Abstract
Betaine-homocysteine methyltransferase (BHMT) regulates homocysteine levels in the liver. We previously reported that the alteration of BHMT is associated with alcoholic liver steatosis and injury. In this study, we tested whether BHMT protects hepatocytes from homocysteine-induced injury and lipid accumulation. Both BHMT transfectants of HepG2 cells and primary mouse hepatocytes with suppressed BHMT were generated. Comparisons were made between the cell models with respect to their response to homocysteine treatments. Homocysteine metabolism was impaired in HepG2 cells, and the expression of BHMT in HepG2 cells ameliorated the impairment and stabilized the levels of intracellular homocysteine after the addition of exogenous homocysteine. BHMT expression inhibited homocysteine-induced glucose-regulated protein 78 (GRP78) and C/EBP-homologous protein (CHOP) and homocysteine-induced cell death. A betaine treatment protected primary mouse hepatocytes from a homocysteine-induced increase in GRP78 and cell death but not a tunicamycin-induced increase. Homocysteine induced greater CHOP expression (2.7-fold) in BHMT small interfering RNA (siRNA)–transfected cells than in a control (1.9-fold). Homocysteine-induced cell death was increased by 40% in the siRNA-treated cells in comparison with the control. ApolipoproteinB(apoB) expression was higher and triglycerides and cholesterol were lower in HepG2 expressing BHMT. In primary mouse hepatocytes, homocysteine induced the accumulation of triglycerides and cholesterol, which was reduced in the presence of betaine. Betaine partially reduced homocysteine-induced sterol regulatory element binding protein 1 expression in HepG2 cells and increased S-adenosylmethionine in primary mouse hepatocytes.
Conclusion
The BHMT/betaine system directly protects hepatocytes from homocysteine-induced injury but not tunicamycin-induced injury, including an endoplasmic reticulum stress response, lipid accumulation, and cell death. This system also exhibits a more generalized effect on liver lipids by inducing ApoB expression and increasing S-adenosylmethionine/ S-adenosylhomocysteine.
Betaine-homocysteine methyltransferase (BHMT) is a cytosolic zinc metalloenzyme that is highly expressed in the liver and kidneys.1–6 BMHT catalyzes methyl transfer from betaine, a product of choline oxidation, to homocysteine, yielding methionine and N,N-dimethylglycine. Homocysteine remethylation is also catalyzed by a cobalamin-dependent enzyme, methionine synthase (MS), with 5-methylfolate as a cosubstrate supplied by 5,10-methylenetetrahydrofolate reductase. Both BHMT and MS have a low Michaelis-Menten constant (Km) for homocysteine. Elevated S-adenosylmethionine (SAM), resulting from a methionine excess, inhibits BHMT and the formation of 5-methyltetrahydrofolate catalyzed by 5,10-methylenetetrahydrofolate reductase.7 Hence, homocysteine remethylation is predominant at low levels of homocysteine and methionine. At high levels, SAM stimulates 2 high-Km enzymes, methionine adenosyltransferase-III and cystathionine β-synthase (CBS). The latter converts homocysteine toward the transsulfuration pathway for the production of cysteine.8,9 Thus, BHMT is a component of the methionine cycle, and when folate-dependent methionine synthesis is impaired by either genetic or environmental factors (for example, a chronic alcohol treatment), the BHMT/betaine system plays a critical role in homocysteine homeostasis.10
Impaired BHMT results in elevated homocysteine levels and could contribute to the risk for vascular, hepatic, and neurological diseases.4,10–13 Betaine supplementation ameliorates the biochemical abnormalities and the clinical course in homocystinuria due to a deficiency of CBS or to several remethylation defects.14 We and others have previously observed that betaine supplementation protects against alcohol-induced fatty liver and endoplasmic reticulum (ER) stress and, at the same time, prevents alcohol-induced hyperhomocysteinemia.15–20 Other potential mechanisms of protection by betaine may contribute to the amelioration of an alcoholic fatty liver/injury, including increasing the SAM to S-adenosylhomocysteine (SAH) ratio and phosphatidylethanolamine methyltransferase activity and acting as a molecular chaperone. In addition, the expression of apolipoprotein B (ApoB) is increased in McArdle RH-7777 expressing BHMTand in rat livers following the in vivo induction of BHMT,21–24 which could increase triglyceride mobilization/secretion to minimize fatty liver. In the liver, BHMT is responsible for 50% of the homocysteine remethylation.3–5,25 A severe reduction of BHMT messenger RNA(mRNA) was found in 90% of patients with hepatitis C virus–induced cirrhosis and in about 50% of patients with chronic alcohol– induced cirrhosis.26
In order to investigate the direct role of the BHMT/betaine system in liver steatosis and injury, we generated BHMT transgenic cell models and silenced BHMT expression in primary mouse hepatocytes. We compared the response of the transgenic models and wild type to homo-cysteine challenge versus other inducers of ER stress and found that BHMT/betaine protected specifically against homocysteine-induced ER stress and cell death in the hepatocytes and decreased hepatocellular lipids, which correlated with decreased sterol regulatory element binding protein 1 (SREBP-1) induction, increased ApoB expression, and restored SAM/SAH.
Materials and Methods
Cloning of Human BHMT and Its Expression in HepG2 Cells
Plasmid Construction
An open reading frame of human BHMT (GeneBank accession number U50929) was blunt-ended and subcloned into the expression vector pcDNA3.1 (Invitrogen, Carlsbad, CA), which contained the cytomegalovirus promoter for constitutive expression in mammalian cell lines and the Zeocin resistance gene for the selection and creation of stable cell lines. The pcDNA3.1-BHMT vector was confirmed with BigDye Terminator sequencing chemistry and maintained and amplified in E. coli. The plasmid DNA was isolated and purified with a QIAwell plasmid purification system (Qiagen, Valencia, CA).
