Abstract
In this study, we generated mice lacking the gene for G-substrate, a specific substrate for cGMP-dependent protein kinase uniquely located in cerebellar Purkinje cells, and explored their specific functional deficits. G-substrate–deficient Purkinje cells in slices obtained at postnatal weeks (PWs) 10–15 maintained electrophysiological properties essentially similar to those from WT littermates. Conjunction of parallel fiber stimulation and depolarizing pulses induced long-term depression (LTD) normally. At younger ages, however, LTD attenuated temporarily at PW6 and recovered thereafter. In parallel with LTD, short-term (1 h) adaptation of optokinetic eye movement response (OKR) temporarily diminished at PW6. Young adult G-substrate knockout mice tested at PW12 exhibited no significant differences from their WT littermates in terms of brain structure, general behavior, locomotor behavior on a rotor rod or treadmill, eyeblink conditioning, dynamic characteristics of OKR, or short-term OKR adaptation. One unique change detected was a modest but significant attenuation in the long-term (5 days) adaptation of OKR. The present results support the concept that LTD is causal to short-term adaptation and reveal the dual functional involvement of G-substrate in neuronal mechanisms of the cerebellum for both short-term and long-term adaptation.
Keywords: cerebellum, long-term depression, optokinetic response, Purkinje cell
The G-substrate purified from rabbit cerebellum is one of the few preferred substrates for cGMP-dependent protein kinase (PKG) (1–8). It is positioned at the downstream end of the cascade linking nitric oxide (NO), soluble guanylate cyclase, cGMP, and PKG. The target(s) for NO is located within Purkinje cells (9–12), where diffusing NO activates soluble guanylate cyclase (13), which, in turn, enhances PKG activity (14). Immunohistochemical studies have revealed that G-substrate is uniquely concentrated in cerebellar Purkinje cells (2, 6, 7, 15, 16). In cerebellar slices, G-substrate in Purkinje cells is effectively phosphorylated in response to a membrane-permeable analogue of cGMP that activates PKG (9). Phosphorylated G-substrate acts as a potent inhibitor of protein phosphatase (PP) 1 and PP2A (6–8). We now have generated G-substrate knockout mice to investigate further the functional roles of G-substrate at cellular and behavioral levels.
Each component of the NO-cGMP-PKG pathway has so far been shown to be required for the induction of cerebellar long-term depression (LTD) (12, 17–24), a characteristic form of synaptic plasticity displayed by Purkinje cells (25). In LTD, synaptic transmission from parallel fibers (PFs) to Purkinje cells is persistently depressed after conjunctive stimulation of the PFs and climbing fibers (CFs). CF stimuli can be replaced by application of depolarizing pulses to the Purkinje cell membrane. Peculiarly, NO is not required for LTD induction in young cultured Purkinje cells (26), suggesting that the NO-cGMP-PKG pathway acts as a modulator whose requirement for LTD may depend on various circumstances (27).
LTD has been considered to provide a cellular mechanism of motor learning (25). Here, we report a distinctive age-dependent deficit of LTD induction in G-substrate–deficient Purkinje cells; LTD occurs at postnatal week (PW) 4 but diminishes at PW5–6 and then recovers at PW10 afterward. We also examined how the age-dependent diminution of LTD is reflected in the age profile of adaptation of optokinetic eye movement response (OKR), a simple form of motor learning. OKR adaptation is an increase of OKR gain induced by continuous oscillation of a screen around a stationary animal and is abolished by gene knockout (28) or pharmacological inhibition of neural NOS (28, 29). As very recently reported, OKR adaptation (30), as well asvestibulo-ocular reflex (VOR) adaptation (31), has 2 distinct phases underlain by different neural mechanisms. The short-term adaptation occurring during 1 h of training has its memory site in the cerebellar cortex of the flocculus, whereas the long-term adaptation accumulated during repeated 5-day training sessions is an event that takes place somewhere else, because the latter is maintained even after a glutamate antagonist (31) or lidocaine (30) has blocked cerebellar cortical activity. There is some evidence indicating that long-term OKR adaptation has its memory site in vestibular nuclear neurons (30).
In this study, we demonstrate in G-substrate knockout mice that the short-term OKR adaptation diminishes in an age-dependent manner in parallel with LTD amplitude reduction. G-substrate knockout affects the LTD induction and short-term OKR adaptation only temporarily around PW6; however, young adult mice at PW12 prove to be free of these deficits. We demonstrate that they are also free of deficits in other motor learning tasks, including eyeblink conditioning (32), motor coordination (33), and adaptive locomotion (34). Eventually, a significant depression of the long-term OKR adaptation is the only deficit exhibited by PW12 G-substrate knockout mice. Thus, the functional role of G-substrate gene is defined by its differential involvement in LTD-driven short-term OKR adaptation and otherwise initiated long-term OKR adaptation.
Results
Generation of G-Substrate Knockout Mice.
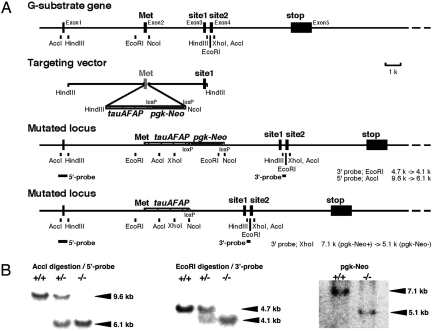
The G-substrate gene consists of 5 exons and 4 introns (Fig. 1A). The initiation Met is in exon 2, and the 2 PKG phosphorylation sites are in separate exons (exons 3 and 4). For the generation of G-substrate knockout mice, the G-substrate gene was disrupted by insertion of DNA encoding a selection marker (pgk-neo cassette) and tau-AFAP in the first coding exon (exon 2) (Fig. 1A). Correct recombination was confirmed by Southern blot analysis of ES cells and F2 mice using 5′- and 3′-probes (Fig. 1B). The removal of the pgk-neo cassette, the selection marker in ES cells, was confirmed by Southern blot analysis (Fig. 1B). Homozygous mice deficient in G-substrate were obtained by mating heterozygous mice and had normal Mendelian distribution, showing lack of embryonic lethality. The homozygous G-substrate knockout mice appeared to develop and reproduce normally.
