Abstract
Background
Inhaled anaesthetics (IAs) produce multiple dose-dependent behavioural effects including amnesia, hypnosis, and immobility in response to painful stimuli that are mediated by distinct anatomical, cellular, and molecular mechanisms. Amnesia is produced at lower anaesthetic concentrations compared with hypnosis or immobility. Nicotinic acetylcholine receptors (nAChRs) modulate hippocampal neural network correlates of memory and are highly sensitive to IAs. Activation of hippocampal nAChRs stimulates the release of norepinephrine (NE), a neurotransmitter implicated in modulating hippocampal synaptic plasticity. We tested the hypothesis that IAs disrupt hippocampal synaptic mechanisms critical to memory by determining the effects of isoflurane on NE release from hippocampal nerve terminals.
Methods
Isolated nerve terminals prepared from adult male Sprague–Dawley rat hippocampus were radiolabelled with [3H]NE and either [14C]GABA or [14C]glutamate and superfused at 37°C. Release evoked by a 2 min pulse of 100 µM nicotine or 5 µM 4-aminopyridine was evaluated in the presence or absence of isoflurane and/or selective antagonists.
Results
Nicotine-evoked NE release from rat hippocampal nerve terminals was nAChR- and Ca2+-dependent, involved both α7 and non-α7 subunit-containing nAChRs, and was partially dependent on voltage-gated Na+ channel activation based on sensitivities to various antagonists. Isoflurane inhibited nicotine-evoked NE release (IC50=0.18 mM) more potently than depolarization-evoked NE release (IC50=0.27 mM, P=0.014), consistent with distinct presynaptic mechanisms of IA action.
Conclusions
Inhibition of hippocampal nAChR-dependent NE release by subanaesthetic concentrations of isoflurane supports a role in IA-induced amnesia.
Keywords: anaesthetics volatile, isoflurane; brain, anaesthesia, molecular effects; brain, hippocampus; ions, ion channels, ligand-gated; nerve, neurotransmitters
General anaesthesia is a complex behavioural response to a number of chemically and pharmacologically diverse drugs, which is characterized by dose-, region-, and mechanism-specific endpoints.1 Amnesia occurs at low (subanaesthetic) concentrations, and immobility requires relatively higher concentrations of halogenated inhaled (volatile) anaesthetics (IAs), which supports the involvement of distinct neurophysiological mechanisms with different anaesthetic sensitivities.2 Whether amnesia and immobility result from graded effects on the same molecular/cellular target(s) or from separate targets with distinct anaesthetic sensitivities is unclear. The immobilizing effect has been localized to spinal networks, although the precise molecular and cellular mechanisms remain unknown.3 In contrast, amnesia involves forebrain structures such as amygdala and hippocampus, which are central to memory acquisition and consolidation.4
Changes in synaptic efficacy such as hippocampal long-term potentiation or depression are neuronal network correlates of memory that are modulated by both norepinephrine (NE)5,6 and nicotinic acetylcholine receptors (nAChRs).7 The nerve terminal is a potential site of action for anaesthetic effects on synaptic transmission.8 Selective inhibition of depolarization-evoked release of excitatory glutamate vs release of inhibitory GABA from neocortical nerve terminals by clinically relevant concentrations of IAs supports an overall depression of excitatory transmission during anaesthesia.9,10 Acetylcholine and NE are excitatory neurotransmitters implicated in memory mechanisms.11 Moreover, hippocampal activation of nAChRs stimulates NE release12 and facilitates long-term potentiation.13 As heterologously expressed neuronal nAChRs are highly sensitive to IAs at subanaesthetic concentrations,14,15 we determined the effects of isoflurane on NE release as a potential neurochemical model for the amnesic effects of IAs. We hypothesized that nAChR-evoked NE release from isolated hippocampal nerve terminals would be more sensitive to inhibition by IAs than depolarization-evoked release if involved in anaesthetic-induced amnesia.
