Abstract
Neuropeptides released from axons of primary afferent nociceptive neurons are the key elements for the incidence of neurogenic inflammation and their release is associated with dorsal root reflexes (DRRs). However, whether the release is due to the triggering of DRRs and plays a role in inflammation-induced pain still remain to be determined. The present study assessed the role of calcitonin gene-related peptide (CGRP) in sensitization of primary afferent nociceptors induced by activation of transient receptor potential vanilloid-1 (TRPV1) following intradermal injection of capsaicin and determined if this release is due to activation of primary afferent neurons antidromically by triggering of DRRs. Under dorsal root intact conditions, primary afferent nociceptive fibers recorded in anesthetized rats could be sensitized by capsaicin injection, as shown by an increase in afferent responses and lowering of the response threshold to mechanical stimuli. After DRRs were removed by dorsal rhizotomy, the capsaicin-evoked sensitization was significantly reduced. In dorsal root intact rats, peripheral pretreatment with a CGRP receptor antagonist could dose-dependently reduce the capsaicin-induced sensitization. Peripheral post-treatment with CGRP could dose-dependently restore the capsaicin-induced sensitization under dorsal rhizotomized conditions. Capsaicin injection evoked increases in numbers of single and double labeled TRPV1 and CGRP neurons in ipsilateral dorsal root ganglia (DRG). After dorsal rhizotomy, these evoked expressions were significantly inhibited.
Perspective
These data indicate that the DRR-mediated neurogenic inflammation enhances sensitization of primary afferent nociceptors induced by capsaicin injection. The underlying mechanism involves antidromic activation of DRG neurons via up-regulation of TRPV1 receptors whereby CGRP is released peripherally.
Keywords: Inflammatory pain, dorsal root reflex, nociceptors, TRPV1, neuropeptides, capsaicin
Neurogenic inflammation is the process by which inflammatory mediators, such as neuropeptides, are released from primary afferent terminal (mainly nociceptors) and produce inflammation in their target tissue 51,64. Pain evoked experimentally by intradermal capsaicin (CAP) injection has been well known to be inflammatory and this process is mediated by a neurogenic mechanism 21,57,58. The transient receptor potential vanilloid-1 (TRPV1) located in primary afferent nociceptors plays a key role in neurogenic inflammation when activated by CAP. The underlying mechanism involves sensitization of primary afferent nociceptors due to activation of TRPV1 receptors, which may lead to the release of neuropeptides, such as calcitonin gene-related peptide (CGRP) and substance P (SP), from the nociceptors to initiate neurogenic inflammation that would presumably promote nociception 20,24,25,40,55,57,58. This is anatomically supported by evidence that many primary afferent nociceptive neurons and their axons are peptidergic with the capability to release inflammatory peptides 2,15,28 and TRPV1 receptors frequently co-localize with CGRP and SP in primary afferent neurons 3,18,44.
Antidromic activation of primary nociceptive afferent endings (efferent function) is well established to be a mechanism of driving the mediator release leading to neurogenic inflammation 27,34. Inflammatory responses (vasodilation and edema) can be evoked experimentally by antidromic stimulation of either small myelinated or unmyelinated primary afferent axons 22,56. There is evidence that a release of CGRP and SP is evoked by antidromic stimulation of primary afferents 19,27. Data from our and other groups using a rat model of CAP injection have clearly revealed that antidromic activity in primary afferent nociceptive fibers (C- and some Aδ-) is centrally mediated by dorsal root reflexes (DRRs) 14,37, which accompany the spread of flare and edema in the paw 14,36. Release of CGRP and SP from axons of primary afferent neurons is suggested to be driven by DRRs 14,35. We hypothesize that sensitization of primary afferent nociceptors evoked by activation of TRPV1 receptors following CAP injection 49,50 involves the triggering of DRRs spinally, and these DRRs would antidromically activate primary afferent neurons to release neuropeptides peripherally. However, whether the release from axons of primary afferent neurons is due to the triggering of DRRs has not been determined, nor has the role of the released neuropeptides resulting from DRRs in the development and maintenance of the inflammatory pain state initiated by CAP injection been investigated.
Using a rat model of neurogenic inflammatory pain induced by intradermal CAP injection 36,49, this study examined electrophysiologically if sensitization of primary afferent nociceptors induced by intradermal injection of CAP is due partially to neurogenic inflammation that underlies an activation of neuropeptidergic receptors by the released neuropeptides from axons of primary afferent nociceptive neurons, and to determine if this process is driven by DRRs. CGRP, one of the neuropeptides, and its receptor antagonist were pharmacologically analyzed for their role in the CAP-evoked sensitization. Using immunohistochemistry, functional activity of dorsal root ganglion (DRG) neurons was evaluated by visualizing changes in the CAP-evoked expression of TRPV1 and/or CGRP in these neurons after elimination of DRRs. Preliminary data were previously presented in annual meetings of the Society for Neuroscience and American Pain Society 48,68.
Materials and Methods
Adult male Sprague-Dawley rats weighing 250-350 g were used in this study. All experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch and were consistent with the guidelines of the National Institutes of Health and the International Association for the Study of Pain. Efforts were made to minimize the number of animals used and their suffering.
Inflammatory pain model
CAP was dissolved in Tween 80 (7%) and saline (93%) to a concentration of 0.15% (pH=7.4). A volume of 20 μl was injected intradermally into the glabrous skin of the hindpaw on one side in anesthetized rats (see anesthesia procedure in the following sections). For control purposes, vehicle (7% Tween 80 and 73% saline) was injected intradermally.
Dorsal Rhizotomy
Dorsal rhizotomy was performed on anesthetized rats to remove DRRs surgically as described previously 36,46. Briefly, a laminectomy was performed to expose dorsal roots of L3-S1 at the T13-L2 vertebral level. After the dura was incised, a small piece of cotton containing 2% lidocaine was applied to the dorsal roots for about 1-2 min at the site where the roots were to be cut to minimize injury discharges. When the dorsal roots at L3-S1 were cut, the exposed spinal cord was covered with a layer of cotton soaked in warm mineral oil, another layer soaked in saline, and a top layer was dry cotton. For the sham-surgery, the same procedure was performed except that dorsal roots were kept intact. The surgery was done by a person who did not perform the experiments. Thus, the experimenter was blind to the type of animal preparation (dorsal rhizotomized or sham-operated).
General preparation and anesthesia for electrophysiological experiments
Animals were first anesthetized with sodium pentobarbital (50 mg/kg) by i.p. injection to perform surgery. Anesthesia was then maintained by a continuous i.v. infusion of a saline solution containing sodium pentobarbital. The infusion rate was adjusted (5-8 mg/kg/h) depending upon the depth of anesthesia. The level of anesthesia was judged as being sufficiently deep when withdrawal responses to noxious limb stimulation and/or the eye-blink reflex to air-puffs were absent. Once a stable level of anesthesia was reached, the animals were paralyzed with pancuronium (0.3-0.4 mg/h, i.v.) and artificially ventilated. Expiratory CO2 was monitored via a tracheal cannula and kept between 3.5 and 4.5%. The adequacy of the depth of anesthesia during an experiment was evaluated by examination of pupillary reflexes and assessing the stability of the expired CO2. Core body temperature was monitored by a rectal probe and maintained near 37°C by a servo-controlled heating blanket.
Single-fiber recordings of afferent activity
Single-fiber recordings of afferent activity were conducted using the procedure that has been described in detail in our previous report 49. Briefly, the tibial nerve was exposed in one hindpaw and then the incised skin was retracted and bound around a frame to form a pool, which was then filled with warm mineral oil covering the exposed tissue. The tibial nerve was carefully freed from surrounding tissues and part of the nerve (one fourth to one third) was removed after a longitudinal cut. The distal cut end of the tibial nerve was then teased into small filaments with fine-tipped forceps on a small mirror-based platform under a dissecting microscope until single-fiber activity of afferents from a fine nerve filament could be isolated on the basis of spike amplitude and waveform. Recorded action potentials and their responses to peripheral mechanical stimuli were amplified and displayed on a digital oscilloscope (TDS-3012B). This allowed us to ensure that the same unit was being recorded throughout the experiment. Action potentials were recorded and then converted into analog signals or histograms that were available to be quantified by Spike-2 software (Cambridge Electronic Design) 49,50. The fiber types of single units were classified by conduction velocity (Aδ, 2-19.9 m/s; C, <2 m/s). This was determined using two parameters: the propagation distance between the stimulating and recording electrodes and the response latency to the single stimulating pulses, as previously described in detail 32,49.
Peripheral administration of CGRP or CGRP8-37 for electrophysiological experiments
Close-by intra-arterial injection was used to administer CGRP and CGRP8-37 locally. To do this, a fine branch of the femoral artery was cannulated by a polyethylene-10 catheter connected to a 50 μl syringe for drug delivery to the hind paw 49,50. CGRP (1, 3, or 10 μg dissolved in saline, TOCRIS) was administered intra-arterially in a volume of 25 μl 10 min after CAP injection in dorsal rhizotomized rats. Under dorsal root intact conditions, CGRP8-37 (a CGRP receptor antagonist, 3, 10 or 30 μg dissolved in saline, TOCRIS) was injected intra-arterially in a volume of 25 μl 5 min prior to CAP injection. For control purposes, saline (25 μl) was given using the same procedure.
