Abstract
It is generally acknowledged that cytotoxic T-lymphocyte–associated antigen-4 (CTLA-4/CD152) plays a pivotal role in the regulation of T-cell activation and the establishment of self-tolerance in the periphery. CTLA-4–deficient (CTLA-4KO) mice develop a lymphoproliferative disorder and die within 4 weeks of birth, suggesting a role for CTLA-4 in T-cell homeostasis or the development and activity of T-regulatory (Treg) cells. To study the role of CTLA-4 in the control of experimental autoimmune encephalomyelitis (EAE), we have generated a CTLA-4KO mouse in which >90% of all CD4+ T cells bear a Vβ8.2 transgenic T-cell receptor that is specific for myelin basic protein peptide Ac1–9 (ASQKRPSQR). These mice do not develop spontaneous lymphoproliferative disease or EAE and are resistant to disease induction. This correlates with a higher frequency of functional FoxP3+ Treg cells in the spleen and thymus of CTLA-4KO mice. The absence of CTLA-4–mediated suppression of CD28 signaling resulted in the early expression of FoxP3 on double-positive cells in the thymic cortex. We conclude that CTLA-4 is not essential for the peripheral function of FoxP3+ Treg cells but plays a pivotal role in their thymic selection.
Keywords: thymus suppression, experimental autoimmune encephalomyelitis, costimulation
Cytotoxic T-lymphocyte–associated antigen-4 (CTLA-4, also known as CD152) has long been known as an important mediator of costimulatory signals, but its precise role remains a subject of intense debate. Mice deficient in CTLA-4 develop spontaneous lymphoproliferative disease with multiorgan lymphocytic infiltration within 3–4 weeks of age (1, 2), suggesting either aberrant homeostatic control or diminished function of T-regulatory (Treg) cells. CTLA-4 has been suggested to function by increasing the activation threshold of T cells specific for endogenous but not exogenous antigen (3, 4). CTLA-4 deficiency would therefore lead to disproportionate T-cell activation and proliferation. Schneider et al. (5, 6), however, have suggested that CTLA-4 could have a role in T-cell activation through up-regulation of lymphocyte function-associated antigen-1 (LFA-1) and reversal of the T-cell receptor (TCR) stop signal. Besides playing a role in the activation of responder T cells, the excessive lymphoproliferation observed in CTLA-4KO mice may also be attributable to diminished suppressive activity of Treg cells. Indeed, the lymphoproliferative disorder characteristic of CTLA-4KO mice is not dissimilar to that observed in mice deficient in FoxP3, the key regulator of Treg-cell function (7, 8). Furthermore, CTLA-4 has been shown to be expressed constitutively on the cell surface and in the cytoplasm of peripheral CD4+CD25+ Treg cells, whereas CD4+CD25− conventional T cells only express CTLA-4 after activation (9). Several studies using neutralizing anti-CTLA-4 antibodies have suggested a role for CTLA-4 in Treg-cell function in vivo (9, 10). In each of these studies, blocking CTLA-4 with a specific monoclonal antibody led to exacerbation of autoimmune disease (e.g., colitis, gastritis), presumably through inhibition of Treg-cell function. The mechanism by which CTLA-4 might contribute to Treg-cell function remains unclear, although factors such as ligand competition with CD28 (11), direct inhibition of CD28 signaling (11, 12), up-regulation of indoleamine 2,3-dioxygenase production by antigen-presenting cells (13), or regulation of immunological synapse formation (14) have been suggested to play a role.
Despite extensive reports on the essential role of CTLA-4 in Treg function, several studies refute these claims. Waterhouse et al. (1) showed that CTLA-4–deficient TCR transgenic mice exhibit normal viability and decreased lymphoproliferation, thus suggesting that the lymphoproliferation observed in nontransgenic mice is antigen specific and does not reflect a general deficiency in T-cell regulation. This was confirmed in DO11+Rag2−/−CTLA-4−/− mice that only have T cells with TCR specific for the exogenous antigen ovalbumin (OVA), and therefore do not show signs of spontaneous lymphoproliferation, lymphadenopathy, or splenomegaly (15). In contrast to their findings with anti-CTLA-4–treated mice, Read et al. (16) found that in B7–1/B7–2/CTLA-4 KO mice, 2.5–3.0% of CD4+ splenocytes express FoxP3 and represent Treg cells that are functional in vivo. In support of this finding, mice overexpressing a truncated mutant of CTLA-4 still produced Treg cells with in vitro suppressive activity (17).
To address the conflicting data concerning the role of CTLA-4 in the control of autoimmune disease, we have created a CTLA-4KO mouse in which >90% of all CD4+ T cells bear a Vβ8.2 transgenic TCR (Tg4) specific for myelin basic protein (MBP) peptide Ac1–9 (ASQKRPSQR) (18). These mice are healthy and are surprisingly resistant to the induction of CNS autoimmune disease because of enhanced thymic selection of functional FoxP3+ Treg cells.
Results
CTLA-4KO Mice Are Resistant to Experimental Autoimmune Encephalomyelitis Induction.