Methods
All drugs were obtained from Sigma-Aldrich Chemical Co. (St Louis, MO, USA) unless otherwise indicated. Isolated nerve terminals (synaptosomes) were prepared from adult male Sprague–Dawley rats (200–280 g, n=42) as described previously10 under National Institutes of Health guidelines as approved by the Weill Cornell Medical College IACUC. Briefly, brains were rapidly removed from rats anaesthetized with 80% CO2/20% O2, the hippocampus was removed and homogenized in ice-cold 0.32 M sucrose with a motor-driven (500 rpm) Teflon-glass homogenizer, the homogenate was centrifuged for 2 min at 4000 g, and the resulting supernatant was centrifuged through a sucrose gradient to demyelinate and pellet the isolated hippocampal nerve terminals. Synaptosomes were radiolabelled with 20 nM [3H]NE (American Radiolabeled Chemicals, St Louis, MO, USA) for 60 min at 35°C in the presence of 1 µM GBR 12935 (a dopamine transport inhibitor to prevent the labelling of dopamine terminals) in 10 ml Krebs–HEPES buffer (KHB, composition in mM: NaCl 140, KCl 5, HEPES 20, MgCl2 1, Na2HPO4 1.2, NaHCO3 5, CaCl2 2, EGTA 0.1, and d-glucose 10, pH 7.4 with NaOH). Synaptosomes were co-labelled with either [14C]glutamate (1 µM; American Radiolabeled Chemicals) or [14C]GABA (2 µM; PerkinElmer, Boston, MA, USA), collected by centrifugation for 10 min at 20 000 g at 4°C, loaded into release chambers, and superfused at 37°C with KHB using a modified Brandel SF-12 apparatus.10 Release was evoked by 2 min pulses of 100 µM (–) nicotine or 5 µM 4-aminopyridine (4AP) in the presence of varying concentrations of isoflurane, nAChR antagonists, or the Na+ channel antagonist tetrodotoxin (TTX), all applied 12 min before the release stimulus. Radioactivity in 1 min fractions was determined by liquid scintillation spectrometry using BioSafe II scintillation cocktail (RPI, Mt Prospect, IL, USA) with quench correction (Beckman-Coulter, Fullerton, CA, USA).
Isoflurane concentrations were sampled and quantified by gas chromatography as described.10 Release was quantified as summed fractional release above baseline (sum ΔFR), normalized to the mean of all assay controls, and analysed using anova or the Student t-test. Concentration–effect data were fitted to sigmoidal curves using Prism version 5.0a (GraphPad, San Diego, CA, USA). Significant differences between IC50 values were determined by F-test comparison of best-fit values derived from independent curve fits.
Results
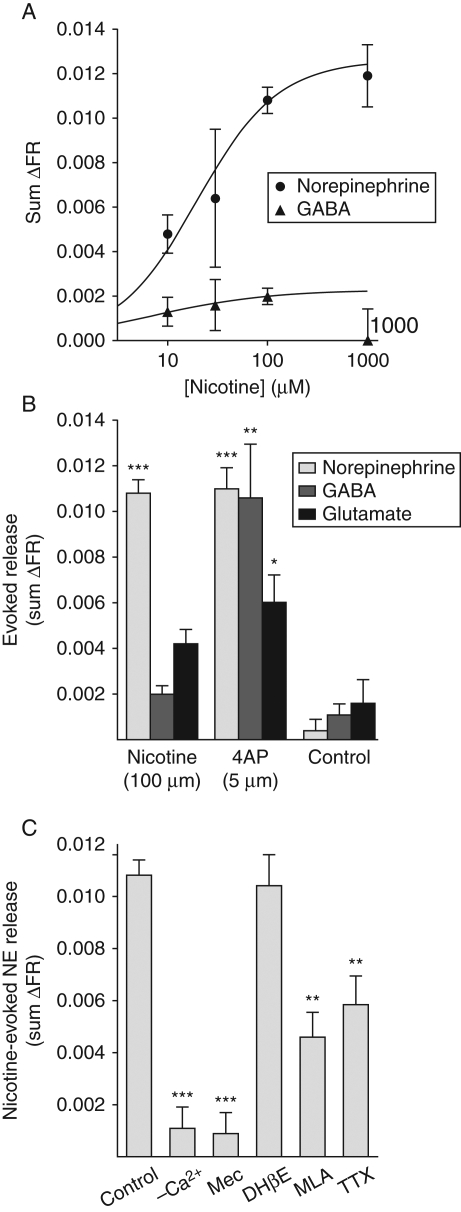