Experimental protocol for electrophysiological experiments
Since our previous studies 49,50 showed in the same model that spontaneous activity was seen in only a few afferent fibers and there was no significant change in spontaneous activity after drug treatments, we only recorded and analyzed the mechanically evoked activity and response thresholds in the present study. A single unit was determined by moving a von Frey filament with a bending force that produced suprathreshold mechanical stimulation over the surface of the glabrous skin of the hindpaw, whereby the mechanical receptive field of an individual single fiber on the plantar surface was located. Mechanically evoked responses of a single Aδ- or C-afferent fiber were then elicited by applying a set of calibrated von Frey filaments (Stoelting, Wood Dale, IL) to the most sensitive spot of the receptive field 49,53. The smallest von Frey filament that elicited at least 5 spikes within 10 s in ≥ 50% of the trials was taken as threshold 1,53. The appropriate set of von Frey filaments (three filaments with graded bending forces) was chosen in each experiment according to different response thresholds. The first filament was that with the threshold bending force and two additional filaments had progressively stronger bending forces. Each filament was applied repetitively for 10 s with enough force to cause slight bending and this force was held for about 1 s followed by a 10-s pause before the next filament was used. To decrease a minimum possible “human factor” bias, mechanical stimuli were applied without observation of the oscilloscope or computerized record so the experimenter was unaware of the response frequencies.
Aδ- and C-afferent fibers with mechanical thresholds of 20-147 mN have previously been demonstrated to be nociceptors 32. Our previous studies have shown that these fibers were sensitized by intradermal injection of CAP 49,50. This phenomenon is also supported by studies of other groups that a high expression of TRPV1 receptors, which are nociceptive molecules, is evident in fibers that are CAP-sensitive 41,59. Therefore, only Aδ and C-afferent fibers with mechanical thresholds of 20-147 mN that were identified as nociceptors were recorded and analyzed in this study.
CAP was injected intradermally near the edge of the receptive field to evoke an acute cutaneous inflammation after baseline responses were recorded 49. For the changes in evoked activity, the responses were sampled at 15, 30, 45, and 60 min after CAP injection. For the changes in response threshold, responses were sampled when they reached the nadir. Changes in mechanically evoked activity and response threshold of single Aδ and C-afferent fibers after CAP injection were recorded and compared between sham surgery rats and dorsal rhizotomized rats.
To investigate whether CGRP affected the sensitization of primary afferents induced by intradermal injection of CAP, CGRP at 3 different doses (1, 3, or 10 μg) was administered intra-arterially into the hindpaw in a volume of 25 μl 10 min after CAP injection in three groups of dorsal rhizotomized rats. Changes in responses to mechanical stimuli after CAP injection were recorded for 60 min. To examine whether a CGRP receptor antagonist could block the CAP-induced sensitization of nociceptors, CGRP8-37 at 3 different doses (3, 10 or 30 μg) was given intra-arterially 5 min prior to CAP injection in three groups of sham surgery (dorsal root intact) rats. As controls, saline (25 μl) was given in the same manner. Using a laser Doppler flow meter for cutaneous blood flow measurements on the foot skin, our recent study of the effects of local injection of CGRP or SP on cutaneous vasodilation has shown that intra-arterial injection of these neuropepetides in the hindpaw did not produce a significant change in blood flow in the forepaw 35.
Immunohistochemistry
Single and/or double labeling for TRPV1 and/or CGRP in DRG neurons was performed using immunohistochemistry and fluorescence tags. DRG tissue at L4-5 on the side ipsilateral to the CAP injection was sampled at 30 and 60 min after intradermal injection of CAP or vehicle under sham dorsal rhizotomized or dorsal rhizotomized conditions. To do this, anesthetized rats were perfused through the left ventricle with 4% paraformaldehyde in 0.1M phosphate-buffered solution (PB, pH 7.3, 4°C). DRGs were removed and placed in the same fixative for 4 h. The fixative was then replaced with 30% sucrose solution overnight. Frozen sections were cut at 10 μm and then blocked with 10% normal goat serum in PBS/0.3% Triton X-100 for 1 h at room temperature. Immunofluorescence staining for TRPV1 receptors (1:1,000, guinea-pig polyclonal, from Chemicon Inc.) and for CGRP (1:1,000, mouse monoclonal, from Chemicon, Inc.) was performed. Sections were incubated with a mixture of the two primary antibodies for 24 h at 4°C. Then the sections were transferred to a secondary antibody solution containing Alexa Fluor® 488 (1:200, goat anti-guinea-pig IgG, from Molecular Probes) and Alexa Fluor® 568 (1:200, goat anti-mouse IgG, from Molecular Probes) for 1 h respectively at room temperature. Controls for specificity included: 1) staining DRG sections as described above but omitting the primary antibody, which resulted in no detectable labeling; 2) incubation with a single primary antibody followed by the appropriate secondary antibody, to ensure that the labeling pattern for each substance in the double-stained sections was similar to that observed in the single-labeled section; 3) incubation with a single primary antibody, followed by a mixture of 2 secondary antibodies, in order to test the species specificity of the secondary antibodies used.
Following staining, labeled sections were examined and processed by confocal laser scanning microscopy (Nikon EZ-C1) for staining of TRPV1- and CGRP-immunofluorescence. Two fluorescence filters (Fluor 488 filter for green and Fluor 568 filter for red) were used to separate individual wave lengths for staining. Thus, digitized images were obtained of two different colors for TRPV1 and CGRP, respectively. Analysis of co-staining was done by matching of corresponding regions. A neuronal profile was considered as positively double labeled if it showed two color codes overlapping in space. For quantification, the numbers of single- and double-labeled neuronal profiles with different labeling were then calculated using Metamorph software. Profiles were counted in 10 sections that were separated by at least 50 μm between consecutive sections. Sections were selected from each animal for counting and averaging cell profile numbers. Two counts were made of 1) the numbers of TRPV1 and CGRP positive neuronal profiles; 2) the number of double labeled neuronal profiles.
Statistical analysis
Electrophysiological data
Recorded fiber activity was analyzed on- or off-line from peristimulus time histograms to obtain the average rate of evoked discharges. All responses evoked by stimulation using three calibrated von Frey filaments with graded bending forces were calculated by subtracting the background discharges during a given period of time from the total number of action potentials that occurred during each stimulus to produce a net increase in discharge rate. In each group, discharge frequencies were converted into percentage data by setting the responses to mechanical stimuli before CAP injection (pre-CAP, baseline) as 100% and the responses to mechanical stimuli after CAP injection as percentage changes from the pre-CAP value. Statistical significance was tested using one-way ANOVA with repeated measures followed by a Bonferroni-corrected t-test in the same group to compare differences between the baseline (pre-CAP injection) and responses after CAP injection at different time points. One-way ANOVA followed by Student-Newman-Keuls test was used to compare the differences in responses between groups having different treatments at the same time point.
The differences of mechanical response threshold was first assessed by comparing the values of bending forces (the weakest von Frey hair that elicited a response) before and after CAP injection in the same group using a Wilcoxon Signed Rank test. The differences of threshold between groups having different treatments were then assessed by comparing percent changes in threshold using a Mann-Whitney Rank Sum Test. To do this, the response threshold before CAP injection was set as 100% and the threshold after CAP injection was expressed as a percentage change from the pre-CAP value in the same group.
Immunochemistry data
Five animals were included in each group. The numbers of immunoreactive positive (single and double labeled) neuronal profiles were counted using Metamorph offline software. Statistical differences between groups were determined by one-way ANOVA followed with Dunnet’s post-test.
Values are presented as means±SE. P<0.05 was taken as significant.
Results
A total of 93 Aδ- and 80 C-afferent nociceptive fibers were recorded from the tibial nerve in 173 rats (i.e. only one fiber was recorded from each rat). Of these fibers, 47 Aδ- and 42 C-fibers were from sham surgery rats, and 46 Aδ- and 38 C-fibers from dorsal rhizotomized rats.