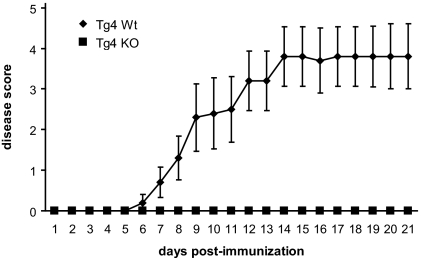
CTLA-4KO mice were bred with Rag−/− Tg4 TCR transgenic mice to generate both Rag+/− and Rag−/− CTLA-4–deficient Tg4 mice. Neither mouse strain suffered from spontaneous lymphoproliferative disease. We induced experimental autoimmune encephalomyelitis (EAE) in Tg4Rag+/−CTLA-4−/− (hereafter termed Tg4 KO) mice and in Tg4Rag+/−CTLA-4+/+ (Tg4 WT) control mice by immunization with MBP peptide Ac1–9 in complete Freund's adjuvant (CFA) to examine a possible role for CTLA-4 in the control of CNS autoimmune disease. Surprisingly, whereas the Tg4 WT mice consistently developed rather severe disease after day 7, the Tg4 KO mice did not develop disease during a follow-up of up to 4 weeks (Fig. 1). Based on the available literature, this could be the result of unresponsiveness of CD4+CD25− T cells because of increased deletion of autoreactive T cells in the thymus (19, 20). Alternatively, the lack of disease could result from augmented activity of Treg cells. We therefore proceeded to investigate both possibilities.
Fig. 1.
Tg4 KO mice are resistant to induction of EAE. Tg4 WT (n = 5) and Tg4 KO (n = 3) mice were immunized with MBP Ac1–9 and pertussis toxin and monitored daily for EAE. Mortality rates were 3 of 5 for Tg4 WT mice and 0 of 3 for Tg4 KO mice. Data are represented as mean ± SEM.
CD4+CD25− T Cells from Tg4 WT and KO Mice Are Equally or More Responsive.
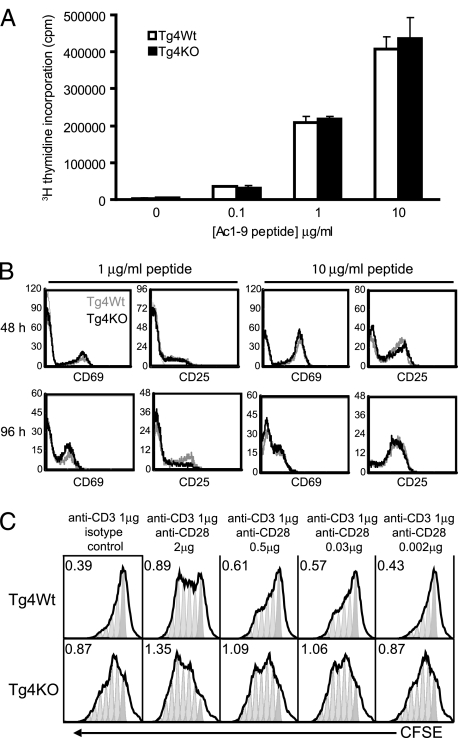
To determine if the striking reduction in disease observed in the Tg4 KO mice was the result of a reduced ability of their T-effector (Teff) cells to respond to antigen, CD4+CD25− T cells were isolated from both Tg4 WT and Tg4 KO mice and were stimulated with MBP Ac1–9 peptide in vitro. After 72 h, the proliferation in response to titrated doses of antigen was determined, revealing no hindrance in the response of Tg4 KO CD4+CD25− T cells (Fig. 2A). This was confirmed by comparing the expression of the activation markers CD25 and CD69 on CD4+CD25− T cells from Tg4 WT and Tg4 KO mice in response to titrated doses of MBP Ac1–9 peptide over a 96-h time period (Fig. 2B). In addition to antigen-specific proliferation, we compared the response of Tg4 WT and Tg4 KO CD4+CD25− T cells to latex microspheres coated with anti-CD3 and anti-CD28 mAb in [3H]thymidine incorporation and carboxyfluorescein succinimidyl ester dilution experiments. Conditions with a constant amount of anti-CD3 and titrated levels of anti-CD28 mAb revealed that Tg4 KO CD4+CD25− T cells respond to anti-CD3 stimulation alone, whereas Tg4 WT CD4+CD25− T cells require a certain threshold of CD28 stimulation (Fig. 2C). Therefore, not only were Tg4 KO CD4+CD25− T cells shown to be able to respond to antigen in conditions with ample costimulation, but these cells exhibited a limited response even in the absence of CD28, whereas Tg4 WT CD4+CD25− T cells remain quiescent. These results confirm previous observations on the role of CTLA-4 in balancing CD28 signaling (11, 12).
Fig. 2.
CD4+CD25− T cells from Tg4 KO mice are not functionally inhibited. (A) CD4+CD25− T cells from Tg4 WT and Tg4 KO mice were cultured in vitro for 72 h in the presence of titrated doses of MBP Ac1–9 peptide in the presence of irradiated APC and then pulsed with [3H]thymidine overnight. Representative data are shown as mean ± SD. (B) CD4+CD25− T cells from Tg4 WT and Tg4 KO mice were cultured in vitro for up to 96 h in the presence of 1 or 10 μg/mL MBP Ac1–9 peptide and stained at regular intervals for CD25 and CD69. The faint gray lines show CD25 and CD69 expression before stimulation. (C) Carboxyfluorescein succinimidyl ester (CFSE)-labeled CD4+CD25− T cells from Tg4 WT and Tg4 KO mice were stimulated in vitro for 72 h with latex microspheres coated with 1 μg/mL anti-CD3 and titrated concentrations of anti-CD28 or isotype control antibody, as indicated. Results are displayed on arbitrary scales, where the x axis shows CFSE fluorescence intensity and the y axis shows the number of cells at each intensity. Division peaks and division indexes (the average number of times that each responding cell has divided) have been added using FlowJo software (Tree Star) to demonstrate the differences in response. All data are representative of 3 or more independent experiments.
The Spleen and Thymus of Tg4 KO Mice Display Higher Frequencies of Functional Treg Cells.