Stimulation of superfused rat hippocampal nerve terminals with a maximally effective concentration of nicotine (Fig. 1a), a non-selective nAChR agonist,13 selectively evoked NE release compared with GABA or glutamate release (Fig. 1b). In contrast, depolarization by 4AP, a K+ channel blocker that mimics repetitive action-potential-mediated depolarization,16 evoked significant release of NE, GABA, and glutamate (Fig. 1b). Comparable fractions of NE release were evoked by either 100 µM nicotine or 5 µM 4AP (Fig. 1b), which were 10-fold less than the fraction evoked by 1 mM 4AP, a near maximally effective concentration that is routinely used for in vitro studies of transmitter release (data not shown). Labelling of dopaminergic nerve terminals with [3H]NE17 was prevented by including GBR12935, a selective dopamine transporter blocker, during the radiolabelling procedure. Specific labelling of noradrenergic terminals was confirmed by the failure of dihyro-β-erythroidine (DHβE) to inhibit NE release (Fig. 1c), as DHβE potently inhibits nicotine-evoked dopamine release.12
Fig 1.
Nicotine-evoked NE release from rat hippocampal nerve terminals. (a) Nicotine-evoked NE release (sum ΔFR) was concentration-dependent and transmitter-selective, and maximally stimulated by 100 µM nicotine (n=4). (b) Nicotine selectively evoked NE (n=61) compared with GABA (n=43) and glutamate (n=35) release, although all three transmitters were evoked by stimulation with 5 µM 4AP (n=13, 4, 17, respectively), which evoked fractional release of NE comparable with that evoked by nicotine. Statistical comparisons of sum ΔFR values [mean (sem)] were performed by one-way anova (*P<0.05; **P<0.01; ***P<0.001 vs control). (c) Nicotine-evoked NE release (n=61) required extracellular Ca2+ (n=8) and was nAChR-dependent as indicated by inhibition by the non-selective nAChR blocker mecamylamine (Mec, 10 µM, n=5). Release was not inhibited by the selective β2 antagonist DHβE (30 µM, n=5) and was partially inhibited by the selective α7 antagonist MLA (10 µM, n=8) or the Na+ channel blocker TTX (1 µM, n=12). Statistical comparison of sum ΔFR values [mean (sem)] with control was performed by one-way anova (**P<0.01; ***P<0.001).
Nicotine-evoked NE release was dependent on extracellular Ca2+ and was completely blocked by the non-selective nAChR antagonist mecamylamine (Fig. 1c). Release was insensitive to the heteromeric β2-subunit-preferring nAChR antagonist DHβE,18 partially blocked by the homomeric α7 nAChR antagonist methyllycaconitine (MLA),19 and partially blocked by the selective voltage-gated Na+ channel blocker TTX (Fig. 1c).
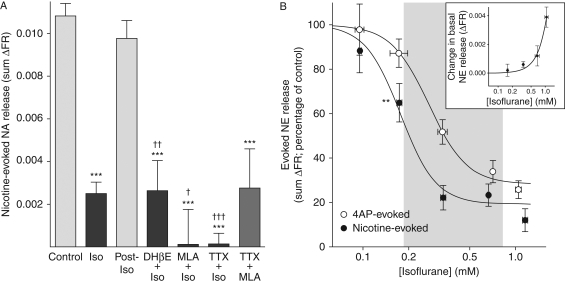
Nicotine-evoked NE release was inhibited by isoflurane; release returned to control levels after 12 min of isoflurane-free perfusion (which reduced isoflurane concentrations to undetectable levels; Fig. 2a). The single-drug and two-drug combination data were compared by the Student t-test after F-test determination of matching standard deviations. Maximal inhibition of nicotine-evoked NE release by isoflurane (achieved with 0.7 mM) was unaffected by the addition of DHβE, but was significantly increased by the addition of MLA or TTX (Fig. 2a). Combined MLA and TTX did not increase inhibition compared with either TTX or MLA alone.