Effects of dorsal root reflex removal on capsaicin-evoked sensitization of primary afferent nociceptive fibers
Mechanically evoked activity and response threshold of Aδ- and C-afferent nociceptive fibers to CAP injection were examined in the groups of sham surgery and dorsal rhizotomized rats, respectively. Panels A and B in Fig. 1 are grouped data in which the effects of CAP injection on mechanically evoked responses and time course are summarized. The responses to mechanical stimuli before CAP injection served as a baseline in each group (100%, pre-CAP injection). The evoked responses of both Aδ- and C-afferent fibers were tested at 15, 30, 45 and 60 min after intradermal injection of CAP in sham surgery and dorsal rhizotomized rats. Consistent with our previous reports 49,50, in sham surgery (dorsal root intact) rats, a significant enhancement of response activity was seen in C-fibers at 15 min (124.0±6.6%, P<0.01, vs. pre-CAP level), both in Aδ- and C-fibers at 30 min (122.2±4.3% and 142.9±8.0%, P<0.001 and P<0.001, vs. pre-CAP level) and 45 min (122.6±5.4% and 157.8±11.7%, P<0.01 and P<0.001, vs. pre-CAP level), and in C-fibers at 60 min (147.4±14.7%, P<0.01, vs. pre-CAP level) after CAP injection. Thus, the magnitude and duration of the increase seen in C-fibers was greater than in Aδ-fibers. The enhancement of the responses reached its peak at 30-45 min after CAP injection in both Aδ- and C-fibers, and recovered toward the baseline at 60 min after CAP injection (Fig. 1A,B). In contrast, the CAP-evoked enhancement of responses of Aδ- and C-afferent fibers to mechanical stimuli was dramatically reduced after dorsal rhizotomy (Fig. 1A,B). The peak increase at 45 min after CAP injection was 105.2±2.9% for Aδ-fibers and 116.2±2.4% (P<0.01, vs. pre-CAP) for C-fibers. The response magnitudes recorded from Aδ-fibers at 30 and 45 min after CAP injection and from C-fibers at 15, 30, 45 and 60 min after CAP injection were significantly reduced compared to those in group of sham surgery rats (see asterisks in Fig. 1A,B). Traces in Fig. 2 are examples of rate histograms of evoked action potentials recorded from single C-fibers showing the effects of CAP injection under dorsal root intact (A) and dorsal rhizotomized (B) conditions.
Figure 1.
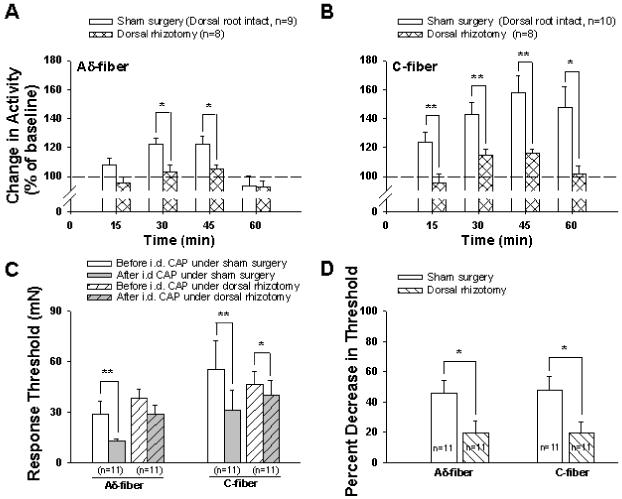
Grouped data summarize the mean effects of dorsal rhizotomy on afferent responses and response threshold of single Aδ- and C-fibers to mechanical stimuli after intradermal injection of CAP. Enhanced responses to mechanical stimuli after CAP injection were seen in Aδ-fibers (A) and C-fibers (B) in sham surgery (dorsal root intact) rats. However, the enhanced responses induced by CAP injection were significantly reduced after dorsal rhizotomy. Baseline level (before CAP injection) was set as 100%. * and **, P<0.05 and P<0.01, dorsal rhizotomized group vs. sham surgery group. C: Changes in response threshold in Aδ- and C-fibers after CAP injection in sham surgery and dorsal rhizotomized rats. *, **: P<0.05, P<0.01, vs. before CAP injection. D: A comparison of changes in percent decrease in response threshold due to CAP injection between sham surgery and dorsal rhizotomized rats. *, P<0.05, vs. sham surgery group.
Figure 2.
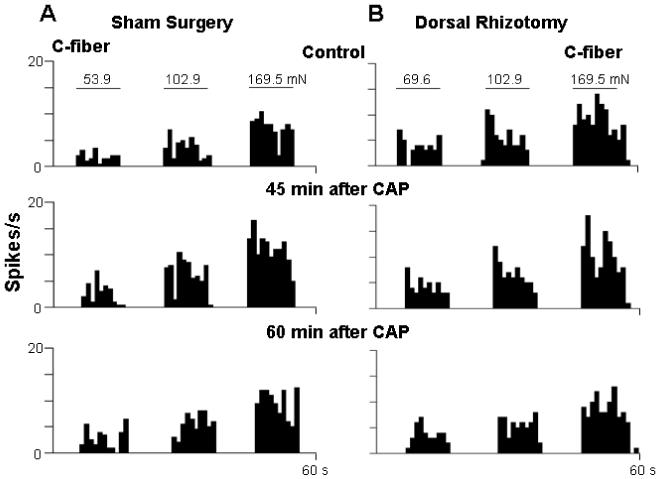
Rate histograms show the effect of intact dorsal roots (Sham surgery, A) and dorsal rhizotomy (B) on the responses of single C-fibers to mechanical stimuli after CAP injection. Top row: Responses to mechanical stimuli before CAP injection. Second row: 45 min after CAP injection; note that an obviously enhanced response to mechanical stimuli was seen in the C-fiber under sham surgery conditions (A). After dorsal rhizotomy, the enhanced responses were completely inhibited (B). Bottom row: 60 min after CAP injection. Horizontal lines above histograms indicate time of application of von Frey hairs. Bending forces are shown above the horizontal lines. Bin width: 1 s.
Changes in the response threshold to mechanical stimuli of Aδ- and C-fibers due to CAP injection were sampled when they reached the nadir in each group (Fig. 1C) and then compared between sham surgery and dorsal rhizotomized groups (Fig. 1D). In the sham surgery group of rats, the response threshold to mechanical stimuli was decreased from 29.0±7.4 mN to 12.6±1.5 mN in Aδ-fibers (p<0.01), and from 55.2±17.5 mN to 31.2±11.5 mN in C-fiber (P<0.01) after CAP injection. After dorsal rhizotomy, response threshold before CAP injection was slightly increased in Aδ-fibers and slightly decreased in C-fibers without a statistically significant difference (Fig. 1C). However, the reduction in response threshold due to the same dose of CAP injection became less. The threshold decreased slightly from 38.2±5.6 mN to 29.1±5.1 mN (p=0.063) in Aδ-fibers and from 46.7±7.8 mN to 40.1±8.8 mN (P<0.05) in C-fibers, respectively. (Fig. 1C). A further comparison was made to examine whether there was a statistically significant difference between sham surgery and dorsal rhizotomized groups on the percent decrease in the response threshold after CAP injection (Fig. 1D). The control (response threshold before CAP injection) was set as 100% and the bars in Fig. 1D show the percentage of control after CAP injection. In the dorsal rhizotomized group, the percent decrease in threshold after CAP injection was significantly less than that in the sham surgery group both in Aδ- and C-fibers (P<0.05 and P<0.05) (Fig. 1D).
Thus, DRR removal led to an attenuation in sensitization of primary afferent nociceptive fibers induced by intradermal injection of CAP.
Effects of blockade of peripheral CGRP receptors on the sensitization of primary afferent nociceptive fibers induced by CAP injection under dorsal root intact conditions
This experiment was to examine further whether blockade of CGRP receptors in the periphery by intra-arterial injection of CGRP8-37 would reduce or block the sensitization of Aδ- and C-fibers induced by CAP injection in dorsal root intact (sham surgery) rats. In the groups of Aδ- and C-fibers with saline pre-treatment, CAP injection produced a significant enhancement of responses to mechanical stimuli. Consistent with the data shown in Fig. 1A,B, a significant increase in evoked responses was seen in Aδ-fibers (Fig. 3A) at 30 and 45 min after CAP injection (120.8±4.1%, P<0.001 and 120.0±5.5%, P<0.01, vs. pre-CAP level) and in C-fibers (Fig. 3B) at 15, 30, 45 and 60 min after CAP injection (120.0±5.8%, P<0.01; 141.6±8.8%, P<0.01; 157.0±13.1%, P<0.01 and 142.2±15.3%, P<0.05, vs. pre-CAP level). In contrast, the CAP-evoked enhancement of responses to mechanical stimuli of Aδ-fibers and C-fibers were dose dependently decreased after pretreatment with different doses of CGRP8-37. In the presence of 3 μg CGRP8-37, a significant reduction in the CAP-induced enhancement was seen only in C-fibers at 45 min after CAP injection (see asterisk in Fig. 3B, P<0.05 vs. saline group). When the dose reached 10 μg, a significant reduction was obtained in both types of fibers at 30 and 45 min after CAP injection. Pretreatment with 30 μg of CGRP8-37 peripherally nearly completely blocked the CAP-induced enhancement of evoked activity (see asterisks in Fig. 3A,B, P<0.01 and P<0.001, vs. saline group). Traces A and B in Fig. 4 are examples of rate histograms of evoked action potentials recorded from single Aδ- and C-fibers showing the effect of 30 μg CGRP8-37 on the CAP-induced enhancement.
Figure 3.