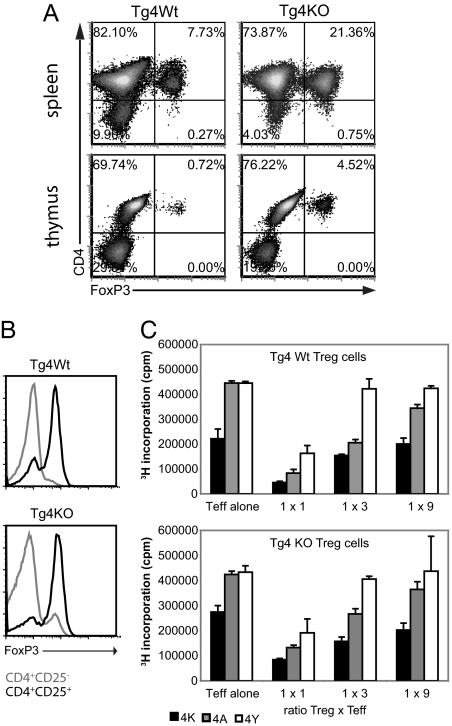
The number of FoxP3+ Treg cells in the spleen and thymus of Tg4 WT and Tg4 KO mice was compared, and the latter consistently displayed a ≈3–4-fold increase in the proportion of Treg cells, both in the spleen and in the thymus (Fig. 3A). The spleen and thymus size, as well as the total number of T cells, did not differ significantly between the 2 groups, and FoxP3 expression was only detected on CD4+ cells. The total number of FoxP3+ CD4+ T cells per spleen averages approximately 3.106 in the Tg4 WT mice and approximately 10.106 in the Tg4 KO mice. Interestingly, the CTLA-4 deficiency in Tg4 KO mice not only led to enhanced numbers of CD4+CD25+FoxP3+ Treg cells but showed a similar increase in FoxP3 expression by CD4+CD25− cells (Fig. 3B). This finding of augmented Treg-cell numbers in Tg4 KO mice contrasts with previous reports on FoxP3 expression in CTLA-4KO mice, which showed equal or reduced presence of peripheral Treg cells (16, 17). To examine if it was indeed the greater frequency of FoxP3+ Treg cells and not a difference in suppressive quality that was responsible for the superior disease resistance of Tg4 KO mice, we isolated CD4+CD25+ Treg cells from the spleen of Tg4 KO and Tg4 WT mice by magnetic cell separation. These Treg cells were then placed directly in culture with various ratios of CD4+CD25− Tg4 WT Teff cells and subsequently stimulated with peptides of differing antigenic strength for the Tg4 TCR. Besides the natural form of MBP Ac1–9 (4K), peptides with altered amino acids at position 4 were used, 4A and 4Y, which have ≈100- and ≈100,000-fold higher affinity for MHC class II (Au) than 4K, respectively (21). As shown in Fig. 3C, Treg cells from both Tg4 WT and Tg4 KO mice suppressed proliferation effectively, with no distinction between the activities of either type of Treg cell. Interestingly, both types of Treg cell were capable of suppressing proliferation of CD4+CD25− T cells in response to peptide, with an affinity up to 100,000 times higher than that of the peptide they were selected for in the thymus.
Fig. 3.
Tg4 KO mice display higher frequencies of functional Treg cells. (A) Isolated splenocytes enriched for CD4 expression by magnetic cell sorting and total thymocytes from Tg4 WT and Tg4 KO mice were stained for CD4 and FoxP3 directly ex vivo. (B) FoxP3 expression on splenocytes from Tg4 WT and Tg4 KO mice was gated on CD4+CD25− and CD4+CD25+ directly ex vivo. (C) CD4+CD25− T cells from Tg4 WT mice were stimulated in vitro for 72 h with MBP Ac1–9 (4K) or its higher MHC II affinity analogues 4A and 4Y. The suppressive activity of Tg4 WT (Upper) or Tg4 KO (Lower) CD4+CD25+ Treg cells at various T-cell ratios is shown. All data are representative of 3 or more independent experiments.
Genetic Depletion of FoxP3+ Treg Cells from Tg4 KO Mice Culminates in Spontaneous EAE.
