Abstract
Background
Fluid resuscitation following traumatic injury causes haemodilution and can contribute to coagulopathy. Coagulation factor replacement may be necessary to prevent bleeding complications of dilutional coagulopathy. Compared with fresh frozen plasma (FFP), prothrombin complex concentrate (PCC) may potentially offer a more rapid and effective means of normalizing coagulation factor levels.
Methods
In anaesthetized mildly hypothermic pigs, 65–70% of total blood volume was substituted in phases with hydroxyethyl starch and red cells. Animals were then treated with 15 ml kg−1 isotonic saline placebo, 25 IU kg−1 PCC, or 15 ml kg−1 FFP. Immediately thereafter, either a standardized femur or spleen injury was inflicted, and coagulation function, including thrombin generation, and bleeding were assessed. An additional group received high-dose FFP (40 ml kg−1) before femur injury.
Results
Haemodilution markedly prolonged prothrombin time and reduced peak thrombin generation. PCC, but not FFP, fully reversed those effects. Compared with 15 ml kg−1 FFP, PCC shortened the time to haemostasis after either bone (P=0.001) or spleen (P=0.028) trauma and reduced the volume of blood lost (P<0.001 and P=0.015, respectively). Subsequent to bone injury, PCC also accelerated haemostasis (P=0.003) and diminished blood loss (P=0.006) vs 40 ml kg−1 FFP.
Conclusions
PCC was effective in correcting dilutional coagulopathy and controlling bleeding in an in vivo large-animal trauma model. In light of its suitability for more rapid administration than FFP, PCC merits further investigation as a therapy for dilutional coagulopathy in trauma and surgery.
Keywords: blood, haemodilution; complications, haemorrhagic disorder; complications, trauma; fresh frozen plasma; prothrombin complex concentrate
Uncontrolled bleeding associated with major trauma and surgery is often life-threatening. Acquired coagulopathy of trauma is responsible for the majority of postoperative traumatic haemorrhagic fatalities, and the onset of acute coagulopathy is associated with increased overall mortality.1 The coagulopathy of trauma is a manifestation of the combined effects of blood loss and dilution, coagulation factor and platelet consumption, hypothermic platelet dysfunction, acidosis-induced decreases in coagulation factor activity, and fibrinolysis.2
A recognized complication of massive transfusion is dilutional coagulopathy, which occurs when lost blood is replaced with fluids that do not contain coagulation factors. Dilutional coagulopathy is distinct from the more recently recognized phenomenon of acute traumatic coagulopathy, which is independent of i.v. fluid administration and appears to be mediated through activation of the protein C pathway.3 The shift from whole blood transfusion in the past to the current practice of specific blood component therapy has resulted in earlier occurrence of thrombocytopenia and clotting factor deficiency in trauma patients.4,5 Trauma resuscitation usually starts with crystalloid or colloid solutions followed by red blood cell concentrates, with resultant coagulation factor dilution.2 A vicious cycle can ensue, in which further haemorrhage necessitates additional resuscitation fluid with resultant exacerbation of haemodilution, coagulopathy, and blood loss. A recent German analysis demonstrated a high frequency of established coagulopathy in multiple injury patients upon emergency room admission that was associated with the amount of prior i.v. fluids administered.6 Consequently, a treatment strategy of earlier coagulation factor replacement has been advocated.4,7,8
Fresh frozen plasma (FFP) is a source of all the coagulation factors, including the labile factors, and its use has been recommended either in massive bleeding or significant bleeding complicated by coagulopathy, as indicated by >1.5-fold prolongation of prothrombin time (PT) and activated partial thromboplastin time (aPTT).9 However, FFP has certain drawbacks for rapid reversal of coagulopathy. FFP may require ABO compatibility, and is typically available only after the blood type has been established and plasma has been thawed. These preliminary procedures may take more than an hour in some facilities and may jeopardize the opportunity to prevent rapid deterioration in the clinical condition of the patient.2 Although the overall risks of FFP are low, complications can include immunological reactions such as allergy/anaphylaxis, transfusion-related acute lung injury (TRALI), and haemolysis due to anti-A or anti-B if FFP is transfused across ABO groups.10,11 TRALI, an acute syndrome of dyspnoea, hypoxia, and pulmonary ‘white-out’, is a major cause of transfusion-related death.10
Delivery of coagulation factors in concentrated form may offer advantages in overcoming the coagulopathic effects of large resuscitation fluid volumes.2 Prothrombin complex concentrate (PCC), which contains vitamin K-dependent coagulation factors, can be administered rapidly without the need for matching the blood group or thawing the product. PCC has increasingly been employed for rapid reversal of coumarin oral anticoagulant therapy.12–21 The most recent update from the British Committee for Standards in Haematology recommends that, for reversal of anticoagulation in patients with major bleeding, PCC should be administered in preference to FFP.22 The use of PCC in trauma patients has not been reported.