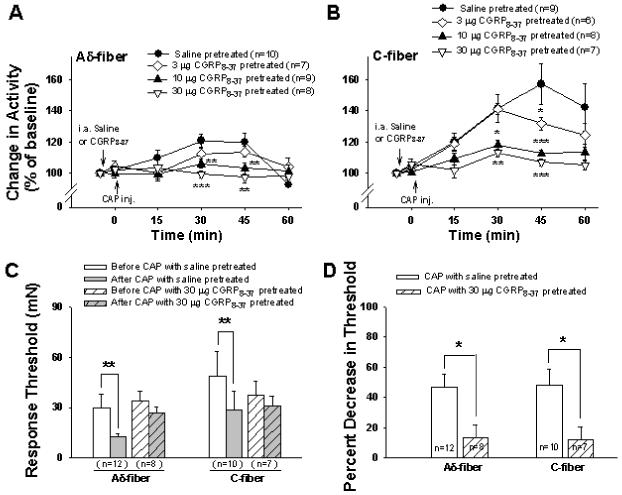
Effects of blockade of peripheral CGRP receptors by intra-arterial injection of CGRP8-37 on afferent responses and response threshold to mechanical stimuli induced by CAP injection in dorsal root intact (sham surgery) rats. A and B: Grouped data summarize the effects of pretreatment with three doses of CGRP8-37 on the CAP-induced enhancement of responses of Aδ- (A) and C-fibers (B) to mechanical stimuli under dorsal root intact conditions. * P < 0.05, ** P < 0.01, and *** P<0.001 vs. saline group. C: Effects of 30 μg CGRP8-37 on changes in response threshold in Aδ- and C-fibers due to CAP injection in sham surgery rats. **: P<0.01, vs. before CAP injection. D: A comparison of changes in percent decrease in response threshold due to CAP injection between saline and 30 μg CGRP8-37 treated groups in sham surgery rats. *: P<0.05, vs. saline treated group.
Figure 4.
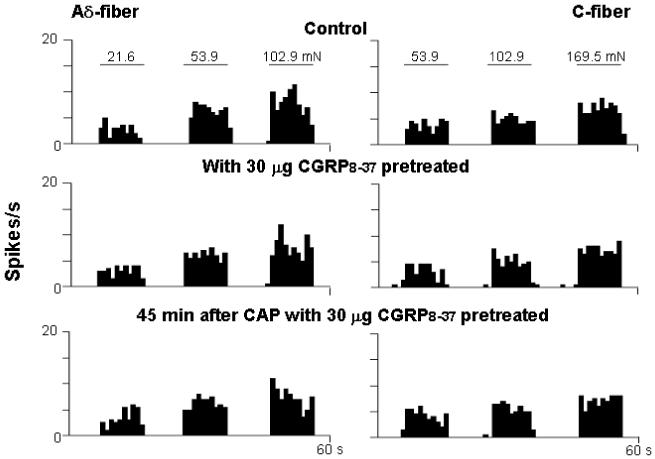
Rate histograms show the effect of CGRP8-37 (30 μg) pre-treatment on CAP-induced sensitization of responses of single Aδ- and C-fibers to mechanical stimuli under dorsal root intact conditions. Top row: Responses to mechanical stimuli before CAP injection. Second row: 5 min after CGRP8-37 pre-treatment. Bottom row: 45 min after CAP injection with CGRP8-37 pre-treatment. Bin width: 1 s.
The effects of blockade of CGRP receptors on changes in the response threshold induced by CAP injection were examined by using 30 μg of CGRP8-37, because the CAP-induced enhancement of evoked activity was nearly completely inhibited at this dose. CAP injection lowered significantly the response threshold to mechanical stimuli in both Aδ- and C-primary afferent nociceptors in the saline pre-treatment group (Fig. 3C). When pre-treated with CGRP8-37 peripherally, however, no statistically significant difference was seen in response threshold before and after CAP injection (Fig. 3C). A comparison was further made between saline- and CGRP8-37-treated groups on the percent decrease in the response threshold after CAP injection (Fig. 3D). In CGRP8-37-treated group, the percent decrease in threshold after CAP injection was significantly less than that in saline treated group both in Aδ- and C-fibers (see asterisks Fig. 3D, P<0.05 and P<0.05, vs. saline treated group).
Thus, dose-response analysis of the antagonistic effect of CGRP8-37 reveals that the CAP-induced sensitization could be inhibited by the blockade of CGRP receptors in a dose-dependent manner.
Effects of activation of peripheral CGRP receptors on capsaicin evoked activity and response threshold of primary afferent nociceptive fibers under dorsal rhizotomized conditions
An attenuation of the CAP-induced sensitization was observed by DRR removal (dorsal rhizotomy) in the first section, which indicates that a neurogenic component driven by DRRs contributes to sensitization. Furthermore, a reduction in the CAP-induced sensitization was seen after blockade of peripheral CGRP receptors in the second section, suggesting that the process of sensitization after TRPV1 activation involves the release of CGRP which then activates CGRP receptors. In this section, we wanted to test pharmacologically whether CGRP release plays a role in the CAP-evoked sensitization. To do this, CGRP was given exogenously in the periphery as a post-treatment to activate CGRP receptors under the conditions of dorsal rhizotomy that removed DRRs. In the presence of CGRP peripherally, the sensitization of afferent fibers following CAP injection was rekindled in a dose-dependent manner. The rekindling was more obvious in C-fibers. Fig. 5A,B is the grouped data summarizing the effects of activation of CGRP receptors on CAP evoked activity under dorsal rhizotomized conditions. Either saline or CGRP at one of three doses (1, 3, 10 μg) was given intra-arterially 10 min after CAP injection in each group of dorsal rhizotomized rats. Consistent with the result shown in Fig. 1, CAP injection under dorsal rhizotomized conditions produced only a slight increase in responses of Aδ- and C-afferent nociceptive fibers to mechanical stimuli (Fig. 5A,B). Post-treatment with saline did not obviously change the CAP effect because the time-course recordings of responses to CAP injection were similar to those shown in Fig. 1A,B. The dose-response tests for CGRP administration showed that a low dose (1 μg) produced no significant effect. When the periphery was post-treated with CGRP at 3 μg, the sensitization of C-fibers (Fig. 5B) was slightly but significantly rekindled when recorded at 30 min (139.5±6.9%, P<0.01 vs. saline group at the same time point) and 45 min (138.4±6.1%, P<0.01 vs. saline group at the same time point) after CAP injection. In the presence of 10 μg CGRP, the sensitization of Aδ-fibers was rekindled at 45 min (125.5±4.3%, P<0.01 vs. saline group) after CAP injection (Fig. 5A), and sensitization of C-fibers was greatly rekindled at 15, 30 and 45 min (151.8±11.7%, P<0.001, 165.3±15.4%, P<0.01, and 161.6±22.5%, P<0.05, vs. saline group) after CAP injection (Fig. 5B). The magnitude of rekindled sensitization seen in C-fibers in the presence of 10 μg CGRP was comparable to that induced by CAP injection only under the condition in which dorsal roots were intact (see Fig. 1). Traces A and B in Fig. 6 are records from two single fibers (Aδ- and C-) of dorsal rhizotomized rats showing the rekindling effect by 10 μg CGRP given as a post-treatment.
Figure 5.
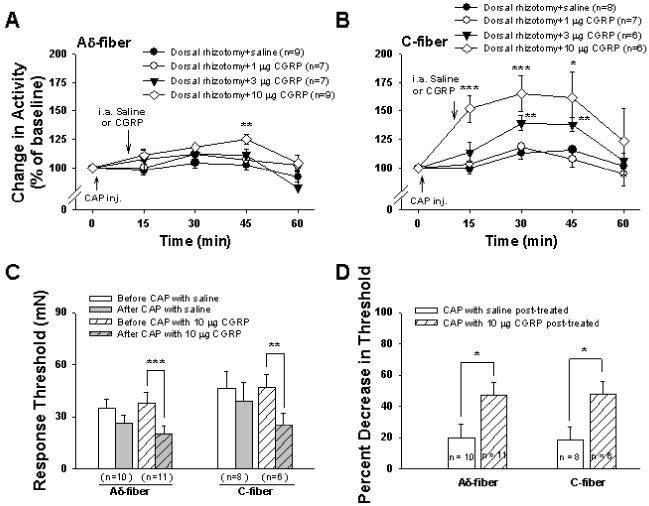
Effects of CGRP post-treatment on the reduction in the CAP-induced sensitization of responses and threshold of single Aδ- and C-fibers to mechanical stimuli due to dorsal rhizotomy. A and B: Grouped data summarize the mean effects of three doses of CGRP on responses of single Aδ- (A) and C-fibers (B) to mechanical stimuli after CAP injection. * P<0.05, ** P<0.01, *** P<0.001 vs. saline group. C: Effects of 10 μg CGRP on changes in response threshold in Aδ- and C-fibers after CAP injection in dorsal rhizotomized rats. **, ***: P<0.01, P<0.001, vs. before CAP injection. D: A comparison of changes in percent decrease in response threshold due to CAP injection between saline and CGRP treated groups in dorsal rhizotomized rats. *: P<0.05, vs. saline treated group.
Figure 6.