BHMT Expression in HepG2
The calcium phosphate transfection kit from Invitrogen was used to transfect HepG2 cells. Briefly, HepG2 cells were plated in a 60-mm dish (5×105 cells/dish) and incubated at 37°C in a humidified CO2 incubator overnight. Dulbecco’s modified Eagle’s medium (high glucose) from Gibco was used, and it contained 10% fetal bovine serum, 1% penicillin-streptomycin-neomycin antibiotics, 1% pyruvate, 0.5% (wt/vol) betaine, and 1% nonessential amino acids. The medium in the dish was changed 4 hours prior to the transfection. A transfection mixture (300 µL in all) was first prepared that contained 18 µL of 2 M CaCl2, 10 µg of pcDNA3.1-BHMT or a vector used only as a mock control, 122 µL of H2O, and 150 µL of 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid– buffered saline. The mixture was then added dropwise to the HepG2 cells, which continued to be incubated overnight at 37°C in a humidified C2O incubator in the 60-mm dish. The cells were washed the following day with 1× phosphate-buffered saline and incubated with 2 mL of a fresh 15% glycerol stock solution in 1× 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid– buffered saline for exactly 2 minutes. Fresh medium was added to the dish, and the cells were incubated for another 48 hours. The medium was then replaced, and the cells were maintained in a selective medium containing the antibiotic Zeocin (500 µg/mL; Invitrogen) for 2 weeks. Zeocin eliminated pcDNA3.1-BHMT–negative HepG2 cells, and pcDNA3.1-BHMT integrants [HepG2 cells transfected with a betaine-homocysteine methyltransferase expression vector (HepG2-BHMT cells)] were obtained by the examination of the expression of BHMT.
ER Stress Induction and Cell Death Assessment
The isolation and culturing of mouse primary hepatocytes were described previously.18,19 Primary human hepatocytes were provided by Cell Culture Core (Gastroenterology/ Liver Division, Keck School of Medicine, University of Southern California). The primary hepatocytes, HepG2 and HepG2-BHMT cells, and Huh-7 cells were plated in a 60-mm dish (1.2 × 106 cells/dish) and treated with the ER stress–inducing agent tunicamycin (10 µg/mL, Sigma) or homocysteine (2–10 mM) for 24–36 hours. The range of homocysteine concentrations was selected on the basis of publications by others16 and our preliminary tests. Homocysteine at concentrations lower than 2 mM did not induce a detectable ER stress response in a western blot analysis. Homocysteine at concentrations higher than 10 mM was toxic and caused the detachment of the cultured cells during a period of 24 hours. The treated cells were either washed 3 times with cold 1× phosphate-buffered saline and scratched off for the extraction of DNA, RNA, and proteins or stained for cell death. The cells were doubly stained with Sytox green (1 µM; Molecular Probes, Eugene, OR) and Hoechst 33258 dye (8 µg/ mL; Sigma) for 30 minutes at 37°C. Cell death (a combination of necrosis and apoptosis) was counted according to a previously described method.24
Transfection of Hepatocytes with BHMT Small Interfering RNA (siRNA)
RNAiFect transfection kits from Qiagen were used with modification for the suppression of BHMT expression in vitro. Briefly, 4×105 mouse primary hepatocytes per well were seeded in a 6-well plate in a 1.9-mL medium. Transfection complexes of 200 µL containing 5 µg of BHMT siRNA (double strands) were added dropwise onto the cells, and the plate was gently swirled to ensure a uniform distribution of the transfection complexes. The cells were then incubated for 24–48 hours. The forward siRNA sequence was 5′-GUGAAGACAAGCUGGAAAAd(TT)-3′, and the reverse RNA sequence was 3′-d(TT) CACUUCUGUUCGACCUUUU-5′, which targeted the BHMT sequence of AAGTGAAGACAAGCTGGAAAA. siRNA without any known adverse effects (provided by Qiagen) was used as a negative control.
RNA Isolation and Analysis
The total RNA was isolated from the cultured hepatocytes (1.2 × 106 cells) with the TRIzol reagent from Invitrogen and purified with the RNeasy mini kit from Qiagen according to the manufacturer’s instructions and with the addition of 500 units of a ribonuclease inhibitor (RNAguard, Amersham Pharmacia Biotech) to the starting materials. RNA was stored at−80°C until use. The Qiagen OneStep RT-PCR kit was used for reverse transcription-PCR. The primer sequences were as follows: mouse apolipoprotein (ApoB), TCAAGGACGCAAAGGCAGAA and GCACTAACTTGTATGAAGGCACC; BHMT, GCGTGAGCCAGACGCCTTCATACCTTAG and CCTTTCTGGGGCCAACTCCTCTGCAATC; MS, CCGAGGGATGGAAGCCATTCGAGAAGCA and GTGGCCAACAGCCTTCTTCATGACACGG; CBS, GGACCTCCCAGAGTATCCCTTTGGCCT and CCAATGACCTTCAAATGGAGGCCCGTGG; SREBP1, ACACTCAGCAGCCACCATCTA and TCTCCACCACTTCGGGTTTCA; human ApoA1, TGCTCAAAGACAGCGGCAGA and ATCTCCTCCTGCCACTTCT; human ApoB, AACTTCTTCCACGAGTCGGG and AGAAGCAGTTTGGCAGGCGA; human ApoE, GCGTTGCTGGTCACATTCCT and AGTGATTGTCGCTGGGCACA; human microsomal triglyceride transfer protein, TCAGCAGAGAGGAGAGAAGAGC and CAAATCCACAGGCATAGAGAAACC; human BHMT, CTGTGTGGGCAGTTGAAACC and TGCTGCTCAGTTGTGGCTTC; human CBS, GCGGCTGAAGAACGAAATCC and GCGTCACCATTCCCAGGATT; and human MS, ATGTCACCCGCG CTCCAAGAC and TCCAGAAGTCCTTTGGCCTGC. The polymerase chain reaction optimal cycle number for each gene was predetermined to obtain a detectable but nonsaturating polymerase chain reaction product. The relative expression was normalized to the expression of β-actin or 18S in that same sample and compared with the control.