Fig. 1.
Generation of G-substrate knockout mice. (A) Gene structure of mouse G-substrate and targeting vector for the generation of G-substrate knockout mice. (B) Confirmation of G-substrate knockout by Southern blot analysis. Restriction-enzyme–digested genomic DNA was subjected to Southern blot hybridization analysis. The band shift attributable to the homologous recombination was confirmed by probing the blot with 32P-labeled 3′- and 5′-probes, as indicated in the figure. The removal of the selection marker, pgk-Neo cassette, was also confirmed by Southern blot analysis using the 32P-labeled 3′-probe.
In samples from WT mice, mRNA was observed as a single band of 1.7 kb on Northern blot analysis; however, there was complete absence of G-substrate mRNA and immunoreactivity in the homozygous knockout mice [supporting information (SI) Fig. S1A and B]. Furthermore, no detectable G-substrate protein was observed in homogenates prepared from G-substrate knockout mice as demonstrated by immunoblotting of immunoprecipitates obtained using G-substrate antibody (Fig. S1C).
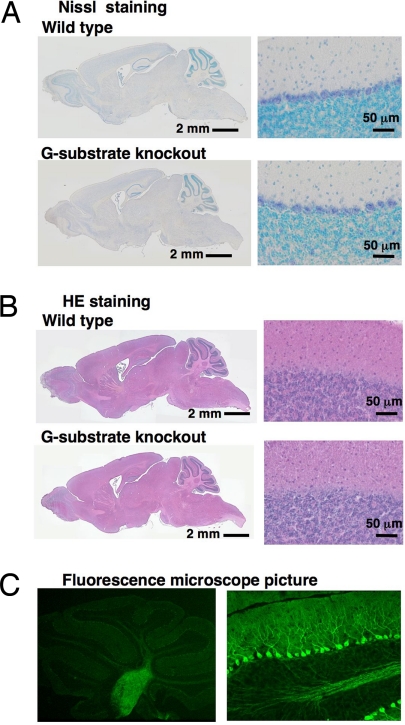
The results suggest that the homozygous G-substrate gene deletion led to a complete loss of the G-substrate mRNA and protein expression in the cerebellum. No major morphological changes were observed in the cerebellum or whole brain of homozygote mice as assessed by light microscopy (Fig. 2 A and B). The layer structures of the cerebellum were indistinguishable in WT and G-substrate knockout mice, as shown by Nissl staining of cerebellar slices. There were no apparent changes in the density, size, or shape of cerebellar Purkinje cells. In G-substrate knockout mice, the shape and path of Purkinje cells were easily visualized by fluorescence microscopy (Fig. 2C), given that AFAP, a GFP derivative, was expressed in G-substrate knockout mice under the control of the G-substrate promoter (Fig. 1). Primary and secondary dendrites and axon bundles were observed with bright fluorescence. Furthermore, axon bundles originating from Purkinje cells were clearly marked by AFAP. These axons course to the deep cerebellar nucleus and vestibular nucleus, as in normal mice.
Fig. 2.
Structures of cerebellum in WT and G-substrate knockout mice. (A) Nissl staining of cerebellar slices obtained from WT and G-substrate knockout mice. The thin sections (30 μm) were obtained from paraformaldehyde-fixed brain using a cryostat and were subjected to Nissl staining. (B) Hematoxylin and Eosin staining of brain slices obtained from WT and G-substrate knockout mice. (C) Fluorescence images of slices obtained from G-substrate knockout mice. The frozen thin sections (20 μm) were observed under a fluorescence microscope. AFAP, a GFP derivative, was expressed under the control of the G-substrate promoter.
Cerebellar LTD.
In current clamp configuration with patch pipettes, we recorded from 120 Purkinje cells in acute slices from G-substrate knockout mice and from 118 Purkinje cells in acute slices from WT littermates. We confirmed that there were no statistically significant differences in membrane potential, membrane resistance, time course of PF-evoked excitatory postsynaptic potentials (EPSP), or waveform of complex spikes, except for a modest difference in paired-pulse facilitation of PF-EPSPs (Table S1). Stimulation of the white matter evoked full-sized CF responses in an all-or-none manner, and there was no evidence for multiple CF innervations of Purkinje cells.