Fig 2.
Nicotinic receptor-evoked NE release from rat hippocampal nerve terminals was potently inhibited by isoflurane. (a) Isoflurane [Iso, 0.68 (0.01) mM, n=13] inhibited nicotine (100 µM)-evoked NE release (n=61), which was reversible [no effect after 12 min washout of 0.72 (0.003) mM isoflurane, Post-Iso, n=3]. Residual release in the presence of isoflurane [0.68 (0.02) mM] was not further reduced by DHβE (30 µM, n=6), whereas MLA (10 µM, n=9) with isoflurane [0.76 (0.01) mM] and TTX (1 µM, n=9) with isoflurane [0.70 (0.01) mM] additively inhibited release. The combination of TTX with MLA (n=9) did not produce greater inhibition than TTX or MLA alone. Statistical comparison of sum ΔFR values [mean (sem)] with control was performed by one-way anova (***P<0.001), and comparisons between drug vs drug combinations were performed by the Student t-test (†P<0.05; ††P<0.01; †††P<0.001). (b) Isoflurane inhibited nicotine (100 µM)-evoked NE release [IC50=0.18 (0.02) mM] more potently (P=0.02) than NE release evoked by 5 µM 4AP [IC50=0.27 (0.03) mM]. Shaded area highlights the clinical concentration range of isoflurane between 0.5 and 2 times the MAC (0.35 mM).37 Only nicotine-evoked NE was significantly inhibited by less than clinical concentrations of isoflurane (F-test comparison with 100%, **P<0.01). Significant increases in basal NE release by isoflurane alone were evident only at concentrations >0.4 mM (∼1 MAC; inset). Data are presented as mean (sem).
Inhibition of nicotine- or 4AP-evoked NE release by isoflurane was concentration-dependent (Fig. 2b). Nicotine-evoked NE release was significantly more sensitive (P=0.02) to inhibition by isoflurane [IC50=0.18 (sd 0.02) mM] than was 4AP-evoked NE release [IC50=0.27 (0.03) mM; Fig. 2b]. Significant inhibition occurred at subanaesthetic isoflurane concentrations [35 (9)% at 0.18 mM, P=0.002] based on its minimum alveolar concentration (MAC) for immobilization in response to a noxious stimulus in rats (0.35 mM).20 Isoflurane at concentrations >0.4 mM produced sustained increases in basal NE release (Fig. 2b, inset), as described previously for halothane.21
Discussion
Nerve terminals are potential sites of IA effects on synaptic transmission.8–10 nAChRs are ligand-gated ion channels that can modulate transmitter release by mediating presynaptic Na+ and Ca2+ entry.22 Some subtypes of nAChRs are potently blocked by IAs,14,15,23 but the possible role of this mechanism in presynaptic anaesthetic effects had not been determined. We tested the hypothesis that the IA isoflurane selectively inhibits nAChR-evoked vs depolarization-evoked NE release. Isoflurane inhibited nicotine-evoked NA release from isolated rat hippocampal nerve terminals at significantly lower concentrations compared with NE release evoked by the non-selective depolarizing agent 4AP. This suggests that isoflurane inhibits nicotine-evoked NE release by a distinct mechanism from that involved in its inhibition of generalized depolarization-evoked release. This differential sensitivity of nAChR- vs depolarization-evoked release to inhibition by isoflurane correlates with the lower doses of isoflurane required for amnesia vs immobility in vivo, such that these synaptic effects could be relevant mechanisms for distinct dose-dependent end-points of isoflurane anaesthesia.