In coagulopathic trauma patients, a major obstacle to optimal coagulation factor replacement therapy has been the lack of suitable assays that can reflect the overall haemostatic balance. The calibrated automated thrombin generation assay (TGA) is a global coagulation test being used to monitor both hypocoagulable and hypercoagulable states. Thrombin generation, which plays a central role in activating coagulation enzymes, inhibitors, and platelets and in cleaving fibrinogen to fibrin monomers, has been proposed as an overall function test of the haemostatic–thrombotic system.23 Thrombin generation has been investigated as a means to monitor treatment with bypassing agents such as factor VIII inhibitor bypass activity (FEIBA), which is an activated PCC, and recombinant factor VIIa in haemophilia patients with inhibitors.24–27 PCC-mediated oral anticoagulant reversal in vitro has recently been evaluated using TGA.28
An animal model of dilutional coagulopathy and major haemorrhage in pigs has been developed.29 In that study, PCC was effective in normalizing coagulation and improving haemostasis compared with an inactive placebo; however, an active control fluid containing coagulation factors was not evaluated. The model was used in the present study to compare the effects of PCC and FFP on haemorrhage after femur or spleen injury. In addition, the impact of those agents on coagulation function in vivo, including thrombin generation, was examined.
Methods
Animals
As described previously in detail, dilutional coagulopathy was induced in anaesthetized mildly hypothermic (36°C) pigs.29 Forty-seven castrated male pigs (large white×German noble) were obtained from a local breeding farm (Schlosser, Schwalmtal, Germany). The animals were 3–4 months old and weighed 21–32 kg. The study was performed in accordance with the German Animal Welfare law and approved by the regional government authorities. Animal housing and care were furnished in compliance with current regulations of the European Union.
Anaesthesia
After an overnight fast with free access to water, pigs were premedicated i.m. with a mixture of 2 mg kg−1 azaperone (Stresnil®, Janssen-Cilag GmbH, Neuss, Germany), 15 mg kg−1 ketamine (Ketavet, Pharmacia & Upjohn, Erlangen, Germany), and 0.02 mg kg−1 atropine sulfate (Atropinsulfate, B. Braun, Melsungen, Germany). Anaesthesia was induced by 10 mg kg−1 thiopental sodium via an ear vein. The animals were placed on a Heyer Access ventilator after tracheal intubation. Inhaled anaesthesia was maintained by isoflurane (Forane, Abbott Laboratories Inc., Abbott Park, IL, USA) at a concentration of 1–2%. A 20-gauge catheter was placed into a femoral artery for continuous arterial blood pressure measurements, and body temperature was monitored by a rectal thermometer. Attainment and maintenance of deep anaesthesia were confirmed by an absent pedal withdrawal reflex.
Haemodilution
Vascular access was secured through a 14-gauge catheter in the external jugular vein. Phased blood withdrawal (total 60 ml kg−1, 65–70% of calculated total blood volume), salvaged erythrocyte retransfusion (total 20 ml kg−1), and volume substitution with a total of 40 ml kg−1 hydroxyethyl starch 200/0.5 (Infukoll 6%, Schwarz Pharma AG, Mannheim, Germany) were performed over a period of 80 min after induction of anaesthesia.29
Treatment
Study treatments were administered 115 min after initiation of blood withdrawal (25 min after completion of phased haemodilution). The pigs were randomly assigned to receive 15 ml kg−1 isotonic saline, 25 IU kg−1 PCC (Beriplex® P/N, CSL Behring GmbH, Marburg, Germany), or standard-dose (15 ml kg−1) or high-dose (40 ml kg−1) porcine FFP via the indwelling jugular catheter. The 40 ml kg−1 FFP dose was selected to maximize efficacy without unacceptable haemodynamic disturbances. Beriplex P/N is a biochemically well-characterized balanced PCC containing coagulation factors II (FII), VII (FVII), IX (FIX), and X (FX), as well as the anticoagulant proteins C and S.30 The administered PCC dose of 25 IU kg−1, based on FIX content, is equivalent to 0.8 ml kg−1.
The respective mean (sd) concentrations of FII, FVII, FIX, and FX in Beriplex P/N are 31.0 (3.4), 16.2 (1.9), 28.9 (2.2), and 40.5 (3.3) IU ml−1.31 Corresponding values in porcine plasma are 0.50 (0.04), 0.48 (0.06), 2.3 (0.42), and 0.58 (0.07) U ml−1.29 Therefore, the administered 0.8 ml kg−1 PCC volume contained doses of those coagulation factors, respectively, 3.3, 1.8, 0.67, and 3.7-fold those of the 15 ml kg−1 porcine PCC volume and 1.2, 0.68, 0.25, and 1.4-fold the 40 ml kg−1 volume.
Porcine rather than human FFP was selected as control fluid, because in pilot experiments unpredictable and sometimes serious transfusion reactions were encountered in pigs receiving human FFP. For preparation of porcine FFP, blood was withdrawn from the carotid artery of healthy donor pigs through custom-made connection tubing. The anticoagulated blood was centrifuged 10 min at 3000 g, and the plasma was frozen and stored at −70°C until treatment. Prior to porcine FFP infusions, haemagglutination and haemolysis of individual animal erythrocyte specimens were tested to lessen the risk of transfusion reactions.
Experimental trauma
At 5 min following administration of study treatments, standardized wounds were created either by drilling a 3 mm hole into the femur or making a 7 cm long and 1 cm deep incision into the spleen. Time to haemostasis and blood loss were monitored for 120 min after the experimental injuries were inflicted. Groups of 5–7 pigs had received each of the test treatments before femur injury. Group size for spleen injury was six animals, with the exception that seven animals had received saline placebo prior to spleen incision.