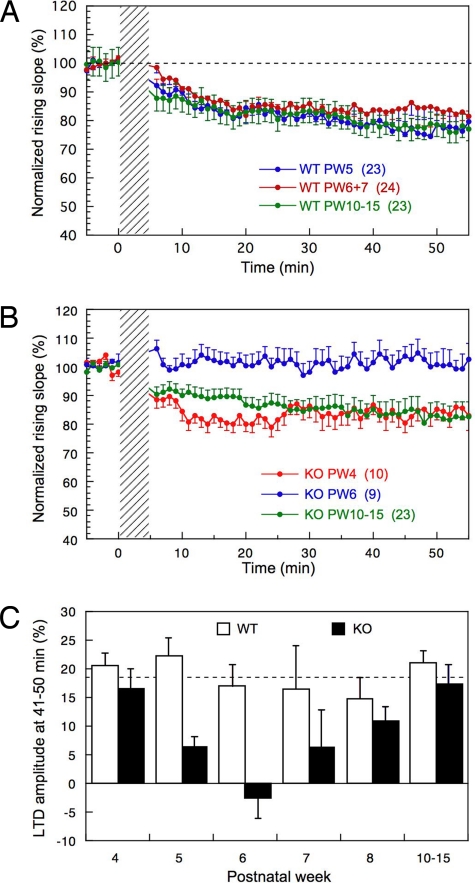
Cerebellar LTD was induced by conjunction of PF stimulation with depolarizing pulses at 1 Hz for 5 min (see Materials and Methods). The induced LTD developed during the initial 20 min and was followed by a slow phase proceeding for another 40 min (Fig. 3A). Because our provisional tests showed variation in the expression of LTD in Purkinje cells deficient of G-substrate, we paid special attention to the possible age-dependent variation of LTD and examined mice at PW4 to PW15. As shown in Fig. 3A, WT Purkinje cells showed virtually identical average LTD time courses throughout life up to PW15. The magnitudes of LTD measured 41–50 min after the onset of 5 min of conjunction were about 20%, on average (Fig. 3A). In contrast, Purkinje cells lacking G-substrate showed virtually normal LTD at PW4, which then diminished to zero at PW6; thereafter it recovered to normal levels from PW10 onward (Fig. 3B). The histogram in Fig. 3C compares the LTD magnitude between G-substrate–deficient and WT Purkinje cells from PW4 to PW15. There was a significant difference in the LTD magnitude between the 2 genotypes of Purkinje cells (2-way factorial ANOVA: F1, 158 = 13.19, P = 0.004). The LTD magnitude in G-substrate knockout mice showed a clear age-dependency (1-way ANOVA: F5, 68 = 2.91, P = 0.019), whereas the incidence and extent of LTD in WT mice did not (F5, 90 = 0.671, P = 0.647). The Dunnett post hoc test in Fig. 3C revealed that the LTD magnitude in G-substrate knockout Purkinje cells was significantly smaller than that in WT Purkinje cells at PW6 (P < 0.01) and PW5 (P < 0.05). LTD magnitudes in G-substrate knockout mice were also smaller at PW7 and PW8 (Fig. 3C), but these decreases were not statistically significant (P > 0.05).
Fig. 3.
Age-dependent expression of cerebellar LTD in G-substrate–lacking Purkinje cells. (A) Averaged time profile of 3 groups of WT Purkinje cells is shown with different colors. The ordinate illustrates the rising slope of PF-EPSPs relative to the average measured 5 min before conjunction, and the abscissa illustrates time. The oblique-shaded band indicates the application of conjunction of PF stimulation and depolarizing pulses. Number of Purkinje cells tested is shown in brackets. Vertical bars, SEs. (B) Similar to A but for G-substrate–deficient Purkinje cells. (C) Histograms showing LTD amplitude at 41–50 min for different PWs in WT and G-substrate–deficient Purkinje cells. Filled columns are G-substrate knockout mice, and empty columns are WT littermates. Vertical bars, SEs. The numbers of cells used for the experiments are as follows: for WT mice, PW4 (12), PW5 (23), PW6 (14), PW7 (10), PW8 (14), and PW10–15 (23); for G-substrate knockout mice, PW4 (10), PW5 (9), PW6 (9), PW7 (6), PW8 (11), and PW10–15 (29).
Dynamics and Short-Term Adaptation of OKR.
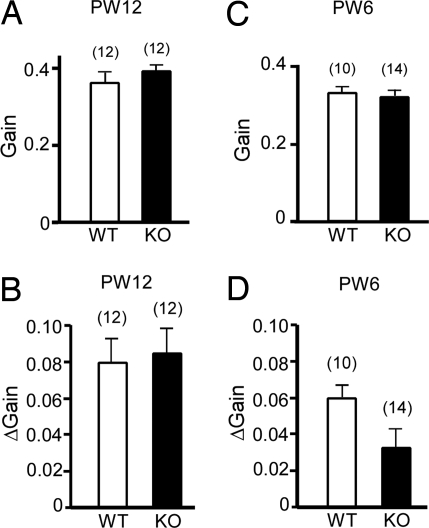
First, we examined the dynamic characteristics of the OKR (gain, phase, and screen frequency dependence) using sinusoidal screen oscillations (15° peak-to-peak, 0.11–0.33 Hz) in the light in 9 G-substrate knockout mice and 7 WT littermate mice at PW12 (Fig. S2). We observed no differences in the gains of OKR between G-substrate knockout and WT littermates (two-way repeated measures ANOVA: F1, 14 = 0.082, P = 0.78). Then, we examined the adaptation of OKR in 12 G-substrate knockout mice and 12 WT littermates at PW12. As shown in Fig. 4A, no difference was observed in the “start gain” of OKR measured by sinusoidal screen oscillation (15° peak-to-peak, 0.17 Hz, maximum screen velocity of 7.9°/sec) between the 2 genotypes at PW12. These mice were subjected to 1-hr continuous screen oscillation with the same stimulus parameters to induce short-term OKR adaptation. The “end gain” of OKR was measured immediately after the 1-hr screen oscillation. The increase in the OKR gain during the 1-hr screen oscillation ([end gain] − [start gain]) represents the magnitude of the short-term OKR adaptation. As shown in Fig. 4B, no significant difference was detected in short-term OKR adaptation in G-substrate knockout mice and WT littermates at PW12 [Student's t test: t (22) = 0.26, P = 0.797].