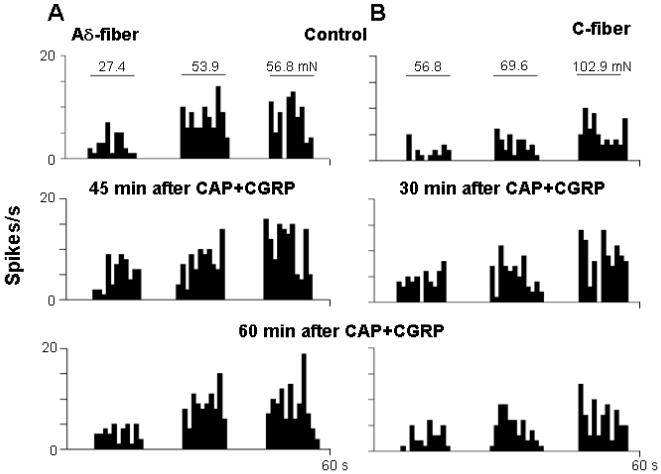
Rate histograms show the effect of CGRP (10 μg) post-treatment on the responses of single Aδ- (A) and C-fibers (B) to mechanical stimuli after CAP injection under dorsal rhizotomized conditions. Top row: Responses to mechanical stimuli before CAP injection. Second row: 45 min in Aδ-fiber and 30 min in C-fiber after CAP injection, an obviously enhanced response to mechanical stimuli was seen after intra-arterial CGRP treatment 10 min after CAP injection under dorsal rhizotomized conditions. Bottom row: 60 min after CAP injection, the enhanced response to mechanical stimuli decreased. Bin width: 1 s.
Based on the above observation that 10 μg of CGRP produced a significant rekindling of CAP-evoked sensitization, the effects of activation of CGRP receptors on changes in the response threshold following CAP injection in dorsal rhizotomized rats were examined using 10 μg of CGRP. In group of dorsal rhizotomized rats, no significant effect was seen of saline post-treatment on the change in response threshold of Aδ- and C-afferent nociceptive fibers induced by CAP injection (Fig. 5C), i.e. the response threshold showed no significant difference before and after CAP injection. However, when CGRP (10 μg) was given as post-treatment, a significant decrease in response threshold was seen in both Aδ- (P<0.001) and C-(P<0.01) fibers after CAP injection (Fig. 5C). A further comparison was made between saline and CGRP treated groups on the percent decrease in the response threshold after CAP injection (Fig. 5D). The control (response threshold before CAP injection) was set as 100% and the bars in Fig. 5D show the percentage of control after CAP injection. In the CGRP treated group, the percent decrease in threshold after CAP injection was significantly more than that in the saline treated group both in Aδ- and C-fibers (P<0.05 and P<0.05) (Fig. 5D).
Effects of dorsal root reflex removal on capsaicin-evoked expression of TRPV1 and CGRP in dorsal root ganglion neurons
Fig. 7 shows representative confocal immunofluorescence images of single labeling of TRPV1 and CGRP and double labeling of these two molecules in L4 DRG neurons in the same section. Single staining of TRPV1 and CGRP and their double staining were seen preferentially in small and medium sized primary afferent neurons in DRG, consistent with previous reports 8,18,43. A1-A3 are immunofluorescence labeling of these two molecules at 30 min after intradermal vehicle injection under sham dorsal rhizotomized conditions (intact dorsal roots). B1-B3 show changes in expression and co-expression of these two molecules in L4 DRG neurons 30 min after intradermal injection of CAP under sham dorsal rhizotomized conditions. There were substantial increases in numbers of the neurons with both single and double staining for these molecules at 30 min after CAP injection. In contrast, CAP-evoked expression of these molecules was inhibited after dorsal rhizotomy (C1-C3). Fig. 8 is the grouped data summarizing the changes in the CAP-evoked expressions of TRPV1 and CGRP in L4-5 DRG due to dorsal rhizotomy that eliminated DRRs. Experiments that served as controls for examining the effects of DRR removal were performed by intradermal injection of vehicle in the sham-dorsal rhizotomized rats. Increases in the number of single and double stained neuronal profiles due to unilateral intradermal injection of CAP peaked at 30 min after CAP injection, and recovered partially at 60 min after injection under conditions where dorsal roots were intact. After DRRs were removed by dorsal rhizotomy, CAP-evoked expression and co-expression were significantly inhibited.
Figure 7.
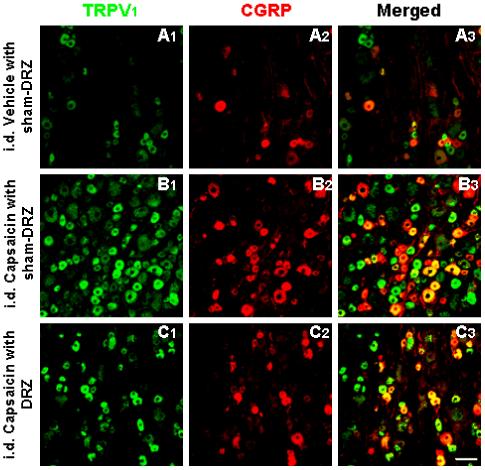
Confocal immunofluorescence images showing TRPV1 positive profiles (A1, B1, C1), CGRP positive profiles (A2, B2, C2) and neurons double-labeled for TRPV1 and CGRP (A3, B3, C3) in L4 DRG on the side ipsilateral to intradermal (i.d.) vehicle or CAP injection. Tissue was collected 30 min after vehicle or CAP injection. A1-A3: intradermal injection of vehicle under sham dorsal rhizotomized (sham-DRZ) conditions. B1-B3: intradermal injection of CAP under sham dorsal rhizotomized (sham-DRZ) conditions. C1-C3: intradermal injection CAP under dorsal rhizotomized (DRZ) conditions. Scale bar=50 μm.
Figure 8.
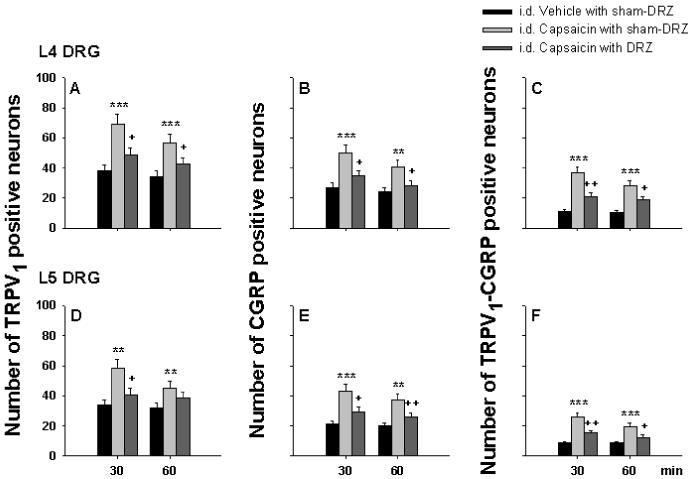
Grouped data summarizing the effects of dorsal rhizotomy (DRR removal) on the CAP-evoked expressions of TRPV1 and CGRP, and their co-expression in L4 (A-C) and L5 (D-F) DRG on the side ipsilateral to intradermal (i.d.) injection. Observations were made in intradermal vehicle and CAP injection groups under sham dorsal rhizotomized (i.d. Vehicle with sham-DRZ and i.d. Capsaicin with sham-DRZ) conditions and in the intradermal CAP injection group under dorsal rhizotomized (i.d. Capsaicin with DRZ) conditions, respectively. DRG tissue was sampled at 30 and 60 min after vehicle or CAP injection. ** and ***: P<0.01 and P<0.001, compared with i.d. Vehicle with sham-DRZ. + and ++: P<0.05 and P<0.01, compared with i.d. Capsaicin with sham-DRZ.
Discussion
The main findings of this study in a rat model of acute inflammatory pain induced by intradermal injection of CAP are that we demonstrate for the first time that the neurogenic mechanism involved in inflammation of somatic tissue, such as the skin, contributes critically to the sensitization of primary afferent nociceptive fibers. This neurogenic mechanism is operated mainly by DRRs that antidromically activate primary afferent neurons in DRG to release neuropeptides peripherally. One of the released neuropeptides, CGRP, is demonstrated in the current study to participate in sensitization of primary afferent nociceptive fibers following CAP injection. Thus, the data obtained from the present study have shed light on the mechanisms of pathological pain due to the sensitization of primary afferent nociceptors.