Skin bleeding time (SBT) was determined in duplicate from a 5 mm long and 1 mm deep incision at a shaved inner site on the ear using a standard cutting device (Surgicutt®, International Technidyne Corp., Edison, NJ, USA). Shed blood was blotted with a filter paper from the edge of the wound. The time from incision to cessation of blood flow was recorded as the SBT. Determinations of time to haemostasis, blood loss, and SBT were made by observers blinded to the group assignments of the animals. While remaining under deep anaesthesia, the animals were humanely killed upon conclusion of the study experimental procedures by injecting embutramide, mebenzonium iodide, and tetracaine hydrochloride (T61®, Intervet Deutschland GmbH, Unterschleißheim, Germany).
Laboratory assays
Blood samples were collected from the carotid artery at baseline, after the completion of phased haemodilution (80 min) and 5 min after study treatment administration (120 min). Coagulation factors were measured in coagulation factor-deficient plasma (Dade Behring, Marburg, Germany) with a Schnitger and Gross coagulometer (Heinrich Amelung GmbH, Lemgo, Germany). FII, FVII, and FX were determined by PT assay, and FIX was measured by aPTT assay. PT was determined with a Schnitger & Gross coagulometer using the Thromborel reagent (Dade Behring).
TGA was performed by calibrated automated thrombinography (CAT, Thrombinoscope B.V., Maastricht, the Netherlands) in diluted plasma according to the method of Hemker and colleagues.32 The concentrations of recombinant relipidated tissue factor (Dade Behring) and phospholipids were 5 pM and 4 µM, respectively. The peak molar quantity of thrombin present in clotting plasma was calculated using the Thrombinoscope software version 3.0.0.29.
Statistical analysis
Median differences and their 95% confidence intervals (CI) were determined by exact Hodges–Lehmann estimation. Time to haemostasis was analysed by the Kaplan–Meier product-limit method and exact logrank test. Between-group differences in the volume of blood lost were evaluated by exact Wilcoxon test. Analyses were performed using R version 2.6.2 (The R Foundation for Statistical Computing, Vienna, Austria) and StatXact 7.0 (Cytel Software Corp., Cambridge, MA, USA) statistical software.
Results
Coagulation factors
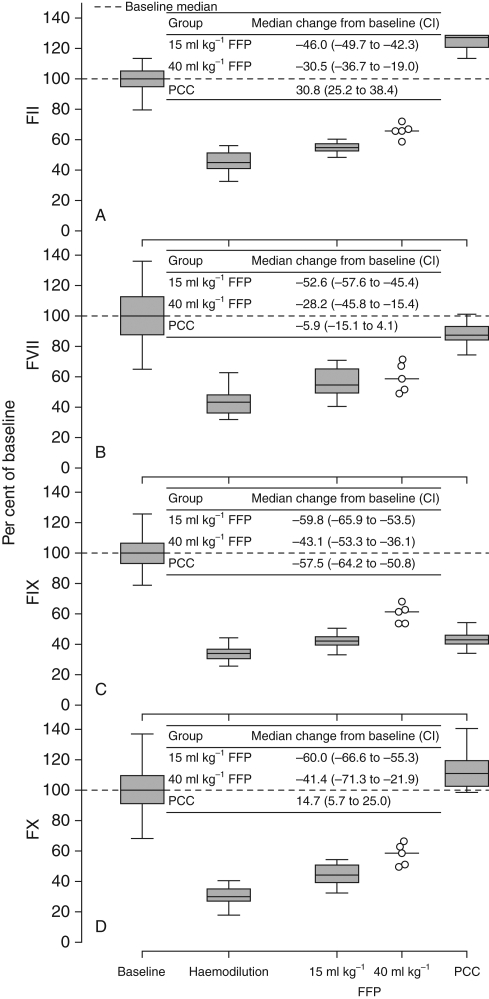
Upon completion of phased haemodilution (80 min), median rectal temperature fell to 36.2°C with an interquartile range (IQR) of 35.9–36.9°C from 37.8°C (IQR, 37.6–38.5°C) at baseline. Haemodilution profoundly diminished circulating concentrations of FII, FVII, FIX, and FX, with median values declining more than 50% (Fig. 1). The impact of standard- or high-dose FFP administration was minor, and all four coagulation factors remained markedly lower than at baseline. In contrast, PCC fully normalized FII, FVII, and FX. While the effect was smaller, PCC did also increase FIX concentration by a median of 7.8% (CI 6.4–9.4%) from the post-haemodilution level.
Fig 1.
Levels of (a) FII, (b) FVII, (c) FIX, and (d) FX at baseline and after haemodilution and subsequent administration of FFP or PCC. Concentration values expressed as a percentage of the baseline median. In each panel is shown the median change from baseline in animals receiving FFP and PCC and corresponding CI. Horizontal lines within boxes indicate the medians, lower and upper box boundaries the 25th and 75th percentiles, respectively, and lower and upper error bars the 10th and 90th percentiles. Individual animal data points and horizontal lines representing the medians are displayed for the 40 ml kg−1 FFP group. CI, 95% confidence interval; FFP, fresh frozen plasma; FII, factor II; FVII, factor VII; FIX, factor IX; FX, factor X; PCC, prothrombin complex concentrate.