Pentameric nAChRs comprise multiple subunit isoforms (α2–10 and β2–4), but most nAChRs in the central nervous system are composed of heteromeric α4β2 or homomeric α7 subunits, both of which appear to be critical for memory function.24 The α3β4 subtype is predominantly peripheral, but is also found in adult rat hippocampus25 where it contributes to nicotine-evoked NE release from rat hippocampal synaptosomes.26,27 The α4β2 and α3β4 nAChR subtypes selectively gate Na+, whereas α7 nAChRs have a higher ratio of Ca2+ to Na+ permeability.13 Use of selective receptor blockers showed that 43% of nicotine-evoked NE release was mediated by MLA-sensitive α7-subunit-containing nAChRs, whereas DHβE-sensitive heteromeric nAChRs were not involved. Although selective inhibitors of β4-containing nAChRs are unavailable, the use of selective α-conotoxins suggests that non-α7-dependent NE release in hippocampus is probably mediated by α3β4 nAChRs.26,27
NE release evoked by nicotine via nAChR activation was compared with NE release evoked by 4AP, a secretegogue that indirectly activates presynaptic Na+ channels and mimics action potential stimulation of neurotransmitter release.9,16 Nicotine evoked NE release, but not glutamate or GABA release, consistent with selective localization of nAChRs on noradrenergic terminals. Voltage-gated Na+ channel block by TTX, which prevents depolarization-dependent activation of downstream voltage-gated Ca2+ channels, partially inhibited nicotine-evoked NE release. This suggests that nAChR activation can trigger downstream Na+ channel activation to produce further depolarization and Ca2+-dependent NE release. Presynaptic nAChR effects are region-specific, as nicotinic receptor agonists evoke excitatory amino acid release from rat prefrontal cortex28 and GABA release from mouse whole-brain29 synaptosomes. In addition to differences in nAChR-subtype distribution between brain regions,30,31 differences in nAChR-subtypes exist between the same brain region of different species.27,32 Identification of the specific nAChR subtypes involved in regulating hippocampal NE release and their individual anaesthetic sensitivities will require more selective nAChR-subtype ligands and/or targeted genetic deletion studies.
Hippocampal long-term potentiation, a form of synaptic plasticity that is widely studied as a cellular correlate of memory,33 is modulated by both norepinephrine34 and nAChRs.24 Neuronal nAChR-mediated NE release in hippocampus plays a critical role in memory formation35 and is a likely site of anaesthetic-induced amnesia.1 Neuronal nAChRs are sensitive to IAs at subanaesthetic concentrations that are sufficient to produce amnesia23 but insufficient for immobilization in response to a painful stimulus as defined by the MAC.2,36 Our observations that isoflurane at subanaesthetic concentrations inhibits nAChR-dependent release of NE from rat hippocampal nerve terminals are consistent with the ability of IAs to disrupt hippocampal memory mechanisms.37 Inhibition of NE release evoked by 4AP-induced depolarization required higher isoflurane concentrations comparable with those that inhibit 4AP-evoked glutamate release from rat9 and mouse38 cortical nerve terminals. These less potent effects of isoflurane on depolarization-evoked transmitter release involve effects on presynaptic voltage-gated Na+ channels,39 Ca2+ channels,40 and/or TREK-1 channels.41 Taken together, these findings indicate that both ligand-gated and voltage-gated ion channels are presynaptic targets for dose-dependent transmitter-selective effects of IAs on transmitter release from mammalian nerve terminals.