We also examined 14 G-substrate knockout mice and 10 WT mice at PW6 (all different from the mice examined previously at PW12). Fig 4C shows that there was no significant difference in the start OKR gain between the 2 genotypes at PW6. However, a significant difference was detected in the short-term OKR adaptation between the 2 genotypes at PW6 (Fig. 4D); the magnitude of short-term OKR adaptation was smaller in G-substrate knockout mice than in WT mice [Student's t test: t (22) = 2.094, P = 0.048]. Furthermore, examination of the mice at PW4 and PW5 revealed a low OKR gain and poor short-term OKR adaptation for both G-substrate knockout and WT littermates (data not shown). It appears that the OKR neuronal circuit is immature at PW4 and PW5, such that short-term OKR adaptation occurs only rudimentarily. The results in Fig. 4 B and D suggest that the OKR neuronal circuit has matured considerably by PW6, although it might not be as complete as that at PW12, and that it expresses a deficit caused by G-substrate knockout. These interpretations are consistent with the results shown in Fig. 3C (i.e., LTD attenuates at PW6 but recovers at PW12).
Fig. 4.
OKR gain and short-term OKR adaptation. Mice were subjected to sinusoidal screen oscillation by 15° (peak-to-peak) at 0.17 Hz (maximum velocity, 7.9°/sec) in the light for 1 hr. (A) Start OKR gain before 1-hr screen oscillation at PW12. (B) OKR gain change obtained during 1-hr sustained screen oscillation at PW12. (C) Similar to A but at PW6. (D) Similar to B but at PW6. Filled columns represent G-substrate knockout (KO) mice, and empty columns represent WT mice. Vertical bars, SEs. The number of mice used is shown in brackets.
Long-Term OKR Adaptation.
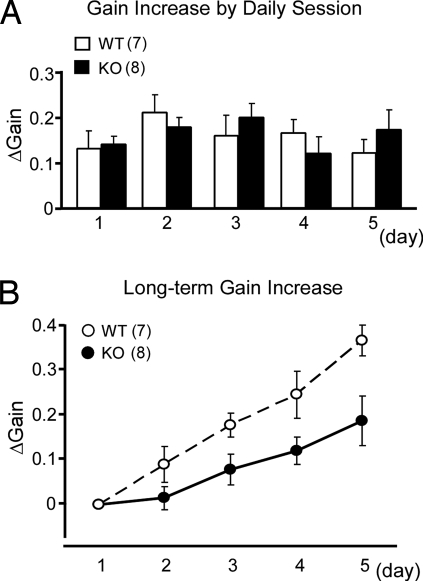
Next, we examined long-term OKR adaptation by carrying out 1 session of 1-hr screen oscillation per day for 5 successive days. Fig. 5A shows that the daily gain changes ([end gain] − [start gain]) did not show significant differences between the WT and G-substrate knockout mice throughout 5 days (two-way repeated measures ANOVA: F1, 13 = 0.019, P = 0.893). The increase in the daily gain recovered within 24 hr; however, the start gain before the 1-hr oscillation gradually increased at days 2–5 compared with the start gain at day 1, which we call long-term adaptation (30). Fig. 5B compares the gain increase induced by long-term OKR adaptation between the 2 genotypes. The gain increase of the G-substrate knockout mice was smaller than that of the WT mice throughout the 5-day session (two-way repeated measures ANOVA: F1, 13 = 6.508, P = 0.024) (Fig. 5B). At the end of day 5, the start gain increased by 0.19 in G-substrate knockout mice, whereas it increased by 0.37 in the WT mice (Fig. 5B). Thus, G-substrate knockout mice are characterized by a partial but significant decrease in the rate of long-term OKR adaptation. The long-term OKR adaptation is not an accumulation of residuals of short-term adaptation, because the former remained intact with local application of lidocaine to the flocculus (30), which blocks the latter. The present results showing that, at PW12, long-term OKR adaptation attenuates, whereas short-term OKR adaptation occurs normally provide another line of evidence that the 2 types of OKR adaptation are underlain by different neural mechanisms.
Fig. 5.
Long-term adaptation of OKR. WT (n = 7) and G-substrate knockout (KO) mice (n = 8) at PW12–15 were exposed to 1-hr sustained screen oscillation every day for 5 days. The mice were kept in the dark, except during the screen oscillation. (A) Daily OKR adaptation. The ordinate illustrates ΔGain, gain changes obtained after 1-hr oscillation on each day. Filled columns are G-substrate KO mice, and empty columns are WT littermates. (B) Cumulative OKR gain changes induced through 5-day sessions. The ordinate illustrates ΔGain, increases of the start gains on each day as measured from the start gain at day 1. Hollow circles represent WT mice, and solid circles represent G-substrate KO mice.
General Behavior.
Behavioral tests described in this article were carried out on young adult mice at PW12, unless otherwise stated. The overall behavioral properties of G-substrate knockout mice were not different from those of WT mice (summarized in Table S2). G-substrate knockout mice exhibited some decrease in locomotor activity in the open-field test; however, the data did not reach statistical significance, except for the distance moved in the dark condition (Fig. S3). The decreased distance moved in the open field might imply an emotional change in G-substrate knockout mice. However, we confirmed that G-substrate knockout mice have normal sensory systems, and they behaved normally in a variety of other emotional tests carried out in the entire test battery (Table S2). A further detailed analysis is required to identify the possible emotional change implied by the open-field test results.
Rotor Rod Test and Gait Analyses.