Haemodilution was also attended by a major decrease in circulating fibrinogen concentration, from a median of 3.34 to 1.82 g litre−1 (Table 1). Nonetheless, fibrinogen level equalled or exceeded 1 g litre−1 in all animals subsequent to haemodilution. After FFP or PCC administration, median fibrinogen remained near the post-haemodilution level. In no recipient of either FFP or PCC did fibrinogen level fall below 1 g litre−1.
Table 1.
Fibrinogen. FFP, fresh frozen plasma; IQR, interquartile range; PCC, prothrombin complex concentrate. *Fibrinogen concentration was not determined in the placebo group. Baseline fibrinogen was not evaluable for one animal in the PCC group due to technical difficulties with the plasma sample
| Category | n | Fibrinogen (g litre−1), median (IQR) |
|---|---|---|
| Baseline | 31* | 3.34 (2.94–3.82) |
| Haemodilution | 32 | 1.82 (1.52–2.04) |
| 15 ml kg−1 FFP | 14 | 2.04 (2.01–2.18) |
| 40 ml kg−1 FFP | 5 | 2.33 (2.26–2.37) |
| PCC | 13 | 1.79 (1.54–1.91) |
Prothrombin time
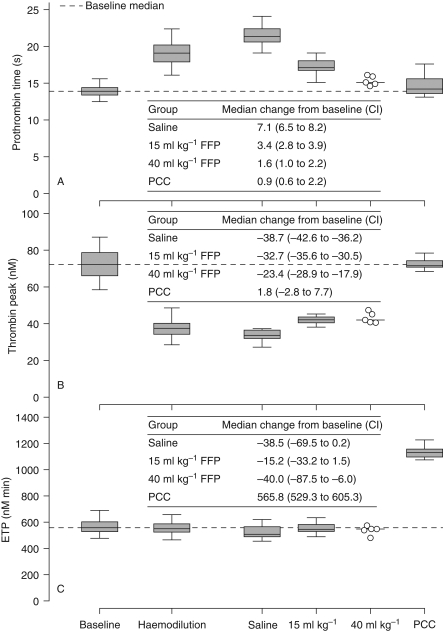
Haemodilution prolonged PT (Fig. 2a). Saline administration further lengthened PT, whereas FFP partially reversed the haemodilution-induced PT prolongation. Of the tested fluids, PCC was the only one to normalize PT fully.
Fig 2.
(a) Prothrombin time, (b) peak thrombin generation, and (c) ETP after haemodilution and subsequent administration of saline, FFP, or PCC. Graphic conventions as in Figure 1. CI, 95% confidence interval; FFP, fresh frozen plasma; ETP, endogenous thrombin potential; PCC, prothrombin complex concentrate.
Platelets
Platelet counts were markedly reduced by haemodilution. The median value declined to 161×109 litre−1 (IQR 138–197×109 litre−1) from 444×109 litre−1 (IQR 374–506×109 litre−1) at baseline. Substantial depletion in platelets persisted after administration of all test fluids.
Thrombin generation
Haemodilution strongly attenuated peak thrombin generation (Fig. 2b). The haemodilution-induced decline in peak thrombin generation was not reversed by either saline or FFP (Fig. 2b). In contrast, PCC restored peak thrombin generation to a level not significantly different from that at baseline.
Despite the observed attenuation of the thrombin peak, the impact of haemodilution on endogenous thrombin potential (ETP) was negligible (Fig. 2c). This observation reflected the persistence of higher post-peak thrombin concentrations, thus offsetting the reduction in peak values. The effects of saline and FFP on ETP were both minimal. PCC, however, augmented ETP by a median of nearly 600 nM min above baseline.
Haemostasis
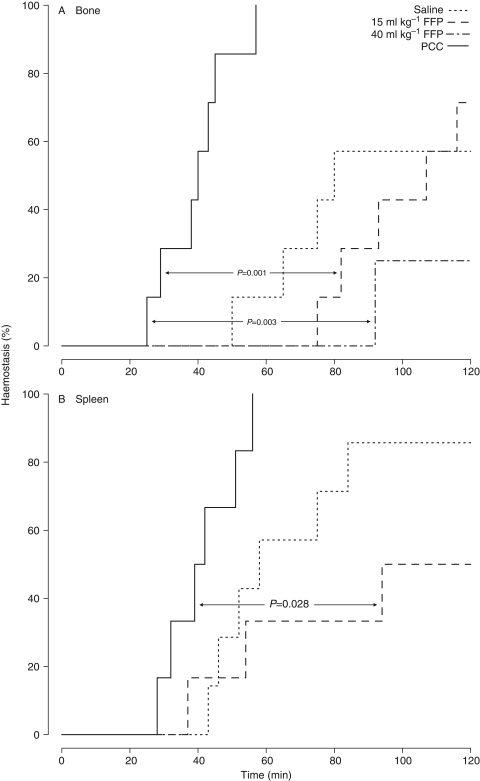
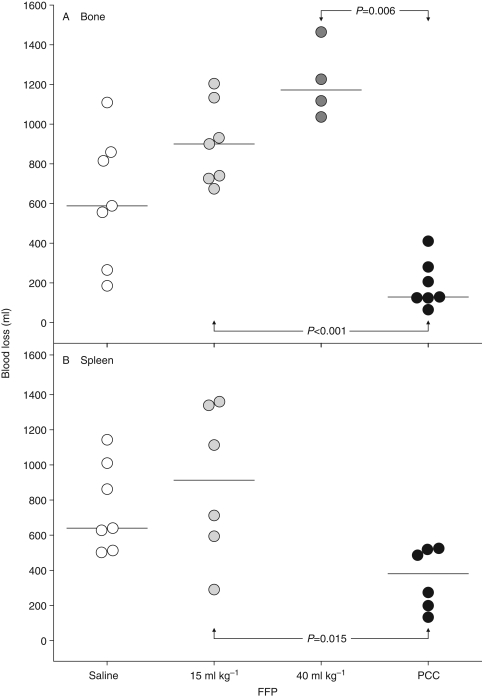
After either experimental bone or spleen trauma, PCC significantly shortened time to haemostasis compared with FFP (Fig. 3). Haemostasis was achieved by all 13 PCC-treated animals. Over the course of the 120 min observation period, bleeding failed to cease in four of 14 saline recipients (29%), five of 13 animals in the 15 ml kg−1 FFP group (38%), and three of four evaluable pigs (75%) treated with 40 ml kg−1 FFP. Compared with FFP, PCC also significantly lowered the volume of blood lost following either bone or spleen trauma (Fig. 4).