Protein Extraction and Analysis
Proteins were extracted according to the method previously reported.18 Nuclear proteins were extracted with a nuclear extract kit from Active Motif (Carlsbad, CA). Proteins were routinely analyzed through immunoblotting with the horseradish peroxidase conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) or alkaline phosphatase–labeled (Cell Signaling Technology, Danvers, MA). Proteins were visualized with LumiGLO reagent (Cell Signaling Technology) on CL-Xposure films (Pierce, Rockford, IL) at an optimized time. Antibodies against ApoB, glucose-regulated protein 78 (GRP78), SREBP-1, nuclear factor kappa B (NF-κB) p65, NF-κB p50, and C/EBP-homologous protein (CHOP) were purchased from Santa Cruz Biotechnology. The intensity of the protein bands on the western blots was quantified with ImageQuant version 5.2 (Molecular Dynamics, Sunnyvale, CA). Polyclonal rabbit anti- BHMT antibodies were generated with an antigenic peptide with a sequence of SEDKLENRGNYVLEKI. The BHMT antibodies were purified with a protein A antibody purification kit (Sigma).
Lipid Extraction and Analysis
Lipids from 106 cells were extracted with 200 µL of chloroform-methanol (2:1). The extraction mixture was spun for 10 minutes at the top speed in a micro-centrifuge tube. The organic phase was collected and vacuum-dried. The dried lipids were dissolved in 20 µL of 2-propanol containing 10% Triton X-100 as assay samples. Triglycerides were determined with a Sigma Diagnostics triglyceride reagent, and cholesterol was determined with a cholesterol quantitation kit from BioVision (Mountain View, CA).
Statistical Analysis
The studies were replicated 2–4 times, with the data shown as the means ± the standard error of the mean. A statistical analysis was performed with a Student t test for unpaired data or an analysis of variance and post hoc Tukey-Kramer multiple-comparison test. A P value lower than 0.05 was considered significant.
Results
BHMT Corrects the Impairment of Homocysteine Metabolism in Hepatoma Cells
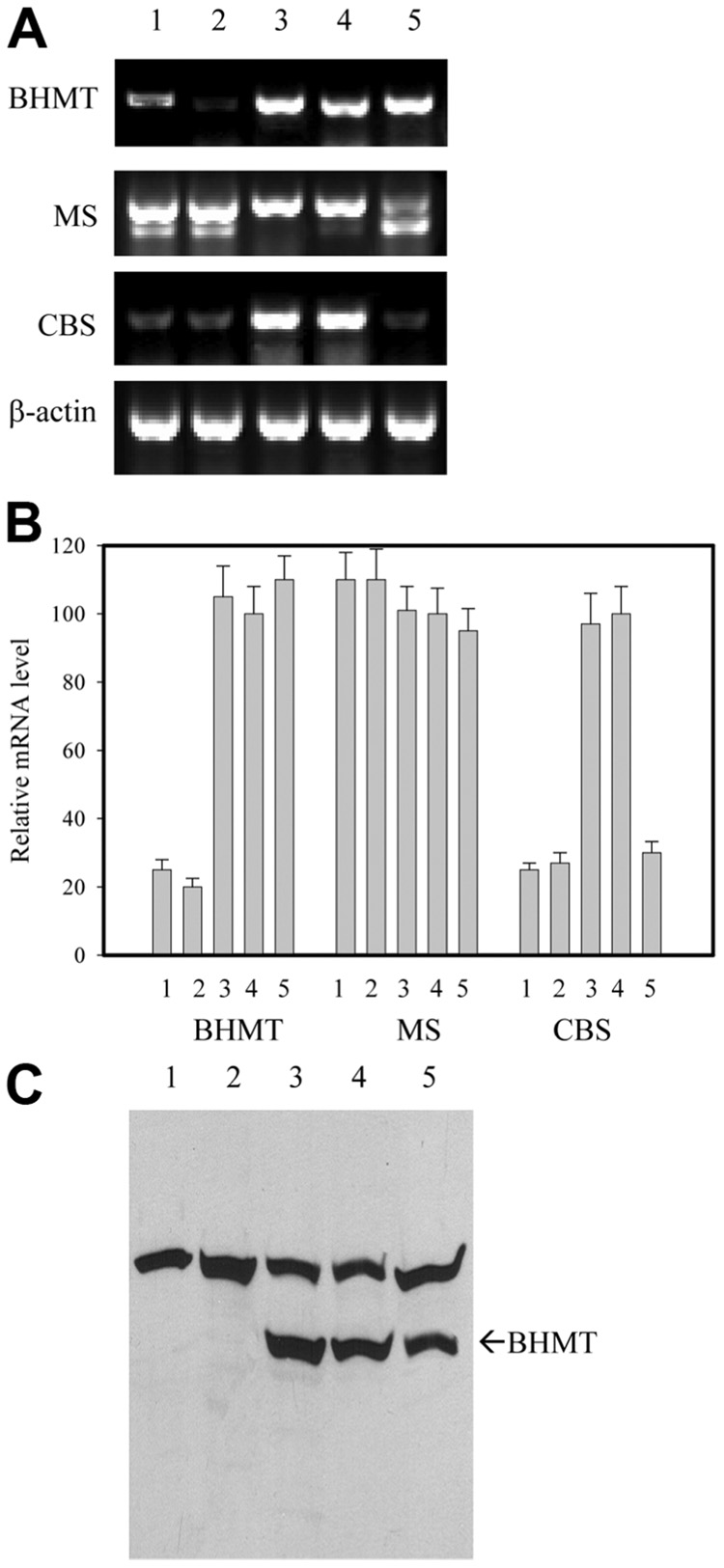
The endogenous BHMT mRNA expression in the HepG2 and Huh-7 cells was less than 30% of the expression in primary human hepatocytes (Fig. 1A,B). In the HepG2-BHMT cells, the BHMT expression at both the mRNA and protein levels was restored to levels comparable to those of primary human and mouse hepatocytes (Fig. 1). The expression of BHMT did not alter the endogenous expression of MS, which was not significantly altered in any of the cell types tested, or the expression of CBS, which was impaired in the hepatoma cell lines. There was no significant difference in the basal levels of intracellular homocysteine in the control HepG2 and HepG2-BHMT cells (Fig. 2A). The addition of exogenous homocysteine (2 mM or 5 mM) to the cell culture transiently increased the intracellular homocysteine in both cell types over a period of 24 hours (Fig. 2). The peak values of intracellular homocysteine were lower and declined faster in the HepG2-BHMT cells than in the control HepG2 cells (27 ± 3.5 nmol/mg of protein). The addition of betaine reduced further the peak values in the HepG2- BHMT cells but not in the control HepG2 cells, and this indicated that the homocysteine metabolism was impaired in the control HepG2 cells and that the expression of BHMT in HepG2 ameliorated the impairment. Primary mouse hepatocytes had comparable basal homocysteine levels but exhibited less homocysteine accumulation after the addition of 2 or 5 mM homocysteine (Fig. 2C). Interestingly, despite the comparable expression of BHMT between primary mouse hepatocytes and HepG2-BHMT, the former accumulated less homocysteine, particularly upon exposure to the high concentration (5 mM) of homocysteine and in the presence of betaine. This could be due to the CBS pathway, but we cannot exclude a difference in betaine production or availability in primary mouse hepatocytes versus HepG2 cells.