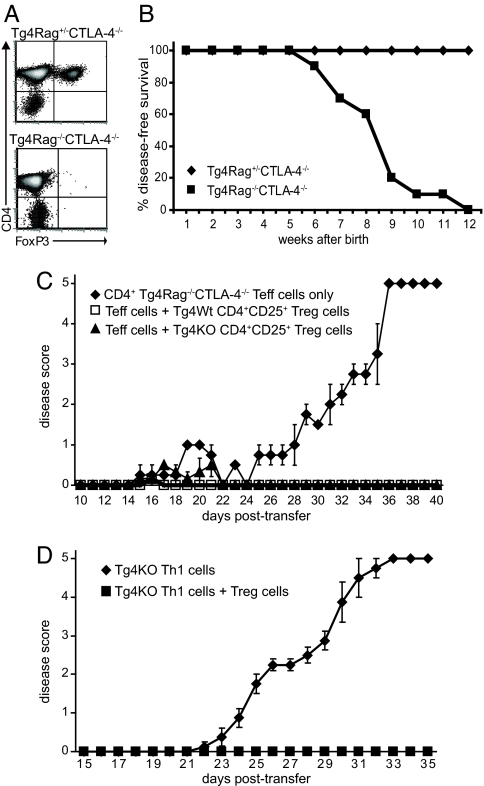
To confirm in vivo that the resistance of Tg4 KO mice to induced disease is attributable to the elevated frequency of Treg cells rather than to unresponsiveness of Teff cells, we backcrossed the Tg4 KO mice onto a Rag−/− background. Although similar to Tg4 KO mice, Tg4Rag−/−CTLA-4−/− mice did not contain any FoxP3+ cells (Fig. 4A), thus providing a model devoid of natural Treg cells. Unmanipulated, Tg4Rag−/−CTLA-4−/− mice remained free of disease for 7–9 weeks after birth, after which they developed spontaneous EAE, without general lymphoproliferative disease (Fig. 4B). In comparison, Tg4Rag−/−CTLA-4+/+ mice developed spontaneous disease at 11–12 weeks after birth, whereas Tg4Rag+/−CTLA-4+/+ mice remained free of disease for life (data not shown). Tg4 mice therefore resemble similar MBP-specific TCR transgenics in which Rag−/− animals develop spontaneous disease (22). The difference in spontaneous disease induction between CD4+ T cells from Tg4Rag−/−CTLA-4−/− and Tg4Rag−/−CTLA-4+/+ mice was further confirmed in an adoptive transfer model. When only 1.106 CD4+ Tg4Rag−/−CTLA-4−/− T cells were transferred to B10.PL(H-2u)Rag−/− mice, these animals developed EAE within 4 weeks after transfer, without peptide and/or adjuvant challenge (Fig. 4C). When, however, 1.106 CD4+ Tg4Rag−/−CTLA-4+/+ T cells were transferred, this did not lead to the spontaneous generation of disease within a follow-up of 7 weeks (data not shown). Although Tg4Rag−/−CTLA-4−/− CD4+ T cells are clearly autoreactive, they remain susceptible to suppression by CD4+CD25+ Treg cells. As shown in Fig. 4C, the cotransfer of 1.106 CD4+ Tg4Rag−/−CTLA-4−/− T cells with 2.105 Tg4 WT or Tg4 KO CD4+CD25+ Treg cells successfully protected B10.PLRag−/− mice from the spontaneous manifestation of EAE. The chosen ratio of Teff to Treg cells reflects that found in Tg4 KO mice. This experiment was repeated with in vitro stimulated CD4+CD25− Tg4 KO T cells to demonstrate that Rag-sufficient, CTLA-4–deficient, CD4+ T cells can cause disease if allowed to be activated. Preactivated Tg4 KO T helper 1 (Th1) cells induced progressive EAE on transfer to B10.PLRag−/− recipients (Fig. 4D). Importantly, cotransfer of Tg4 KO CD4+CD25+ Treg cells at a 1 to 5 ratio protected effectively against disease, further demonstrating that the expression of CTLA-4 on neither Teff nor Treg cells is essential for successful suppression. Together, these findings corroborate our belief that the increase in FoxP3+ Treg cells was responsible for the resistance against induced disease in Tg4 KO mice, rather than a direct negative effect on the activity or development of Teff cells.
Fig. 4.
Treg cells protect Tg4Rag+/−CTLA-4−/− from spontaneous disease. (A) Splenocytes of Tg4Rag+/−CTLA-4−/− and Tg4Rag−/−CTLA-4−/− were stained with anti-CD4 and anti-FoxP3 directly ex vivo and analyzed by flow cytometry. The data are representative of at least 3 independent experiments. (B) Tg4Rag+/−CTLA-4−/− (n = 7) and Tg4Rag−/−CTLA-4−/− (n = 10) were kept in specific pathogen-free conditions and monitored for the spontaneous development of EAE over a period of up to 4 months. (C) B10.PL(H-2u)Rag−/− mice received a transfer of 1.106 CD4+ Tg4Rag−/−CTLA-4−/− T cells with 2.105 Tg4 WT or Tg4 KO CD4+CD25+ Treg cells and were monitored for EAE. Tg4Rag−/− CTLA-4−/− CD4+ T cells only, n = 2; Tg4Rag−/−CTLA-4−/− CD4+ T cells + Tg4 WT Treg cells, n = 3; Tg4Rag−/−CTLA-4−/− CD4+ T cells + Tg4 KO Treg cells, n = 3. (D) B10.PL(H-2u)Rag−/− mice received a transfer of 3.106 in vitro activated Tg4 KO Th1 cells, with or without 6.105 Tg4 KO CD4+CD25+ Treg cells and were monitored for EAE. Th1 only, n = 4; Th1 + Treg, n = 3. Data are represented as mean ± SEM.
Additional FoxP3+ Treg Cells Are Selected in the Thymus of Tg4 KO Mice.