Motor coordination was examined in the G-substrate knockout and WT mice using the rotor rod test at a speed of 8 rotations per min (rpm) (Fig. S4A) and 12 rpm (data not shown). G-substrate knockout mice and WT mice indistinguishably improved their retention time before falling during repeated trials (two-way repeated measures ANOVA: F1, 9 = 0.015, P = 0.907). Furthermore, the results obtained 24 hr after the initial 10 trials at 8 rpm were not different between WT and G-substrate knockout mice (data not shown).
We examined the kinematics of gait using high-speed video recordings during treadmill locomotion to characterize locomotor movements. No significant differences were observed between WT and G-substrate knockout mice in terms of temporal parameters such as step cycle duration, swing phase duration, stance phase duration, and bisupport phase duration (Table S3). As the treadmill speed increased, the step cycle duration and stance phase duration decreased, although the swing phase duration remained nearly constant in both lines of mice. The angular excursions of the knee and ankle were similar in the WT and knockout mice, although the joints stayed in slightly extended positions in G-substrate knockout mice (Fig. S4 B and C). G-substrate knockout mice did not show any appreciable abnormality in motor coordination and hind limb kinematics during treadmill locomotion, and it is concluded that the gait in G-substrate knockout mice is not ataxic.
Eyeblink Conditioning.
This is a well-established form of cerebellum-dependent motor learning (35), and some genetically modified mouse lines with impaired cerebellar LTD have been reported to show abnormality in eyeblink conditioning (36–40). In this study, G-substrate knockout mice acquired the task as quickly as the WT mice in delay eyeblink conditioning tasks (Fig. S5A) and in trace eyeblink conditioning tasks (Fig. S5B).
Discussion
Homozygotic G-substrate–deficient mice survived and were normal in terms of morphology of the cerebellum (Fig. 2) and other brain areas, general behaviors (Table S1 and Figs. S2 and S3), and reproduction. G-substrate knockout mice thus share a disturbance-free phenotype with Purkinje cell-specific (PKGI) knockout mice (24). This is partly because both PKG and G-substrate are localized in Purkinje cells, but it is also because the deletion of PKGI (24) or G-substrate (see Results) does not cause multiple innervations of Purkinje cells by CFs, which may lead to ataxia or seizure.
The temporary hiatus of LTD and short-term OKR adaptation in G-substrate knockout mice around PW6 may suggest that the NO-cGMP-PKG-G-substrate pathway becomes essential during this crucial stage of development. At other times, it plays an accessory role rather than an essential role. At the end of this pathway, PKG-phosphorylated G-substrate acts as a potent inhibitor of PP1 and PP2A (6–8). Complex involvement of PPs in cerebellar LTD has been observed in cultured Purkinje cells; myosin/moesin phosphatase (containing PP1 catalytic subunit in its complex) plays a major role at 9–16 days in vitro (DIV) (41), but at 22–35 DIV, PP2A takes over (42). Hence, it is possible that, underlying the age profile of LTD (Fig. 3C), G-substrate comes into play as a preferential inhibitor of PP2A with a delay of 6 weeks, and then is probably replaced by PW10 with another PP inhibitor, which is currently unknown. The results indicating that G-substrate knockout attenuates both cerebellar LTD and short-term OKR adaptation temporarily at the early stage of development (Figs. 3C and 4D) conform to previous results showing that blockade of LTD leads to impairment of short-term OKR adaptation (28). Together, these results consistently support the current view that LTD is an essential mechanism of cerebellum-dependent learning. In this context, a close association was also reported very recently in mice lacking delphilin in Purkinje cells, whose LTD induction and OKR adaptation were both enhanced (43). Temporary diminution of LTD, which is now shown to be associated with attenuation of short-term OKR adaptation in G-substrate knockout mice, may also impair other forms of motor learning such as motor coordination on the rotor rod and eyeblink conditioning. We leave analyses of this association to future study until the dual-phase mechanism, which is the basis of the present analysis of OKR adaptation, is also defined for other forms of motor learning.
In G-substrate knockout mice at PW12, LTD induction and short-term OKR adaptation occurred normally (Figs. 3A and 4B) but long-term adaptation was significantly impaired (Fig. 5B). This long-term adaptation is caused by a slowly developed potentiation of synaptic transmission or an intrinsic excitability in vestibular relay neurons (30). The partial impairment of long-term VOR adaptation was also reported to occur in Purkinje cell-specific PKGI-deficient mice with normal short-term adaptation (24). Therefore, the NO-cGMP-PKG-G-substrate cascade appears to play an essential role in the induction of long-term VOR and OKR adaptations, which cannot be compensated for by another pathway. Long-term VOR and OKR adaptations would share a common synaptic mechanism at vestibular relay neurons (44). How the lack of G-substrate in Purkinje cells impairs the adaptive mechanism in vestibular relay neurons is presently unknown. Preliminary results suggest that G-substrate undergoes intracellular translocation from the cell nucleus to cytosol in response to the membrane-permeable analogue of cGMP, 8-bromoguanosine 3′: 5′-cyclic monophosphate (M.S. and S.E., unpublished observation). Recently, the DARPP-32, a PP inhibitor in striatal neurons, was reported to translocate to the cell nuclei, and the translocation was associated with increased histone H3 phosphorylation, an important component of nucleosomal response such as transcription (45). G-substrate translocation may lead to changes in the state of protein phosphorylation in the nucleus and cytosol, or it may affect transcription-translation systems. These effects potentially affect molecular events in the axon terminals of Purkinje cells, which would, in turn, act on vestibular relay neurons transsynaptically.