Fig. 1. Expression of BHMT in hepatocytes.
(A) RT-PCR analysis of mRNA, (B) relative mRNA levels normalized to β-actin, and (C) western blotting with anti-BHMT antiserum. The samples were from (1) HepG2, (2) Huh-7, (3) primary mouse hepatocytes, (4) primary human hepatocytes, and (5) HepG2 transfected with a BHMT expression vector. Abbreviations: BHMT, betaine-homocysteine methyltransferase; CBS, cystathionine β-synthase; mRNA, messenger RNA; MS, methionine synthase.
Fig. 2. Effects of transgenic BHMT on intracellular homocysteine levels.
(A) basal levels of intracellular homocysteine, (B) time course of intracellular homocysteine levels after the addition of homocysteine and/or betaine (1 mM), and (C) time course of intracellular homocysteine levels in primary mouse hepatocytes after the addition of homocysteine and/or betaine. Abbreviations: BHMT, betaine-homocysteine methyltransferase; HepG2-BHMT, HepG2 transfected with a betaine-homocysteine methyltransferase expression vector.
BHMT Protects Cells from Homocysteine-Induced ER Stress and Cell Death
In order to determine whether transgenic BHMT protected HepG2 cells from homocysteine-induced ER stress and cell death, we challenged the HepG2-BHMT cells, the control HepG2 cells, and the primary mouse hepatocytes with tunicamycin (ER stress–inducing agent) or homocysteine. The induction of GRP78 and CHOP by tunicamycin was observed in all cell types (Fig. 3A). The induction of GRP78 by homocysteine was moderate. The induction of CHOP by homocysteine occurred in the control HepG2 cells and was comparable to the induction by tunicamycin. However, the induction of CHOP by homocysteine was less in the HepG2-BHMT cells. The transgenic BHMT reduced the homocysteine-induced CHOP by 50%. Tunicamycin induced 25% cell death in all cell types. Homocysteine-induced cell death was less in the HepG2-BHMT cells (11%±3%) and in the primary mouse hepatocytes (9% ± 3%) than in the control HepG2 cells (18% ± 4%), and this indicated that the expression of BHMT partially protected hepatocytes from a homocysteine-induced ER stress response. The betaine treatment significantly reduced homocysteine-induced GRP78 expression in the primary mouse hepatocytes (Fig. 3B).
Fig. 3. Effects of betaine and BHMT on homocysteine-induced ER stress.
(A) Protein levels normalized to β-actin. αP < 0.05 versus the control; β P < 0.05 versus wild-type HepG2. (B) Western blot of samples from primary mouse hepatocytes. Betaine was added 1 hour before ER stress induction; the bar graph shows the quantitation of GRP78 protein levels. *P < 0.05 and **P < 0.01 versus the control; #P < 0.05 versus the absence of betaine. The betaine concentration was 1 mM. Abbreviations: BHMT, betaine-homocysteine methyltransferase; C, control; CHOP, C/EBP homologous protein; ER, endoplasmic reticulum; GRP78, glucose-regulated protein 78; Hcy, homocysteine (10 mM); HepG2-BHMT, HepG2 transfected with a betaine-homocysteine methyltransferase expression vector; Tm, tunicamycin (10 µg/mL).
The suppression of BHMT in the primary mouse hepatocytes through RNA interference potentiated the homocysteine-induced ER stress response. BHMT siRNA transfection reduced BHMT mRNA by 45% (Fig.4A). The homocysteine treatment induced CHOP expression by 2.7-fold in the BHMT siRNA–transfected cells but only by 1.9-fold in control small interfering RNA (csiRNA)–treated cells (Fig. 4B). Homocysteine-induced cell death was also increased in siRNA-treated cells versus csiRNA-treated cells (Fig. 4C). Tunicamycin-induced CHOP expression and cell death in the siRNA-treated cells were not different from those of the csiRNA-treated cells.