Isoflurane inhibited nAChR-mediated NE release by up to 77% (but this maximal effect is probably limited by the stimulatory effect of isoflurane on basal NE release), implicating effects on both heteromeric and α7-subunit-containing receptors. The α7-subunit-selective blocker MLA produced 67% inhibition of nicotine-evoked NE release. Both TTX- and MLA-insensitive nicotine-evoked NE release were fully inhibited by isoflurane, demonstrating IA effects on targets in addition to voltage-gated Na+ channels and α7-containing nAChRs, probably including heteromeric nAChR subtypes such as α3β4 as discussed earlier. This conclusion is further supported by the incomplete block of nicotine-evoked NE release by the combination of TTX and MLA. Recently, inhibition of nicotine-evoked NE release from spinal cord slices has also been shown to involve α7 nAChRs, and not β2-subunit containing nAChRs.23 Earlier studies had suggested that α7 nAChRs were resistant to isoflurane,14 but subsequent studies indicated that both α7 and non-α7-subunit containing nAChRs are indeed inhibited by isoflurane.23,42
In summary, isoflurane inhibited nAChR-dependent NE release from isolated rat hippocampal nerve terminals at subanaesthetic concentrations in the range of its amnesic effects. This correlates with the greater sensitivity to inhibition by IAs of nAChR-dependent NE release compared with depolarization-evoked transmitter release. These findings are consistent with distinct presynaptic mechanisms for anaesthetic-induced amnesia, involving nAChR inhibition, and for immobility, involving the inhibition of Na+ channels and for possibly other targets at higher doses. Presynaptic anaesthetic mechanisms include concentration-dependent inhibition of both ligand-gated and voltage-gated ion channels coupled to neurotransmitter-selective inhibition of transmitter release.
Funding
Supported by National Institutes of Health grant GM58055. We thank Pamela Flood for helpful discussions.
References
- 1.Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–20. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- 2.Eger EI, II, Koblin DD, Harris RA, et al. Hypothesis: inhaled anesthetics produce immobility and amnesia by different mechanisms at different sites. Anesth Analg. 1997;84:915–8. doi: 10.1097/00000539-199704000-00039. [DOI] [PubMed] [Google Scholar]
- 3.Sonner J, Antognini JF, Dutton RC, et al. Inhaled anesthetics and immobility: mechanisms, mysteries, and minimum alveolar anesthetic concentration. Anesth Analg. 2003;97:718–40. doi: 10.1213/01.ANE.0000081063.76651.33. [DOI] [PubMed] [Google Scholar]
- 4.McGaugh JL. The amygdala modulates the consolidation of memories of emotional arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 5.Scheiderer CL, Dobrunz LE, McMahon LL. Novel form of long-term synaptic depression in rat hippocampus induced by activation of alpha 1 adrenergic receptors. J Neurophysiol. 2004;91:1071–7. doi: 10.1152/jn.00420.2003. [DOI] [PubMed] [Google Scholar]
- 6.Strange BA, Dolan RJ. β-Adrenergic modulation of amygdala and hippocampal responses. Proc Natl Acad Sci USA. 2004;101:11454–8. doi: 10.1073/pnas.0404282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKay BE, Placzek AN, Dani JA. Regulation of synaptic transmission and plasticity by neuronal nicotinic acetylcholine receptors. Biochem Pharmacol. 2007;74:1120–33. doi: 10.1016/j.bcp.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemmings HC, Jr, Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26:503–10. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Westphalen RI, Hemmings HC., Jr Volatile anesthetic effects on glutamate versus GABA release from isolated rat cortical nerve terminals: 4-aminopyridine evoked release. J Pharmacol Exp Ther. 2006;316:216–23. doi: 10.1124/jpet.105.090662. [DOI] [PubMed] [Google Scholar]