Fig 3.
Time to haemostasis following experimental (a) bone or (b) spleen trauma in animals treated with saline, FFP, or PCC. FFP, fresh frozen plasma; PCC, prothrombin complex concentrate.
Fig 4.
Individual animal blood losses following experimental (a) bone or (b) spleen trauma in groups treated with saline, FFP or PCC. Horizontal lines depict the medians. FFP, fresh frozen plasma; PCC, prothrombin complex concentrate.
From a baseline median of 117 s, SBT was more than doubled by haemodilution (Table 2). Saline placebo and FFP showed little effect in shortening the prolonged SBT. However, PCC normalized SBT.
Table 2.
Skin bleeding time. FFP, fresh frozen plasma; IQR, interquartile range; PCC, prothrombin complex concentrate; SBT, skin bleeding time. *One animal excluded due to the development of hypotension
| Category | n | SBT (s), median (IQR) |
|---|---|---|
| Baseline | 47 | 117 (108–127) |
| Haemodilution | 47 | 305 (279–329) |
| Placebo | 14 | 288 (273–311) |
| 15 ml kg−1 FFP | 15 | 250 (219–280) |
| 40 ml kg−1 FFP | 4* | 234 (177–244) |
| PCC | 13 | 137 (126–152) |
Discussion
Acquired coagulopathy following trauma is an important determinant of outcome. Coagulopathic patients, including those with head injury, experience worse outcomes than patients with the same injury severity but no clotting disturbance.9 Therefore, a major clinical need exists for modalities that quickly and effectively reverse the dilutional coagulopathy occurring over the course of trauma treatment. Pharmacological treatment options consist of antifibrinolytics, fresh whole blood and plasma, FFP, cryoprecipitate, and coagulation factor concentrates.2,9,33 This is the first study to compare PCC with FFP for correction of coagulopathy under conditions of haemodilution and haemorrhage in a porcine trauma model.
Time to haemostasis and blood loss following either femur or spleen injury were both significantly diminished by PCC compared with FFP treatment. The ineffectiveness of FFP with respect to these primary endpoints appears to reflect persistent coagulation factor deficiency after administration of a standard FFP dose (15 ml kg−1) comparable to the typical adult dose used in clinical practice, as well as after a much higher dose (40 ml kg−1).34 In the absence of ongoing coagulation factor consumption or loss, a dose of 10–15 ml kg−1 FFP is expected to raise factor levels by 25% in humans.33 In the porcine model, smaller increases were observed, possibly due to the use of human coagulation factor-deficient plasma in the porcine coagulation factor assays. The availability of species-specific coagulation factor-deficient plasma would be needed to assess the contribution, if any, of assay method to the relatively small increases in coagulation factor levels following FFP administration. On the other hand, the normalization of FII, FVII, and FX levels by PCC in the study animals suggests that assay inaccuracy may not explain the lack of FFP effect in restoring levels of those coagulation factors. In any case, high-dose FFP proved to be no more effective than the standard dose in speeding haemostasis and restricting blood loss after bone injury.
In patients, greater restoration of coagulation factor levels with FFP is hampered by the risk of fluid overload. Additionally, during preparation of FFP for clinical use, coagulation factors are diluted by ∼15% with citrate, and further losses are believed to occur during freezing and thawing.35,36 Due to the high coagulation factor concentrations in PCC, FII, FVII, and FX rebounded fully to baseline levels after PCC administration in the model system. The small FIX rise produced by PCC was similar in magnitude to that by 15 ml kg−1 porcine FFP. This observation reflects the comparatively low FIX dose in the administered 0.8 ml kg−1 PCC volume. Whereas the doses of the other three coagulation factors in the administered PCC volume were 2–4-fold those in 15 ml kg−1 porcine FFP, the corresponding dose of FIX was 33% lower. In any case, PCC did produce a small but statistically significant increase in FIX which, in concert with full normalization of the other three coagulation factors, proved sufficient to enhance haemostasis.