Fig. 4. Effects of the suppression of BHMT by siRNA on the Hcy-induced ER stress response and cell death in primary hepatocytes.
(A) RT-PCR of BHMT expression. (B) Western blots and quantitation (right panel). The cells were treated with Tm (10 µg/mL) or Hcy (10 mM) 24 hours after siRNA transfection. (C) Quantitation of cell death. *P < 0.05 versus csiRNA. Abbreviations: BHMT, betaine-homocysteine methyltransferase; CHOP, C/EBP-homologous protein; csiRNA, control small interfering RNA (that is, no suppression effects); Hcy, homocysteine; NC, control cells without any small interfering RNA or drug treatments; siRNA, small interfering RNA; Tm, tunicamycin.
Expression of BHMT Increases the ApoB Expression and Reduces the Lipid Content
To determine the effects of transgenic BHMT on apolipoproteins, which are important not only in maintaining the structural integrity of lipoproteins but also in facilitating the solubilization and transport of lipids, we examined the expression of ApoA1, ApoB, ApoE, and microsomal triglyceride transfer protein at mRNA levels. No significant difference was found in the expression of ApoA1, ApoE, and microsomal triglyceride transfer protein between control HepG2 and HepG2-BHMT cells or in the presence of homocysteine (Fig. 5A). However, there was an increase in ApoB expression in HepG2- BHMT cells (Fig. 5B). Remarkably, in comparison with wild-type HepG2, the level of ApoB was nearly doubled in HepG2-BHMT cells treated with betaine and homocysteine. In primary mouse hepatocytes, the homocysteine treatment reduced the expression of ApoB, which was recovered in the presence of betaine (Fig. 5B), and in parallel, homocysteine reduced BHMT expression, which was restored by betaine (Fig. 5C); this indicated that the expression of ApoB correlated with the expression of BHMT. There was a trend in which betaine plus homocysteine increased BHMT expression. At present, we do not understand the mechanism for this increase. In addition, homocysteine did not have a consistent effect on NF-κB (p65 or p50; data not shown).
Fig. 5. Expression of apolipoproteins.
(A) RT-PCR analysis of ApoA1, ApoB, ApoE, and MTTP. (B) Relative levels of mRNA of ApoB. αP < 0.05 versus the control; βP < 0.05 versus the absence of betaine; γP < 0.05 versus the wild type. (C) Expression of BHMT in mouse primary hepatocytes. Abbreviations: ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; ApoE, apolipoprotein E; BHMT, betaine-homocysteine methyltransferase; C, control; H, homocysteine (10 mM); HepG2-BHMT, HepG2 transfected with a betainehomocysteine methyltransferase expression vector; MTTP, microsomal triglyceride transfer protein; T, tunicamycin (8 µg/mL).
In parallel with the increased expression of ApoB, the basal levels of lipids were somewhat lower in HepG2-BHMT cells in comparison with the wild type, being significant for cholesterol and having a trend for triglycerides (Fig. 6). The betaine treatment in the absence of tunicamycin and homocysteine caused a slight further reduction in both lipids in the transgenic cells but had no effect on lipids in the wild type, which also correlated with a further increase in ApoB expression (Fig. 5B). Homocysteine caused a significant and similar proportional increase in cholesterol and triglyceride in the cell types in comparison with the control levels, and tunicamycin caused a similar proportional increase in cholesterol with a trend of increasing triglycerides, which was not statistically significant. However, the betaine treatment prevented the increase in both cholesterol and triglycerides in HepG2-BHMT cells but not the wild type. In primary cells, there was a trend for increased triglycerides and cholesterol in response to tunicamycin and a significant increase in triglycerides in response to homocysteine, but betaine blunted these changes only in the homocysteine-treated cells that expressed BHMT (Fig. 6B,D).
Fig. 6. Effects of betaine on intracellular triglycerides and cholesterol.
(A,B) intracellular levels of triglycerides and (C,D) intracellular levels of cholesterol. αP < 0.05 versus the control; βP < 0.05 versus the absence of betaine; γP < 0.05 versus the wild type (n = 3). Abbreviations: C, control; H, homocysteine (5 mM); HepG2-BHMT, HepG2 transfected with a betaine-homocysteine methyltransferase expression vector; T, tunicamycin (8 µg/mL).
There was no difference in the SREBP-1 expression under basal conditions in the wild-type cells versus the BHMT-expressing cells. The SREBP-1 mRNA was increased by homocysteine in the wild type and HepG2- BHMT (Fig. 7). The increase was slightly less in response to tunicamycin, and this might account for a somewhat milder triglyceride increase. Betaine blunted the homocysteine-induced increase in SREBP-1 only when BHMT was expressed. In primary hepatocytes, a similar trend was observed, that is, an increase in SREBP-1 with both ER stressors and protection by betaine only in the case of homocysteine. Of note, the SAM/SAH ratio in the control primary mouse hepatocytes (2.48 ± 0.18) was increased by betaine (3.24 ± 0.17, P < 0.05), decreased by homocysteine (1.39 ± 0.16, P < 0.05), and restored to the control level when betaine was added to homocysteine (2.5 ± 0.32). In addition, there was no difference in the SAM/SAH ratio between the control (0.28 ± 0.04) and betaine-treated HepG2 cells (0.29 ± 0.03). However, the ratio in the HepG2-BHMT cells (0.43 ± 0.09) was increased by betaine (0.68 ± 0.11, P < 0.05), decreased by homocysteine (0.11 ± 0.06, P < 0.05), and restored partially when betaine was added to homocysteine (0.35 ± 0.06). The lower SAM/SAH ratio in the HepG2 cells in comparison with the primary hepatocytes probably reflects the known absence of methionine adenosyltransferase-1A expression in the former.