- 10.Westphalen RI, Hemmings HC., Jr Volatile anesthetic effects on glutamate versus GABA release from isolated rat cortical nerve terminals: basal release. J Pharmacol Exp Ther. 2006;316:208–15. doi: 10.1124/jpet.105.090647. [DOI] [PubMed] [Google Scholar]
- 11.Canal CE, Chang Q, Gold PE. Intra-amygdala injections of CREB antisense impair inhibitory avoidance memory: role of norepinephrine and acetylcholine. Learn Mem. 2008;15:677–86. doi: 10.1101/lm.904308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clarke PB, Reuben M. Release of [3H]noradrenaline from rat hippocampal synaptosomes by nicotine: mediation by different nicotonic receptor subtypes from striatal [3H]-dopamine release. Br J Pharmacol. 1996;117:595–606. doi: 10.1111/j.1476-5381.1996.tb15232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu Rev Pharmacol Toxicol. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- 14.Flood P, Ramirez-Latorre J, Role L. α4β2 Neuronal nicotinic avetylcholine receptors in the central nervous system are inhibited by isoflurane and propofol, but α7-type nicotinic acetylcholine receptors are unaffected. Anesthesiology. 1997;86:859–65. doi: 10.1097/00000542-199704000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Violet JM, Downie DL, Nakisa RC, Lieb WR, Franks NP. Differential sensitivities of mammalian neuronal and muscle nicotinic acetylcholine receptors to general anesthetics. Anesthesiology. 1997;86:866–74. doi: 10.1097/00000542-199704000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Tibbs GR, Barrie AP, Van Mieghem FJE, McMahon HT, Nicholls DG. Repetitive action potentials in isolated nerve terminals in the presence of 4-aminopyridine: effects on cytosolic free Ca2+ and glutamate release. J Neurochem. 1989;53:1693–9. doi: 10.1111/j.1471-4159.1989.tb09232.x. [DOI] [PubMed] [Google Scholar]
- 17.Rothman RB, Bauman MH, Dersch CM, et al. Amphetamine-type central nervous system stimulates release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliot KJ, Johnson EC. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors hα2β2, hα2β4, hα3β2, hα3β4, hα4β2, hα4β4 and hα7 expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1997;280:346–56. [PubMed] [Google Scholar]
- 19.Barik J, Wonnacott S. Indirect modulation by α7 nicotinic acetylcholinereceptors of noradrenaline release in rat hippocampal slices: interaction with glutamate and GABA systems and effect of nicotine withdrawal. Mol Pharmacol. 2006;69:618–28. doi: 10.1124/mol.105.018184. [DOI] [PubMed] [Google Scholar]
- 20.Taheri S, Halsey MJ, Liu J, Eger EI, II, Koblin DD, Laster MJ. What solvent best represents the site of action on inhaled anesthetics in humans, rats, and dogs. Anesth Analg. 1991;72:627–34. doi: 10.1213/00000539-199105000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Pashkov VN, Hemmings HC., Jr The effects of general anesthetics on norepinephrine release from isolated rat cortical nerve terminals. Anesth Analg. 2002;95:1274–81. doi: 10.1097/00000539-200211000-00032. [DOI] [PubMed] [Google Scholar]
- 22.McDermott AB, Role LW, Siegelbaum SA. Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci. 1999;22:443–85. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- 23.Rowley TJ, Flood P. Isoflurane prevents nicotine-evoked norepinephrine release from the mouse spinal cord at low clinical concentrations. Anesth Analg. 2008;107:885–9. doi: 10.1213/01.ane.0000287646.85834.1a. [DOI] [PubMed] [Google Scholar]
- 24.Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psycopharmacology. 2006;184:523–39. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- 25.Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Res. 2008;84:30–44. doi: 10.1002/bdrc.20118. [DOI] [PubMed] [Google Scholar]