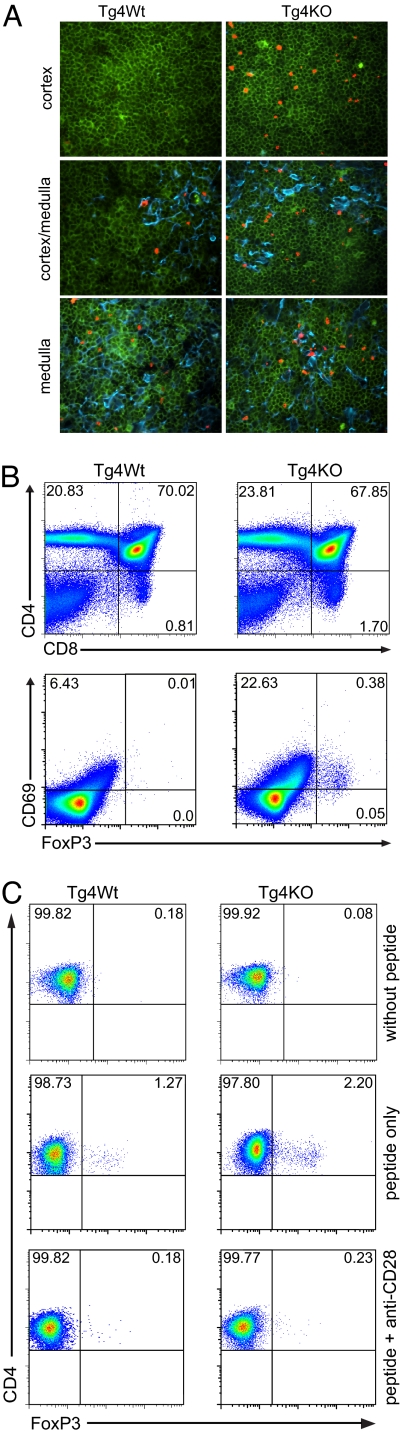
The next step in our investigation was to determine if the higher frequency of FoxP3+ Treg cells in Tg4 KO mice was the result of increased peripheral expansion or if it was derived from an augmentation in thymic selection. The higher number of FoxP3+ cells in the thymus as well as the spleen was an indication toward the latter option, but one cannot exclude the possibility that peripheral Treg cells might migrate back into the thymus. Peripherally expanded T cells will almost exclusively be present as single-positive (SP) cells in the medulla of the thymus (23), and we therefore compared the expression of FoxP3 in the thymic cortex and medulla of Tg4 WT and Tg4 KO mice. A distinction between areas of the thymus was made by the presence of cytokeratin 5, which forms part of the matrix in the medulla but not in the cortex (24). Immunofluorescent staining of frozen sections of thymi from 5–7-week-old Tg4 WT and Tg4 KO mice revealed that both showed ample expression of FoxP3 on CD4+ cells in the medulla (Fig. 5A). The most striking difference between the 2 thymi lies in the presence of significantly higher numbers of FoxP3+ cells in the cortical area. In the Tg4 WT thymus, FoxP3+ cells were almost exclusively present in and surrounding the medulla, similar to the expression pattern of FoxP3gfp in C57BL/6 × 129 mice, as previously reported by Fontenot et al. (25). In the Tg4 KO mice, however, there were clear niche areas in the cortex, where FoxP3+ cells were relatively abundant. Flow cytometric analysis confirmed the enhanced frequency of FoxP3+ CD4CD8 double-positive (DP) T cells in the thymus of Tg4 KO mice (Fig. 5B, Lower), whereas the relative and absolute numbers of CD4SP, CD8SP, and CD4CD8DP cells were not affected (Fig. 5B, Upper and data not shown). To confirm that the FoxP3+ cells in the cortex were indeed newly formed and had not returned to the thymus after peripheral expansion, we subsequently double-stained thymus sections for CD4 and CD8. This revealed that all FoxP3+ cells in the thymic medulla of both Tg4 WT and Tg4 KO mice were CD4 SP cells, whereas the FoxP3-expressing cells in the cortex of Tg4 KO mice were DP for CD4 and CD8 (Fig. S1). Finally, fetal thymus organ cultures were used to determine the mechanism by which CTLA-4 regulates the development of FoxP3+ Treg cells. E17 thymi were cultured in vitro for 7 days in the presence of 20 ng/mL MBP Ac1–9 peptide, leading to the expression of FoxP3 by CD4SP cells. The amount of peptide added to the culture is paramount, because no FoxP3+ Treg cells were selected without peptide and higher concentrations led to exacerbated loss of CD4SP T cells (Fig. 5C and Fig. S2). This is consistent with recent findings in the nonobese diabetic mouse model (26). The addition of a blocking anti-CD28 mAb to the culture successfully abrogated the expression of FoxP3 both in Tg4 WT and Tg4 KO thymi (Fig. 5D), demonstrating that CTLA-4 regulates the CD28-dependent expression of FoxP3 by developing thymocytes.
Fig. 5.
Enhanced selection of FoxP3+ Treg cells in the thymus of Tg4 KO mice. (A) Frozen sections of Tg4 WT and Tg4 KO thymi were stained for CD4 (green), FoxP3 (red), and cytokeratin 5 (blue) to discriminate between the cortex and medulla. Niche sites with FoxP3+ Treg cells were found in the cortex of Tg4 KO but not Tg4 WT mice. (B) FoxP3 and CD69 expression on thymocytes from Tg4 WT and Tg4 KO mice (Lower) was gated on dual expression of CD4 and CD8 (Upper). One result representative of 3 identical experiments is shown. (C) FoxP3 expression on CD4SP thymocytes after 7-day fetal thymus organ culture of Tg4 WT and Tg4 KO E17 embryos without peptide (n = 9 and n = 4, respectively), with 20 ng/mL MBP Ac1–9 peptide (n = 11 and n = 5, respectively) or with peptide plus 10 μg/mL blocking anti-CD28 antibody (n = 9 and n = 4, respectively).
Discussion
The role of CTLA-4 in thymic selection, like many aspects of CTLA-4 activity, so far remains controversial. Early reports suggested that thymic selection in CTLA-4KO mice was normal and that any findings of an altered thymic phenotype were the result of inclusion of the parathymic lymph nodes or the infiltration of the thymus by activated peripheral T cells (1, 27). More recently, a role for CTLA-4 in the negative selection of DP thymocytes has been suggested (19, 20). In addition to TCR signals, the deletion of autoreactive DP T cells in the thymus requires a costimulatory signal that can be provided by CD28 (28). Activation of DP thymocytes induces the expression of CTLA-4 (29), which can counteract the CD28-driven apoptosis of autoreactive cells following the encounter of high-dose, but not low-dose, specific antigen (20).