Another advantage of PCC is viral safety. Most FFP preparations are not subjected to viral inactivation. Prepared from plasma screened by polymerase chain reaction, Beriplex P/N is pasteurized and nanofiltrated to eliminate viruses.30,37 In clinical trials there has been no evidence of viral transmission following Beriplex P/N administration.20,31
Although the coagulation factor increase was relatively small, FFP did partially reverse the haemodilution-induced PT prolongation. PCC, however, entirely normalized PT. These findings are consistent with prior data. In a prospective audit of FFP transfusion in patients with mild PT prolongation (13–17 s), halfway normalization of PT was accomplished in only 15% of cases and full correction in <1%.38 In contrast, Beriplex P/N administration effectively normalized PT in critically ill patients with moderately reduced coagulation activity.39 In a porcine model of dilutional coagulopathy similar to that in the present study, PCC plus fibrinogen normalized PT, while normal saline was ineffective.40
It is generally recognized that both PT and aPTT are insensitive measures for detecting hypocoagulant conditions.36 The value of SBT in monitoring the coagulation system and directing therapeutic inventions remains to be established. In this study, the SBT results closely coincided with those for time to haemostasis and volume of blood loss after bone or spleen trauma. While platelet function is a major determinant of SBT, the coagulation cascade may also play a role. In rats, the direct thrombin inhibitor melagatran has been shown to prolong SBT, and the prolongation could be reversed by PCC.41
TGAs are being actively investigated for their utility in characterizing and monitoring both hypocoagulable and hypercoagulable states. In the present study, TGA data were consistent with the bleeding endpoint results. Thus, PCC normalized the thrombin peak, whereas FFP had negligible effect.
TGA has been used to monitor the response to FFP administration in surgery patients.36 Thrombin generation parameters and fibrinogen levels were higher in post-transfusion plasma from patients who stopped bleeding than from those with ongoing haemorrhage. An in vitro study has demonstrated the ability of PCC to restore thrombin generation in plasma from orally anticoagulated patients.42 TGA has also been used to monitor the effects of activated PCC.25,43,44
Both platelet count and fibrinogen concentration were substantially reduced by haemodilution and did not recover in response to PCC or FFP treatment. However, the platelet number and fibrinogen concentration were not below the thresholds to sustain the competence of the coagulation system, as demonstrated by the ability of PCC to control femur and spleen bleeding.
The porcine model of dilutional coagulopathy employed in the present study has been previously established for evaluating the effectiveness of i.v. fluids in normalizing coagulation function and reducing bleeding resulting from a subsequent haemorrhagic challenge in the form of a standardized experimental femur or spleen injury.29 The model simulates clinical situations in which, after initial traumatic haemorrhagic shock and resuscitation, haemostasis has been secured, but dilutional coagulopathy needs to be corrected to prevent excessive bleeding during further surgical interventions the patient may require. A similar porcine dilutional coagulopathy model has been described, in which placebo or coagulation factors were administered prior to a standardized hepatic laceration, and subsequent blood loss and survival were assessed.40
In this porcine trauma model of dilutional coagulopathy and haemorrhage, PCC proved superior to FFP in normalizing PT, SBT, and peak thrombin generation and controlling bleeding. These findings support a potential role for PCC in coagulopathic trauma and surgery patients. In view of the unmet clinical need for more efficacious haemostatic agents in such patients, clinical studies are now justified to confirm the observed favourable effects of PCC in the present preclinical model system.
Funding
The authors received an unrestricted grant from CSL Behring. Funding to Pay the Open Access Charge was provided by CSL Behring.
Acknowledgements
Prothrombin complex concentrate for use in this study was provided by CSL Behring GmbH, Marburg, Germany, the commercial supplier of that product and sponsor of the study.
References
- 1.Kauvar DS, Wade CE. The epidemiology and modern management of traumatic hemorrhage: US and international perspectives. Crit Care. 2005;9:S1–S9. doi: 10.1186/cc3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hess JR. Blood and coagulation support in trauma care. Hematology Am Soc Hematol Educ Program. 2007:187–91. doi: 10.1182/asheducation-2007.1.187. [DOI] [PubMed] [Google Scholar]
- 3.Brohi K, Cohen MJ, Davenport RA. Acute coagulopathy of trauma: mechanism, identification and effect. Curr Opin Crit Care. 2007;13:680–5. doi: 10.1097/MCC.0b013e3282f1e78f. [DOI] [PubMed] [Google Scholar]