Fig. 7. Effects of betaine on the mRNA levels of SREBP.
αP < 0.05 versus the control; βP < 0.05 versus the absence of betaine (n = 3). Abbreviations: C, control; H, homocysteine (5 mM); HepG2-BHMT, HepG2 transfected with a betaine-homocysteine methyltransferase expression vector; mRNA, messenger RNA; SREBP, sterol regulatory element binding protein; Tm, tunicamycin (8 µg/mL).
Discussion
Excessive homocysteine has many harmful consequences. 27,28 BHMT catalyzes the remethylation of homocysteine back to methionine and therefore contributes to the regulation of homocysteine levels in the liver. We reported previously that the alteration of BHMT is associated with an alcohol-induced elevation of homocysteine and liver steatosis and injury in a mouse model.19 In this study, we further demonstrated that BHMT protects hepatocytes from homocysteine-induced injury by expressing the BHMT gene in HepG2 cells that have an impaired metabolism of homocysteine. The expression of BHMT in HepG2 ameliorated the impairment of homocysteine metabolism, and this was evidenced by the more quickly stabilized levels of intracellular homocysteine after the addition of exogenous homocysteine in HepG2 expressing BHMT. In particular, the expression of BHMT inhibited a homocysteine-induced increase in the ER stress markers GRP78 and CHOP and cell death. The betaine treatment also protected primary mouse hepatocytes from a homocysteine-induced increase in the GRP78 protein and cell death but not a tunicamycin-induced increase. Furthermore, suppression of BHMT expression in primary mouse hepatocytes with siRNA specifically worsened the homocysteine-induced ER stress response and cell death. The evidence points to an important role of the betaine/BHMT system in maintaining cellular homocysteine and/or methionine homeostasis.
In agreement with the findings by others in cultured rat hepatocytes,21–23 we also detected increased expression of ApoB in HepG2 cells expressing BHMT. How the BHMT expression increases the expression of ApoB is not clear. It is possible that altered BHMT levels of the intracellular SAM and SAM/SAH in BHMT-expressing cells or other unknown metabolites regulate the expression of ApoB. The greater increase in SAM, SAM/SAH, and ApoB by the betaine treatment of BHMT-expressing cells indirectly supports this possibility. Because the BHMT promoter contains regulatory elements for several hormones, including hydrocortisone, cortisol, and triamcinolone,2,3,29 it is also possible that perturbations of hormone homeostasis resulting from the abnormal expression of BHMT may contribute to the up-regulation of ApoB. In addition, betaine/BHMT influences the levels of homocysteine, which inhibit ApoB expression not only transcriptionally, as shown in our experiments, with primary hepatocytes treated with homocysteine but also posttranscriptionally by, for example, ApoB lipidation and very low density lipoprotein assembly and secretion due to the interactive thiol group of homocysteine.30 Thus, the increased expression of ApoB might result from a feedback mechanism. Regardless of how BHMT regulates ApoB, it is known that betaine increases BHMT expression, and this may account for protection by betaine against decreased ApoB in primary mouse hepatocytes. Because some studies have suggested that betaine can protect against bile acid– induced ER stress and liver injury,31 it is conceivable that betaine acts as a chemical chaperone. However, we observed that the protection was specific for homocysteine-induced ER stress and cell death, whereas betaine exerted no protection against tunicamycin-induced effects. Therefore, at least in cell culture models, betaine does not appear to act as a nonspecific chemical chaperone.
An interesting question is how the betaine/BHMT system reduces the accumulation of triglycerides and cholesterol in the hepatocytes. We previously hypothesized that the up-regulation of SREBPs, which are activated by ER stress and regulate the expression of lipogenic genes, contributes to lipid accumulation. 18,20,27 Indeed, both homocysteine and tunicamycin induced a moderate increase in SREBP-1 expression. The betaine/BHMT system lowered intracellular homocysteine and therefore reduced homocysteine- induced ER stress and lipid accumulation. Thus, the data continue to support the involvement of ER stress –induced SREBPs in cellular lipogenesis. However, the current data also suggest that multiple mechanisms may be involved in the lipid reduction by the betaine/BHMT system. For instance, ApoB is not regulated by SREBPs and is involved in lipid secretion, which also contributes to the reduction of lipids inside the hepatocytes. As noted by others, the increased SAM/SAH will enhance phosphatidylethanolamine methyltransferase activity, which contributes to increased lipid secretion.32,33 Also, because SAM affects numerous methylation reactions, including lipogenic genes or proteins, it is conceivable that betaine’s enhancement of SAM also plays an epigenetic role in the reduction of the homocysteine-induced accumulation of triglycerides and cholesterol.
Of note, the treatment of primary mouse hepatocytes with homocysteine decreased the expression of BHMT. Others have shown that increased SAM, mainly via 5-methylthioadenosine (MTA) formation, decreases BHMT expression by activating a repressive effect of NF-κB on the BHMT promoter.34 Because a high level of homocysteine in a culture might increase the MTA level and active NF-κB or directly activate NF-κB (for example, oxidative stress), we examined nuclear p50 and p65 in primary hepatocytes exposed to homocysteine with or without betaine, and we did not find any significant differences between the treatments; this suggests that the down-regulation of BHMT may not involve NF-κB activation. Because a high level of homocysteine induces ER stress, which attenuates protein synthesis,16,19 it is possible that the attenuation of regulatory proteins other than NF-κB down-regulates BHMT. Further work is required to define the mechanism of the down-regulation of BHMT by homocysteine.
In summary, the BHMT/betaine system directly protects hepatocytes from homocysteine-induced injury but not tunicamycin-induced injury, including an ER stress response, lipid accumulation, and cell death. In addition to protecting against the effects of homocysteine, this system may exhibit a more generalized effect on liver lipids by increasing SAM and inducing ApoB expression.