In addition to regulating the negative selection of autoreactive T cells, CD28 has been reported to be responsible for the induction of FoxP3 expression and differentiation of Treg cells from DP cells in the thymus (30). In this report, we not only confirm this finding but elaborate by showing that CTLA-4 regulates the CD28-dependent expression of FoxP3 in the thymus. Whether the effect of CTLA-4 on FoxP3 expression results from a direct influence on CD28 signaling so far remains unclear, although several mechanisms have previously been suggested. First, CTLA-4 and CD28 share the ligands B7.1 (CD80) and B7.2 (CD80), but the affinity of CTLA-4 for these ligands, particularly CD80, is much higher. CTLA-4 expression on the cell surface would therefore lead to ligand competition resulting in reduced CD28 signaling (11). Second, several studies, including a recent report by Jackman et al. (14), have suggested that CTLA-4 may disrupt immunological synapse formation, leading to reduced signaling through (co-)stimulatory receptors such as CD28. Similarly, CTLA-4 has been suggested to affect the expression of the adhesion molecule LFA-1 (5) as well as the TCR stop signal (6), resulting in enhanced motility of T cells, and therefore reduced interaction time of CD28 with its ligands. Finally, it has been reported that signaling through the cytoplasmic tail of CTLA-4 directly inhibits CD28 signaling events (11), possibly mediated by phosphatases such as SHP-2 or PP2A.
Recent human and mouse studies using anti-CTLA-4 antibody have demonstrated that blockade of CTLA-4 leads to peripheral expansion of the FoxP3+ Treg-cell population (31, 32). Both groups report that FoxP3+ T cells demonstrate a greater steady-state turnover than FoxP3− T cells. The population of rapidly dividing FoxP3+ T cells up-regulates CTLA-4 through a CD28-driven mechanism (32). Blockade of CTLA-4 therefore removes the brake from these expanding cells, rather than lowering the activation threshold of anergic cells. Both studies show that blockade of CTLA-4 with specific antibody leads to augmented proliferation of both FoxP3+ and FoxP3− cells. The increased numbers of FoxP3+ Treg cells in the periphery after CTLA-4 blockade is thus not attributable to an effect of the antibody on Treg cells in particular but merely results from the naturally greater homeostatic expansion of FoxP3+ Treg cells compared with FoxP3− cells.
We now propose that CTLA-4 controls the frequency of FoxP3+ Treg cells in 2 separate ways. First, CTLA-4 regulates the CD28-dependent expression of FoxP3 by developing thymocytes. CTLA-4 deficiency therefore leads to an enhanced frequency of self-antigen–specific Treg cells in the thymus and periphery. Second, CTLA-4 acts as a brake on peripherally expanding T cells. FoxP3+ Treg cells display greater steady-state expansion than FoxP3− T cells, leading to augmented numbers of FoxP3+ cells in the periphery when CTLA-4 is blocked or deleted.
The dominance of the thymic effect of CTLA-4 in the Tg4 TCR-transgenic model raises the question as to whether the steady-state expansion in nontransgenic animals is the result of activation by nonthymically expressed antigens. This might explain why normal CTLA-4KO mice develop a lethal lymphoproliferative disorder, whereas Tg4 KO mice remain healthy. In the Tg4 model, nearly all T cells are specific for MBP Ac1–9 peptide. Particularly in the case of CTLA-4 deficiency, large numbers of antigen-specific FoxP3+ Treg cells are produced in addition to autoreactive Teff cells. Despite the ability of CTLA-4–deficient autoreactive T cells to cause CNS autoimmune disease, the augmented level of antigen-specific Treg cells clearly controls autoreactive responses in the periphery. In nontransgenic CTLA-4KO models, the TCR diversity is far greater and not every antigen is present in the thymus in sufficient quantities to induce the selection of adequate numbers of Treg cells specific for each antigen. As a result, CTLA-4 deficiency in mice with a broad TCR repertoire gives rise to the antigen-dependent lymphoproliferative disease common in these mice.
In conclusion, we demonstrate here that FoxP3+ Treg cells from TCR transgenic CTLA-4KO mice display a normal suppressive capacity, both in vitro and in vivo. Moreover, we show that CTLA-4 deficiency leads to enhanced selection of FoxP3+ Treg cells in the thymus, resulting in an augmented frequency in the periphery. We therefore reveal a role for CTLA-4 in the thymic generation of FoxP3+ Treg cells.
Materials and Methods
Mice.
All mice were bred under specific pathogen-free conditions. The Tg4 mouse strain was established as previously described (18) and backcrossed onto the B10.PL (H-2u) background. Tg4 Rag−/− mice were generated by breeding Tg4 mice with a Rag2−/− B10 strain (33). CTLA-4–deficient mice were created by gene targeting in embryonic stem cells, as previously described (2). All experiments were carried out under a United Kingdom Home Office Project License and were subject to assessment by the University of Bristol Ethical Review Committee.
Peptides.
The acetylated N-terminal peptide of murine MBP, Ac1–9, and the higher MHC affinity analogues with substitutions at position 4 were synthesized using standard Fmoc chemistry on an AMS 422 multiple peptide synthesizer (Abimed).
Induction and Scoring of EAE.
Age- and gender-matched mice were primed s.c. at the base of the tail with 200 μg of MBP Ac1–9 in 0.1 mL of a sonicated emulsion consisting of an equal volume of CFA and PBS containing 4 mg/mL heat-killed Mycobacterium tuberculosis (Becton Dickinson Microbiology Systems). On days 0 and 2, 200 ng of pertussis toxin (Sigma Aldrich) was administered i.p. in 0.5 mL of PBS. EAE was assessed daily for up to 4 weeks with the following scoring system: 0, no signs; 1, flaccid tail; 2, impaired righting reflex and/or gait; 3, hind limb paralysis; 4, forelimb and hind limb paralysis; and 5, moribund or dead.