Conclusion
We have shown that G-substrate knockout causes dual deficits in motor learning. It attenuates cerebellar LTD and associated short-term adaptation temporarily at the early stage of development, consistent with the current view that LTD is an essential mechanism of cerebellum-dependent learning, and it also persistently impairs long-term adaptation of OKR. Otherwise, the G-substrate knockout mice are surprisingly free of disturbances in neuronal functions or behaviors. These mice provide a good model for the investigation of cellular, molecular, and genetic mechanisms underlying the short-term and long-term adaptations of motor behaviors.
Materials and Methods
Isolation and Targeted Disruption of Mouse G-Substrate Gene.
The cDNA for mouse G-substrate was obtained from mouse cerebellum by PCR using the primer sets based on human (6) and rat (7) G-substrate cDNA. Then, a mouse C57BL/6 genomic library (46) constructed in a BAC plasmid was screened using a random-primed cDNA probe for mouse G-substrate. Positive clones were analyzed by restriction mapping and sequencing using a GPS system (NEB).
The standard technique for gene targeting (47) was used. Targeting vectors were constructed in pBluescript to replace exon 2 of the G-substrate gene, which encodes the initiation site Met, with the tau-AFAP-pgk-Neo cassette (Fig. 1). Detailed methods for confirmation of positive clones and generation of chimeric mice and mice harboring the knockout allele are provided in SI Text and in Fig. 1. Total RNA was isolated from the cerebellum using Sepasol (Nakalai Tesque) and was analyzed by Northern blotting analysis using a 32P-labeled mouse G-substrate probe (corresponding to nucleotides 129–608 of AF071562). The blots were hybridized in QuikHyb hybridization solution (Stratagene) at 65 °C overnight and washed with 0.2× SSC containing 0.1% SDS. The hybridization signals on the blots were analyzed using a phosphorimager BAS5000 (Fuji Film).
Immunohistochemistry.
Paraformaldehyde-fixed brain slices were stained with affinity-purified anti-G-substrate antibodies (7). Immunoreaction was visualized with Alexa Fluor 546-conjugated anti-mouse IgG (Molecular Probes). Fluorescent image stainings were obtained using an FX1000 confocal fluorescence microscope (Olympus).
Immunoprecipitation and Immunoblots.
Mouse cerebella were homogenized in extraction buffer containing 50 mM Tris-HCl (pH 7.5), protease inhibitor mixture (Roche Diagnostics), 25 mM β-glycerophosphate, and 1% Nonidet P-40. The homogenates were centrifuged at 100,000 × g for 1 h, and the resulting supernatants were then subjected to immunoprecipitation. Affinity-purified rabbit anti-G-substrate antibodies against the amino terminus portion or the carboxyl terminus portion of G-substrate were used for the immunoprecipitation (7). Immunoprecipitates were subjected to SDS/PAGE, followed by immunoblot analysis. The protein concentration was determined by the method of Bradford (48) using BSA as the standard.
Slice Experiments.
Under general anesthesia by ether inhalation, mice were decapitated and the cerebellum was excised. Sagittal slices of 300 μm thickness were prepared from the vermis. The recording chamber was perfused with oxygenated Ringer's solution containing 100 μM picrotoxin at 30–31 °C. Under an upright microscope, whole-cell patch-clamp recordings were performed using borosilicate pipettes (resistance, 3–5 MΩ). A Multiclamp700A amplifier (Axon) and pClamp 9 software (Axon) were used. PFs were focally stimulated through a glass pipette. To induce LTD, depolarizing pulses of 200 msec duration were applied to Purkinje cell membrane and adjusted (within 2 nA) to evoke at least 1 Ca2+ spike. PFs were stimulated with double pulses (each 0.1 msec in duration) paired at 50-msec intervals timed in such a way that the first pulse fell 30 msec later than the onset of each depolarizing pulse. The combination of double PF stimuli and a depolarizing pulse was repeated at 1 Hz for 5 min (300 pulses) in each trial of LTD induction.
Behavioral Analysis.
For this purpose, mice were backcrossed with C57BL/6 for at least 5 generations. Protocols for all animal experiments were approved by the animal experiment committees of the RIKEN Brain Science Institute, Okinawa Institute of Science and Technology, and the other authors' institutions. Maximum efforts were made to reduce the stress of the mice. Detailed methods for the behavioral analyses, including general behaviors, eye movement, eyeblink conditioning, and rotor rod and treadmill locomotion, are provided in SI Text.
Supplementary Material
Acknowledgments.
We thank Ms. Masako Suzuki for the generation of G-substrate knockout mice and Dr. Mariko Sumi and Ms. Yumiko Motoyama for other technical assistance. MS12 cells were a generous gift from the Meiji Dairies Corporation. This work was supported by a grant for collaboration of the Okinawa Institute of Science and Technology and RIKEN Brain Science Institute and by grants from Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0813341106/DCSupplemental.