- 4.Ho AM, Karmakar MK, Dion PW. Are we giving enough coagulation factors during major trauma resuscitation? Am J Surg. 2005;190:479–84. doi: 10.1016/j.amjsurg.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 5.Spahn DR, Rossaint R. Coagulopathy and blood component transfusion in trauma. Br J Anaesth. 2005;95:130–9. doi: 10.1093/bja/aei169. [DOI] [PubMed] [Google Scholar]
- 6.Maegele M, Lefering R, Yucel N, et al. Early coagulopathy in multiple injury: an analysis from the German Trauma Registry on 8724 patients. Injury. 2007;38:298–304. doi: 10.1016/j.injury.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Borgman MA, Spinella PC, Perkins JG, et al. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63:805–13. doi: 10.1097/TA.0b013e3181271ba3. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez EA, Moore FA, Holcomb JB, et al. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62:112–9. doi: 10.1097/01.ta.0000250497.08101.8b. [DOI] [PubMed] [Google Scholar]
- 9.Spahn DR, Cerny V, Coats TJ, et al. Management of bleeding following major trauma: a European guideline. Crit Care. 2007;11:R17. doi: 10.1186/cc5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacLennan S, Williamson LM. Risks of fresh frozen plasma and platelets. J Trauma. 2006;60:S46–S50. doi: 10.1097/01.ta.0000199546.22925.31. [DOI] [PubMed] [Google Scholar]
- 11.Khan H, Belsher J, Yilmaz M, et al. Fresh-frozen plasma and platelet transfusions are associated with development of acute lung injury in critically ill medical patients. Chest. 2007;131:1308–14. doi: 10.1378/chest.06-3048. [DOI] [PubMed] [Google Scholar]
- 12.Makris M, Greaves M, Phillips WS, Kitchen S, Rosendaal FR, Preston EF. Emergency oral anticoagulant reversal: the relative efficacy of infusions of fresh frozen plasma and clotting factor concentrate on correction of the coagulopathy. Thromb Haemost. 1997;77:477–80. [PubMed] [Google Scholar]
- 13.Evans G, Luddington R, Baglin T. Beriplex P/N reverses severe warfarin-induced overanticoagulation immediately and completely in patients presenting with major bleeding. Br J Haematol. 2001;115:998–1001. doi: 10.1046/j.1365-2141.2001.03214.x. [DOI] [PubMed] [Google Scholar]
- 14.Preston FE, Laidlaw ST, Sampson B, Kitchen S. Rapid reversal of oral anticoagulation with warfarin by a prothrombin complex concentrate (Beriplex): efficacy and safety in 42 patients. Br J Haematol. 2002;116:619–24. doi: 10.1046/j.0007-1048.2001.03295.x. [DOI] [PubMed] [Google Scholar]
- 15.Sharples R, Burnett C, Murphy M, Hanley JP. Prospective evaluation of a regional protocol for a rapid warfarin reversal in major haemorrhage. Br J Haematol. 2003;121:3–4. [Google Scholar]
- 16.van Aart L, Eijkhout HW, Kamphuis JS, et al. Individualized dosing regimen for prothrombin complex concentrate more effective than standard treatment in the reversal of oral anticoagulant therapy: An open, prospective randomized controlled trial. Thromb Res. 2006;118:313–20. doi: 10.1016/j.thromres.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Bhardwaj M, Bunsell R. Beriplex P/N: an alternative to fresh frozen plasma in severe haemorrhage. Anaesthesia. 2007;62:832–4. doi: 10.1111/j.1365-2044.2007.05134.x. [DOI] [PubMed] [Google Scholar]
- 18.Riess HB, Meier-Hellmann A, Motsch J, Elias M, Kursten FW, Dempfle CE. Prothrombin complex concentrate (Octaplex®) in patients requiring immediate reversal of oral anticoagulation. Thromb Res. 2007;121:9–16. doi: 10.1016/j.thromres.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Vigué B, Ract C, Tremey B, et al. Ultra-rapid management of oral anticoagulant therapy-related surgical intracranial hemorrhage. Intensive Care Med. 2007;33:721–5. doi: 10.1007/s00134-007-0528-z. [DOI] [PubMed] [Google Scholar]
- 20.Pabinger I, Brenner B, Kalina U, Knaub S, Nagy A, Ostermann H. Prothrombin complex concentrate (Beriplex® P/N) for emergency anticoagulation reversal: A prospective multinational clinical trial. J Thromb Haemost. 2008;6:622–31. doi: 10.1111/j.1538-7836.2008.02904.x. [DOI] [PubMed] [Google Scholar]
- 21.Pabinger-Fasching I. Warfarin-reversal: results of a phase III study with pasteurised, nanofiltrated prothrombin complex concentrate. Thromb Res. 2008;122:S19–S22. doi: 10.1016/S0049-3848(08)70005-7. [DOI] [PubMed] [Google Scholar]
- 22.Baglin TP, Keeling DM, Watson HG. Guidelines on oral anticoagulation (warfarin): third edition – 2005 update. Br J Haematol. 2006;132:277–85. doi: 10.1111/j.1365-2141.2005.05856.x. [DOI] [PubMed] [Google Scholar]
- 23.Hemker HC, Al Dieri R, De Smedt E, Beguin S. Thrombin generation, a function test of the haemostatic-thrombotic system. Thromb Haemost. 2006;96:553–61. [PubMed] [Google Scholar]
- 24.Turecek PL, Váradi K, Keil B, et al. Factor VIII inhibitor-bypassing agents act by inducing thrombin generation and can be monitored by a thrombin generation assay. Pathophysiol Haemost Thromb. 2003;33:16–22. doi: 10.1159/000071637. [DOI] [PubMed] [Google Scholar]