Peripheral sensitization of nociceptors is thought to be responsible for the primary mechanical allodynia and hyperalgesia that may occur following injury 30,42. One of the major mechanisms by which nociceptors are sensitized is that a variety of inflammatory mediators released after tissue injury act on receptors contained in the surface membranes of the nociceptors 10. These receptors are coupled to intracellular signal transduction cascades 17,26, and protein kinases in these cascades can phosphorylate transduction molecules or ion channels in the membranes of nociceptors. Phosphorylation of these membrane proteins may result in a lowered threshold to stimuli, a process called peripheral sensitization 13,16,39. The TRPV1 receptor serves as an important nociceptive molecule and plays a critical role in sensitization of primary afferent nociceptors because it is expressed predominantly in small to medium sized primary afferent neurons and their axon terminals, and is the basis on which these nociceptive terminals are CAP-sensitive. It has been confirmed by previous and present studies in our and other groups that intradermal CAP injection leads to sensitization of primary afferent nociceptive fibers 29,49,50. In addition to the enhanced responses of Aδ- and C-nociceptive fibers to mechanical stimuli, the present study provides further evidence that the response threshold to mechanical stimuli of these nociceptive fibers is lowered after CAP injection. It is generally accepted that the mechanisms underlying sensitization of primary afferent nociceptors induced by CAP include a direct action of CAP by which TRPV1 receptors are activated to cause influx of Ca2+ and/or Na+ to produce depolarization of neuronal membranes and the generation of action potentials 4. Furthermore, up-regulation of TRPV1 receptors by protein phosphorylation that is triggered by signal transduction cascades due to Ca2+ influx 23 would then enhance the activity of TRPV1 receptors either by increasing protein expression or sensitizing the receptors.
Activation or sensitization of TRPV1 receptors also has an efferent function by means of axon reflexes. This is based on the facts that TRPV1 receptors frequently co-localize with neuropeptides in primary afferent nociceptive neurons and their axons 3,18,44 and that activation of TRPV1 receptors induces Ca2+-dependent CGRP release 24,52. Thus, a contribution of neurogenic inflammation to sensitization of nociceptors is implied. Studies by our and other groups have demonstrated that the efferent function by TRPV1 receptors to initiate neurogenic inflammation is mediated by triggering DRRs spinally 14,35,36. Thus, what we wanted to address in the present study was whether the CAP-induced sensitization of primary afferent nociceptors involved a neurogenic component, and if so, how the neurogenic component was involved in the pathological basis of sensitization? We propose that neurogenic inflammation is initiated by activation of TRPV1 receptors in primary afferent nociceptors to trigger DRRs in the spinal cord, which would antidromically activate DRG neurons to release inflammatory neuropeptides that help sensitize primary afferent nociceptors. To test this hypothesis, electrophysiological recordings of primary afferent nociceptive fibers were conducted in vivo to evaluate the sensitization that followed intradermal injection of CAP in animal preparations in which DRRs were eliminated surgically by dorsal rhizotomy. Previous studies by our and other groups have demonstrated that dorsal rhizotomy is an effective means to eliminate DRRs 14,36,46. We found that an enhanced mechanically evoked activity and lowered response threshold induced by CAP injection were dramatically inhibited after dorsal rhizotomy. These data strongly indicate that a neurogenic mechanism contributes importantly to sensitization, and a DRR-driven antidromic release of inflammatory mediators is strongly suggested.
A potential release of CGRP and SP driven by DRRs and its role in the CAP-induced neurogenic inflammation have been suggested by our and other groups using the same model as we used in the current experiments 14,35. Therefore, we have then analyzed whether the released CGRP contributed a critical component to the CAP-induced sensitization. Under dorsal rhizotomized conditions that removed DRRs, CGRP was exogenously given right after CAP injection to mimic the endogenous release of CGRP that was initiated by CAP injection and then driven by DRRs. The results show that CGRP could dose-dependently restore the CAP-induced sensitization of primary afferent nociceptive fibers. An experiment using a CGRP receptor antagonist in dorsal root intact animals has further shown that the blockade of CGRP receptors could significantly attenuate the CAP-induced sensitization in a dose-dependent manner. Thus, CGRP released in the periphery participates in sensitization of primary afferent nociceptors evoked by activation of TRPV1 receptors following CAP injection. The process of CGRP release is largely due to DRRs, even though a direct action by TRPV1 activation to induce Ca2+ influx-dependent release of CGRP also contributes 24,52,66.
It has been demonstrated pharmacologically that DRRs are generated in spinal cord GABAergic interneuronal circuits following CAP injection 36,47,54, and that GABAergic interneurons are sensitized by CAP injection 69. DRRs are then conveyed antidromically along primary afferents to DRG neurons whereby DRRs are propagated towards periphery 9,63,65. We presume that the above process should involve an antidromic activation of DRG neurons via up-regulation of some nociceptive molecules, such as TRPV1 receptors, which would lead to a hyperactive synthesis of neuropeptides in neurons to participate in neurogenic inflammation. Using immunofluorescence in this study, the CAP-evoked expression of TRPV1 and CGRP in DRG neurons was visualized to determine the effects of dorsal rhizotomy in order to evaluate the contribution of DRRs to activation of DRG neurons. Observations made at 30 and 60 min after CAP injection show that evoked increases in expression were most obvious at 30 min after injection. This rapid up-regulation after noxious stimulation was proposed to be afferent activity dependent but remains to be further investigated 7,45. Critically, our data reveal that the CAP-evoked expressions were partially but significantly inhibited after DRRs were eliminated. Pathophysiologically, these data help elucidate the mechanisms of the DRR-mediated acute neurogenic inflammation induced by CAP injection examined by our and other groups 14,35,36,60. Thus, new evidence is provided to indicate strongly that DRRs drive the release of CGRP from primary afferent terminals by sensitization of DRG neurons via TRPV1 receptors following CAP injection.
CGRP released from peripheral nociceptive terminals has been proposed to be a factor in promoting pain sensation, but the study of its action and mechanisms has been indirect and inconsistent 11,12,33,62,67. A migraine-like headache could be induced by administration of CGRP 31, and alleviated by a CGRP antagonist 11. In a rat model of meningeal nociception, the endogenous release of CGRP in the trigeminal nucleus was suggested to play an important role in the maintenance of neuronal activity, and blockade of CGRP receptors significantly attenuated the neuronal activity 12. However, no evidence is available that CGRP sensitizes primary afferent nociceptive neurons and their axons directly, even though in vitro studies show that activation of TRPV1 receptors produces a Ca2+-dependent CGRP release 24,52. Currently, the most prominent hypothesis suggests that, as a result of its vasodilative effect, CGRP can lead to the liberation of many pro-inflammatory agents, such as bradykinin and cytokines, which are potent stimulants of nociceptors 5,6. There is evidence that one mechanism by which afferent nociceptors are sensitized is that these inflammatory agents sensitize TRPV1 receptors 38,61. This seems to be supported by the data obtained from our present study and other groups that TRPV1 and neuropeptides were co-expressed in DRG neurons and their axons 3,18,44,62. Thus, sensitization of primary afferent nociceptors involves a neurogenic mechanism by which inflammatory mediators released by antidromic drive may up-regulate TRPV1 receptors.
To conclude, neurogenic inflammation initiated by activation of TRPV1 receptors following intradermal injection of CAP and mediated by the generation of DRRs makes a major contribution to the sensitization of primary afferent nociceptors by antidromic activation of DRG neurons to drive the release of inflammatory neuropeptides, such as CGRP. In this process, TRPV1 receptors are functionally up-regulated. Based on this pilot study, an extended and in-depth investigation will be conducted to examine if other inflammatory mediators are involved in DRR-mediated inflammatory pain and the interactions among these mediators in causing nociceptor sensitization.
Acknowledgements
We are grateful to Drs. RE Coggeshall and WD Willis for their valuable comments and advice on the preparation of this manuscript and to Dr. GC Ji for his generous technical help in neural activity recordings. This work was supported by National Institute of Neurological Disorders and Stroke grant R01 NS-40723 to Q. Lin, P01 Grant NS011255 and National Institute of Dental Research Grant R03 DE14814 to L. Fang.