References
- 1.Schlichter DJ, Casnellie JE, Greengard P. An endogenous substrate for cGMP-dependent protein kinase in mammalian cerebellum. Nature. 1978;273:61–62. doi: 10.1038/273061a0. [DOI] [PubMed] [Google Scholar]
- 2.Schlichter DJ, et al. Localization of cyclic GMP-dependent protein kinase and substrate in mammalian cerebellum. Proc Natl Acad Sci USA. 1980;77:5537–5541. doi: 10.1073/pnas.77.9.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aswad DW, Greengard P. A specific substrate from rabbit cerebellum for guanosine 3′:5′-monophosphate-dependent protein kinase. I. Purification and characterization. J Biol Chem. 1981;256:3487–3493. [PubMed] [Google Scholar]
- 4.Aswad DW, Greengard P. A specific substrate from rabbit cerebellum for guanosine 3′:5′-monophosphate-dependent protein kinase. II. Kinetic studies on its phosphorylation by guanosine 3′:5′-monophosphate-dependent and adenosine 3′:5′-monophosphate-dependent protein kinases. J Biol Chem. 1981;256:3494–3500. [PubMed] [Google Scholar]
- 5.Aitken A, et al. A specific substrate from rabbit cerebellum for guanosine-3′:5′- monophosphate-dependent protein kinase. III. Amino acid sequences at the two phosphorylation sites. J Biol Chem. 1981;256:3501–3506. [PubMed] [Google Scholar]
- 6.Endo S, et al. Molecular identification of human G-substrate, a possible downstream component of the cGMP-dependent protein kinase cascade in cerebellar Purkinje cells. Proc Natl Acad Sci USA. 1999;96:2467–2472. doi: 10.1073/pnas.96.5.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Endo S, Nairn AC, Greengard P, Ito M. Thr123 of rat G-substrate contributes to its action as a protein phosphatase inhibitor. Neurosci Res (NY) 2003;45:79–89. doi: 10.1016/s0168-0102(02)00199-2. [DOI] [PubMed] [Google Scholar]
- 8.Hall KU, et al. Phosphorylation-dependent inhibition of protein phosphatase-1 by G-substrate. A Purkinje cell substrate of the cyclic GMP-dependent protein kinase. J Biol Chem. 1999;274:3485–3495. doi: 10.1074/jbc.274.6.3485. [DOI] [PubMed] [Google Scholar]
- 9.Hartell NA. cGMP acts within cerebellar Purkinje cells to produce long term depression via mechanisms involving PKC and PKG. NeuroReport. 1994;5:833–836. doi: 10.1097/00001756-199403000-00024. [DOI] [PubMed] [Google Scholar]
- 10.Lev-Ram V, et al. Long-term depression in cerebellar Purkinje neurons results from coincidence of nitric oxide and depolarization-induced Ca2+ transients. Neuron. 1995;15:407–415. doi: 10.1016/0896-6273(95)90044-6. [DOI] [PubMed] [Google Scholar]
- 11.Lev-Ram V, et al. Synergies and coincidence requirements between NO, cGMP, and Ca2+ in the induction of cerebellar long-term depression. Neuron. 1997;18:1025–1038. doi: 10.1016/s0896-6273(00)80340-2. [DOI] [PubMed] [Google Scholar]
- 12.Shin JH, Linden DJ. An NMDA receptor/nitric oxide cascade is involved in cerebellar LTD but is not localized to the parallel fiber terminal. J Neurophysiol. 2005;94:4281–4289. doi: 10.1152/jn.00661.2005. [DOI] [PubMed] [Google Scholar]
- 13.Stone JR, Marlette MA. Soluble guanylate cyclase from bovine lung: Activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous and ferric states. Biochemistry. 1996;35:1094–1099. doi: 10.1021/bi00184a036. [DOI] [PubMed] [Google Scholar]
- 14.Hartell NA, Furuya S, Jacoby S, Okada D. Intercellular action of nitric oxide increases cGMP in cerebellar Purkinje cells. NeuroReport. 2001;12:25–28. doi: 10.1097/00001756-200101220-00013. [DOI] [PubMed] [Google Scholar]
- 15.Detre JA, Nairn AC, Aswad DW, Greengard P. Localization in mammalian brain of G-substrate, a specific substrate for guanosine 3′, 5′-cyclic monophosphate-dependent protein kinase. J Neurosci. 1984;4:2843–2849. doi: 10.1523/JNEUROSCI.04-11-02843.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian Y, et al. cGMP-dependent protein kinase in dorsal root ganglion: Relationship with nitric oxide synthase and nociceptive neurons. J Neurosci. 1996;16:3130–3138. doi: 10.1523/JNEUROSCI.16-10-03130.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crepel F, Jaillard D. Protein kinases, nitric oxide and long-term depression of synapses in the cerebellum. NeuroReport. 1990;1:122–136. doi: 10.1097/00001756-199010000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Ito M, Karachot L. Messengers mediating long-term desensitization in cerebellar Purkinje cells. NeuroReport. 1990;1:129–132. doi: 10.1097/00001756-199010000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Shibuki K, Okada D. Endogenous nitric oxide release required for long-term synaptic depression in the cerebellum. Nature. 1991;349:326–328. doi: 10.1038/349326a0. [DOI] [PubMed] [Google Scholar]
- 20.Daniel H, Hemart N, Jaillard D, Crepel F. Long-term depression requires nitric oxide and guanosine 3′:5′ cyclic monophosphate production in rat cerebellar Purkinje cells. Eur J Neurosci. 1993;5:1079–1082. doi: 10.1111/j.1460-9568.1993.tb00961.x. [DOI] [PubMed] [Google Scholar]
- 21.Lev-Ram V, et al. Absence of cerebellar long-term depression in mice lacking neuronal nitric oxide synthase. Learn Mem. 1997;4:169–717. doi: 10.1101/lm.4.1.169. [DOI] [PubMed] [Google Scholar]
- 22.Boxall AR, Garthwaite J. Long-term depression in rat cerebellum requires both NO synthase and NO-sensitive guanylyl cyclase. Eur J Neurosci. 1996;8:2209–2212. doi: 10.1111/j.1460-9568.1996.tb00743.x. [DOI] [PubMed] [Google Scholar]