Acknowledgments
Supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases (pilot project P30DK048522-12 to C.J.) and by the U.S. National Institute on Alcohol Abuse and Alcoholism (R01 AA014428-04 to N.K. and C.J. and P50AA11999).
Abbreviations
- Apo
apolipoprotein
- BHMT
betaine-homocysteine methyltransferase
- C
control
- CBS
cystathionine β-synthase
- CHOP
C/EBP-homologous protein
- csiRNA
control small interfering RNA
- ER
endoplasmic reticulum
- GRP78
glucose-regulated protein 78
- H
homocysteine
- Hcy
homocysteine
- HepG2-BHMT
HepG2 transfected with a betaine-homocysteine methyltransferase expression vector
- mRNA
messenger RNA
- MS
methionine synthase
- MTTP
microsomal triglyceride transfer protein
- NC
control cells without any small interfering RNA or drug treatments
- NF-κB
nuclear factor kappa B
- SAH
S-adenosylhomocysteine
- SAM
S-adenosylmethionine
- siRNA
small interfering RNA
- SREBP
sterol regulatory element binding protein
- Tm
tunicamycin
Footnotes
Published online in Wiley InterScience (www.interscience.wiley.com).
Potential conflict of interest: Nothing to report.
References
- 1.Garrow TA. Purification, kinetic properties, and cDNA cloning of mammalian betaine-homocysteine methyltransferase. J Biol Chem. 1996;271:22831–22838. doi: 10.1074/jbc.271.37.22831. [DOI] [PubMed] [Google Scholar]
- 2.Millian NS, Garrow TA. Human betaine-homocysteine methyltransferase is a zinc metalloenzyme. Arch Biochem Biophys. 1998;356:93–98. doi: 10.1006/abbi.1998.0757. [DOI] [PubMed] [Google Scholar]
- 3.Neece DJ, Griffiths MA, Garrow TA. Isolation and characterization of a mouse betaine-homocysteine S-methyltransferase gene and pseudogene. Gene. 2000;250:31–40. doi: 10.1016/s0378-1119(00)00191-8. [DOI] [PubMed] [Google Scholar]
- 4.Finkelstein JD. Methionine metabolism in liver diseases. Am J Clin Nutr. 2003;77:1094–1095. doi: 10.1093/ajcn/77.5.1094. [DOI] [PubMed] [Google Scholar]
- 5.Finkelstein JD. Inborn errors of sulfur-containing amino acid metabolism. J Nutr. 2006;136 suppl 6:S1750–S1754. doi: 10.1093/jn/136.6.1750S. [DOI] [PubMed] [Google Scholar]
- 6.Emmert JL, Webel DM, Biehl RR, Griffiths MA, Garrow LS, Garrow TA, et al. Hepatic and renal betaine-homocysteine methyltransferase activity in pigs as affected by dietary intakes of sulfur amino acids, choline, and betaine. J Anim Sci. 1998;76:606–610. doi: 10.2527/1998.762606x. [DOI] [PubMed] [Google Scholar]
- 7.Finkelstein JD, Martin JJ. Methionine metabolism in mammals. Distribution of homocysteine between competing pathways. J Biol Chem. 1984;259:9508–9513. [PubMed] [Google Scholar]
- 8.Selhub J, Miller JW. The pathogenesis of homocysteinemia: interruption of the coordinate regulation by sadenosylmethionine of the remethylation and transsulfuration of homocysteine. Am J Clin Nutr. 1992;55:131–138. doi: 10.1093/ajcn/55.1.131. [DOI] [PubMed] [Google Scholar]
- 9.Reed MC, Nijhout HF, Sparks R, Ulrich CM. A mathematical model of the methionine cycle. J Theor Biol. 2004;226:33–43. doi: 10.1016/j.jtbi.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Weisberg IS, Park E, Ballman KV, Berger P, Nunn M, Suh DS, et al. Investigations of a common genetic methyltransferase (BHMT) variant in betaine-homocysteine in coronary artery disease. Atherosclerosis. 2003;167:205–214. doi: 10.1016/s0021-9150(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 11.Heil SG, Lievers KJ, Boers GH, Verhoef P, den Heijer M, Trijbels FJ, et al. Betaine-homocysteine methyltransferase (BHMT): genomic sequencing and relevance to hyperhomocysteinemia and vascular disease in humans. Mol Genet Metab. 2000;71:511–519. doi: 10.1006/mgme.2000.3078. [DOI] [PubMed] [Google Scholar]
- 12.Zhu HP, Curry S, Wen S, Wicker NJ, Shaw GM, Lammer EJ, et al. Are the betaine-homocysteine methyltransferase (BHMT and BHMT2) genes risk factors for spina bifida and orofacial clefts? Am J Med Genet A. 2005;135:274–277. doi: 10.1002/ajmg.a.30739. [DOI] [PubMed] [Google Scholar]
- 13.Boyles AL, Billups AV, Deak KL, Siegel DG, Mehltretter L, Slifer SH, et al. Neural tube defects and folate pathway genes: family-based association tests of gene-gene and gene-environment interactions. Environ Health Perspect. 2006;114:1547–1552. doi: 10.1289/ehp.9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Malinow MR, et al. Mice deficient in cystathionine beta-synthase: animal models for mild and severe homocyst(e)inemia. Proc Natl Acad Sci U S A. 1995;92:1585–1589. doi: 10.1073/pnas.92.5.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barak AJ, Beckenhauer HC, Junnila M, Tuma DJ. Dietary betaine promotes generation of hepatic S-adenosylmethionine and protects the liver from ethanol-induced fatty infiltration. Alcohol Clin Exp Res. 1993;17:552–555. doi: 10.1111/j.1530-0277.1993.tb00798.x. [DOI] [PubMed] [Google Scholar]