References
- 1.Ali Z, Ringkamp M, Hartke TV, Chien HF, Flavahan NA, Campbell JN, Meyer RA. Uninjured C-fiber nociceptors develop spontaneous activity and alpha-adrenergic sensitivity following L6 spinal nerve ligation in monkey. J Neurophysiol. 1999;81:455–466. doi: 10.1152/jn.1999.81.2.455. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez FJ, Cervantes C, Blasco I, Villalba R, Martinez-Murillo R, Polak JM, Rodrigo J. Presence of calcitonin gene-related peptide (CGRP) and substance P (SP) immunoreactivity in intraepidermal free nerve endings of cat skin. Brain Res. 1988;442:391–395. doi: 10.1016/0006-8993(88)91532-6. [DOI] [PubMed] [Google Scholar]
- 3.Aoki Y, Ohtori S, Takahashi K, Ino H, Douya H, Ozawa T, Saito T, Moriya H. Expression and Co-expression of VR1, CGRP, and IB-4-binding glycoprotein in dorsal root ganglion neurons in rats: differences between the disc afferents and the cutaneous afferents. Spine. 2005;30:1496–1500. doi: 10.1097/01.brs.0000167532.96540.31. [DOI] [PubMed] [Google Scholar]
- 4.Bevan S, Szolcsanyi J. Sensory neuron-specific actions of capsaicin: mechanisms and applications. Trends Neurosci. 1990;11:330–333. doi: 10.1016/0165-6147(90)90237-3. [DOI] [PubMed] [Google Scholar]
- 5.Buckley TL, Brain SD, Rampart M, Williams TJ. Time-dependent synergistic interactions between the vasodilator neuropeptide, calcitonin gene-related peptide (CGRP) and mediators of inflammation. Br J Pharmacol. 1991;103:1515–1519. doi: 10.1111/j.1476-5381.1991.tb09819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckley TL, Brain SD, Collins PD, Williams TJ. Inflammatory edema induced by interactions between IL-1 and the neuropeptide calcitonin gene-related peptide. J Immunol. 1991;146:3424–3430. [PubMed] [Google Scholar]
- 7.Bulling DGS, Kelly D, Bond S, McQueen DS, Seckl JR. Adjuvant-induced joint inflammation causes very rapid transcription of β-preprotachykinin and α-CGRP genes in innervating sensory ganglia. J Neurochem. 2001;77:373–382. doi: 10.1046/j.1471-4159.2001.00175.x. [DOI] [PubMed] [Google Scholar]
- 8.Carlton SM, Hargett GL. Stereological analysis of Ca2+/calmodulin-dependent protein kinase IIα-containing dorsal root ganglion neurons in the rat: colocalization with isolectin Griffonia simplicifolia, calcitonin gene-related peptide, or vanilloid receptor 1. J Comp Neurol. 2002;448:102–110. doi: 10.1002/cne.10250. [DOI] [PubMed] [Google Scholar]
- 9.Cervero F, Laird JM. Mechanisms of touch-evoked pain (allodynia): a new model. Pain. 1996;68:13–23. doi: 10.1016/S0304-3959(96)03165-X. [DOI] [PubMed] [Google Scholar]
- 10.Coggeshall RE, Carlton SM. Receptor localization in the mammalian dorsal horn and primary afferent neurons. Brain Res Rev. 1997;24:28–66. doi: 10.1016/s0165-0173(97)00010-6. [DOI] [PubMed] [Google Scholar]
- 11.Durham PL. CGRP-receptor antagonists --- A fresh approach to migraine therapy. N Engl J Med. 2004;350:1073–1075. doi: 10.1056/NEJMp048016. [DOI] [PubMed] [Google Scholar]
- 12.Fischer MJ, Koulchitsky S, Messlinger K. The nonpeptide calcitonin gene-related peptide receptor antagonist BIBN4096BS lowers the activity of neurons with meningeal input in the rat spinal trigeminal nucleus. J Neurosci. 2005;25:5877–5883. doi: 10.1523/JNEUROSCI.0869-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fitzgerald EM, Okuse K, Wood JN, Dolphin AC, Moss SJ. cAMP-dependent phosphorylation of the tetrodotoxin-resistant voltage-dependent sodium channel SNS. J Physiol (Lond) 1999;516:433–446. doi: 10.1111/j.1469-7793.1999.0433v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Nicas E, Laird J, Cervero F. Vasodilatation in hyperalgesic rat skin evoked by stimulation of afferent Aβ-fibers: further evidence for a role of dorsal root reflexes in allodynia. Pain. 2001;94:283–291. doi: 10.1016/S0304-3959(01)00365-7. [DOI] [PubMed] [Google Scholar]
- 15.Gibbins IL, Furness JB, Costa M, MacIntyre I, Hillyard CJ, Girgis S. Co-localization of calcitonin gene-related peptide-like immunoreactivity with substance P in cutaneous, vascular and visceral sensory neurons of guinea pig. Neurosci Lett. 1985;57:125–130. doi: 10.1016/0304-3940(85)90050-3. [DOI] [PubMed] [Google Scholar]
- 16.Gold MS, Levine JD, Correa AM. Modulation of TTX-R INa by PKC and PKA and their role in PGE2-induced sensitization of rat sensory neurons in vitro. J Neurosci. 1998;18:10345–10355. doi: 10.1523/JNEUROSCI.18-24-10345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guenther S, Reeh PW, Kress M. Rises in [Ca2+]i mediate capsaicin- and proton-induced heat sensitization of rat primary nociceptor neurons. Eur J Neurosci. 1999;11:3143–3150. doi: 10.1046/j.1460-9568.1999.00734.x. [DOI] [PubMed] [Google Scholar]
- 18.Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 19.Habler HJ, Timmermann L, Stegmann JU, Jänig W. Involvement of neurokinins in antidromic vasodilatation in hairy and hairless skin of the rat hindlimb. Neuroscience. 1999;89:1259–1268. doi: 10.1016/s0306-4522(98)00322-4. [DOI] [PubMed] [Google Scholar]
- 20.Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- 21.Jancso N, Jancso-Gabor A, Szolcsanyi J. Direct evidence for neurogenic inflammation and its prevention by denervation and pretreatment with capsaicin. Br J Pharmacol. 1967;31:138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jänig W, Lisney SJW. Small diameter mylinated afferents produce vasodilatation but not plasma extravasation in rat skin. J Physiol (Lond) 1989;415:477–486. doi: 10.1113/jphysiol.1989.sp017732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung J, Shin JS, Lee SY, Hwang SW, Koo J, Cho H, Oh U. Phosphorylation of vanilloid receptor 1 by Ca+2/calmodulin-dependent kinase II regulates its vanilloid binding. J Biol Chem. 2004;279:7048–7054. doi: 10.1074/jbc.M311448200. [DOI] [PubMed] [Google Scholar]
- 24.Kessler F, Habelt B, Averbeck B, Reeh PW, Kress M. Heat-induced release of CGRP from isolated rat skin and effects of bradykinin and the protein kinase C activator PMA. Pain. 1999;83:289–295. doi: 10.1016/s0304-3959(99)00108-6. [DOI] [PubMed] [Google Scholar]
- 25.Kilo S, Harding-Rose C, Hargreaves KM, Flores CM. Peripheral CGRP release as a marker for neurogenic inflammation: a model system for the study of neuropeptide secretion in rat paw skin. Pain. 1997;73:201–207. doi: 10.1016/S0304-3959(97)00108-5. [DOI] [PubMed] [Google Scholar]
- 26.Kress M, Guenther S. Role of [Ca2+]i in the ATP-induced heat sensitization process of rat nociceptive neurons. J Neurophysiol. 1999;81:2612–2619. doi: 10.1152/jn.1999.81.6.2612. [DOI] [PubMed] [Google Scholar]
- 27.Kress M, Guthmann C, Averbeck B, Reeh PW. Calcitonin gene-related peptide and prostaglandin E2 but not substance P release induced by antidromic nerve stimulation from rat skin in vitro. Neuroscience. 1999;89:303–310. doi: 10.1016/s0306-4522(98)00280-2. [DOI] [PubMed] [Google Scholar]
- 28.Kruger L, Silverman JD, Mantyh PW, Sternini C, Brecha NC. Peripheral patterns of calcitonin-gene-related peptide general somatic sensory innervation: cutaneous and deep terminations. J Comp Neurol. 1989;280:291–302. doi: 10.1002/cne.902800210. [DOI] [PubMed] [Google Scholar]
- 29.LaMotte RH, Lundberg LE, Torebjörk HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol (Lond) 1992;448:749–764. doi: 10.1113/jphysiol.1992.sp019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LaMotte RH, Thalhammer JG, Torebjörk HE, Robinson CJ. Peripheral neural mechanisms of cutaneous hyperalgesia following mild injury by heat. J Neurosci. 1982;2:765–781. doi: 10.1523/JNEUROSCI.02-06-00765.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lassen LH, Haderslev PA, Jacobsen VB, Iversen HK, Sperling B, Olesen J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- 32.Leem JW, Willis WD, Chung JM. Cutaneous sensory receptors in rat foot. J Neurophysiol. 1993;69:1684–1699. doi: 10.1152/jn.1993.69.5.1684. [DOI] [PubMed] [Google Scholar]
- 33.Levy D, Burstein R, Strassman AM. Calcitonin gene-related peptide does not excite or sensitize meningeal nociceptors: implication for the pathophysiology of migraine. Ann Neurol. 2005;58:698–705. doi: 10.1002/ana.20619. [DOI] [PubMed] [Google Scholar]