T-Cell Separation and Differentiation.
CD4+CD25+ Treg cells were isolated by magnetic separation using the CD4+CD25+ regulatory T-cell isolation kit (Milenyi Biotec) according to the manufacturer's recommended protocol. CD4+CD25− cells were skewed toward a Th1 phenotype by stimulation with 10 μg/mL MBP Ac1–9 peptide in the presence of 25 ng/mL recombinant murine (rm) IL-12 and 25 ng/mL rmIL-18 (both from Peprotech) for 10 days. On day 3, 20 U/mL rhIL-2 (R&D Systems) was added. Cells were restimulated with plate-bound anti-CD3e (1 μg/mL) and anti-CD28 (2 μg/mL), both from BD Biosciences, for 3 days before i.p. transfer.
T-Cell Proliferation Assays.
A total of 5.104 T cells/well were cultured in complete RPMI medium in a 96-well plate at 37 °C in the presence of different concentrations of MBP Ac1–9 or its analogues. Irradiated B10.PL splenocytes or CD4-depleted Tg4 splenocytes were used as antigen-presenting cells (APC). At the times indicated, cells were pulsed with 0.5 μCi of [3H]thymidine overnight and the incorporated radioactivity was measured on a liquid scintillation β-counter (1450 Microbeta; Wallac). Alternatively, CD4+CD25− T cells were labeled with carboxyfluorescein diacetate, succinimidyl ester (Molecular Probes) and stimulated with 5-μm latex microspheres (Molecular Probes) coated with affinity-purified anti-CD3e and anti-CD28 or hamster IgG1 isotype control (anti-trinitrophenol; all from BD Biosciences).
Flow Cytometry.
Cells were surface stained with anti-CD4-Alexa700 (Invitrogen), CD25-APC, and CD69-FITC (both from BD Biosciences) and intracellularly with either anti-FoxP3-PE or rat IgG2a isotype control antibody, using permeabilization buffers recommended by the manufacturer (eBioscience). Fluorescence intensity was measured on a FACSCalibur flow cytometer (BD Biosciences) and analyzed using FCS Express 2 (De Novo Software) or FlowJo 7.2.2 (Tree Star) FACS analysis software.
In Situ Immunofluorescent Staining.
Thymi from mice 5–7 weeks of age were frozen in liquid nitrogen immediately after removal. Seven-micrometer sections were fixed in acetone and washed in PBS containing 0.5 mM MgCl2 and 1 mM CaCl2. Sections were then blocked with 2% (vol/vol) normal donkey serum (Jackson ImmunoResearch Laboratories) in PBS containing 0.5 mM MgCl2, 1 mM CaCl2, 2% (vol/vol) FCS, and 0.2% (vol/vol) saponin. Subsequently, sections were stained with a combination of anti-CD4-biotin, anti-CD4-FITC, anti-CD8-FITC (all from BD Bioscience), affinity-purified anti-FoxP3 (clone NRRF-30; eBioscience), or anti-cytokeratin 5 (Covance Research Products), followed by streptavidin-coumarin (Perkin-Elmer Life Sciences), Fab2′ donkey anti-rat-rhodamine, or donkey anti-rabbit 7-amino-4-methylcoumarin-3-acetic acid (both from Jackson Immunoresearch). Images were obtained on an Openlab system (Improvision) with a cooled CCD camera attached to a DM IRB inverted epifluorescence microscope (Leica Microsystems) and analyzed using Volocity 4.0 software (Improvision).
Fetal Thymus Organ Cultures.
Thymic lobes from day 15–17 embryos were cultured for 7 days as previously described (34) with or without MBP Ac1–9 peptide in the medium. After 24 h, 10 μg/mL anti-CD28 antibody (E18, NA/LE; Serotec) was added to the medium of indicated cultures.
Supplementary Material
Acknowledgments.
We thank Dr. L.S.K. Walker for critically reading the manuscript and Mr. M. Schiaulini, Miss L.E.L. Falk, and Mrs. J.D. Radcliffe for assistance with the breeding and handling of mice. Furthermore, we thank the Medical Research Council for providing an Infrastructure Award to establish the School of Medical Sciences Cell Imaging Facility. This work was supported by a Wellcome Trust Program grant. L.G. was supported by a studentship from the Multiple Sclerosis Society of Great Britain and Northern Ireland. C.A.S. was supported by the Batchworth Trust.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803186106/DCSupplemental.
