly over a 30-min period and was largely complete after 2 h. Bound, labeled polysaccharide was slowly eluted from Cryptococcus neoformans after the addition of unlabeled polysaccharide, indicating reversibility of binding. Non-encapsulated cryptococci bound polysaccharide in two ways. Specific binding to the yeast was saturable by ca. 82 ng of polysaccharide per 10(6) yeast cells. Nonspecific binding also occurred which was not saturable under the conditions used in our experiments. Phagocytosis of the non-encapsulated yeast strain was inhibited when the specific binding was ca. 50% saturated. Binding of polysaccharide to an encapsulated strain showed nonspecific, nonsaturable binding, but little specific binding occurred. Presumably the specific binding sites were saturated in the encapsulated strain. Polysaccharides obtained from a hypocapsular mutant (A61) and a normally encapsulated strain competed effectively with labeled serotype D polysaccharide for binding sites on non-encapsulated cryptococci and had identical phagocytosis-inhibiting properties. Similarly, polysaccharides from all four cryptococcal serotypes competed effectively with labeled serotype D polysaccharide for binding sites on the non-encapsulated strain, and all four polysaccharides inhibited phagocytosis of non-encapsulated Cryptococcus neoformans. Unmodified, de-O-acetylated, carboxyl-reduced, periodate-oxidized and reduced (polyalcohol), and Smith-degraded polysaccharides competed with labeled polysaccharide for binding sites on the cell. The unmodified, de-O-acetylated and carboxyl-reduced polysaccharides inhibited phagocytosis of non-encapsulated cells, but the polyalcohol and Smith product were unable to inhibit phagocytosis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend W. P., Mannik M. The macrophage receptor for IgG: number and affinity of binding sites. J Immunol. 1973 Jun;110(6):1455–1463. [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A. K., Kwon-Chung K. J., Glaudemans C. P. On the structure of the capsular polysaccharide from Cryptococcus neoformans serotype C. Immunochemistry. 1978 Sep;15(9):673–679. doi: 10.2196/40846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee A. K., Kwon-Chung K. J., Glaudemans C. P. Structural studies on the major, capsular polysaccharide from Cryptococcus bacillisporus serotype B. Carbohydr Res. 1980 Jun;82(1):103–111. doi: 10.1016/s0008-6215(00)85524-x. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee A. K., Kwon-Chung K. J., Glaudemans C. P. The structure of the capsular polysaccharide from Cryptococcus neoformans serotype D. Carbohydr Res. 1979 Aug;73:183–192. doi: 10.1016/s0008-6215(00)85488-9. [DOI] [PubMed] [Google Scholar]
- Brandt J., Andersson L. O., Porath J. Covalent attachment of proteins to polysaccharide carriers by means of benzoquinone. Biochim Biophys Acta. 1975 Mar 28;386(1):196–202. doi: 10.1016/0005-2795(75)90259-7. [DOI] [PubMed] [Google Scholar]
- Bulmer G. S., Sans M. D. Cryptococcus neoformans. 3. Inhibition of phagocytosis. J Bacteriol. 1968 Jan;95(1):5–8. doi: 10.1128/jb.95.1.5-8.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M. R., Gordon M. A., Lapa E. W., Ghiorse W. C. Micromorphology of Cryptococcus neoformans. J Bacteriol. 1967 Sep;94(3):766–777. doi: 10.1128/jb.94.3.766-777.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GADEBUSCH H. H., WARD P. A., FRENKEL E. P. NATURAL HOST RESISTANCE TO INFECTION WITH CRYPTOCOCCUS NEOFORMANS. III. THE EFFECT OF CRYPTOCOCCAL POLYSACCHARIDE UPON THE PHYSIOLOGY OF THE RETICULOENDOTHELIAL SYSTEM OF LABORATORY ANIMALS. J Infect Dis. 1964 Feb;114:95–106. doi: 10.1093/infdis/114.1.95. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Goren M. B., Middlebrook G. M. Protein conjugates of polysaccharide from Cryptococcus neoforman s. J Immunol. 1967 May;98(5):901–913. [PubMed] [Google Scholar]
- Gotschlich E. C., Fraser B. A., Nishimura O., Robbins J. B., Liu T. Y. Lipid on capsular polysaccharides of gram-negative bacteria. J Biol Chem. 1981 Sep 10;256(17):8915–8921. [PubMed] [Google Scholar]
- Grimes W. J., Greegor S. Carbohydrate compositions of normal, spontaneously transformed, and virally transformed cells derived from BALB/c mice. Cancer Res. 1976 Nov;36(11 Pt 1):3905–3910. [PubMed] [Google Scholar]
- Jacobson E. S., Ayers D. J., Harrell A. C., Nicholas C. C. Genetic and phenotypic characterization of capsule mutants of Cryptococcus neoformans. J Bacteriol. 1982 Jun;150(3):1292–1296. doi: 10.1128/jb.150.3.1292-1296.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Cazin J. Nonencapsulated Variant of Cryptococcus neoformans I. Virulence Studies and Characterization of Soluble Polysaccharide. Infect Immun. 1971 Feb;3(2):287–294. doi: 10.1128/iai.3.2.287-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Gotschlich E. C. The capsule of cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J Immunol. 1982 Oct;129(4):1675–1680. [PubMed] [Google Scholar]
- Kozel T. R., Mastroianni R. P. Inhibition of phagocytosis by cryptococcal polysaccharide: dissociation of the attachment and ingestion phases of phagocytosis. Infect Immun. 1976 Jul;14(1):62–67. doi: 10.1128/iai.14.1.62-67.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R. Non-encapsulated variant of Cryptococcus neoformans. II. Surface receptors for cryptococcal polysaccharide and their role in inhibition of phagocytosis by polysaccharide. Infect Immun. 1977 Apr;16(1):99–106. doi: 10.1128/iai.16.1.99-106.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross A., Taylor I. E. Extracellular glycoprotein from virulent and avirulent Cryptococcus species. Infect Immun. 1981 Mar;31(3):911–918. doi: 10.1128/iai.31.3.911-918.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. L., Conrad H. E. Stoichiometric depolymerization of polyuronides and glycosaminoglycuronans to monosaccharides following reduction of their carbodiimide-activated carboxyl groups. Biochemistry. 1972 Apr 11;11(8):1383–1388. doi: 10.1021/bi00758a009. [DOI] [PubMed] [Google Scholar]
- Ternynck T., Avrameas S. A new method using p-benzoquinone for coupling antigens and antibodies to marker substances. Ann Immunol (Paris) 1976 Mar-Apr;127(2):197–208. [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]