- 16.Werstuck GH, Lentz SR, Dayal S, Hossain GS, Sood SK, Shi YY, et al. Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways. J Clin Invest. 2001;107:1263–1273. doi: 10.1172/JCI11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esfandiari F, Villanueva JA, Wong DH, French SW, Halsted CH. Chronic ethanol feeding and folate deficiency activate hepatic endoplasmic reticulum stress pathway in micropigs. Am J Physiol Gastrointest Liver Physiol. 2005;289:G54–G63. doi: 10.1152/ajpgi.00542.2004. [DOI] [PubMed] [Google Scholar]
- 18.Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. 2003;124:1488–1499. doi: 10.1016/s0016-5085(03)00276-2. [DOI] [PubMed] [Google Scholar]
- 19.Ji C, Deng Q, Kaplowitz N. Role of TNF-alpha in ethanol-induced hyperhomocysteinemia and murine alcoholic liver injury. HEPATOLOGY. 2004;40:442–451. doi: 10.1002/hep.20309. [DOI] [PubMed] [Google Scholar]
- 20.Ji C, Chan C, Kaplowitz N. Predominant role of sterol response element binding proteins (SREBP) lipogenic pathways in hepatic steatosis in the murine intragastric ethanol feeding model. J Hepatol. 2006;45:717–724. doi: 10.1016/j.jhep.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Sowden MP, Collins HL, Smith HC, Garrow TA, Sparks JD, Sparks CE. Apolipoprotein B mRNA and lipoprotein secretion are increased in McArdle RH-7777 cells by expression of betaine-homocysteine S-methyl-transferase. Biochem J. 1999;341(pt 3):639–645. [PMC free article] [PubMed] [Google Scholar]
- 22.Collins HL, Sparks CE, Sparks JD. B48 is preferentially translated over B100 in cells with increased endogenous apo B mRNA. Biochem Biophys Res Commun. 2000;273:1156–1160. doi: 10.1006/bbrc.2000.3074. [DOI] [PubMed] [Google Scholar]
- 23.Sparks JD, Collins HL, Chirieac DV, Cianci J, Jokinen J, Sowden MP, et al. Hepatic very-low-density lipoprotein and apolipoprotein B production are increased following in vivo induction of betaine-homocysteine S-methyltransferase. Biochem J. 2006;395:363–371. doi: 10.1042/BJ20051966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumaru K, Ji C, Kaplowitz N, et al. Mechanisms for sensitization to TNF-induced apoptosis by acute glutathione depletion in murine hepatocytes. HEPATOLOGY. 2003;37:1425–1434. doi: 10.1053/jhep.2003.50230. [DOI] [PubMed] [Google Scholar]
- 25.Mudd SH, Brosnan JT, Brosnan ME, Jacobs RL, Stabler SP, Allen RH, et al. Methyl balance and transmethylation .uxes in humans. Am J Clin Nutr. 2007;85:19–25. doi: 10.1093/ajcn/85.1.19. [DOI] [PubMed] [Google Scholar]
- 26.Avila MA, Berasain C, Torres L, Martin-Duce A, Corrales FJ, Yang H, et al. Reduced mRNA abundance of the main enzymes involved in methionine metabolism in human liver cirrhosis and hepatocellular carcinoma. J Hepatol. 2000;33:907–914. doi: 10.1016/s0168-8278(00)80122-1. [DOI] [PubMed] [Google Scholar]
- 27.Ji C, Kaplowitz N. ER stress: Can the liver cope? J Hepatol. 2006;45:321–333. doi: 10.1016/j.jhep.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Jakubowski H. Pathophysiological consequences of homocysteine excess. J Nutr. 2006;136(6 suppl):1741S–1749S. doi: 10.1093/jn/136.6.1741S. [DOI] [PubMed] [Google Scholar]
- 29.Pajares MA, Perez-Sala D. Betaine homocysteine S-methyltransferase: just a regulator of homocysteine metabolism? Cell Mol Life Sci. 2006;63:2792–2803. doi: 10.1007/s00018-006-6249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Herscovitz H. Nascent lipidated apolipoprotein B is transported to the Golgi as an incompletely folded intermediate as probed by its association with network of endoplasmic reticulum molecular chaperones, GRP94, ERp72, BiP,calreticulin, and cyclophilin B. J Biol Chem. 2003;278:7459–7468. doi: 10.1074/jbc.M207976200. [DOI] [PubMed] [Google Scholar]
- 31.Graf D, Kurz AK, Reinehr R, Fischer R, Kircheis G, Haussinger D. Prevention of bile acid-induced apoptosis by betaine in rat liver. HEPATOLOGY. 2002;36(4 pt 1):829–839. doi: 10.1053/jhep.2002.35536. [DOI] [PubMed] [Google Scholar]
- 32.Kharbanda KK, Mailliard ME, Baldwin CR, Beckenhauer HC, Sorrell MF, Tuma DJ. Betaine attenuates alcoholic steatosis by restoring phosphatidylcholine generation via the phosphatidylethanolamine methyltransferase pathway. J Hepatol. 2007;46:314–321. doi: 10.1016/j.jhep.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Agellon LB, Allen TM, Umeda M, Jewell L, Mason A, et al. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab. 2006;3:321–331. doi: 10.1016/j.cmet.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Ou X, Yang H, Ramani K, Ara AI, Chen H, Mato JM, et al. Inhibition of human betaine-homocysteine methyltransferase expression by S-adenosylmethionine and methylthioadenosine. Biochem J. 2007;401:87–96. doi: 10.1042/BJ20061119. [DOI] [PMC free article] [PubMed] [Google Scholar]