References
- 1.Waterhouse P, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 2.Tivol EA, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 3.Eggena MP, et al. Cooperative roles of CTLA-4 and regulatory T cells in tolerance to an islet cell antigen. J Exp Med. 2004;199:1725–1730. doi: 10.1084/jem.20040124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walker LS, Ausubel LJ, Chodos A, Bekarian N, Abbas AK. CTLA-4 differentially regulates T cell responses to endogenous tissue protein versus exogenous immunogen. J Immunol. 2002;169:6202–6209. doi: 10.4049/jimmunol.169.11.6202. [DOI] [PubMed] [Google Scholar]
- 5.Schneider H, Valk E, da Rocha Dias S, Wei B, Rudd CE. CTLA-4 up-regulation of lymphocyte function-associated antigen 1 adhesion and clustering as an alternate basis for coreceptor function. Proc Natl Acad Sci USA. 2005;102:12861–12866. doi: 10.1073/pnas.0505802102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider H, et al. Reversal of the TCR stop signal by CTLA-4. Science. 2006;313:1972–1975. doi: 10.1126/science.1131078. [DOI] [PubMed] [Google Scholar]
- 7.Lee KM, et al. Molecular basis of T cell inactivation by CTLA-4. Science. 1998;282:2263–2266. doi: 10.1126/science.282.5397.2263. [DOI] [PubMed] [Google Scholar]
- 8.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi T, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carreno BM, et al. CTLA-4 (CD152) can inhibit T cell activation by two different mechanisms depending on its level of cell surface expression. J Immunol. 2000;165:1352–1356. doi: 10.4049/jimmunol.165.3.1352. [DOI] [PubMed] [Google Scholar]
- 12.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grohmann U, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 14.Jackman RP, Balamuth F, Bottomly K. CTLA-4 differentially regulates the immunological synapse in CD4 T cell subsets. J Immunol. 2007;178:5543–5551. doi: 10.4049/jimmunol.178.9.5543. [DOI] [PubMed] [Google Scholar]
- 15.Greenwald RJ, Boussiotis VA, Lorsbach RB, Abbas AK, Sharpe AH. CTLA-4 regulates induction of anergy in vivo. Immunity. 2001;14:145–155. doi: 10.1016/s1074-7613(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 16.Read S, et al. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol. 2006;177:4376–4383. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kataoka H, et al. CD25(+)CD4(+) regulatory T cells exert in vitro suppressive activity independent of CTLA-4. Int Immunol. 2005;17:421–427. doi: 10.1093/intimm/dxh221. [DOI] [PubMed] [Google Scholar]
- 18.Liu GY, et al. Low avidity recognition of self-antigen by T cells permits escape from central tolerance. Immunity. 1995;3:407–415. doi: 10.1016/1074-7613(95)90170-1. [DOI] [PubMed] [Google Scholar]
- 19.Buhlmann JE, Elkin SK, Sharpe AH. A role for the B7–1/B7–2:CD28/CTLA-4 pathway during negative selection. J Immunol. 2003;170:5421–5428. doi: 10.4049/jimmunol.170.11.5421. [DOI] [PubMed] [Google Scholar]
- 20.Kwon H, Jun HS, Khil LY, Yoon JW. Role of CTLA-4 in the activation of single- and double-positive thymocytes. J Immunol. 2004;173:6645–6653. doi: 10.4049/jimmunol.173.11.6645. [DOI] [PubMed] [Google Scholar]
- 21.Fairchild PJ, Wildgoose R, Atherton E, Webb S, Wraith DC. An autoantigenic T cell epitope forms unstable complexes with class II MHC: A novel route for escape from tolerance induction. Int Immunol. 1993;5:1151–1158. doi: 10.1093/intimm/5.9.1151. [DOI] [PubMed] [Google Scholar]
- 22.Lafaille JJ, Nagashima K, Katsuki M, Tonegawa S. High incidence of spontaneous autoimmune encephalomyelitis in immunodeficient anti-myelin basic protein T cell receptor transgenic mice. Cell. 1994;78:399–408. doi: 10.1016/0092-8674(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 23.Agus DB, Surh CD, Sprent J. Reentry of T cells to the adult thymus is restricted to activated T cells. J Exp Med. 1991;173:1039–1046. doi: 10.1084/jem.173.5.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams JA, et al. Regulated costimulation in the thymus is critical for T cell development: Dysregulated CD28 costimulation can bypass the pre-TCR checkpoint. J Immunol. 2005;175:4199–4207. doi: 10.4049/jimmunol.175.7.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fontenot JD, Dooley JL, Farr AG, Rudensky AY. Developmental regulation of Foxp3 expression during ontogeny. J Exp Med. 2005;202:901–906. doi: 10.1084/jem.20050784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feuerer M, et al. Enhanced thymic selection of FoxP3+ regulatory T cells in the NOD mouse model of autoimmune diabetes. Proc Natl Acad Sci USA. 2007;104:18181–18186. doi: 10.1073/pnas.0708899104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chambers CA, Cado D, Truong T, Allison JP. Thymocyte development is normal in CTLA-4-deficient mice. Proc Natl Acad Sci USA. 1997;94:9296–9301. doi: 10.1073/pnas.94.17.9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Punt JA, Osborne BA, Takahama Y, Sharrow SO, Singer A. Negative selection of CD4+CD8+ thymocytes by T cell receptor-induced apoptosis requires a costimulatory signal that can be provided by CD28. J Exp Med. 1994;179:709–713. doi: 10.1084/jem.179.2.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cilio CM, Daws MR, Malashicheva A, Sentman CL, Holmberg D. Cytotoxic T lymphocyte antigen 4 is induced in the thymus upon in vivo activation and its blockade prevents anti-CD3-mediated depletion of thymocytes. J Exp Med. 1998;188:1239–1246. doi: 10.1084/jem.188.7.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 31.Kavanagh B, et al. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood. 2008;112:1175–1183. doi: 10.1182/blood-2007-11-125435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang AL, et al. CTLA4 expression is an indicator and regulator of steady-state CD4+ FoxP3+ T cell homeostasis. J Immunol. 2008;181:1806–1813. doi: 10.4049/jimmunol.181.3.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicolson KS, et al. Antigen-induced IL-10+ regulatory T cells are independent of CD25+ regulatory cells for their growth, differentiation, and function. J Immunol. 2006;176:5329–5337. doi: 10.4049/jimmunol.176.9.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenkinson EJ, Anderson G, Owen JJ. Studies on T cell maturation on defined thymic stromal cell populations in vitro. J Exp Med. 1992;176:845–853. doi: 10.1084/jem.176.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.