- 34.Lewin GR, Lisney SJW, Mendell LM. Neonatal anti-NGF treatment reduces the A delta and C fiber evoked vasodilator responses in rat skin: evidence that nociceptor afferents mediate antidromic dilatation. Eur J Neurosci. 1992;4:1213–1218. doi: 10.1111/j.1460-9568.1992.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 35.Lin Q, Li D, Xu X, Zou X, Fang L. Roles of TRPV1 and neuropeptidergic receptors in dorsal root reflex-mediated neurogenic inflammation induced by intradermal injection of capsaicin. Mol Pain. 2007;3:30. doi: 10.1186/1744-8069-3-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin Q, Wu J, Willis WD. Dorsal root reflexes and cutaneous neurogenic inflammation following intradermal injection of capsaicin in rats. J Neurophysiol. 1999;82:2602–2611. doi: 10.1152/jn.1999.82.5.2602. [DOI] [PubMed] [Google Scholar]
- 37.Lin Q, Zou X, Willis WD. Aδand C primary afferents convey dorsal root reflexes after intradermal injection of capsaicin in rats. J Neurophysiol. 2000;84:2695–2698. doi: 10.1152/jn.2000.84.5.2695. [DOI] [PubMed] [Google Scholar]
- 38.Linhart O, Obreja O, Kress M. The inflammatory mediators serotonin, prostaglandin E2 and bradykinin evoke calcium influx in rat sensory neurons. Neuroscience. 2003;118:69–74. doi: 10.1016/s0306-4522(02)00960-0. [DOI] [PubMed] [Google Scholar]
- 39.Lopshire JC, Nicol GD. The cAMP transduction cascade mediates the prostaglandin E2 enhancement of the capsaicin-elicited current in rat sensory neurons: Whole cell and single-channel studies. J Neurosci. 1998;18:6081–6092. doi: 10.1523/JNEUROSCI.18-16-06081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lynn B. Capsaicin: actions on nociceptive C-fibres and therapeutic potential. Pain. 1990;41:61–69. doi: 10.1016/0304-3959(90)91110-5. [DOI] [PubMed] [Google Scholar]
- 41.Ma QP. Vanilloid receptor homologue, VR1, is expressed by both A- and C-fiber sensory neurons. NeuroReport. 2001;12:3693–3695. doi: 10.1097/00001756-200112040-00018. [DOI] [PubMed] [Google Scholar]
- 42.Meyer RA, Campbell JN. Myelinated nociceptive afferents account for the hyperalgesia that follows a burn to the hand. Science. 1981;213:1527–1529. doi: 10.1126/science.7280675. [DOI] [PubMed] [Google Scholar]
- 43.Price J. An immunohistochemical and quantitative examination of dorsal root ganglion neuronal subpopulations. J Neurosci. 1985;5:2051–2059. doi: 10.1523/JNEUROSCI.05-08-02051.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Price T, Flores CM. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J Pain. 2007;8:263–272. doi: 10.1016/j.jpain.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Puehler W, Zollner C, Brack A, Shaqura MA, Krause H, Schafer M, Stein C. Rapid upregulation of μ opioid receptor mRNA in dorsal root ganglia in response to peripheral inflammation depends on neuronal conduction. Neuroscience. 2004;129:473–479. doi: 10.1016/j.neuroscience.2004.06.086. [DOI] [PubMed] [Google Scholar]
- 46.Rees H, Sluka KA, Westlund KN, Willis WD. Do dorsal root reflexes augment peripheral inflammation? NeuroReport. 1994;5:821–824. doi: 10.1097/00001756-199403000-00021. [DOI] [PubMed] [Google Scholar]
- 47.Rees H, Sluka KA, Westlund KN, Willis WD. The role of glutamate and GABA receptors in the generation of dorsal root reflexes by acute arthritis in the anaesthetised rat. J Physiol (Lond.) 1995;484:437–445. doi: 10.1113/jphysiol.1995.sp020676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ren Y, Lin Q, Zou X, Fang L, Willis WD. Dorsal root reflexes mediate the sensitization of Aδ and C primary afferent nociceptors induced by intradermal injection of capsaicin, Program No. 407.11, 2004. Soc for Neurosci, San Diego. 2004 Abstract. [Google Scholar]
- 49.Ren Y, Zou X, Fang L, Lin Q. Sympathetic modulation of activity in Aδ and C-primary nociceptive afferents after intradermal injection of capsaicin in rats. J Neurophysiol. 2005;93:365–377. doi: 10.1152/jn.00804.2004. [DOI] [PubMed] [Google Scholar]
- 50.Ren Y, Zou X, Fang L, Lin Q. Involvement of peripheral purinoceptors in sympathetic modulation of capsaicin-Induced sensitization of primary afferent fibers. J Neurophysiol. 2006;96:2207–2216. doi: 10.1152/jn.00502.2006. [DOI] [PubMed] [Google Scholar]
- 51.Richardson JD, Vasko MR. Cellular mechanisms of neurogenic inflammation. J Pharmacol Exp Ther. 2002;302:839–845. doi: 10.1124/jpet.102.032797. [DOI] [PubMed] [Google Scholar]
- 52.Sauer SK, Reeh PW, Bove GM. Noxious heat-induced CGRP release from rat sciatic nerve axons in vitro. Eur J Neurosci. 2001;14:1203–1208. doi: 10.1046/j.0953-816x.2001.01741.x. [DOI] [PubMed] [Google Scholar]
- 53.Shim B, Kim DW, Kim BH, Nam TS, Leem JW, Chung JM. Mechanical and heat sensitization of cutaneous nociceptors in rats with experimental peripheral neuropathy. Neuroscience. 2005;132:193–201. doi: 10.1016/j.neuroscience.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 54.Sluka KA, Willis WD, Westlund KN. Joint inflammation and hyperalgesia are reduced by spinal bicuculline. NeuroReport. 1993;5:109–112. doi: 10.1097/00001756-199311180-00003. [DOI] [PubMed] [Google Scholar]
- 55.Szolcsanyi J. Selective responsiveness of polymodal nociceptors of the rabbit ear to capsaicin, bradykinin and ultra-violet irradiation. J Physiol. 1987;388:9–23. doi: 10.1113/jphysiol.1987.sp016598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szolcsanyi J. Antidromic vasodilatation and neurogenic inflammation. Agents Actions. 1988;23:4–11. doi: 10.1007/BF01967170. [DOI] [PubMed] [Google Scholar]
- 57.Szolcsanyi J. Actions of capsaicin on sensory receptors. In: Wood JN, editor. Capsaicin in the Study of Pain. Academic Press; London: 1993. pp. 1–26. [Google Scholar]
- 58.Szolcsanyi J. Neurogenic inflammation: reevaluation of axon reflex theory. In: Geppetti P, Holzer P, editors. Neurogenic inflammation. CRC Press; New York: 1996. pp. 33–42. [Google Scholar]
- 59.Tohda C, Sasaki M, Konemura T, Sasamura T, Itoh M, Kuraishi Y. Axonal transport of VR1 capsaicin receptor mRNA in primary afferents and its participation in inflammation-induced increase in capsaicin sensitivity. J Neurochem. 2001;76:1628–1635. doi: 10.1046/j.1471-4159.2001.00193.x. [DOI] [PubMed] [Google Scholar]
- 60.Valencia De Ita S, Lawand NB, Lin Q, Castaneda-Hernandez G, Willis WD. The role of the Na+-K+-2Cl- cotransporter in the development of capsaicin-induced neurogenic inflammation. J Neurophysiol. 2006;95:3553–3561. doi: 10.1152/jn.01091.2005. [DOI] [PubMed] [Google Scholar]
- 61.Vyklický L, Knotková-Urbancová H, Vitásková Z, Vlachová V, Kress M, Reeh PW. Inflammatory mediators at acidic pH activate capsaicin receptors in cultured sensory neurons from newborn rats. J Neurophysiol. 1998;79:670–676. doi: 10.1152/jn.1998.79.2.670. [DOI] [PubMed] [Google Scholar]
- 62.Wick EC, Hoge SG, Grahn SW, Kim GE, Divino LA, Grady EF, Bunnett NW, Kirkwood KS. Transient receptor potential vanilloid 1, calcitonin gene-related peptide, and substance P mediate nociception in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G959–G969. doi: 10.1152/ajpgi.00154.2005. [DOI] [PubMed] [Google Scholar]
- 63.Willis WD. Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res. 1999;124:395–421. doi: 10.1007/s002210050637. [DOI] [PubMed] [Google Scholar]
- 64.Willis WD, Coggeshall RE. Sensory mechanisms of the spinal cord. Plenum; New York: 2004. pp. 63–64. [Google Scholar]
- 65.Willis WD, Sluka KA, Rees H, Westlund KN. A contribution of dorsal root reflexes to peripheral inflammation. In: Rudomin P, Romo R, Mendell LM, editors. Presynaptic Inhibition and Neural Control. Oxford University Press; New York: 1998. pp. 407–423. [Google Scholar]
- 66.Zhang Y, Malmberg AB, Yaksh TL, Sjolund B, Sundler F, Hakanson R. Capsaicin-evoked release of pituitary adenylate cyclase activating peptide (PACAP) and calcitonin gene-related peptide (CGRP) from rat spinal cord in vivo. Regul Pept. 1997;69:83–87. doi: 10.1016/s0167-0115(97)02133-2. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Z, Winborn CS, Marquez de Prado B, Russo AF. Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J Neurosci. 2007;27:2693–2703. doi: 10.1523/JNEUROSCI.4542-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zou X, Lin Q. The phosphorylation of PKC and CaMKII contributes to neurogenic inflammation by triggering dorsal root reflex flowing intradermal injection of capsaicin. J Pain (Suppl) 2006;7:S16. [Google Scholar]
- 69.Zou X, Lin Q, Willis WD. NMDA and non-NMDA receptor antagonists attenuate increased Fos expression in spinal dorsal horn GABAergic neurons after intradermal injection of capsaicin in rats. Neuroscience. 2001;106:171–182. doi: 10.1016/s0306-4522(01)00175-0. [DOI] [PubMed] [Google Scholar]