- 26.Luo S, Kulak JM, Cartier GE, et al. α-Conotoxin AuIB selectively blocks α3β4 nicotinic acetylcholine receptors and nicotine-evoked norepinephrine release. J Neurosci. 1998;18:8571–9. doi: 10.1523/JNEUROSCI.18-21-08571.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azam L, McIntosh JM. Characterization of nicotinic acetylcholine receptors that modulate nicotine-evoked [3H]norepinephrine release from mouse hippocampal synaptosomes. Mol Pharmacol. 2006;70:967–76. doi: 10.1124/mol.106.024513. [DOI] [PubMed] [Google Scholar]
- 28.Dickinson JA, Kew JNC, Wonnacott S. Presynaptic α7- and β2-containing nicotinic acetylcholine receptors modulate excitatory amino acid release from rat prefrontal cortex nerve terminals via distinct cellular mechanisms. Mol Pharmacol. 2008;74:348–59. doi: 10.1124/mol.108.046623. [DOI] [PubMed] [Google Scholar]
- 29.Lu Y, Grady S, Marks MJ, Picciotto M, Changeux J-P, Collins AC. Pharmacological characterization of nicotinic receptor-stimulated GABA release from mouse brain synaptsomes. J Pharmacol Exp Ther. 1998;287:648–57. [PubMed] [Google Scholar]
- 30.Fabian-Fine R, Skehel P, Errington ML, et al. Ultrastructural distribution of the α7 nicotinic acetylcholine receptor subunit in rat hippocampus. J Neurosci. 2001;21:7993–8003. doi: 10.1523/JNEUROSCI.21-20-07993.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones IW, Wonnacott S. Precise localization of α7 nicotinic acetylcholine receptors on glutamatergic axon terminals in the rat ventral temental area. J Neurosci. 2004;24:11244–52. doi: 10.1523/JNEUROSCI.3009-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amtage F, Neughebauer B, McIntosh JM, et al. Characterization of nicotinic receptors inducing noradrenaline release and absence of nicotinic autoreceptors in human neocortex. Brain Res Bull. 2004;62:413–23. doi: 10.1016/j.brainresbull.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 33.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–9. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 34.Lee EH, Ma YL. Amphetamine enhances memory retention and facilitates norepinephrine release from hippocampus in rats. Brain Res Bull. 1995;37:411–6. doi: 10.1016/0361-9230(95)00039-9. [DOI] [PubMed] [Google Scholar]
- 35.Fu Y, Matta SG, James TJ, Sharp BM. Nicotine-induced norepinephrine release in the rat amygdala and hippocampus is mediated through brainstem nicotinic cholinergic receptors. J Pharmacol Exp Ther. 1998;284:1188–96. [PubMed] [Google Scholar]
- 36.Flood P, Sonner JM, Gong D, Coates KM. Heteromeric nicotinic inhibition by isoflurane does not mediate MAC or loss of righting reflex. Anesthesiology. 2002;97:902–5. doi: 10.1097/00000542-200210000-00023. [DOI] [PubMed] [Google Scholar]
- 37.Ghoneim MM. Drugs and human memory (part 2) Anesthesiology. 2004;100:1277–97. doi: 10.1097/00000542-200405000-00033. [DOI] [PubMed] [Google Scholar]
- 38.Westphalen RI, Krivitski M, Amarosa A, Guy N, Hemmings HC., Jr Reduced inhibition of cortical glutamate and GABA release by halothane in mice lacking the K+ channel, TREK-1. Br J Pharmacol. 2007;152:939–45. doi: 10.1038/sj.bjp.0707450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ouyang W, Hemmings HC., Jr Depression by isoflurane of the action potential and underlying voltage-gated ion currents in isolated rat neurohypophysial nerve terminals. J Pharmacol Exp Ther. 2005;312:801–8. doi: 10.1124/jpet.104.074609. [DOI] [PubMed] [Google Scholar]
- 40.Miao N, Frazer MJ, Lynch CI. Volatile anethetics depress Ca2+ transients and glutamate release in isolated cerebral synaptosomes. Anesthesiology. 1995;83:593–603. doi: 10.1097/00000542-199509000-00019. [DOI] [PubMed] [Google Scholar]
- 41.Heurteaux C, Guy N, Laigle C, et al. TREK-1, a K+ channel involved in neuroprotection and general anesthesia. EMBO J. 2004;23:2684–95. doi: 10.1038/sj.emboj.7600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flood P, Coates KM. Sensitivity of the α7 nicotinic acetylcholine receptor to isoflurane may depend on receptor inactivation. Anesth Analg. 2002;95:83–7. doi: 10.1097/00000539-200207000-00015. [DOI] [PubMed] [Google Scholar]