- 23.Jacoby S, Sims RE, Hartell NA. Nitric oxide is required for the induction and heterosynaptic spread of cerebellar LTP. J Physiol (London) 2001;535:825–839. doi: 10.1111/j.1469-7793.2001.t01-1-00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feil R, et al. Impairment of LTD and cerebellar learning by Purkinje cell-specific ablation of cGMP-dependent protein kinase I. J Cell Biol. 2003;163:295–302. doi: 10.1083/jcb.200306148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito M. Cerebellar long-term depression: Characterization, signal transduction, and functional roles. Physiol Rev. 2001;81:1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- 26.Linden DJ, Connor JA. Long-term depression of glutamate currents in cultured cerebellar Purkinje neurons does not require nitric oxide signaling. Eur J Neurosci. 1992;4:10–15. doi: 10.1111/j.1460-9568.1992.tb00104.x. [DOI] [PubMed] [Google Scholar]
- 27.Ito M. The molecular organization of cerebellar long-term depression. Nat Rev Neurosci. 2002;3:896–902. doi: 10.1038/nrn962. [DOI] [PubMed] [Google Scholar]
- 28.Katoh A, Kitazawa H, Itohara S, Nagao S. Inhibition of nitric oxide synthesis and gene knockout of neuronal nitric oxide synthase impaired adaptation of mouse optokinetic response eye movement. Learn Mem. 2000;7:220–226. doi: 10.1101/lm.7.4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagao S, Ito M. Subdural application of hemoglobin to the cerebellum blocks vestibuloocular reflex adaptation. NeuroReport. 1991;2:193–196. doi: 10.1097/00001756-199104000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Shutoh F, et al. Memory trace of motor learning shifts transsynaptically from cerebellar cortex to nuclei for consolidation. Neuroscience. 2006;139:767–777. doi: 10.1016/j.neuroscience.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 31.Kassardjian CD, et al. The site of a motor memory shifts with consolidation. J Neurosci. 2005;25:7979–7985. doi: 10.1523/JNEUROSCI.2215-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chapman PF, Atkins CM, Allen MT, Haley JE, Steinmetz JE. Inhibition of nitric oxide synthesis impairs two different forms of learning. NeuroReport. 1992;3:567–570. doi: 10.1097/00001756-199207000-00005. [DOI] [PubMed] [Google Scholar]
- 33.Kriegsfeld LJ, et al. Nocturnal motor coordination deficits in neuronal nitric oxide synthase knock-out mice. Neuroscience. 1991;89:311–315. doi: 10.1016/s0306-4522(98)00614-9. [DOI] [PubMed] [Google Scholar]
- 34.Yanagihara D, Kondo I. Nitric oxide plays a key role in adaptive control of locomotion in cat. Proc Natl Acad Sci USA. 1996;93:13292–13297. doi: 10.1073/pnas.93.23.13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim JJ, Thompson RF. Cerebellar circuits and synaptic mechanisms involved in classical eyeblink conditioning. Trends Neurosci. 1997;20:177–181. doi: 10.1016/s0166-2236(96)10081-3. [DOI] [PubMed] [Google Scholar]
- 36.Aiba A, et al. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. [PubMed] [Google Scholar]
- 37.Kishimoto Y, et al. Impaired delay but normal trace eyeblink conditioning in PLCβ4 mutant mice. NeuroReport. 2001;12:2919–2922. doi: 10.1097/00001756-200109170-00033. [DOI] [PubMed] [Google Scholar]
- 38.Kishimoto Y, et al. Classical eyeblink conditioning in glutamate receptor subunit δ2 mutant mice is impaired in the delay paradigm but not in the trace paradigm. Eur J Neurosci. 2001;13:1249–1253. doi: 10.1046/j.0953-816x.2001.01488.x. [DOI] [PubMed] [Google Scholar]
- 39.Kishimoto Y, et al. mGluR1 in cerebellar Purkinje cells is required for normal association of temporally contiguous stimuli in classical conditioning. Eur J Neurosci. 2002;16:2416–2424. doi: 10.1046/j.1460-9568.2002.02407.x. [DOI] [PubMed] [Google Scholar]
- 40.Shibuki K, et al. Deficient cerebellar long-term depression, impaired eyeblink conditioning, and normal motor coordination in GFAP mutant mice. Neuron. 1996;16:587–599. doi: 10.1016/s0896-6273(00)80078-1. [DOI] [PubMed] [Google Scholar]
- 41.Eto M, Bock R, Brautigan DL, Linden DJ. Cerebellar long-term synaptic depression requires PKC-mediated activation of CPI-17, a myosin/moesin phosphatase inhibitor. Neuron. 2002;36:1145–1158. doi: 10.1016/s0896-6273(02)01107-8. [DOI] [PubMed] [Google Scholar]
- 42.Launey T, et al. Protein phosphatase 2A inhibition induces cerebellar long-term depression and declustering of synaptic AMPA receptor. Proc Natl Acad Sci USA. 2004;101:6766–6781. doi: 10.1073/pnas.0302914101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takeuchi T, et al. Enhancement of both long-term depression induction and optokinetic response adaptation in mice lacking delphilin. PloS ONE. 2008;3:e2297. doi: 10.1371/journal.pone.0002297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78:272–303. doi: 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Stipanovich A, et al. A phosphatase cascade by which rewarding stimuli control nucleosomal response. Nature. 2008;453:879–884. doi: 10.1038/nature06994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Osoegawa K, et al. Bacterial artificial chromosome libraries for mouse sequencing and functional analysis. Genome Res. 2000;10:116–128. [PMC free article] [PubMed] [Google Scholar]
- 47.Gomi H, et al. Mice devoid of the glial fibrillary acidic protein develop normally and are susceptible to scrapie prions. Neuron. 1995;14:29–41. doi: 10.1016/0896-6273(95)90238-4. [DOI] [PubMed] [Google Scholar]
- 48.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.