- 25.Váradi K, Négrier C, Berntorp E, et al. Monitoring the bioavailability of FEIBA with a thrombin generation assay. J Thromb Haemost. 2003;1:2374–80. doi: 10.1046/j.1538-7836.2003.00450.x. [DOI] [PubMed] [Google Scholar]
- 26.Dargaud Y, Béguin S, Lienhart A, et al. Evaluation of thrombin generating capacity in plasma from patients with haemophilia A and B. Thromb Haemost. 2005;93:475–80. doi: 10.1160/TH04-10-0706. [DOI] [PubMed] [Google Scholar]
- 27.van Veen JJ, Gatt A, Bowyer AE, Cooper PC, Kitchen S, Makris M. The effect of tissue factor concentration on calibrated automated thrombography in the presence of inhibitor bypass agents. Int J Lab Hematol. doi: 10.1111/j.1751-553X.2007.01022.x. doi:10.1111/j.1751-553X.2007.01022.x. [DOI] [PubMed] [Google Scholar]
- 28.Jose M, Borrell M, Fontcuberta J, Jorquera JI. Thrombin generation assay (TGA) correction in vitro after addition of therapeutic range doses of prothrombin complex concentrate to plasma samples with INR 5. J Thromb Haemost. 2007;5:P-S-052. [Google Scholar]
- 29.Dickneite G, Doerr B, Kaspereit F. Characterization of the coagulation deficit in porcine dilutional coagulopathy and substitution with a prothrombin complex concentrate. Anesth Analg. 2008;106:1070–7. doi: 10.1213/ane.0b013e318165dfbb. [DOI] [PubMed] [Google Scholar]
- 30.Römisch J, Gröner A, Bernhardt D, et al. Nanofiltration bei der Herstellung von Beriplex® P/N: Erhöhung der Kapazität zur Viruseliminierung unter Beibehaltung der Produktqualität. Beitr Infusionsther Transfusionsmed. 1996;33:220–4. [PubMed] [Google Scholar]
- 31.Ostermann H, Haertel S, Knaub S, Kalina U, Jung K, Pabinger I. Pharmacokinetics of Beriplex P/N prothrombin complex concentrate in healthy volunteers. Thromb Haemost. 2007;98:790–7. [PubMed] [Google Scholar]
- 32.Hemker HC, Giesen P, Al Dieri R, et al. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiol Haemost Thromb. 2003;33:4–15. doi: 10.1159/000071636. [DOI] [PubMed] [Google Scholar]
- 33.Wong MP, Droubatchevskaia N, Chipperfield KM, Wadsworth LD, Ferguson DJ. Guidelines for frozen plasma transfusion. BC Med J. 2007;49:311–9. [Google Scholar]
- 34.Hellstern P, Haubelt H. Indications for plasma in massive transfusion. Thromb Res. 2002;107:S19–S22. doi: 10.1016/s0049-3848(02)00147-0. [DOI] [PubMed] [Google Scholar]
- 35.Chowdhury P, Saayman AG, Paulus U, Findlay GP, Collins PW. Efficacy of standard dose and 30 ml/kg fresh frozen plasma in correcting laboratory parameters of haemostasis in critically ill patients. Br J Haematol. 2004;125:69–73. doi: 10.1111/j.1365-2141.2004.04868.x. [DOI] [PubMed] [Google Scholar]
- 36.Schols SEM, van der Meijden PEJ, van Oerle R, Curvers J, Heemskerk JWM, van Pampus ECM. Increased thrombin generation and fibrinogen level after therapeutic plasma transfusion: relation to bleeding. Thromb Haemost. 2008;99:64–70. doi: 10.1160/TH07-07-0438. [DOI] [PubMed] [Google Scholar]
- 37.Weimer T, Streichert S, Watson C, Groner A. High-titer screening PCR: a successful strategy for reducing the parvovirus B19 load in plasma pools for fractionation. Transfusion. 2001;41:1500–4. doi: 10.1046/j.1537-2995.2001.41121500.x. [DOI] [PubMed] [Google Scholar]
- 38.Abdel-Wahab OI, Healy B, Dzik WH. Effect of fresh-frozen plasma transfusion on prothrombin time and bleeding in patients with mild coagulation abnormalities. Transfusion. 2006;46:1279–85. doi: 10.1111/j.1537-2995.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- 39.Staudinger T, Frass M, Rintelen C, et al. Influence of prothrombin complex concentrates on plasma coagulation in critically ill patients. Intensive Care Med. 1999;25:1105–10. doi: 10.1007/s001340051019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fries D, Haas T, Klingler A, et al. Efficacy of fibrinogen and prothrombin complex concentrate used to reverse dilutional coagulopathy—a porcine model. Br J Anaesth. 2006;97:460–7. doi: 10.1093/bja/ael191. [DOI] [PubMed] [Google Scholar]
- 41.Elg M, Carlsson S, Gustafsson D. Effects of agents, used to treat bleeding disorders, on bleeding time prolonged by a very high dose of a direct thrombin inhibitor in anesthesized rats and rabbits. Thromb Res. 2001;101:159–70. doi: 10.1016/s0049-3848(00)00398-4. [DOI] [PubMed] [Google Scholar]
- 42.Dargaud Y, Desmurs-Clavel H, Marin S, Bordet J-C, Poplavsky JL, Negrier C. Comparison of the capacities of two prothrombin complex concentrates to restore thrombin generation in plasma from orally anticoagulated patients: an in vitro study. J Thromb Haemost. 2008;6:962–8. doi: 10.1111/j.1538-7836.2008.02964.x. [DOI] [PubMed] [Google Scholar]
- 43.Dargaud Y, Lienhart A, Meunier S, et al. Major surgery in a severe haemophilia A patient with high titre inhibitor: use of the thrombin generation test in the therapeutic decision. Haemophilia. 2005;11:552–8. doi: 10.1111/j.1365-2516.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 44.McCormick AN, Sunner T, Rangarajan S, Savidge G. Thrombin generation assay: clinical use in monitoring FVIII bypassing agent therapy during surgery in a severe haemophilia A patient with inhibitor. J Thromb Haemost. 2007;5:P-M-038. [Google Scholar]